Assessment of the Anti-Biofilm Effect of Cefiderocol Against 28 Clinical Strains of Multidrug-Resistant Gram-Negative Bacilli
Abstract
1. Introduction
2. Results
2.1. MIC and MBIC of FDC
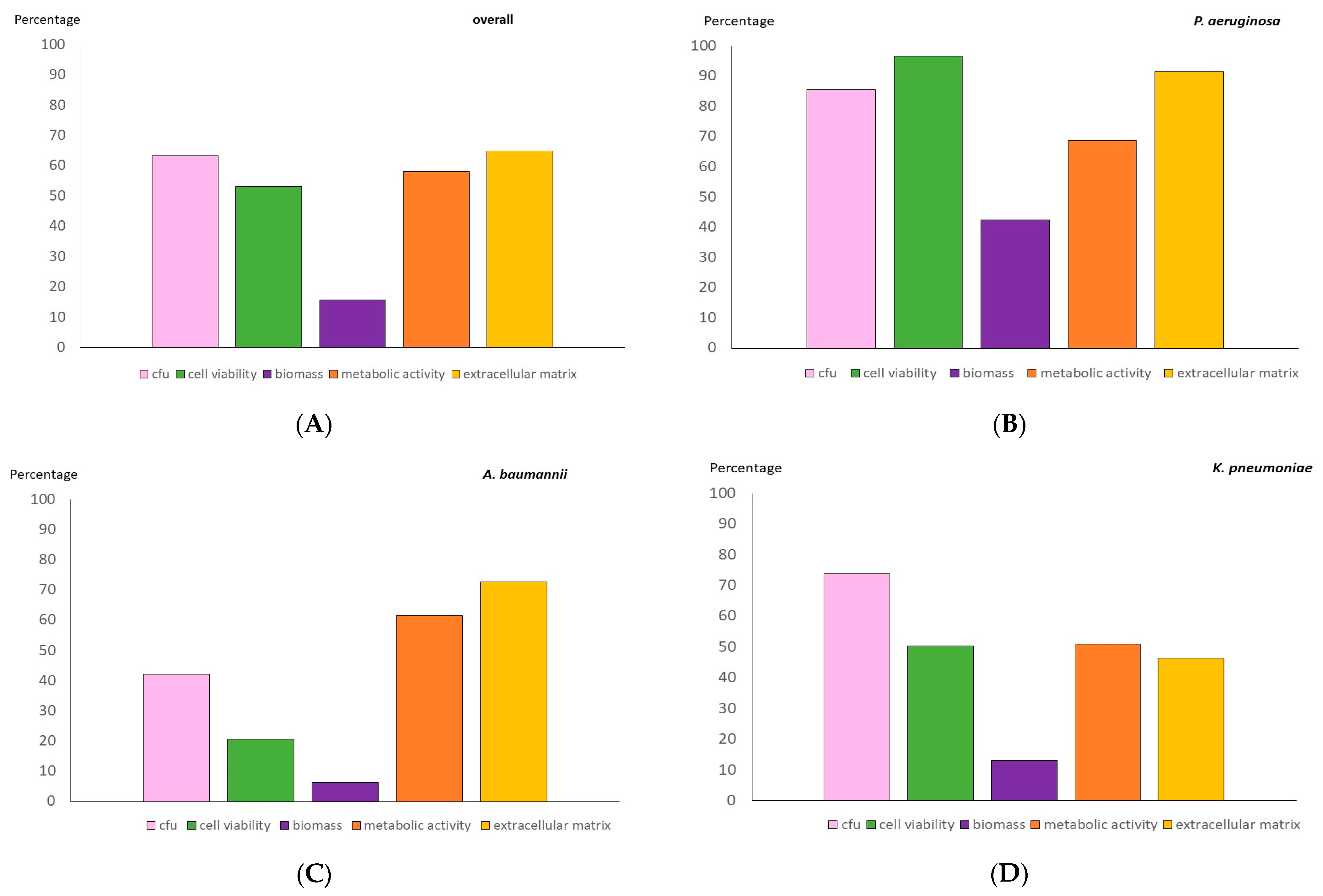
2.2. Anti-Biofilm Testing of an 8 mg/mL FDC Concentration
3. Discussion
4. Materials and Methods
4.1. Biofilm Procedure
4.2. CFU Counts and Cell Viability
4.3. Biomass and Metabolic Activity
4.4. CLSM Analysis of Extracellular Matrix
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Domenico, E.G.; Oliva, A.; Guembe, M. The Current Knowledge on the Pathogenesis of Tissue and Medical Device-Related Biofilm Infections. Microorganisms 2022, 10, 1259. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Moser, C.; Jensen, P.; Kolpen, M.; Qvist, T.; Aanaes, K.; Pressler, T.; Skov, M.; Ciofu, O. Diagnosis of biofilm infections in cystic fibrosis patients. Pathogens 2017, 125, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Manos, J. The human microbiome in disease and pathology. APMIS Acta Pathol. Microbiol. Immunol. Scand. 2022, 130, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Tam, R.Y.; van Dorst, J.M.; McKay, I.; Coffey, M. Intestinal Inflammation and Alterations in the Gut Microbiota in Cystic Fibrosis: A Review of the Current Evidence, Pathophysiology and Future Directions. J. Clin. Med. 2022, 11, 649. [Google Scholar] [CrossRef] [PubMed]
- Domingue, J.C.; Drewes, J.L.; Merlo, C.A.; Housseau, F.; Sears, C.L. Host responses to mucosal biofilms in the lung and gut. Mucosal Immunol. 2020, 13, 413–422. [Google Scholar] [CrossRef] [PubMed]
- Firoz, A.; Haris, M.; Hussain, K.; Raza, M.; Verma, D.; Bouchama, M.; Namiq, K.S.; Khan, S. Can Targeting Iron Help in Combating Chronic Pseudomonas Infection? A Systematic Review. Cureus 2021, 13, e13716. [Google Scholar] [CrossRef] [PubMed]
- Beauruelle, C.; Lamoureux, C.; Mashi, A.; Ramel, S.; Le Bihan, J.; Ropars, T.; Dirou, A.; Banerjee, A.; Tandé, D.; Le Bars, H.; et al. In Vitro Activity of 22 Antibiotics against Achromobacter Isolates from People with Cystic Fibrosis. Are There New Therapeutic Options? Microorganisms 2021, 9, 2473. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Pisi, G.; Fainardi, V.; Principi, N. What is the role of Achromobacter species in patients with cystic fibrosis? Front. Biosci. 2021, 26, 1613–1620. [Google Scholar] [CrossRef] [PubMed]
- Cullen, L.; Weiser, R.; Olszak, T.; Maldonado, R.F.; Moreira, A.S.; Slachmuylders, L.; Brackman, G.; Paunova-Krasteva, T.S.; Zarnowiec, P.; Czerwonka, G.; et al. Phenotypic characterization of an international Pseudomonas aeruginosa reference panel: Strains of cystic fibrosis (CF) origin show less in vivo virulence than non-CF strains. Appl. Environ. Microbiol. 2015, 161, 1961–1977. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.M.; Nørskov-Lauritsen, N.; Bjarnsholt, T.; Meyer, R.L. Achromobacter Species Isolated from Cystic Fibrosis Patients Reveal Distinctly Different Biofilm Morphotypes. Microorganisms 2016, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; El Tabei, L.; Büsker, S.; Krauss, C.; Fuhr, U.; Taubert, M. Clinical Pharmacokinetics and Pharmacodynamics of Cefiderocol. Clin. Pharmacokinet. 2021, 60, 1495–1508. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.T.; López-Medrano, F. Cefiderocol, a new antibiotic against multidrug-resistant Gram-negative bacteria. Rev. Esp. Quimioter. 2021, 34 (Suppl. 1), 41–43. [Google Scholar] [CrossRef] [PubMed]
- Ong’uti, S.; Czech, M.; Robilotti, E.; Holubar, M. Cefiderocol: A New Cephalosporin Stratagem Against Multidrug-Resistant Gram-Negative Bacteria. Clin. Infect. Dis. 2022, 74, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- McCreary, E.K.; Heil, E.L.; Tamma, P.D. New Perspectives on Antimicrobial Agents: Cefiderocol. Molecules 2021, 65, e0217120. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Xie, L.; Ma, Y.; An, R.; Gu, B.; Wang, C. Proteomic and Transcriptomic Analyses Indicate Reduced Biofilm-Forming Abilities in Cefiderocol-Resistant Klebsiella pneumoniae. Front. Microbiol. 2021, 12, 778190. [Google Scholar] [CrossRef] [PubMed]
- Pybus, C.A.; Felder-Scott, C.; Obuekwe, V.; Greenberg, D.E. Cefiderocol Retains Antibiofilm Activity in Multidrug-Resistant Gram-Negative Pathogens. Antimicrob. Agents Chemother. 2021, 65, e01194-20. [Google Scholar] [CrossRef] [PubMed]
- Plaisance, C.J.; Borne, G.E.; Daniel, C.P.; Wagner, M.J.; Shelvan, A.; Mathew, J.; Ahmadzadeh, S.; Paladini, A.; Varrassi, G.; Shekoohi, S.; et al. Cefiderocol (Fetroja) as a Treatment for Hospital-Acquired Pneumonia. Cureus 2024, 16, e52230. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhu, J.; Chen, L.; Du, H. Cefiderocol: Clinical application and emergence of resistance. Drug Resist. Updates 2024, 72, 101034. [Google Scholar] [CrossRef] [PubMed]
- Tascini, C.; Antonelli, A. Infective Endocarditis Associated with Implantable Cardiac Device by Metallo-β-Lactamase-Producing Pseudomonas aeruginosa, Successfully Treated with Source Control and Cefiderocol Plus Imipenem. Antimicrob. Agents Chemother. 2023, 67, e0131322. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, C.; Poma, N.V.; Bernardo, M.; Rindi, L.; Cesta, N.; Tavanti, A.; Tascini, C.; Di Luca, M. Evaluation of antibiofilm activity of cefiderocol alone and in combination with imipenem against Pseudomonas aeruginosa. J. Glob. Antimicrob. Resist. 2024, 37, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Barbier, F.; Hraiech, S.; Kernéis, S.; Veluppillai, N.; Pajot, O.; Poissy, J.; Roux, D.; Zahar, J.R. Rationale and evidence for the use of new beta-lactam/beta-lactamase inhibitor combinations and cefiderocol in critically ill patients. Ann. Intensive Care 2023, 13, 65. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, I.; Oliva, A.; Pages, R.; Sivori, F.; Truglio, M.; Fabrizio, G.; Pasqua, M.; Pimpinelli, F.; Di Domenico, E.G. Acinetobacter baumannii in the critically ill: Complex infections get complicated. Front. Microbiol. 2023, 14, 1196774. [Google Scholar] [CrossRef] [PubMed]
- Karakonstantis, S.; Rousaki, M.; Vassilopoulou, L.; Kritsotakis, E.I. Global prevalence of cefiderocol non-susceptibility in Enterobacterales, Pseudomonas aeruginosa, Acinetobacter baumannii, and Stenotrophomonas maltophilia: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2024, 30, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Alonso, B.; Pérez-Granda, M.J.; Latorre, M.C.; Rodríguez, C.; Sánchez-Carrillo, C.; Muñoz, P.; Guembe, M. Is heparinized 40% ethanol lock solution efficient for reducing bacterial and fungal biofilms in an in vitro model? PLoS ONE 2019, 14, e0219098. [Google Scholar] [CrossRef]
- Li, L.; Mendis, N.; Trigui, H.; Oliver, J.D.; Faucher, S.P. The importance of the viable but non-culturable state in human bacterial pathogens. Front. Microbiol. 2014, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Jose, A.; García Rodríguez, R.C.; Sánchez, J.E.; Gomez-Lus, Mª.L.; Martínez, L.M.; Rodríguez-Avial, C.; Vila, J. Special Methods for Antimicrobial Susceptibility Testing; SEIMC: Madrid, Spain, 2000. [Google Scholar]
- Del Pozo, J.L.; Guinea, J.; Macià, M.D. Diagnóstico microbiológico de las infecciones relacionadas con la formación de biopelículas. 60. Macià Romero, MD (coordinadora). In Procedimientos en Microbiología Clínica; Cercenado Mansilla, E., Cantón Moreno, R., Eds.; Sociedad Española de Enfermedades Infecciosas y Microbiología Clínica (SEIMC): Madrid, Spain, 2017. [Google Scholar]
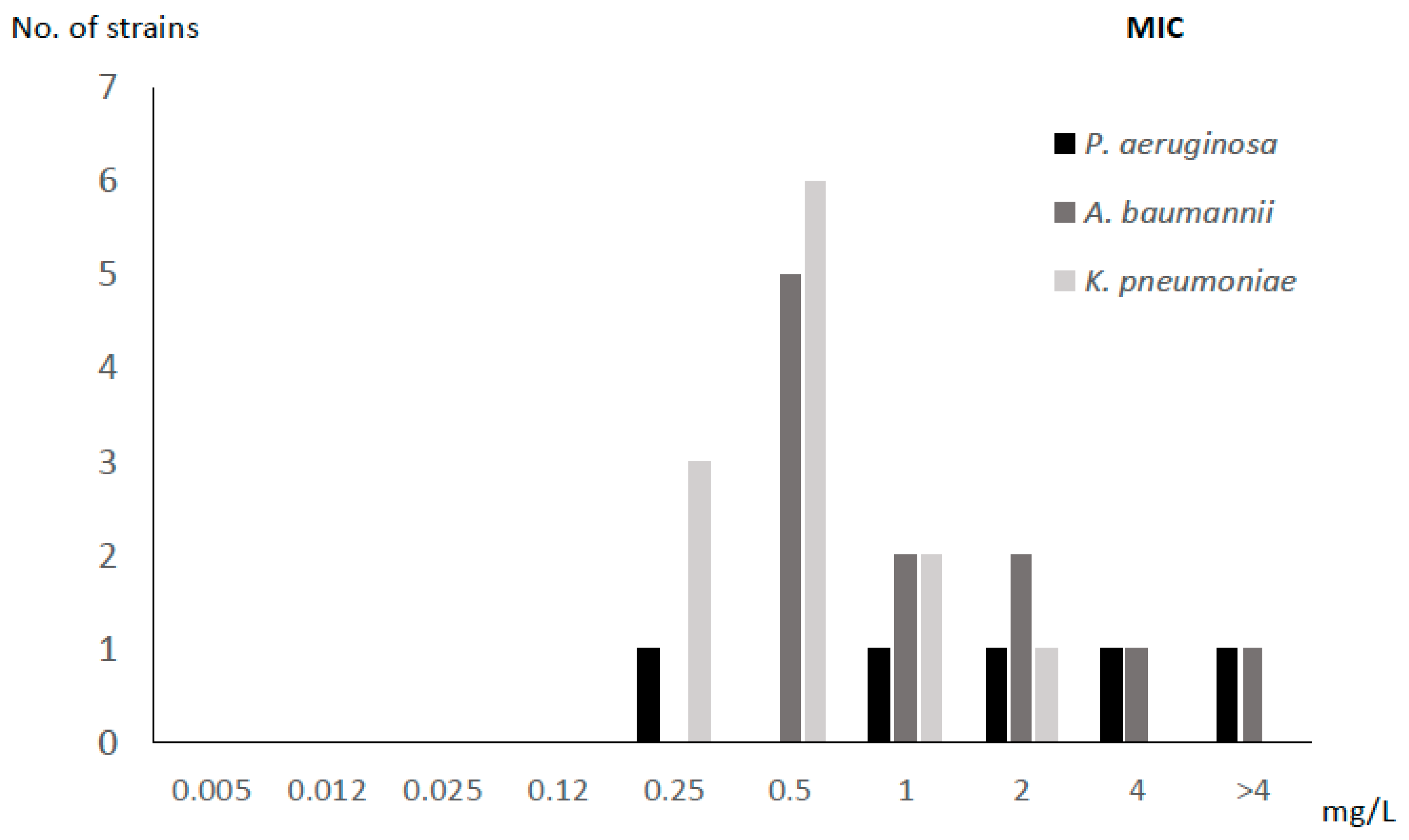
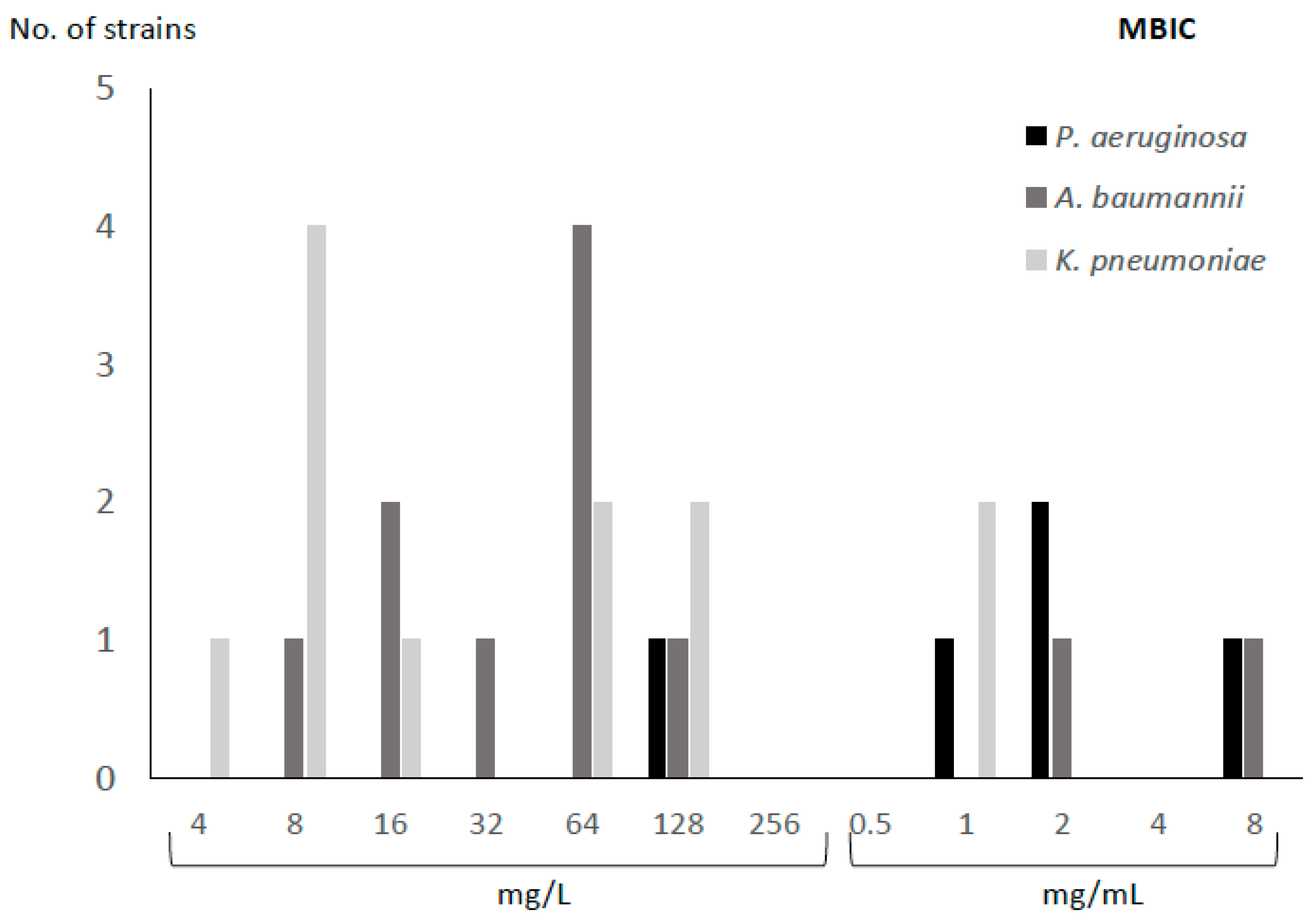

| Species | Code | Resistance Profile | Origin | MIC (mg/L) | MBIC (mg/L) |
|---|---|---|---|---|---|
| Pseudomonas aeruginosa | PA1 | MDR | Blood | 2 | 2000 |
| PA2 | MDR | Blood | 4 | 2000 | |
| PA3 | MDR | Abscess | 0.25 | 128 | |
| PA4 | VIM | Blood | >4 | 8000 | |
| PA5 | MDR | Abscess | 1 | 1000 | |
| Acinetobacter baumannii | AB1 | MDR | Abscess | 0.5 | 64 |
| AB2 | MDR | BAL | 1 | 8 | |
| AB3 | MDR | PE | 2 | 64 | |
| AB4 | MDR | RE | 0.5 | 32 | |
| AB5 | MDR | BA | 2 | 16 | |
| AB6 | MDR | BA | 0.5 | 16 | |
| AB7 | MDR | RE | 0.5 | 64 | |
| AB8 | MDR | RE | 0.5 | 64 | |
| AB9 | MDR | RE | >4 | 2000 | |
| AB10 | MDR | BA | 1 | 128 | |
| AB11 | MDR | Blood | 4 | 8000 | |
| Klebsiella pneumoniae | KP1 | VIM | Blood | 1 | 128 |
| KP2 | KPC | Blood | 2 | 16 | |
| KP3 | KPC | Blood | 1 | 64 | |
| KP4 | KPC | Blood | 0.5 | 64 | |
| KP5 | KPC | Blood | 0.25 | 128 | |
| KP6 | KPC | Blood | 0.5 | 8 | |
| KP7 | KPC | Blood | 0.5 | 8 | |
| KP8 | KPC | Blood | 0.5 | 8 | |
| KP9 | VIM | Blood | 0.25 | 8 | |
| KP10 | KPC | Blood | 0.25 | 4 | |
| KP11 | KPC | Blood | 0.5 | 1000 | |
| KP12 | KPC | Blood | 0.5 | 1000 |
| Strains | Median (IQR) Percentage Reduction | ||||
|---|---|---|---|---|---|
| Log CFU/mL | Cell Viability | Biomass | Metabolic Activity | Extracellular Matrix | |
| Overall | 67.8 (47.7–80.2) | 73.1 (12.4–86.5) | 0.0 (0.0–28.9) | 59.3 (28.1–97.4) | 79.5 (37.3–95.5) |
| Pseudomonas aeruginosa | 86.7 (73.8–96.7) | 93.2 (86.5–98.0) | 47.0 (0.0–82.4) | 61.8 (45.6–95.3) | 94.7 (85.3–95.9) |
| Acinetobacter baumannii | 43.0 (20.7–53.2) | 6.1 (0.8–5.0) | 0.0 (0.0–11.9) | 48.1 (29.8–98.6) | 81.5 (55.0–98.0) |
| Klebsiella pneumoniae | 75.6 (63.1–80.2) | 60.3 (14.3–81.5) | 0.0 (0.0–39.4) | 56.5 (0.0–95.3) | 44.8 (0.2–91.2) |
| Groups | p-Value * | ||||
|---|---|---|---|---|---|
| Log CFU/mL | Cell Viability | Biomass | Metabolic Activity | Extracellular Matrix | |
| PA vs. AB | 0.009 | 0.029 | 0.079 | 0.593 | 0.282 |
| PA vs. KP | 0.082 | 0.008 | 0.190 | 0.426 | 0.05 |
| AB vs. KP | 0.002 | 0.054 | 0.500 | 0.412 | 0.148 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Navarro, M.; Cercenado, E.; Visedo, A.; Marín, M.; Machado, M.; Irigoyen-von-Sierakowski, Á.; Loeches, B.; Cacho-Calvo, J.; García-Rodríguez, J.; Di Domenico, E.G.; et al. Assessment of the Anti-Biofilm Effect of Cefiderocol Against 28 Clinical Strains of Multidrug-Resistant Gram-Negative Bacilli. Antibiotics 2025, 14, 738. https://doi.org/10.3390/antibiotics14080738
Díaz-Navarro M, Cercenado E, Visedo A, Marín M, Machado M, Irigoyen-von-Sierakowski Á, Loeches B, Cacho-Calvo J, García-Rodríguez J, Di Domenico EG, et al. Assessment of the Anti-Biofilm Effect of Cefiderocol Against 28 Clinical Strains of Multidrug-Resistant Gram-Negative Bacilli. Antibiotics. 2025; 14(8):738. https://doi.org/10.3390/antibiotics14080738
Chicago/Turabian StyleDíaz-Navarro, Marta, Emilia Cercenado, Andrés Visedo, Mercedes Marín, Marina Machado, Álvaro Irigoyen-von-Sierakowski, Belén Loeches, Juana Cacho-Calvo, Julio García-Rodríguez, Enea G. Di Domenico, and et al. 2025. "Assessment of the Anti-Biofilm Effect of Cefiderocol Against 28 Clinical Strains of Multidrug-Resistant Gram-Negative Bacilli" Antibiotics 14, no. 8: 738. https://doi.org/10.3390/antibiotics14080738
APA StyleDíaz-Navarro, M., Cercenado, E., Visedo, A., Marín, M., Machado, M., Irigoyen-von-Sierakowski, Á., Loeches, B., Cacho-Calvo, J., García-Rodríguez, J., Di Domenico, E. G., Muñoz, P., & Guembe, M. (2025). Assessment of the Anti-Biofilm Effect of Cefiderocol Against 28 Clinical Strains of Multidrug-Resistant Gram-Negative Bacilli. Antibiotics, 14(8), 738. https://doi.org/10.3390/antibiotics14080738








