Abstract
Background/Objectives: The overuse of antibiotics has led to the emergence of antibiotic-resistant bacteria, posing a global public health challenge. Among these, extended-spectrum β-lactamase and carbapenemase-producing Enterobacteriaceae (ESBL-E and CPE) are of particular concern due to their potential to spread resistance in various environments. Understanding the prevalence and spread of these bacteria in the influent and effluent of wastewater treatment plants is essential. Methods: This study examined ESBL-E and CPE in wastewater from three WWTPs in Ouagadougou, Burkina Faso, and was conducted between February and August 2024. Phenotypic detection of ESBL-E was performed on the isolates using the double-disk synergy test and the combination disk test, whereas the CPE detection employed the combination disk test and the modified Hodge test. Results: A total of 250 Enterobacteriaceae isolates were found, with Escherichia coli, Klebsiella spp., Enterobacter spp., and Buttiauxella spp. the most predominant taxa. Phenotypic analysis revealed a high prevalence of ESBL-E, particularly in influent samples, with rates ranging from 55 to 98% across the WWTPs. CPE detection showed varying prevalence, with higher proportions identified in effluent samples, ranging from 37 to 68%, depending on the plant. These findings highlight the critical role of WWTPs in the persistence and potential spread of antibiotic-resistant bacteria. Conclusions: This study underscores the urgent need for improved wastewater treatment technologies and comprehensive monitoring systems to reduce the dissemination of ESBL-E and CPE in the environment. Addressing these challenges is crucial for mitigating the public health risks associated with antimicrobial resistance.
1. Introduction
Antibiotic resistance is a growing global issue that threatens the medical advances achieved over the past decades [1]. This issue has significant implications for infection treatment, disease duration, morbidity, and mortality [2]. These consequences hinder development by delaying progress toward achieving the Sustainable Development Goals (SDGs), particularly those related to health and well-being [3]. In the specific context of Africa, these impacts are exacerbated by limited technical and financial resources and restricted access to healthcare [4,5].
Systematic reviews and meta-analyses have highlighted alarming rates of antimicrobial resistance in enterobacterales infections among children in sub-Saharan Africa, with a particular focus on third-generation cephalosporin resistance and the growing prevalence of carbapenem-resistant strains [6,7]. Among them, Enterobacteriaceae are the bacteria found in most environments and have been identified by the World Health Organization (WHO) as critical threats to human health [8,9].
Building on this concern, studies in sub-Saharan Africa, particularly in Burkina Faso, have shown that antibiotic-resistant bacteria are present in various environments across the country, like ready-to-eat foods and clinical settings [10,11]. Beyond these sources, environmental factors also play a crucial role in the dissemination of resistance. In recent years, pharmaceutical compounds have been detected in aquatic environments, with wastewater treatment plants (WWTPs) having a significant impact on the dispersion of these pharmaceutical substances [12,13]. A study by Ouédraogo et al. revealed the presence of antibiotic residues in effluents such as amoxicillin, chloramphenicol, and ceftriaxone discharged into the environment, raising concerns not only about the potential toxicity of these compounds to aquatic organisms and humans but also because of the emergence of resistance in pathogenic bacteria [14].
The presence of extended-spectrum β-lactamase-producing Enterobacteriaceae (ESBL-E) in wastewater represents a major public health issue, as these strains can be transmitted through environmental pathways [15,16]. Carbapenemase-producing Enterobacteriaceae (CPE) are particularly concerning due to their resistance to carbapenems, which are commonly used as last-line antibiotics for treating serious infections [17].
In this context, Burkina Faso is deeply affected by the challenges posed by rapid urbanization and population growth, particularly in managing water resources and wastewater [18]. Effective wastewater management is crucial for protecting the environment and public health, especially in rapidly expanding urban areas such as Ouagadougou.
However, current knowledge about antibiotic-resistant bacteria in wastewater from wastewater treatment plants (WWTPs) in Burkina Faso remains very limited, most studies focus on clinical settings. In response to this gap, research has been conducted to provide a deeper understanding of the antibiotic resistance mechanisms of bacteria isolated from WWTPs. The lack of data on the prevalence of antibiotic-resistant bacteria in wastewater treatment plants in Burkina Faso justifies this study, which aims to phenotypically detect and assess the prevalence of ESBL-E and CPE. This study will also help evaluate the effectiveness of current treatment processes in eliminating these resistant microorganisms.
2. Results
2.1. Bacterial Isolation
The total number of Enterobacteriaceae isolates found across the three wastewater treatment plants (WWTPs) was 250. In WWTP 1, isolates were identified in 40% (101/250) of the samples, with 61/101 (60%) from influent samples and 40/101 (40%) from effluent samples. In WWTP 2, isolates were found in 30% (76/250) of the samples, comprising 47/76 (62%) in influent samples and 29/76 (38%) in effluent samples. In WWTP 3, isolates were detected in 29% (73/250) of the samples, with 57/73 (78%) from influent samples and 16/73 (22%) from effluent samples. The most predominant taxa were Escherichia coli, followed by Klebsiella spp., Enterobacter spp., and Buttiauxella spp., as shown in Figure 1 and Table S1.
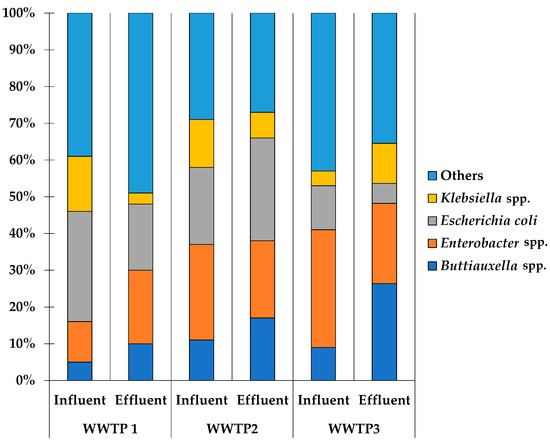
Figure 1.
Proportion of predominant taxa identified in three WWTPs.
2.2. Phenotypic Detection from Three WWTPs
Among the 250 Enterobacteriaceae isolates analyzed, phenotypic detection identified 167 (67%) as ESBL-E. From WWTP 1, 61/101 (60%) isolates were ESBL-E-positive, with a distribution of 67% (41/61) in influent samples and 50% (20/40) in effluent samples. In WWTP 2, 38/76 (50%) isolates were positive, comprising 55% (26/47) of the influent samples and 41% (12/29) of the effluent samples. In WWTP 3, 68/73 (93%) isolates were ESBL-E-positive, with 98% (56/57) in the influent samples and 75% (12/16) in the effluent samples.
Similarly, phenotypic detection identified 109 (44%) isolates as CPE. In WWTP 1, 52/101 (51%) isolates were positive, including 41% (25/61) in the influent samples and 68% (27/40) in the effluent samples. WWTP 2 recorded 30/76 (39%) positive isolates, with 32% (15/47) in the influent samples and 52% (15/29) in the effluent samples. In WWTP 3, 27/73 (37%) isolates were CPE-positive, with 37% (21/57) in influent samples and 38% (6/16) in effluent samples.
An overview of the different phenotypic techniques used for detecting ESBL-E and CPE, such as the double-disk synergy test (DDST), the combination disk test (CDT), and the modified Hodge test (MHT), alongside the predominant taxa identified during the study, is given in Figure 2 and Table S2. This highlights the variability in detection rates across taxa (Buttiauxella spp., Enterobacter spp., Escherichia coli, and Klebsiella spp.) and between influent and effluent samples across the three WWTPs.
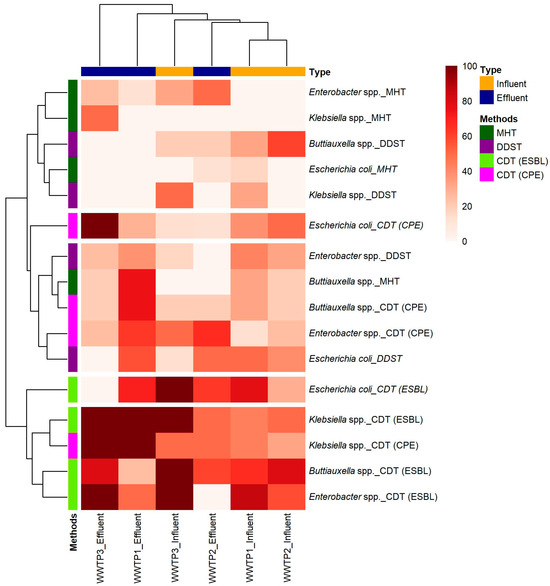
Figure 2.
Heatmap of phenotypic detection of ESBL-E and CPE among predominant bacterial taxa across three WWTPs, based on comparative methodological approaches.
2.3. Co-Occurrence of ESBL-E and CPE
The phenotypic detection revealed the co-occurrence of ESBL-E and CPE within various taxa, including Buttiauxella spp., Enterobacter spp., E. coli, and Klebsiella spp.
Across the WWTPs, Enterobacter spp. were more prevalent in the influent samples, especially in WWTP 3. Escherichia coli was mostly found in influents, while Klebsiella spp. were detected in both influents and effluents, often with higher prevalence in effluents. Buttiauxella spp. were found to be inconsistently distributed, appearing in the effluent of WWTP 1 and only in the influent of WWTP 3.
These findings are summarized in Table 1, which highlights the distribution of taxa positive for both ESBL-E and CPE in influent and effluent samples across the three WWTPs.

Table 1.
Co-occurrence of ESBL-E and CPE in predominant taxa from three WWTPs.
2.4. Determination of the Antimicrobial Susceptibility Pattern of Enterobacteriaceae
The resistance patterns of ESBL-E and CPE to various antibiotics across the three wastewater treatment plants (WWTPs) revealed significant trends, highlighting antibiotics with both high and low resistance rates.
For ESBL-E, high resistance was observed for ampicillin (100% in WWTP 1 influent, and both influent and effluent of WWTP 3), ceftriaxone (ranging from 70 to 100%), cefepime (80–90% across all WWTPs), and imipenem (90–100% in all WWTPs). Conversely, low resistance rates were consistently noted for doripenem (0–20%), MRP (0–20%), and piperacillin-tazobactam (0–20%) across both the influent and effluent samples (Table 2).

Table 2.
Overall percentage resistance of ESBL-E to different antibiotics.
Similarly, the CPE exhibited comparable resistance trends. High resistance percentages were recorded for AMP (70–100% across all the WWTPs), CTR (50–100%), FEP (50–100%), and IMP (90–100%). In contrast, resistance to DOR (10–30%), MRP (10–30%), and TZP (10–30%) remained low in both the influent and effluent samples (Table 3).

Table 3.
Overall percentage resistance of CPE to different antibiotics.
2.5. Multidrug Resistance
During our study, the ESBL and CPE strains demonstrated resistance to more than three antibiotics (Table 4). These findings highlight variations in multiresistance levels among different wastewater treatment plants (WWTPs).

Table 4.
Multidrug resistance profiles of ESBL-E and CPE from three WWTPs.
3. Discussion
The emergence and dissemination of antimicrobial resistance (AMR) have become significant global public health concerns, exacerbated by the overuse and misuse of antibiotics in human, veterinary, and agricultural settings, as well as the discharge of untreated or inadequately treated wastewater into the environment [19,20]. Wastewater treatment plants (WWTPs) serve as both reservoirs and dissemination points for antimicrobial-resistant bacteria (ARB) and antimicrobial resistance genes (ARGs) [21]. The release of these resistant bacteria into receiving waters can facilitate the persistence and spread of AMR within aquatic ecosystems, posing risks to human health and environmental microbial communities [22].
3.1. Bacterial Isolation
In our study, the predominant species identified were Escherichia coli, Klebsiella spp., Buttiauxella spp., and Enterobacter spp. Similarly, a recent study by Urzua-Abad et al. characterized bacterial species isolated from hospital and municipal wastewater in Mexico City, describing several enteric and non-enteric Gram-negative strains [23]. Another study by Nasser-Ali et al. also found human and environmental bacteria in wastewater from urban wastewater treatment plants in Coruña, Spain [24]. Regarding Buttiauxella spp., this species is more commonly found in environmental settings than in humans [25]. However, another study discovered Buttiauxella agrestis present in sewage sludge [26].
3.2. Phenotypic Detection
The overall prevalence of ESBL-E among Enterobacteriaceae isolates in our study (67%) is comparable to studies that reported high ESBL rates in wastewater environments [27]. Our results demonstrate that WWTP 3 had the highest ESBL-E prevalence (93%), particularly in the influent samples (98%). This aligns with a study in which ESBL-producing bacteria were detected in 97.6% of wastewater samples collected from five healthcare centers in Burkina Faso [28]. This highlights the range of antibiotic-resistant bacteria in hospital effluents, often exceeding those found in municipal sewage systems [13,29]. The reduction observed in the effluent samples (75%) aligns with findings from other studies indicating incomplete removal of resistant bacteria during wastewater treatment [20]. However, the persistence of such a high proportion in effluent underscores the limitations of conventional treatment processes in fully eliminating resistant pathogens.
The CPE prevalence in this study (44%) is slightly higher than what some studies have reported but is within the range documented in regions with high antimicrobial usage [27]. Our findings from WWTP 1 (51% CPE-positive isolates) and the notable increase in effluent samples (68%) reflect trends observed in studies suggesting that selective pressure during treatment processes may favor the survival of carbapenemase producers [30]. We can also hypothesize horizontal gene transfer, as well as the insufficient efficiency of wastewater treatment processes [20,24,31]. In comparison, WWTP 3 recorded the lowest CPE prevalence (37%), with minimal variation between the influent and effluent samples, indicating possible differences in influent composition. Industrial processes may contribute more significantly to the prevalence of these resistance genes, as highlighted by Wu et al., where the discharge of heavy metals or other pollutants from industrial activities can be responsible for certain resistance traits [32]. Additionally, the types and quantities of antibiotics consumed by the general population, as well as their subsequent excretion into sewage systems, contribute substantially to shaping the resistance profiles observed in wastewater [33].
3.3. Comparison of Techniques and Taxa Prevalence
The CDT technique demonstrated greater sensitivity for detecting ESBL-E compared with DDST (Figure 2). DDST, which is useful for detecting ESBLs, may be less sensitive in certain contexts [34]. Therefore, CDT is generally more sensitive and can detect a broader range of resistance mechanisms, making it effective for identifying ESBL-E in wastewater samples [35]. A study in India by Sageerabanoo et al. highlighted the superiority of CDT over other methods for ESBL detection, which is consistent with our findings [36].
MHT showed a slightly lower sensitivity than CDT in detecting CPE (Figure 2). However, MHT is particularly useful for identifying CPE but may not provide information on some resistance mechanisms detected by CDT, such as metallo-beta-lactamase (MBL) [37]. Kumudunie et al. emphasized the limitations of MHT, where MHT could detect only 50% of NDM-producing strains, which corroborates our observation that CDT detected a higher proportion of CPE across all taxa [38].
3.4. Dual Resistance
The dominance of Enterobacter spp. and Klebsiella spp. as carriers of both ESBL-E and CPE is consistent with findings in prior studies, such as those by Garba et al. and Haller et al., which highlighted these taxa as significant reservoirs of multidrug resistance in wastewater [28,39]. The persistence of Klebsiella spp. in effluents aligns with observations of their resilience and ability to survive standard treatment processes, such as Klebsiella pneumonia ST101, which can withstand chlorine treatment [40]. Escherichia coli, while frequently detected as ESBL-E, is less commonly found with dual resistance, reflecting its lower environmental resilience compared to Klebsiella spp. [41,42]. The limited data on Buttiauxella spp. in the literature make direct comparisons challenging, but its emerging presence in wastewater, as noted in this study, needs further investigation into its role in AMR dissemination.
3.5. Antibiotic Susceptibility
The widespread resistance to AMP (70–100%) observed in both the ESBL-E and CPE isolates aligns with its frequent use in human and veterinary medicine [43]. A study by Belachew et al. similarly reported high AMP resistance rates, emphasizing the selective pressure exerted by its extensive use [44]. Previous research in West Africa, including studies in Burkina Faso, has identified similar resistance trends in environmental and clinical isolates, underscoring the regional significance of penicillin resistance [5].
Resistance to CTR (70–100%) and FEP (80–90%) highlights the pervasive impact of third-generation cephalosporins on the selection of resistant strains. Notably, ceftriaxone residues were detected in WWTP 1, highlighting the role of wastewater treatment plants as reservoirs for these antibiotics and their contributions to resistance selection [14]. These findings are consistent with studies that link environmental contamination by healthcare and agricultural effluents to increased resistance in aquatic ecosystems [45,46].
Resistance to IMP (90–100%) among CPE isolates is a significant concern. The high resistance levels observed in this study mirror findings from similar wastewater studies globally, where carbapenem-resistant Enterobacteriaceae (CRE) are increasingly reported [47]. This resistance compromises the efficacy of last-resort antibiotics and aligns with studies that have documented carbapenem resistance in treated effluents, raising questions about the effectiveness of current wastewater treatment processes [48].
Conversely, resistance to DOR, MRP, and TZP (0–30%) remained low, indicating that these antibiotics are limited in terms of usage and reduce selective pressure in the studied environments. This can be explained by a 2022 study on the use of WATCH antibiotics prior to hospital admission in rural Burkina Faso, which revealed that ceftriaxone, a WATCH category antibiotic, was used significantly more than carbapenems in hospital settings [49]. The low resistance rates provide optimism for their continued use in combating resistant infections.
3.6. Multidrug Resistance
These findings from Table 4 are consistent with studies from wastewater environments worldwide, where ESBL-E isolates frequently exhibit MDR, due to multiple resistance mechanisms and the presence of plasmids and mobile genetic elements [35,50]. The results in Table 4 also align with global studies reporting that carbapenem-resistant strains are often MDR, driven by plasmid-mediated resistance mechanisms such as carbapenemase genes and additional resistance determinants [51,52,53]. Moreover, the high rates of MDR observed in WWTP 3 suggest significant selective pressure from healthcare effluents, as reported in studies on hospital wastewater systems [13,28,29].
3.7. Limitations of the Study
In this study, we evaluated the performance of the combination disk test and double disk synergy test for detecting extended-spectrum β-lactamases-producing Enterobacteriaceae and the combination disk test and modified Hodge test for detecting carbapenemase-producing Enterobacteriaceae. However, we did not employ molecular methods as reference standards to confirm the presence of extended-spectrum β-lactamase or carbapenemase genes. This could potentially affect the accuracy of our phenotypic detection results, as molecular methods are widely regarded as the gold standard for such analyses. Future studies should incorporate molecular techniques to validate and enhance the reliability of phenotypic detection methods.
4. Materials and Methods
4.1. Sites and Sampling
The study area is in West Africa, in Ouagadougou, the capital of Burkina Faso. This study focuses on three wastewater stabilization ponds serving as wastewater treatment plants in the city of Ouagadougou, Burkina Faso. Most of the raw urban wastewater treated by the first WWTP, the Kossodo treatment plant, comes from various sources, including domestic and hospital wastewater as well as pre-treated (settled) industrial wastewater from the slaughterhouse and the brewery industry, the latter being a major contributor. Only domestic wastewater is treated at the second WWTP, the 2iE Institute Treatment Plant. The third WWTP, the Tengandogo University Hospital Treatment Plant, treats only wastewater from the hospital. Monthly sampling was carried out at each WWTP between February and August 2024. All the WWTPs’ wastewater samples were taken instantaneously at the effluent and as 24 h composites at the influent. A total of 38 liquid effluent samples were collected, 14 from WWTP 1 and 12 from WWTP 1 and WWTP 3. The samples were transported to the laboratory, kept at 4 °C, and processed immediately.
4.2. Bacterial Isolation
After transporting the samples from the treatment plants, tenfold serial dilutions (10–1000 times) of each water sample were made in sterile saline solution. A total of 1 mL of each dilution was then plated onto MacConkey agar supplemented with cefotaxime (1 μg/mL) or meropenem (0.25 μg/mL). After incubation for 24–48 h at 35 ± 2 °C, bacterial colonies with distinct coloration and morphologies were randomly picked and subcultured onto MacConkey agar containing cefotaxime (1 μg/mL) or meropenem (0.25 μg/mL) for further purification. A single colony of purified bacteria was picked and cultured in Trypticase Soy Broth. Each isolate was assigned a unique identification number and stored at −80 °C in 20% (v/v) glycerol for further investigation.
4.3. Identification
The pure isolates were screened for Gram-negative bacteria using a chemical method, the potassium hydroxide (KOH) test [54]. The isolates were subcultured on Eosin Methylene Blue (EMB) agar and Salmonella-Shigella (SS) agar. The plates were incubated at 37 °C for 24 h. Species-level identification of the isolates was performed using standard biochemical test protocols (Table S3). The Gram-negative isolates were tested for catalase activity, motility, indole production, methyl red reaction, Voges-Proskauer reaction, citrate utilization, urease activity, hydrogen sulfide, acid, and gas production in Triple Sugar Iron (TSI) agar. The results were interpreted following Bergey’s Manual of Systematics of Archaea and Bacteria [55].
4.4. Antibiotic Susceptibility Test
Antibiotic susceptibility testing was conducted on the Enterobacteriaceae isolates using twelve antibiotics, including four cephalosporins (cefotaxime 30 µg, ceftriaxone 30 µg, cefepime 30 µg, and ceftazidime 30 µg), four carbapenems (imipenem 10 µg, ertapenem 10 µg, meropenem 10 µg, and doripenem 10 µg), and other antibiotics such as ampicillin 10 µg, amoxicillin-clavulanate 20 µg/10 µg, aztreonam 30 µg, and piperacillin-tazobactam 100 µg/10 µg. The disc diffusion method, as described by Bauer et al., was employed to assess antibiotic susceptibility [56]. A single colony from an overnight bacterial culture was suspended in sterile saline solution and adjusted to a turbidity of 0.5 McFarland. Sterile Mueller-Hinton agar (MHA) plates were inoculated with the bacterial suspension, spread evenly using a sterile swab, and allowed to dry. Antibiotic discs were placed on the agar surface using sterile forceps. The plates were then incubated at 35 °C ± 2 °C for 18–24 h. Following incubation, the diameter of the inhibition zones was measured and interpreted according to the Antibiogram Committee of the French Society for Microbiology in association with the European Committee on Antimicrobial Susceptibility Testing (CA-SFM/EUCAST) [57,58].
4.5. Phenotypic Detection of β-Lactamases
4.5.1. Screening Enterobacteriaceae for ESBL Production
The double disk synergy test (DDST) was employed to confirm the presence of extended-spectrum β-lactamases (ESBL) production in bacterial isolates resistant to cephalosporins and penicillin, following CA-SFM/EUCAST guidelines [57]. The bacterial isolates were adjusted to 0.5 McFarland turbidity standards and evenly inoculated onto Mueller-Hinton agar (MHA) plates. Antibiotic disks containing aztreonam (30 µg), cefepime (30 µg), ceftriaxone (30 µg), ceftazidime (30 µg), and cefotaxime (30 µg) were positioned 30 mm (center to center) from a central amoxicillin-clavulanate (20 µg/10 µg) disk. The plates were incubated aerobically at 37 °C for 18–24 h. Following incubation, the isolates were evaluated for ESBL production. A clear extension of the inhibition zone of any cephalosporin disk toward the central amoxicillin-clavulanate disk, with an observed increase in the zone diameter of at least 5 mm compared with that of the cephalosporin disk alone, was interpreted as indicative of ESBL production.
Following the Clinical and Laboratory Standards Institute (CLSI) guidelines, the combination disk test (CDT) was employed to confirm the ESBL production [59]. Ceftazidime (30 µg) and cefotaxime (30 µg) disks, both with and without clavulanic acid, were placed at appropriate distances on a Muller-Hinton agar (MHA) plate inoculated with a bacterial suspension standardized to 0.5 McFarland turbidity. The plates were incubated aerobically at 37 °C for 16–18 h. An isolate was confirmed as an ESBL producer if the inhibition zone diameter around the combination disk (antibiotic plus clavulanic acid) increased by more than 5 mm compared with the inhibition zone around the antibiotic disk alone.
4.5.2. Screening Enterobacteriaceae for Carbapenemase Production
The combination disk test (CDT) used by Chauhan et al. was performed to confirm carbapenemase production [60]. Imipenem (10 µg) disks, with and without ethylenediaminetetraacetic acid (EDTA), were placed at a standardized distance on Mueller-Hinton agar (MHA) plates inoculated with a bacterial suspension adjusted to 0.5 McFarland turbidity. The plates were incubated aerobically at 37 °C for 16–18 h. Carbapenemase production was confirmed if the inhibition zone diameter around the combination disk (imipenem plus EDTA) was at least 7 mm larger than the inhibition zone around the imipenem disk alone.
The modified Hodge test (MHT) was conducted to detect carbapenemase production following the CLSI guidelines [61]. A suspension of E. coli ATCC 25922 was prepared to a 0.5 McFarland standard and uniformly swabbed onto MHA plates. After the plate was allowed to dry, a 10 μg ertapenem disk was placed at the center, and the test isolates were streaked in a straight line from the edge of the disk to the periphery of the plate. The plates were incubated at 37 °C for 16–18 h. The presence of a cloverleaf-like indentation in the inhibition zone around the ertapenem disk, near the growth of E. coli ATCC 25922, was interpreted as a positive result for carbapenemase production.
4.6. Quality Control
The quality of the media, reagents, and antibiotic discs was ensured by strictly following the manufacturer’s instructions (Liofilchem, Roseto degli Abruzzi, Italy). To verify the reliability of the antibiotic susceptibility tests, phenotypic detection methods, and biochemical assays, the reference strain Escherichia coli ATCC 25922 was employed as a quality control.
4.7. Statistical Analysis
The data were entered, cleaned, and analyzed using Microsoft Excel (version 2024). Descriptive statistics were used to summarize the findings. The prevalence of ESBL-E and CPE, based on comparative methodological approaches, was visualized using a heatmap generated with the pheatmap package in R (RStudio, version 4.5.0).
5. Conclusions
This study highlights the prevalence of extended-spectrum β-lactamase and carbapenemase-producing Enterobacteriaceae in wastewater treatment plants in Ouagadougou, Burkina Faso. The high levels of ESBL strains show the increasing difficulty of treating infections caused by bacteria resistant to common antibiotics, whereas the detection of carbapenemase-producing bacteria signals the growing threat of resistance to last-resort antibiotics. These findings underscore the important contribution of wastewater treatment plants to the spread of antibiotic resistance in the environment, emphasizing the urgent need to upgrade treatment infrastructure through advanced methods such as membrane filtration or UV disinfection, which can improve the removal of resistant bacteria and genes. Moreover, implementing routine surveillance programs for resistant strains in wastewater systems will facilitate early detection and targeted interventions to mitigate environmental dissemination. This research calls for improved wastewater management and public health efforts, involving national health authorities, environmental agencies, municipalities, and wastewater treatment plant operators, in order to resolve the rising problem of antibiotic resistance and protect both human and environmental health.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics14070641/s1, Table S1. Distribution of predominant taxa identified across the Three WWTPs. Table S2. Phenotypic detection of predominant taxa from three WWTPs. Table S3. Biochemical profile of representative species from predominant bacterial taxa isolated from WWTPs.
Author Contributions
Conceptualization, I.H.Y., B.S., B.V.E.J.T.B. and Y.K.; methodology, I.H.Y., B.S., B.V.E.J.T.B. and Y.K.; software, I.H.Y. and B.V.E.J.T.B.; validation, I.H.Y. and Y.K.; formal analysis, B.V.E.J.T.B. and B.S.; investigation, I.H.Y. and B.S.; resources, Y.K.; data curation, I.H.Y. and B.V.E.J.T.B.; writing—original draft preparation, I.H.Y. and Y.K.; writing—review and editing, I.H.Y., B.S., B.V.E.J.T.B. and Y.K.; visualization I.H.Y., B.S. and Y.K.; supervision, Y.K.; project administration, Y.K. and B.S.; funding acquisition, B.S. and Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This study received support from the World Bank through the African Center of Excellence Project-Impact (ACE-Impact) with grant number IDA 6388-BF/D443.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data of this study are available on request from the authors.
Acknowledgments
The authors would like to express their sincere gratitude to the Institut International d’Ingénierie de l’Eau et de l’Environnement (2iE) for its support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Njeru, J.; Odero, J.; Chebore, S.; Ndung’u, M.; Tanui, E.; Wesangula, E.; Ndanyi, R.; Githii, S.; Gunturu, R.; Mwangi, W.; et al. Development, Roll-out and Implementation of an Antimicrobial Resistance Training Curriculum Harmonizes Delivery of in-Service Training to Healthcare Workers in Kenya. Front. Microbiol. 2023, 14, 1142622. [Google Scholar] [CrossRef]
- Dadgostar, P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019, 12, 3903–3910. [Google Scholar] [CrossRef]
- Tang, K.W.K.; Millar, B.C.; Moore, J.E. Antimicrobial Resistance (AMR). Br. J. Biomed. Sci. 2023, 80, 11387. [Google Scholar] [CrossRef]
- Godman, B.; Egwuenu, A.; Wesangula, E.; Schellack, N.; Kalungia, A.C.; Tiroyakgosi, C.; Kgatlwane, J.; Mwita, J.C.; Patrick, O.; Niba, L.L.; et al. Tackling Antimicrobial Resistance across Sub-Saharan Africa: Current Challenges and Implications for the Future. Expert. Opin. Drug Saf. 2022, 21, 1089–1111. [Google Scholar] [CrossRef]
- Siachalinga, L.; Godman, B.; Mwita, J.C.; Sefah, I.A.; Ogunleye, O.O.; Massele, A.; Lee, I.-H. Current Antibiotic Use Among Hospitals in the Sub-Saharan Africa Region; Findings and Implications. Infect. Drug Resist. 2023, 16, 2179–2190. [Google Scholar] [CrossRef]
- Kowalski, M.; Obama, B.M.; Catho, G.; Dewez, J.E.; Merglen, A.; Ruef, M.; Andrey, D.O.; Hassoun-Kheir, N.; de Kraker, M.E.A.; Combescure, C.; et al. Antimicrobial Resistance in Enterobacterales Infections among Children in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. eClinicalMedicine 2024, 70, 102512. [Google Scholar] [CrossRef]
- Ruef, M.; Emonet, S.; Merglen, A.; Dewez, J.E.; Obama, B.M.; Catho, G.; Andrey, D.O.; Kowalski, M.; Harbarth, S.; Combescure, C.; et al. Carriage of Third-Generation Cephalosporin-Resistant and Carbapenem-Resistant Enterobacterales among Children in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. eClinicalMedicine 2024, 70, 102508. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The Changing Face of the Family Enterobacteriaceae (Order: “Enterobacterales”): New Members, Taxonomic Issues, Geographic Expansion, and New Diseases and Disease Syndromes. Clin. Microbiol. Rev. 2021, 34, e00174-20. [Google Scholar] [CrossRef]
- WHO Bacterial Priority Pathogens List 2024: Bacterial Pathogens of Public Health Importance, to Guide Research, Development, and Strategies to Prevent and Control Antimicrobial Resistance, 1st ed.; World Health Organization: Geneva, Switzerland, 2024; ISBN 978-92-4-009346-1.
- Bouniounou, D.Y.P.; Metuor Dabiré, A.; Ouedraogo, N.; Tiemtore, R.Y.; Sougué, S.; Bonkoungou, P.R.; Simporé, J. Multiresistance to Carbapenems by Production of Verona Integron-Encoded Metallo-β-Lactamase (VIM)-Type Carbapenemases in Gram-Negative Bacilli Isolated at the Centre Hospitalier Universitaire de Tengandogo (CHU-T) and the Hôpital Saint Camille de Ouagadougou (HOSCO) in Burkina Faso. Microbes Infect. Dis. 2024, 5, 759–769. [Google Scholar] [CrossRef]
- Soubeiga, A.P.; Kpoda, D.S.; Compaoré, M.K.A.; Somda-Belemlougri, A.; Kaseko, N.; Rouamba, S.S.; Ouedraogo, S.; Traoré, R.; Karfo, P.; Nezien, D.; et al. Molecular Characterization and the Antimicrobial Resistance Profile of Salmonella Spp. Isolated from Ready-to-Eat Foods in Ouagadougou, Burkina Faso. Int. J. Microbiol. 2022, 2022, 9640828. [Google Scholar] [CrossRef]
- Al-Riyami, I.M.; Ahmed, M.; Al-Busaidi, A.; Choudri, B.S. Antibiotics in Wastewaters: A Review with Focus on Oman. Appl. Water Sci. 2018, 8, 199. [Google Scholar] [CrossRef]
- Omuferen, L.O.; Maseko, B.; Olowoyo, J.O. Occurrence of Antibiotics in Wastewater from Hospital and Convectional Wastewater Treatment Plants and Their Impact on the Effluent Receiving Rivers: Current Knowledge between 2010 and 2019. Environ. Monit. Assess. 2022, 194, 306. [Google Scholar] [CrossRef]
- Ouédraogo, G.A.; Cissé, H.; Ouédraogo, H.S.; Kaboré, B.; Traoré, R.; Traoré, Y.; Bassolé, I.H.N.; Tchoumbougnang, F.; Savadogo, A. Research of Antibiotic Residues and Bacterial Strain’s Antibiotic Resistance Profile in the Liquid Effluents Evacuated in Nature by Two CHUs and a Mixed WWTP of Ouagadougou (Burkina Faso). Infect. Drug Resist. 2023, 16, 2537–2547. [Google Scholar] [CrossRef]
- Irfan, M.; Almotiri, A.; AlZeyadi, Z.A. Antimicrobial Resistance and β-Lactamase Production in Clinically Significant Gram-Negative Bacteria Isolated from Hospital and Municipal Wastewater. Antibiotics 2023, 12, 653. [Google Scholar] [CrossRef]
- Cho, S.; Jackson, C.R.; Frye, J.G. Freshwater Environment as a Reservoir of Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae. J. Appl. Microbiol. 2023, 134, lxad034. [Google Scholar] [CrossRef]
- Iovleva, A.; Doi, Y. Carbapenem-Resistant Enterobacteriaceae. Clin. Lab. Med. 2017, 37, 303. [Google Scholar] [CrossRef]
- Wang, H.; Wang, T.; Zhang, B.; Li, F.; Toure, B.; Omosa, I.B.; Chiramba, T.; Abdel-Monem, M.; Pradhan, M. Water and Wastewater Treatment in Africa–Current Practices and Challenges. CLEAN–Soil Air Water 2014, 42, 1029–1035. [Google Scholar] [CrossRef]
- WHO Antimicrobial Resistance: Accelerating National and Global Responses; World Health Organization: Geneva, Switzerland, 2023.
- Owojori, G.O.; Lateef, S.A.; Ana, G.R.E.E. Effectiveness of Wastewater Treatment Plant at the Removal of Nutrients, Pathogenic Bacteria, and Antibiotic-Resistant Bacteria in Wastewater from Hospital Source. Environ. Sci. Pollut. Res. 2024, 31, 10785–10801. [Google Scholar] [CrossRef]
- Drane, K.; Sheehan, M.; Whelan, A.; Ariel, E.; Kinobe, R. The Role of Wastewater Treatment Plants in Dissemination of Antibiotic Resistance: Source, Measurement, Removal and Risk Assessment. Antibiotics 2024, 13, 668. [Google Scholar] [CrossRef]
- Barathan, M.; Ng, S.-L.; Lokanathan, Y.; Ng, M.H.; Law, J.X. Unseen Weapons: Bacterial Extracellular Vesicles and the Spread of Antibiotic Resistance in Aquatic Environments. Int. J. Mol. Sci. 2024, 25, 3080. [Google Scholar] [CrossRef]
- Urzua-Abad, M.M.; Aquino-Andrade, A.; Castelan-Vega, J.A.; Merida-Vieyra, J.; Ribas-Aparicio, R.M.; Belmont-Monroy, L.; Jimenez-Alberto, A.; Aparicio-Ozores, G. Detection of Carbapenemases in Enterobacterales and Other Gram-Negative Bacilli Recovered from Hospital and Municipal Wastewater in Mexico City. Sci. Rep. 2024, 14, 26576. [Google Scholar] [CrossRef]
- Nasser-Ali, M.; Aja-Macaya, P.; Conde-Pérez, K.; Trigo-Tasende, N.; Rumbo-Feal, S.; Fernández-González, A.; Bou, G.; Poza, M.; Vallejo, J.A. Emergence of Carbapenemase Genes in Gram-Negative Bacteria Isolated from the Wastewater Treatment Plant in A Coruña, Spain. Antibiotics 2024, 13, 194. [Google Scholar] [CrossRef]
- Patra, N.; Prakash, M.R.; Patil, S.; Rao, M.B. First Case Report of Surgical Site Infection Due to Buttiauxella agrestis in a Neurocare Center in India. Arch. Med. Health Sci. 2018, 6, 117. [Google Scholar] [CrossRef]
- Wójcik-Fatla, A.; Farian, E.; Kowalczyk, K.; Sroka, J.; Skowron, P.; Siebielec, G.; Zdybel, J.M.; Jadczyszyn, T.; Cencek, T. Enterobacteriaceae in Sewage Sludge and Digestate Intended for Soil Fertilization. Pathogens 2024, 13, 1056. [Google Scholar] [CrossRef]
- Wilson, H.; Török, M.E. Extended-Spectrum β-Lactamase-Producing and Carbapenemase-Producing Enterobacteriaceae. Microb. Genom. 2018, 4, e000197. [Google Scholar] [CrossRef]
- Garba, Z.; Bonkoungou, I.O.J.; Millogo, N.O.; Natama, H.M.; Vokouma, P.A.P.; Bonko, M.d.A.; Karama, I.; Tiendrebeogo, L.A.W.; Haukka, K.; Tinto, H.; et al. Wastewater from Healthcare Centers in Burkina Faso Is a Source of ESBL, AmpC-β-Lactamase and Carbapenemase-Producing Escherichia Coli and Klebsiella Pneumoniae. BMC Microbiol. 2023, 23, 351. [Google Scholar] [CrossRef]
- Hubeny, J.; Ciesielski, S.; Harnisz, M.; Korzeniewska, E.; Dulski, T.; Jałowiecki, Ł.; Płaza, G. Impact of Hospital Wastewater on the Occurrence and Diversity of Beta-Lactamase Genes During Wastewater Treatment with an Emphasis on Carbapenemase Genes: A Metagenomic Approach. Front. Environ. Sci. 2021, 9, 738158. [Google Scholar] [CrossRef]
- Fouz, N.; Pangesti, K.N.A.; Yasir, M.; Al-Malki, A.L.; Azhar, E.I.; Hill-Cawthorne, G.A.; Abd El Ghany, M. The Contribution of Wastewater to the Transmission of Antimicrobial Resistance in the Environment: Implications of Mass Gathering Settings. Trop. Med. Infect. Dis. 2020, 5, 33. [Google Scholar] [CrossRef]
- Liu, Y.; Tong, Z.; Shi, J.; Jia, Y.; Yang, K.; Wang, Z. Correlation between Exogenous Compounds and the Horizontal Transfer of Plasmid-Borne Antibiotic Resistance Genes. Microorganisms 2020, 8, 1211. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, Z.; Wang, S.; Song, L. Occurrence and Prevalence of Antibiotic Resistance Genes and Pathogens in an Industrial Park Wastewater Treatment Plant. Sci. Total Environ. 2023, 880, 163278. [Google Scholar] [CrossRef]
- Nyirenda, T. Antibiotic Resistance Causes More Deaths than Malaria and HIV/Aids Combined. What Africa Is Doing to Fight This Silent Epidemic. Available online: http://theconversation.com/antibiotic-resistance-causes-more-deaths-than-malaria-and-hiv-aids-combined-what-africa-is-doing-to-fight-this-silent-epidemic-217689 (accessed on 25 November 2024).
- Kaur, J.; Chopra, S.; Sheevani; Mahajan, G. Modified Double Disc Synergy Test to Detect ESBL Production in Urinary Isolates of Escherichia Coli and Klebsiella Pneumoniae. J. Clin. Diagn. Res. JCDR 2013, 7, 229. [Google Scholar] [CrossRef]
- Husna, A.; Rahman, M.M.; Badruzzaman, A.T.M.; Sikder, M.H.; Islam, M.R.; Rahman, M.T.; Alam, J.; Ashour, H.M. Extended-Spectrum β-Lactamases (ESBL): Challenges and Opportunities. Biomedicines 2023, 11, 2937. [Google Scholar] [CrossRef]
- Sageerabanoo, S.; Malini, A.; Mangaiyarkarasi, T.; Hemalatha, G. Phenotypic Detection of Extended Spectrum β-Lactamase and Amp-C β-Lactamase Producing Clinical Isolates in a Tertiary Care Hospital: A Preliminary Study. J. Nat. Sci. Biol. Med. 2015, 6, 383–387. [Google Scholar] [CrossRef]
- Sachdeva, R.; Sharma, B.; Sharma, R. Evaluation of Different Phenotypic Tests for Detection of Metallo-β-Lactamases in Imipenem-Resistant Pseudomonas Aeruginosa. J. Lab. Physicians 2017, 9, 249–253. [Google Scholar] [CrossRef]
- Kumudunie, W.G.M.; Wijesooriya, L.I.; Wijayasinghe, Y.S. Comparison of Four Low-Cost Carbapenemase Detection Tests and a Proposal of an Algorithm for Early Detection of Carbapenemase-Producing Enterobacteriaceae in Resource-Limited Settings. PLoS ONE 2021, 16, e0245290. [Google Scholar] [CrossRef]
- Haller, L.; Chen, H.; Ng, C.; Le, T.H.; Koh, T.H.; Barkham, T.; Sobsey, M.; Gin, K.Y.-H. Occurrence and Characteristics of Extended-Spectrum β-Lactamase- and Carbapenemase-Producing Bacteria from Hospital Effluents in Singapore. Sci. Total Environ. 2018, 615, 1119–1125. [Google Scholar] [CrossRef]
- Popa, L.I.; Gheorghe, I.; Barbu, I.C.; Surleac, M.; Paraschiv, S.; Măruţescu, L.; Popa, M.; Pîrcălăbioru, G.G.; Talapan, D.; Niţă, M.; et al. Multidrug Resistant Klebsiella Pneumoniae ST101 Clone Survival Chain from Inpatients to Hospital Effluent After Chlorine Treatment. Front. Microbiol. 2021, 11, 610296. [Google Scholar] [CrossRef]
- Onyedibe, K.I.; Shobowale, E.O.; Okolo, M.O.; Iroezindu, M.O.; Afolaranmi, T.O.; Nwaokorie, F.O.; Opajobi, S.O.; Isa, S.E.; Egah, D.Z. Low Prevalence of Carbapenem Resistance in Clinical Isolates of Extended Spectrum Beta Lactamase (ESBL) Producing Escherichia Coli in North Central, Nigeria. Adv. Infect. Dis. 2018, 8, 109–120. [Google Scholar] [CrossRef][Green Version]
- Ebomah, K.E.; Okoh, A.I. An African Perspective on the Prevalence, Fate and Effects of Carbapenem Resistance Genes in Hospital Effluents and Wastewater Treatment Plant (WWTP) Final Effluents: A Critical Review. Heliyon 2020, 6, e03899. [Google Scholar] [CrossRef]
- Güzelaydın, K.; Yıldırım, M. The Bioavailability of Ampicillins in Some Animals. J. Istanb. Vet. Sci. 2024, 8, 1–4. [Google Scholar] [CrossRef]
- Belachew, T.; Mihret, A.; Legesse, T.; Million, Y.; Desta, K. High Level of Drug Resistance by Gram-Negative Bacteria from Selected Sewage Polluted Urban Rivers in Addis Ababa, Ethiopia. BMC Res. Notes 2018, 11, 524. [Google Scholar] [CrossRef]
- Zhang, L.; Ma, X.; Luo, L.; Hu, N.; Duan, J.; Tang, Z.; Zhong, R.; Li, Y. The Prevalence and Characterization of Extended-Spectrum β-Lactamase- and Carbapenemase-Producing Bacteria from Hospital Sewage, Treated Effluents and Receiving Rivers. Int. J. Environ. Res. Public. Health 2020, 17, 1183. [Google Scholar] [CrossRef]
- Hanna, N.; Tamhankar, A.J.; Lundborg, C.S. Antibiotic Concentrations and Antibiotic Resistance in Aquatic Environments of the WHO Western Pacific and South-East Asia Regions: A Systematic Review and Probabilistic Environmental Hazard Assessment. Lancet Planet. Health 2023, 7, e45–e54. [Google Scholar] [CrossRef]
- Foxman, B.; Salzman, E.; Gesierich, C.; Gardner, S.; Ammerman, M.; Eisenberg, M.; Wigginton, K. Wastewater Surveillance of Antibiotic Resistant Bacteria for Public Health Action: Potential and Challenges. Am. J. Epidemiol. 2024, 194, kwae419. [Google Scholar] [CrossRef]
- Hoelle, J.; Johnson, J.R.; Johnston, B.D.; Kinkle, B.; Boczek, L.; Ryu, H.; Hayes, S. Survey of US Wastewater for Carbapenem-Resistant Enterobacteriaceae. J. Water Health 2019, 17, 219–226. [Google Scholar] [CrossRef]
- Valia, D.; Ingelbeen, B.; Kaboré, B.; Karama, I.; Peeters, M.; Lompo, P.; Vlieghe, E.; Post, A.; Cox, J.; de Mast, Q.; et al. Use of WATCH Antibiotics Prior to Presentation to the Hospital in Rural Burkina Faso. Antimicrob. Resist. Infect. Control 2022, 11, 59. [Google Scholar] [CrossRef]
- Nasrollahian, S.; Graham, J.P.; Halaji, M. A Review of the Mechanisms That Confer Antibiotic Resistance in Pathotypes of E. Coli. Front. Cell. Infect. Microbiol. 2024, 14, 1387497. [Google Scholar] [CrossRef]
- Lascols, C.; Cherney, B.; Conley, A.B.; Rishishwar, L.; Crawford, M.A.; Morse, S.A.; Fisher, D.J.; Anderson, K.; Hodge, D.R.; Pillai, S.P.; et al. Investigation of Multidrug-Resistant Plasmids from Carbapenemase-Producing Klebsiella Pneumoniae Clinical Isolates from Pakistan. Front. Microbiol. 2023, 14, 1192097. [Google Scholar] [CrossRef] [PubMed]
- Kopotsa, K.; Osei Sekyere, J.; Mbelle, N.M. Plasmid Evolution in Carbapenemase-Producing Enterobacteriaceae: A Review. Ann. N. Y. Acad. Sci. 2019, 1457, 61–91. [Google Scholar] [CrossRef]
- Jean, S.-S.; Harnod, D.; Hsueh, P.-R. Global Threat of Carbapenem-Resistant Gram-Negative Bacteria. Front. Cell. Infect. Microbiol. 2022, 12, 823684. [Google Scholar] [CrossRef]
- Akpan, S.N.; Odeniyi, O.A.; Adebowale, O.O.; Alarape, S.A.; Adeyemo, O.K. Antibiotic Resistance Profile of Gram-Negative Bacteria Isolated from Lafenwa Abattoir Effluent and Its Receiving Water (Ogun River) in Abeokuta, Ogun State, Nigeria. Onderstepoort J. Vet. Res. 2020, 87, 1854. [Google Scholar] [CrossRef] [PubMed]
- Whitman, W.B. Bergey’s Manual of Systematics of Archaea and Bacteria; Wiley: Hoboken, NJ, USA, 2015; ISBN 978-1-118-96060-8. [Google Scholar]
- Bauer, A.W.; Kirby, W.M.; Sherris, J.C.; Turck, M. Antibiotic Susceptibility Testing by a Standardized Single Disk Method. Am. J. Clin. Pathol. 1966, 45, 493–496. [Google Scholar] [CrossRef] [PubMed]
- CA-SFM/EUCAST. Comité de l’Antibiograme de la Société Française de Microbiologie; Société Française de Microbiologie: Paris, France, 2024. [Google Scholar]
- Lascols, C.; Legrand, P.; Mérens, A.; Leclercq, R.; Armand-Lefevre, L.; Drugeon, H.B.; Kitzis, M.D.; Muller-Serieys, C.; Reverdy, M.E.; Roussel-Delvallez, M.; et al. In Vitro Antibacterial Activity of Doripenem against Clinical Isolates from French Teaching Hospitals: Proposition of Zone Diameter Breakpoints. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 475–482. [Google Scholar] [CrossRef] [PubMed]
- M100; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2023.
- Chauhan, K.; Pandey, A.; Asthana, A.K.; Madan, M. Evaluation of Phenotypic Tests for Detection of Klebsiella Pneumoniae Carbapenemase and Metallo-Beta-Lactamase in Clinical Isolates of Escherichia Coli and Klebsiella Species. Indian. J. Pathol. Microbiol. 2015, 58, 31–35. [Google Scholar] [CrossRef]
- M100S; Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016; Volume 26.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).