Pharmacokinetics/Pharmacodynamics-Based Repositioning of Cefmetazole and Flomoxef in Extended-Spectrum β-Lactamase-Producing Enterobacterales Treatment: An Injectable Carbapenem-Sparing and Outpatient Strategy
Abstract
1. Introduction
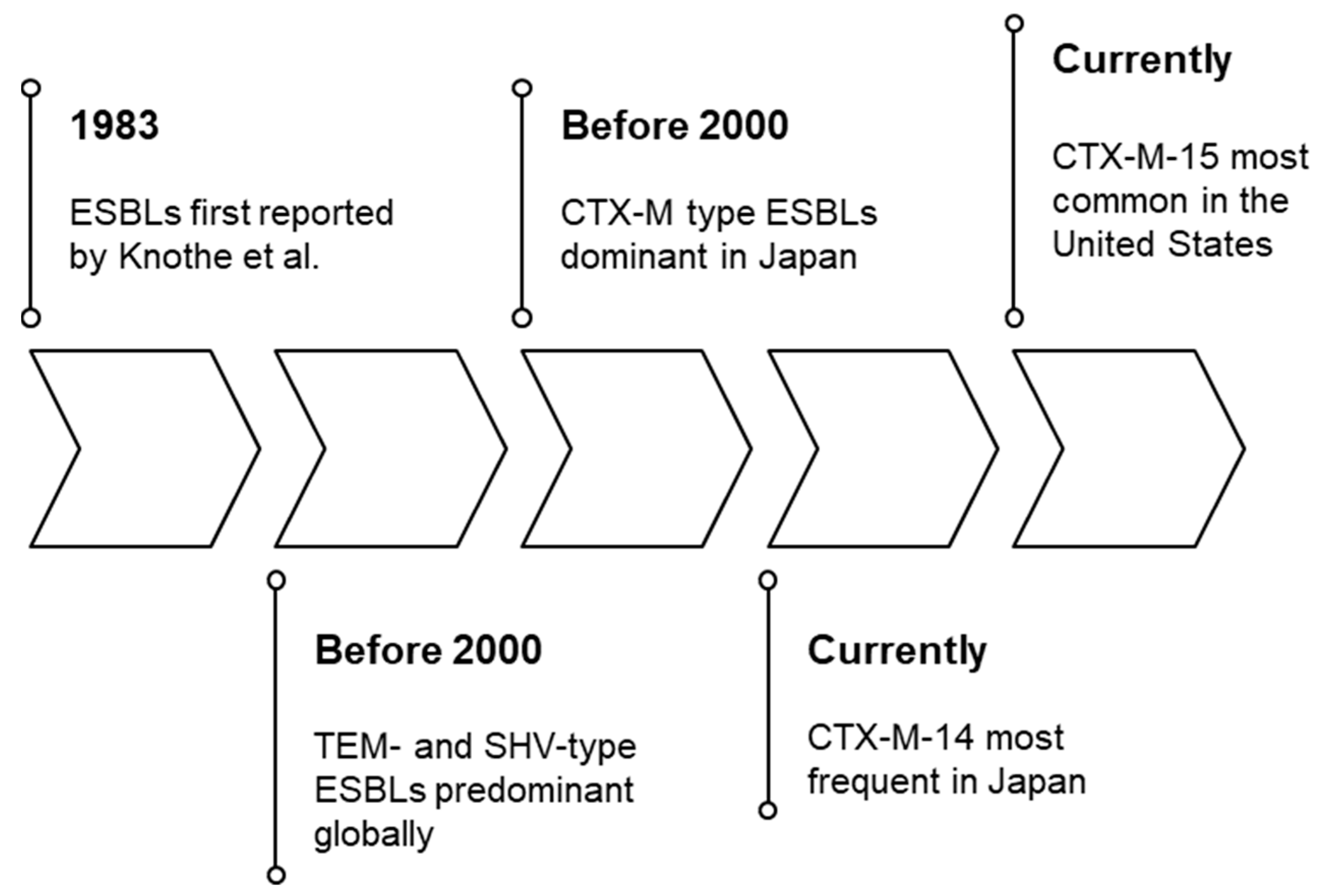
2. Extended-Spectrum β-Lactamases
3. Cefmetazole and Flomoxef: Profiles and Properties
3.1. Cefmetazole (CMZ)
3.2. Flomoxef (FMOX)
3.3. In Vitro Activity and Pharmacodynamic Considerations for ESBL-E
3.4. Pharmacokinetics/Pharmacodynamics (Pks/Pds) of Cefmetazole
3.5. Pharmacokinetics/Pharmacodynamics (Pks/Pds) of Flomoxef
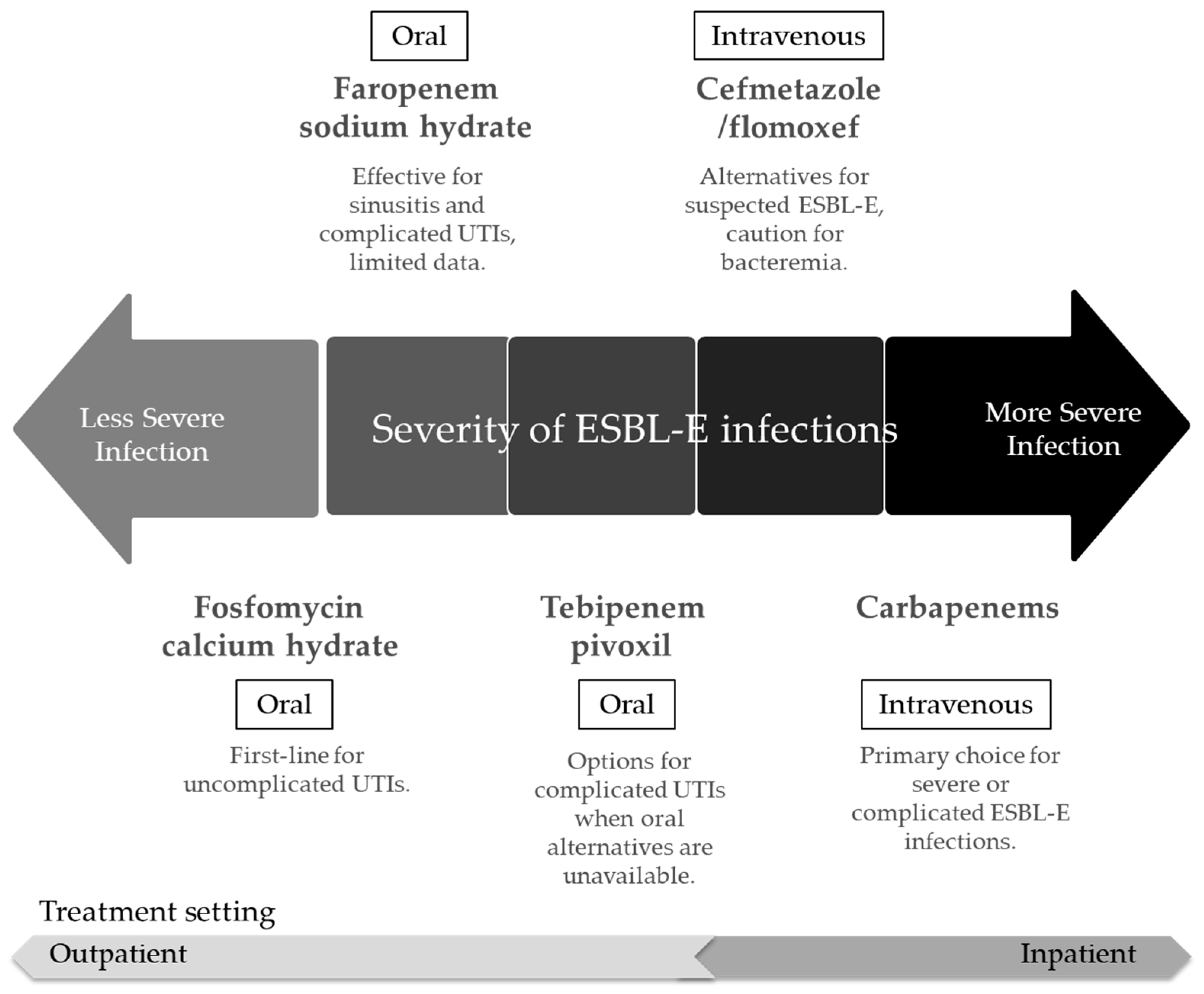
4. Clinical Evidence and Comparison with Other Therapeutic Options
4.1. Cefmetazole and Flomoxef vs. Carbapenems
4.2. Other Therapeutic Options
5. Challenge of Resistance and Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ESBL-Es | extended-spectrum β-lactamase-producing Enterobacterales |
| MIC | minimum inhibitory concentration |
| TMIC | time above minimum inhibitory concentration |
| UTIs | urinary tract infections |
| PTA | probability of target attainment |
| CMZ | cefmetazole |
| FMOX | flomoxef |
| PKs/PDs | pharmacokinetics/pharmacodynamics |
| AUC | area under the drug concentration–time curve |
References
- Cosgrove, S.E. The relationship between antimicrobial resistance and patient outcomes: Mortality, length of hospital stay, and health care costs. Clin. Infect. Dis. 2006, 42, S82–S89. [Google Scholar] [CrossRef] [PubMed]
- Tumbarello, M.; Spanu, T.; Di Bidino, R.; Marchetti, M.; Ruggeri, M.; Trecarichi, E.M.; De Pascale, G.; Proli, E.M.; Cauda, R.; Cicchetti, A.; et al. Costs of bloodstream infections caused by Escherichia coli and influence of extended-spectrum-beta-lactamase production and inadequate initial antibiotic therapy. Antimicrob. Agents Chemother. 2010, 54, 4085–4091. [Google Scholar] [CrossRef] [PubMed]
- Cantón, R.; Novais, A.; Valverde, A.; Machado, E.; Peixe, L.; Baquero, F.; Coque, T.M. Prevalence and spread of extended-spectrum beta-lactamase-producing Enterobacteriaceae in Europe. Clin. Microbiol. Infect. 2008, 14, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Coque, T.M.; Baquero, F.; Canton, R. Increasing prevalence of ESBL-producing Enterobacteriaceae in Europe. Eurosurveillance 2008, 13, 19044. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y. Treatment Options for Carbapenem-resistant Gram-negative Bacterial Infections. Clin. Infect. Dis. 2019, 69, S565–S575. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Heil, E.L.; Justo, J.A.; Mathers, A.J.; Satlin, M.J.; Bonomo, R.A. Infectious Diseases Society of America 2024 Guidance on the Treatment of Antimicrobial-Resistant Gram-Negative Infections. Clin. Infect. Dis. 2024, ciae403. [Google Scholar] [CrossRef] [PubMed]
- Takemura, W.; Tashiro, S.; Hayashi, M.; Igarashi, Y.; Liu, X.; Mizukami, Y.; Kojima, N.; Morita, T.; Enoki, Y.; Taguchi, K.; et al. Cefmetazole as an alternative to carbapenems against extended-spectrum beta-lactamase-producing Escherichia coli infections based on in vitro and in vivo pharmacokinetics/pharmacodynamics experiments. Pharm. Res. 2021, 38, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Knothe, H.; Shah, P.; Krcmery, V.; Antal, M.; Mitsuhashi, S. Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection 1983, 11, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Simner, P.J.; Bradford, P.A. Extended-spectrum β-lactamases: An update on their characteristics, epidemiology and detection. JAC Antimicrob. Resist. 2021, 3, dlab092. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Smith, T.T.; Adebayo, A.; Karaba, S.M.; Jacobs, E.; Wakefield, T.; Nguyen, K.; Whitfield, N.N.; Simner, P.J. Prevalence of blaCTX-M Genes in Gram-Negative Bloodstream Isolates across 66 Hospitals in the United States. J. Clin. Microbiol. 2021, 59, e00127-21. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Kimbrough, J.H.; DeVries, S.; Mendes, R.E.; Sader, H.S. Trends of β-lactamase occurrence among Escherichia coli and Klebsiella pneumoniae in United States hospitals during a 5-year period and activity of antimicrobial agents against isolates stratified by β-lactamase type. Open Forum Infect. Dis. 2023, 10, ofad038. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lievano, A.P.; Cervantes-Flores, F.; Nava-Torres, A.; Carbajal-Morales, P.J.; Villaseñor-Garcia, L.F.; Zavala-Cerna, M.G. Fluoroquinolone resistance in Escherichia coli causing community-acquired urinary tract infections: A systematic review. Microorganisms 2024, 12, 2320. [Google Scholar] [CrossRef] [PubMed]
- Hadi, H.A.; Al-Hail, H.; Aboidris, L.E.; Al-Orphaly, M.; Ahmed, M.A.S.; Samuel, B.G.; Mohamed, H.A.; Sultan, A.A.; Skariah, S. Prevalence and genetic characterization of clinically relevant extended-spectrum β-lactamase-producing Enterobacterales in the Gulf Cooperation Council countries. Front. Antibiot. 2023, 2, 1177954. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Jacoby, G.A. Updated functional classification of beta-lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, M.; Farrell, S.E.; Krause, K.M.; Jones, R.N.; Sader, H.S. Contemporary Diversity of β-lactamases among Enterobacteriaceae in the nine U.S. census regions and ceftazidime-avibactam activity tested against isolates producing the most prevalent β-lactamase groups. Antimicrob. Agents Chemother. 2014, 58, 833–838. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Bradford, P.A. Epidemiology of β-Lactamase-Producing Pathogens. Clin. Microbiol. Rev. 2020, 33, e00047-19. [Google Scholar] [CrossRef] [PubMed]
- Hareza, D.A.; Cosgrove, S.E.; Simner, P.J.; Harris, A.D.; Bergman, Y.; Conzemius, R.; Jacobs, E.; Beisken, S.; Tamma, P.D. Is Carbapenem Therapy Necessary for the Treatment of Non-CTX-M Extended-Spectrum β-Lactamase-Producing Enterobacterales Bloodstream Infections? Clin. Infect. Dis. 2024, 78, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.A. Extended-Spectrum Beta-Lactamases in the 21st Century: Characterization, Epidemiology, and Detection of This Important Resistance Threat. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Yagi, T.; Kurokawa, H.; Senda, K.; Ichiyama, S.; Ito, H.; Ohsuka, S.; Shibayama, K.; Shimokata, K.; Kato, N.; Ohta, M.; et al. Nosocomial Spread of Cephem-resistant Escherichia coli strains Carrying Multiple Toho-1-like Beta-lactamase Genes. Antimicrob. Agents Chemother. 1997, 41, 2606–2611. [Google Scholar] [CrossRef] [PubMed]
- Bauernfeind, A.; Stemplinger, I.; Jungwirth, R.; Ernst, S.; Casellas, J.M. Sequences of Beta-lactamase Genes Encoding CTX-M-1 (MEN-1) and CTX-M-2 and Relationship of their Amino Acid Sequences with those of Other Beta-lactamases. Antimicrob. Agents Chemother. 1996, 40, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Yamashiro, H.; Kasamatsu, Y.; Anan, N.; Takemura, M.; Yamano, Y. In Vitro Efficacy of Humanized Regimen of Flomoxef Against Extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2023, 67, e0025823. [Google Scholar] [CrossRef] [PubMed]
- Takano, C.; Seki, M.; Shiihara, H.; Komine-Aizawa, S.; Kuroda, K.; Takahashi, S.; Ushijima, H.; Hayakawa, S. Frequent Isolation of Extended-spectrum beta-lactamase-producing Bacteria from Fecal Samples of Individuals With Severe Motor and Intellectual Disabilities. J. Infect. Chemother. 2018, 24, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Fukuchi, T.; Iwata, K.; Kobayashi, S.; Nakamura, T.; Ohji, G. Cefmetazole for Bacteremia caused by ESBL-producing Enterobacteriaceae comparing with Carbapenems. BMC Infect. Dis. 2016, 16, 427. [Google Scholar] [CrossRef] [PubMed]
- Sakaeda, K.; Sadahira, T.; Maruyama, Y.; Iwata, T.; Watanabe, M.; Wada, K.; Araki, M. The Genotypic and Phenotypic Characteristics Contributing to Flomoxef Sensitivity in Clinical Isolates of ESBL-Producing E. coli Strains from Urinary Tract Infections. Antibiotics 2023, 12, 522. [Google Scholar] [CrossRef] [PubMed]
- Ohkawa, M.; Orito, M.; Sugata, T.; Shimamura, M.; Sawaki, M.; Nakashita, E.; Kuroda, K.; Sasahara, K. Pharmacokinetics of Cefmetazole in Normal Subjects and in Patients with Impaired Renal Function. Antimicrob. Agents Chemother. 1980, 18, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Halstenson, C.E.; Guay, D.R.; Opsahl, J.A.; Hirata, C.A.; Olanoff, L.S.; Novak, E.; Ko, H.; Cathcart, K.S.; Matzke, G.R. Disposition of Cefmetazole in Healthy Volunteers and Patients with Impaired Renal Function. Antimicrob. Agents Chemother. 1990, 34, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Ito, M.; Ishigami, T. The meaning of the development of flomoxef and clinical experience in Japan. Infection 1991, 19, S253–S257. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Ikawa, K.; Nishikawa, G.; Kobayashi, I.; Tobiume, M.; Sugie, M.; Muramatsu, H.; Morinaga, S.; Kajikawa, K.; Watanabe, M.; et al. Clinical pharmacokinetics of flomoxef in prostate tissue and dosing considerations for prostatitis based on site-specific pharmacodynamic target attainment. J. Infect. Chemother. 2020, 26, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.S.; Salstrom, S.J.; Signs, S.A.; Hoffman, H.E.; File, T.M. Pharmacokinetics of Intravenous Cefmetazole with Emphasis on Comparison Between Predicted Theoretical Levels in Tissue and Actual Skin Window Fluid Levels. Antimicrob. Agents Chemother. 1989, 33, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Namiki, T.; Yokoyama, Y.; Hashi, H.; Oda, R.; Jibiki, A.; Kawazoe, H.; Matsumoto, K.; Suzuki, S.; Nakamura, T. Pharmacokinetics/pharmacodynamics analysis and establishment of optimal dosing regimens using unbound cefmetazole concentration for patients infected with Extended-Spectrum β-lactamase producing Enterobacterales (ESBL-E). Pharmacotherapy 2024, 44, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Yoshida, T.; Oguma TKimura, Y.; Hirauchi, M.; Uchida, K.; Kakushi, H.; Matsubara, T.; Konaka, T. Phase I clinical study on 6315-S, a new oxacephem antibiotic. Chemotherapy 1987, 35, 494–517. [Google Scholar]
- Andrassy, K.; Koderisch, J.; Gorges, K.; Sonntag, H.; Hirauchi, K. Pharmacokinetics and hemostasis following administration of a new, injectable oxacephem (6315-S, flomoxef) in volunteers and in patients with renal insufficiency. Infection 1991, 19, S296–S302. [Google Scholar] [CrossRef] [PubMed]
- Nakao, H.; Yanagisawa, H.; Shimizu, B.; Kaneko, M.; Nagano, M. A new semisynthetic 7alpha-methoxycephalosporin, CS-1170: 7beta-((cyanomethyl)thio)acetamido)-7alpha-methoxy-3-((1-methyl-1H-tetrazol-5-yl)thio)methyl)-3-cephem-4-carboxylic acid. J. Antibiot. 1976, 29, 554–558. [Google Scholar] [CrossRef]
- Tsuji, T.; Satoh, H.; Narisada, M.; Hamashima, Y.; Yoshida, T. Synthesis and antibacterial activity of 6315-S, a new member of the oxacephem antibiotic. J. Antibiot. 1985, 38, 466–476. [Google Scholar] [CrossRef] [PubMed]
- Jacoby, G.A.; Carreras, I. Activities of beta-lactam antibiotics against Escherichia coli strains producing extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 1990, 34, 858–862. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, Y.; Yamamoto, M.; Nagao, M.; Komori, T.; Fujita, N.; Hayashi, A.; Shimizu, T.; Watanabe, H.; Doi, S.; Tanaka, M.; et al. Multicenter retrospective study of cefmetazole and flomoxef for treatment of extended-spectrum-β-lactamase-producing Escherichia coli bacteremia. Antimicrob. Agents Chemother. 2015, 59, 5107–5113. [Google Scholar] [CrossRef] [PubMed]
- Darlow, C.A.; Hope, W. Flomoxef for neonates: Extending options for treatment of neonatal sepsis caused by ESBL-producing Enterobacterales. J. Antimicrob. Chemother. 2022, 77, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Darlow, C.A.; Hope, W. Flomoxef for neonates: Extending options for treatment of neonatal sepsis caused by ESBL-producing Enterobacterales-authors’ response. J. Antimicrob. Chemother. 2022, 77, 2047–2048. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhang, H.; Cheng, J.; Xu, Z.; Xu, Y.; Cao, B.; Kong, H.; Ni, Y.; Yu, Y.; Sun, Z.; et al. In vitro activity of flomoxef and comparators against Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis producing extended-spectrum β-lactamases in China. Int. J. Antimicrob. Agents 2015, 45, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Iio, K.; Hagiya, H.; Higashionna, T.; Otsuka, F. In vitro activity of cefmetazole and flomoxef among extended-spectrum beta-lactamase producing Enterobacterales. New Microbiol. 2024, 46, 348–353. [Google Scholar] [PubMed]
- Delattre, I.K.; Taccone, F.S.; Jacobs, F.; Hites, M.; Dugernier, T.; Spapen, H.; Laterre, P.F.; Wallemacq, P.E.; Van Bambeke, F.; Tulkens, P.M. Optimizing β-lactams treatment in critically-ill patients using pharmacokinetics/pharmacodynamics targets: Are first conventional doses effective? Expert Rev. Anti-Infect. Ther. 2017, 15, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Ohge, H.; Ikawa, K.; Uegami, S.; Watadani, Y.; Shigemoto, N.; Yoshimura, K.; Kitagawa, H.; Kaiki, Y.; Morikawa, N.; et al. Pharmacokinetics of flomoxef in plasma, peritoneal fluid, peritoneum, and subcutaneous adipose tissue of patients undergoing lower gastrointestinal surgery: Dosing considerations based on site-specific pharmacodynamic target attainment. J. Infect. Chemother. 2023, 29, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Matsumura, Y.; Nagashima, M.; Akazawa, T.; Doi, Y.; Hayakawa, K. Retrospective evaluation of appropriate dosing of cefmetazole for invasive urinary tract infection due to extended-spectrum β-lactamase-producing Escherichia coli. J. Infect. Chemother. 2021, 27, 1602–1606. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Tatsumi, Y.M.; Wajima, T.; Nakamura, R.; Tsuji, M. Evaluation of antibacterial activities of flomoxef against ESBL producing Enterobacteriaceae analyzed by Monte Carlo simulation. Jpn. J. Antibiot. 2013, 66, 71–86. [Google Scholar] [PubMed]
- Hamada, Y.; Kasai, H.; Suzuki-Ito, M.; Matsumura, Y.; Doi, Y.; Hayakawa, K. Pharmacokinetic/Pharmacodynamic Analysis and Dose Optimization of Cefmetazole and Flomoxef against Extended-Spectrum β-Lactamase-Producing Enterobacterales in Patients with Invasive Urinary Tract Infection Considering Renal Function. Antibiotics 2022, 11, 456. [Google Scholar] [CrossRef] [PubMed]
- Kaiki, Y.; Ohge, H.; Ikawa, K.; Uegami, S.; Watadani, Y.; Shigemoto, N.; Hirano, T.; Yoshimura, K.; Kitagawa, H.; Morikawa, N.; et al. Pharmacokinetics of cefmetazole in plasma, peritoneal fluid, peritoneum, and subcutaneous adipose tissue of patients scheduled for lower gastrointestinal surgery: Dosing considerations based on site-specific pharmacodynamic target attainment. J. Infect. Chemother. 2023, 29, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, K.; Matsumura, Y.; Uemura, K.; Tsuzuki, S.; Sakurai, A.; Tanizaki, R.; Shinohara, K.; Hashimoto, T.; Hase, R.; Matono, T.; et al. Effectiveness of cefmetazole versus meropenem for invasive urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Antimicrob. Agents Chemother. 2023, 67, e0051023. [Google Scholar] [CrossRef] [PubMed]
- Kashihara, E.; Sada, R.M.; Tsugihashi, Y.; Obayashi, H.; Nakamura, A.; Abe, N.; Miyake, H.; Akebo, H. Efficacy and safety of cefmetazole for bacteremia caused by extended-spectrum β-lactamase-producing enterobacterales vs carbapenems: A retrospective study. Open Forum Infect. Dis. 2023, 10, ofad502. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Su, L.H.; Tang, Y.F.; Liu, J.W. Treatment of ESBL-producing Klebsiella pneumoniae bacteraemia with carbapenems or flomoxef: A retrospective study and laboratory analysis of the isolates. J. Antimicrob. Chemother. 2006, 58, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Su, L.H.; Chen, F.J.; Tang, Y.F.; Li, C.C.; Chien, C.C.; Liu, J.W. Comparative effectiveness of flomoxef versus carbapenems in the treatment of bacteraemia due to extended-spectrum β-lactamase-producing Escherichia coli or Klebsiella pneumoniae with emphasis on minimum inhibitory concentration of flomoxef: A retrospective study. Int. J. Antimicrob. Agents 2015, 46, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Ito, R.; Kawamura, M.; Sato, T.; Fujimura, S. Cefmetazole Resistance Mechanism for Escherichia coli Including ESBL-Producing Strains. Infect. Drug Resist. 2022, 15, 5867–5878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Li, Q.; Zhou, X. The Clinical Efficacy and Safety of Intravenous Cefmetazole in Treatment of 1522 Patients with Aspiration Pneumonia. Zhonghua Nei Ke Za Zhi 2011, 50, 295–298. [Google Scholar] [PubMed]
- Yangco, B.G.; Kenyon, V.S.; Halkias, K.D.; Toney, J.F.; Chmel, H. Comparative Evaluation of Safety and Efficacy of Cefmetazole and Cefoxitin in Lower Respiratory Tract Infections. J. Antimicrob. Chemother. 1989, 23, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Shimada, J.; Hayashi, Y.; Nakamura, K. Cefmetazole: Clinical evaluation of efficacy and safety in Japan. Drugs Exp. Clin. Res. 1985, 11, 181–194. [Google Scholar] [PubMed]
- Moe, J.B.; Piper, R.C.; Tanase, H.; Sotani, K.; Manabe, J. Preclinical safety studies of cefmetazole. J. Antimicrob. Chemother. 1989, 23, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Kodama, T.; Takesue, Y.; Fujimoto, M.; Hiyama, E.; Ichikawa, T. Studies on antibacterial activity of flomoxef and its distribution to serum and intraperitoneal exudate in surgery. Jpn. J. Antibiot. 1987, 40, 1809–1819. [Google Scholar] [PubMed]
- Sato, H.; Narita, A.; Nakazawa, S.; Suzuki, H.; Mastumoto, K.; Nakanishi, Y.; Nakazawa, S.; Niino, K.; Nakada, Y. The study of flomoxef in the pediatric field. Jpn. J. Antibiot. 1987, 40, 1349–1363. [Google Scholar] [PubMed]
- Chen, Y.C.; Hung, C.C.; Lin, S.F.; Chang, S.C.; Hsieh, W.C. Comparison of flomoxef with latamoxef in the treatment of sepsis and/or Gram-negative bacteremia in adult patients. Int. J. Antimicrob. Agents 1996, 7, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Uchida, K.; Matsubara, T. Effect of flomoxef on blood coagulation and alcohol metabolism. Infection 1991, 19, S284–S295. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, S.; Hayashi, M.; Takemura, W.; Igarashi, Y.; Liu, X.; Mizukami, Y.; Kojima, N.; Enoki, Y.; Taguchi, K.; Yokoyama, Y.; et al. Pharmacokinetics/pharmacodynamics evaluation of flomoxef against extended-spectrum beta-lactamase-producing Escherichia coli in vitro and in vivo in a murine thigh infection model. Pharm. Res. 2021, 38, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Le Terrier, C.; Nordmann, P.; Bouvier, M.; Poirel, L. Impact of acquired broad-spectrum β-lactamases on susceptibility to oral penems/carbapenems (tebipenem, sulopenem, and faropenem) alone or in combination with avibactam and taniborbactam β-lactamase inhibitors in Escherichia coli. Antimicrob. Agents Chemother. 2023, 67, e0054723. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Muir, L.; Critchley, I.A.; Walpole, S.; Kwak, H.; Phelan, A.M.; Moore, G.; Jain, A.; Keutzer, T.; Dane, A.; et al. Oral tebipenem pivoxil hydrobromide in complicated urinary tract infection. N. Engl. J. Med. 2022, 386, 1327–1338. [Google Scholar] [CrossRef] [PubMed]
- Ebihara, F.; Maruyama, T.; Kasai, H.; Shiokawa, M.; Matsunaga, N.; Hamada, Y. Preclinical study of pharmacokinetic/pharmacodynamic analysis of tebipenem using monte carlo simulation for extended-spectrum β-lactamase-producing bacterial urinary tract infections in japanese patients according to renal function. Antibiotics 2025, 14, 648. [Google Scholar] [CrossRef]
- Ishikawa, K.; Uehara, Y.; Mori, N.; Mikami, Y.; Tokioka, S.; Kobayashi, D.; Goke, H.; Inukai, T.; Sakurai, A.; Doi, Y.; et al. In vitro activity and clinical efficacy of faropenem against third-generation cephalosporin-resistant Escherichia coli and Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2022, 66, e0012522. [Google Scholar] [CrossRef] [PubMed]
- Gandra, S.; Takahashi, S.; Mitrani-Gold, F.S.; Mulgirigama, A.; Ferrinho, D.A. A systematic scoping review of faropenem and other oral penems: Treatment of Enterobacterales infections, development of resistance and cross-resistance to carbapenems. JAC Antimicrob. Resist. 2022, 4, dlac125. [Google Scholar] [CrossRef] [PubMed]
- Gandra, S.; Choi, J.; McElvania, E.; Green, S.J.; Harazin, M.; Thomson, R.B.; Dantas, G.; Singh, K.S.; Das, S. Faropenem resistance causes in vitro cross-resistance to carbapenems in ESBL-producing Escherichia coli. Int. J. Antimicrob. Agents 2020, 55, 105902. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.T.; May, D.B. Potential role of fosfomycin in the treatment of community-acquired lower urinary tract infections caused by extended-spectrum β-lactamase-producing Escherichia coli. Am. J. Ther. 2013, 20, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Bader, M.S.; Loeb, M.; Brooks, A.A. An update on the management of urinary tract infections in the era of antimicrobial resistance. Postgrad. Med. 2017, 129, 242–258. [Google Scholar] [CrossRef] [PubMed]
- Bader, M.S.; Loeb, M.; Leto, D.; Brooks, A.A. Treatment of urinary tract infections in the era of antimicrobial resistance and new antimicrobial agents. Postgrad. Med. 2020, 132, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Gutiérrez, B.; Rodríguez-Baño, J. Current options for the treatment of infections due to extended-spectrum beta-lactamase-producing Enterobacteriaceae in different groups of patients. Clin. Microbiol. Infect. 2019, 25, 932–942. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Ucha, J.C.; Seoane-Estévez, A.; Rodiño-Janeiro, B.K.; González-Bardanca, M.; Conde-Pérez, K.; Martínez-Guitián, M.; Alvarez-Fraga, L.; Arca-Suárez, J.; Lasarte-Monterrubio, C.; Gut, M.; et al. Activity of imipenem/relebactam against a Spanish nationwide collection of carbapenemase-producing Enterobacterales. J. Antimicrob. Chemother. 2021, 76, 1498–1510. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, N.E.; Price, V.; Cunningham-Oakes, E.; Tsang, K.K.; Nunn, J.G.; Midega, J.T.; Anjum, M.F.; Wade, M.J.; Feasey, N.A.; Peacock, S.J.; et al. Innovations in genomic antimicrobial resistance surveillance. Lancet Microbe 2023, 4, e1063–e1070. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yan, T. Metagenomic next generation sequencing for studying antibiotic resistance genes in the environment. Adv. Appl. Microbiol. 2023, 123, 41–89. [Google Scholar] [CrossRef] [PubMed]
| Category | Characteristic | Cefmetazole (CMZ) | Flomoxef (FMOX) |
|---|---|---|---|
| Pharmacokinetics (PKs) | Half-life | Approx. 0.8–1.8 h [25,26]. Prolonged as renal function declines [26]. | Approx. 49.2 min following a 1 g intravenous dose [27]. |
| Volume of distribution | 0.165 ± 0.025 L/kg [25]. | Central compartment: approximately 6.6 L [28]. Peripheral compartment: approximately 4.88 L [28]. | |
| Protein binding | High: 65–79% [29]. Free (unbound) fraction is pharmacologically active [29,30]. | Low: 35% [31]. | |
| Excretion | High urinary excretion rate (approx. 87% in 24 h) [25]. | 85% of the dose is excreted unchanged in urine within 6 h [27]. | |
| Effect of renal impairment/dialysis | Half-life is prolonged in patients with impaired renal function [26]. Readily removed by hemodialysis [26,30]. | Dosing adjustments may be necessary in renal insufficiency [32]. | |
| Pharmacodynamics (PDs) | Spectrum of activity | Broad-spectrum cephamycin [33]. Not active against Pseudomonas aeruginosa [33]. | Broad-spectrum oxacephem against Gram-positive, Gram-negative, and anaerobic bacteria [34,35]. Stable against ESBL-E [24,35,36,37,38,39]. |
| In vitro activity (vs. ESBL-E) | Minimum inhibitory concentration (MIC90): 4 mg/L [21]. Susceptibility: For Escherichia coli, 57–84%; MIC of 1 mg/L [21]. For Klebsiella pneumoniae, 50–92%; MIC of 1 mg/L [21]. Enterobacter cloacae often exhibits high resistance (MIC ≥32 mg/L) [40]. | MIC90: 0.5 mg/L [21]. Susceptibility: For E. coli, 97–100%; MIC of 1 mg/L [21]. For K. pneumoniae, 80–100%; MIC of 1 mg/L [21]. E. cloacae often exhibits high resistance (MIC ≥32 mg/L) [40]. | |
| PK/PD | Primary PK/PD index | Percentage of dosing interval during which the free drug concentration remains above the MIC (fTMIC) [30]. Clinical application is a challenge. | Percentage of the dosing interval during which the drug concentration remains above TMIC [41,42]. |
| PK/PD target | TMIC > 70% has been proposed for complicated urinary tract infections (cUTIs) [43]. | TMIC > 70% [41,44]. | |
| Dosing and target attainment | Adjusted dosing achieves 90% PTA for an MIC of 4 mg/L [45]. Dose of 2 g q6h is needed for strains with MIC of 8 mg/L [45]. In hemodialysis patients, a 1 g post-dialysis dose is effective against strains with MIC ≤4 mg/L [30]. | 1 g q8h (2 h infusion) or 1 g q6h (1 h infusion) achieves TMIC > 70% [41,44]. 1 g q6h provides sufficient bactericidal effect against strains with MICs ≤4 mg/L [21]. | |
| Tissue penetration | Free concentration is important for distribution volume [30]. Mean AUC0–3.5 h ratios to plasma are 60% in peritoneal fluid, 36% in peritoneum, and 11% in subcutaneous adipose tissue. [46]. | Good tissue penetration. Achieves PK/PD targets in ascitic fluid, peritoneal tissue, subcutaneous fat, and prostatic tissue [28,42]. | |
| Clinical application and precautions | Clinical efficacy (vs. carbapenems for ESBL-E) | No significant difference in clinical effectiveness or mortality for E. coli-related UTIs [47]. No significant difference in 90-day or 30-day mortality for bacteremia [36,48]. | A propensity-score-matched study showed no difference in 30-day mortality for bacteremia [36]. One retrospective study reported a higher mortality rate with FMOX vs. carbapenems, particularly for isolates with MICs of 2–8 mg/L [49,50]. |
| Precautions and considerations | Considered a carbapenem-sparing alternative [23]. Caution is warranted in severe infections or for isolates with high MICs [47]. | Use should be guided by MIC testing, especially in severe infections like bacteremia [49,50]. A valuable carbapenem-sparing option [36]. | |
| Resistance mechanisms | Resistance in E. coli is linked to a reversible porin-dependent mechanism (decreased OmpF and OmpC expression) [51]. | Mechanisms underlying resistance to FMOX have not been investigated. | |
| Safety | Adverse effects | Generally well-tolerated. Common effects include gastrointestinal disturbances [52,53,54]. These are typically reversible [52,53,54,55]. | Adverse effects are infrequent but can include eosinophilia, elevated transaminases, and leukopenia [56,57,58]. |
| Specific warnings | Potential for a disulfiram-like reaction with alcohol [52,53,54,55]. | Exhibits minor and clinically insignificant effect on prothrombin time [32,59]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, T.; Yagi, Y.; Maruyama, T.; Hamada, Y. Pharmacokinetics/Pharmacodynamics-Based Repositioning of Cefmetazole and Flomoxef in Extended-Spectrum β-Lactamase-Producing Enterobacterales Treatment: An Injectable Carbapenem-Sparing and Outpatient Strategy. Antibiotics 2025, 14, 737. https://doi.org/10.3390/antibiotics14080737
Kato T, Yagi Y, Maruyama T, Hamada Y. Pharmacokinetics/Pharmacodynamics-Based Repositioning of Cefmetazole and Flomoxef in Extended-Spectrum β-Lactamase-Producing Enterobacterales Treatment: An Injectable Carbapenem-Sparing and Outpatient Strategy. Antibiotics. 2025; 14(8):737. https://doi.org/10.3390/antibiotics14080737
Chicago/Turabian StyleKato, Takahiro, Yusuke Yagi, Takumi Maruyama, and Yukihiro Hamada. 2025. "Pharmacokinetics/Pharmacodynamics-Based Repositioning of Cefmetazole and Flomoxef in Extended-Spectrum β-Lactamase-Producing Enterobacterales Treatment: An Injectable Carbapenem-Sparing and Outpatient Strategy" Antibiotics 14, no. 8: 737. https://doi.org/10.3390/antibiotics14080737
APA StyleKato, T., Yagi, Y., Maruyama, T., & Hamada, Y. (2025). Pharmacokinetics/Pharmacodynamics-Based Repositioning of Cefmetazole and Flomoxef in Extended-Spectrum β-Lactamase-Producing Enterobacterales Treatment: An Injectable Carbapenem-Sparing and Outpatient Strategy. Antibiotics, 14(8), 737. https://doi.org/10.3390/antibiotics14080737






