Dual Redox Targeting by Pyrroloformamide A and Silver Ions Enhances Antibacterial and Anti-Biofilm Activity Against Carbapenem-Resistant Klebsiella pneumoniae
Abstract
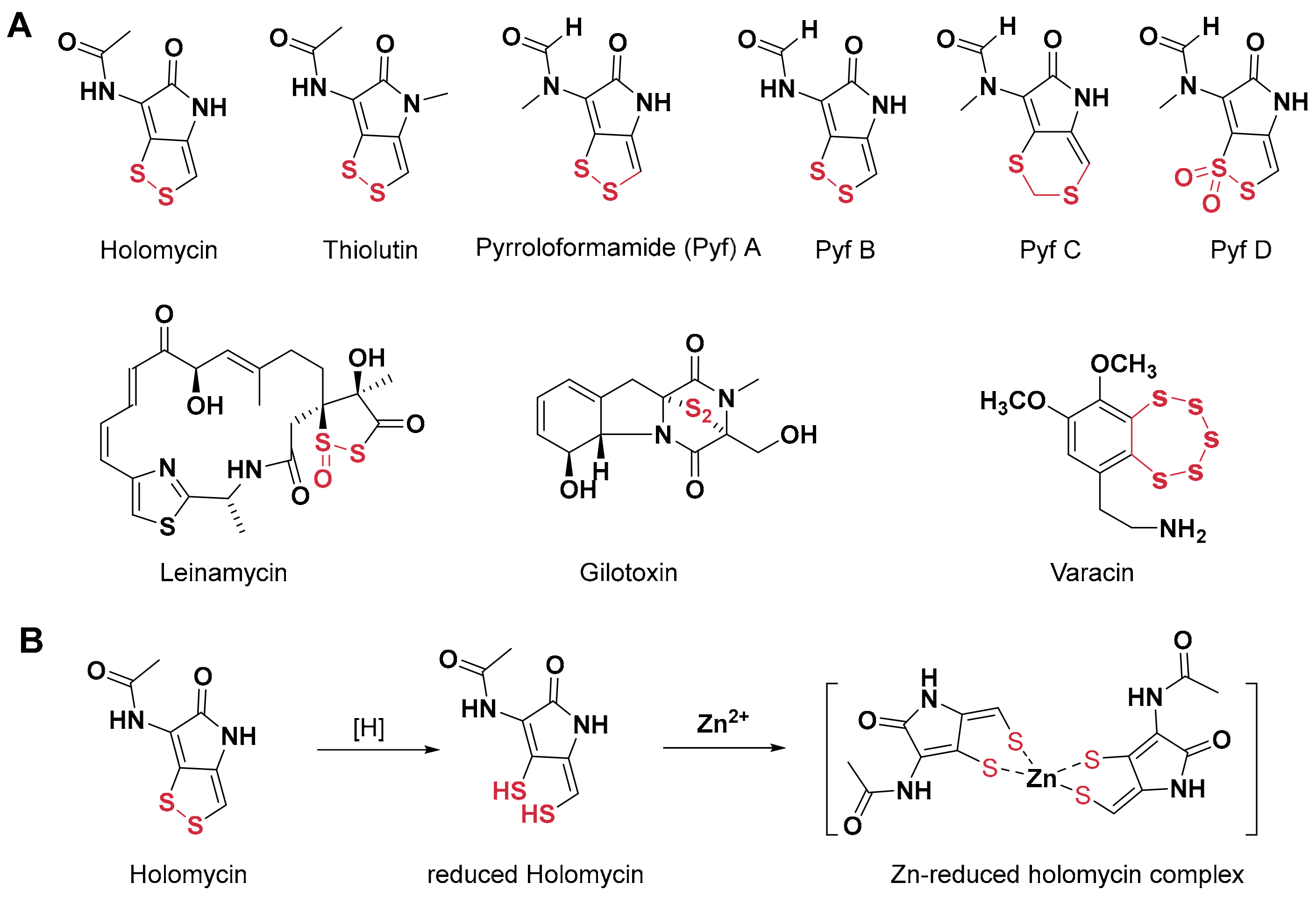
1. Introduction
2. Results
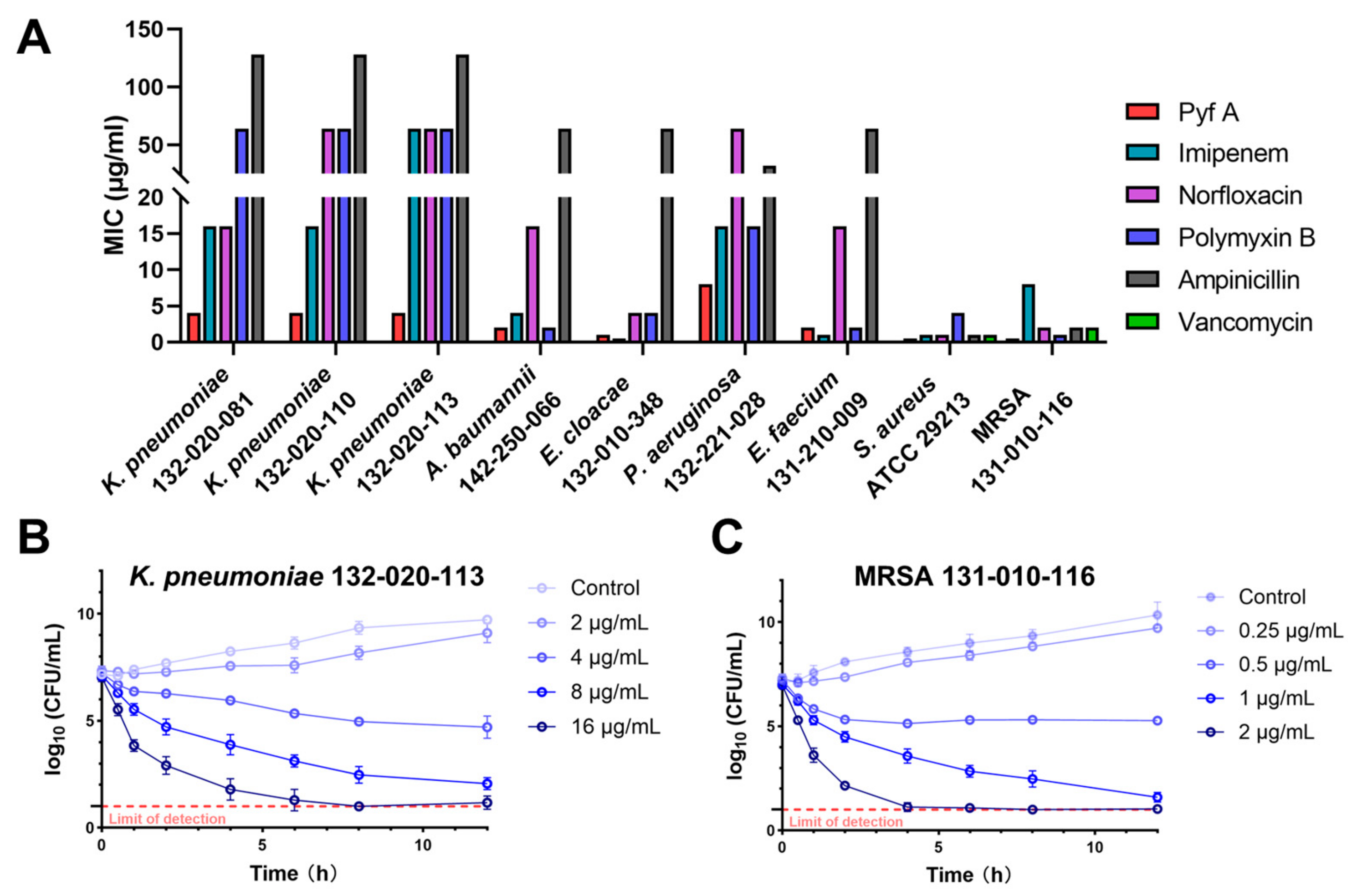
2.1. Pyf A Exhibits Potent Antimicrobial Activity Against CRKP
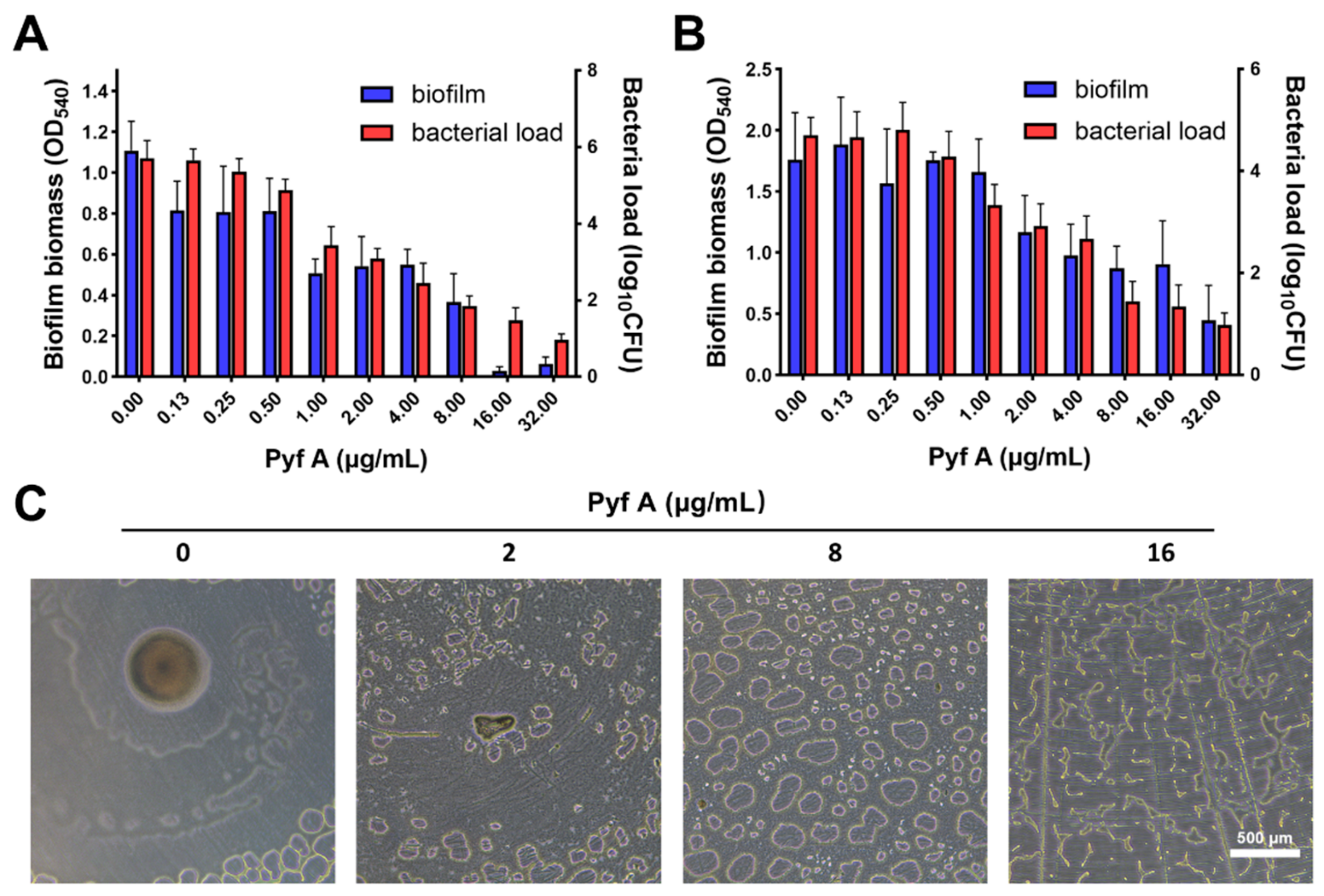
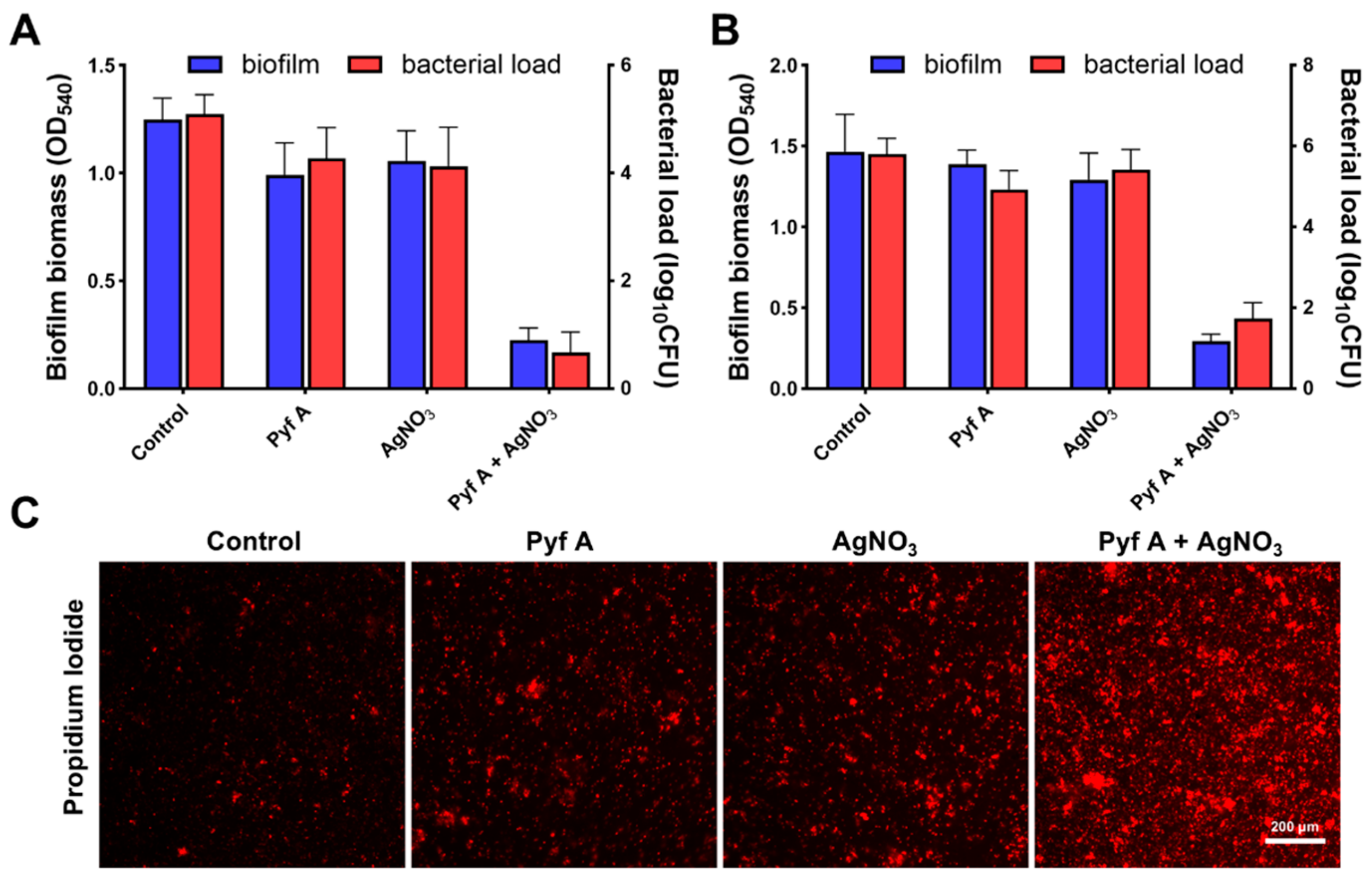
2.2. Pyf A Inhibits Biofilm Formation and Disrupts Established Biofilms
2.3. Pyf A Compromises Bacterial Membrane Integrity
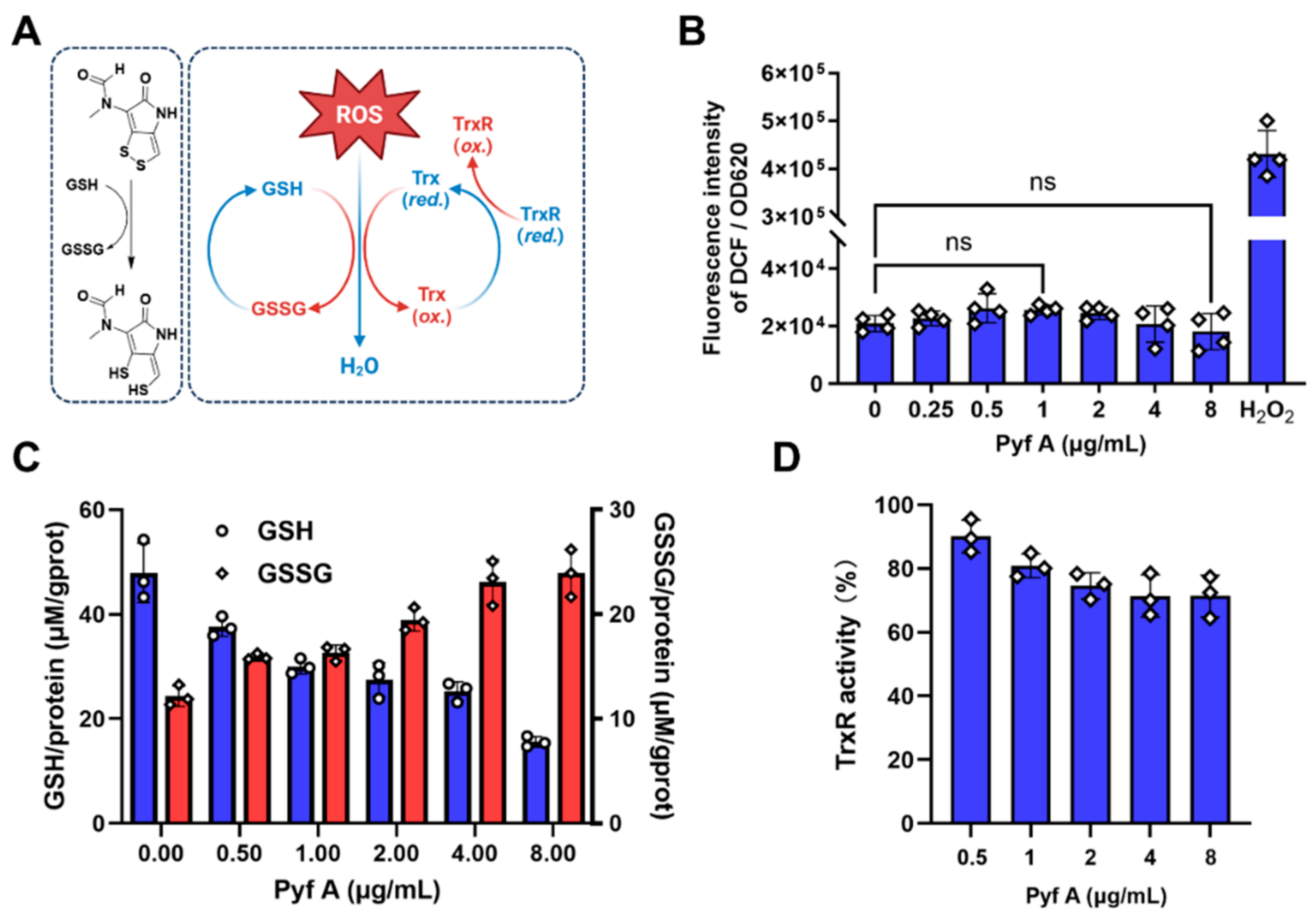
2.4. Pyf A Reduces Intracellular GSH Without Elevating ROS
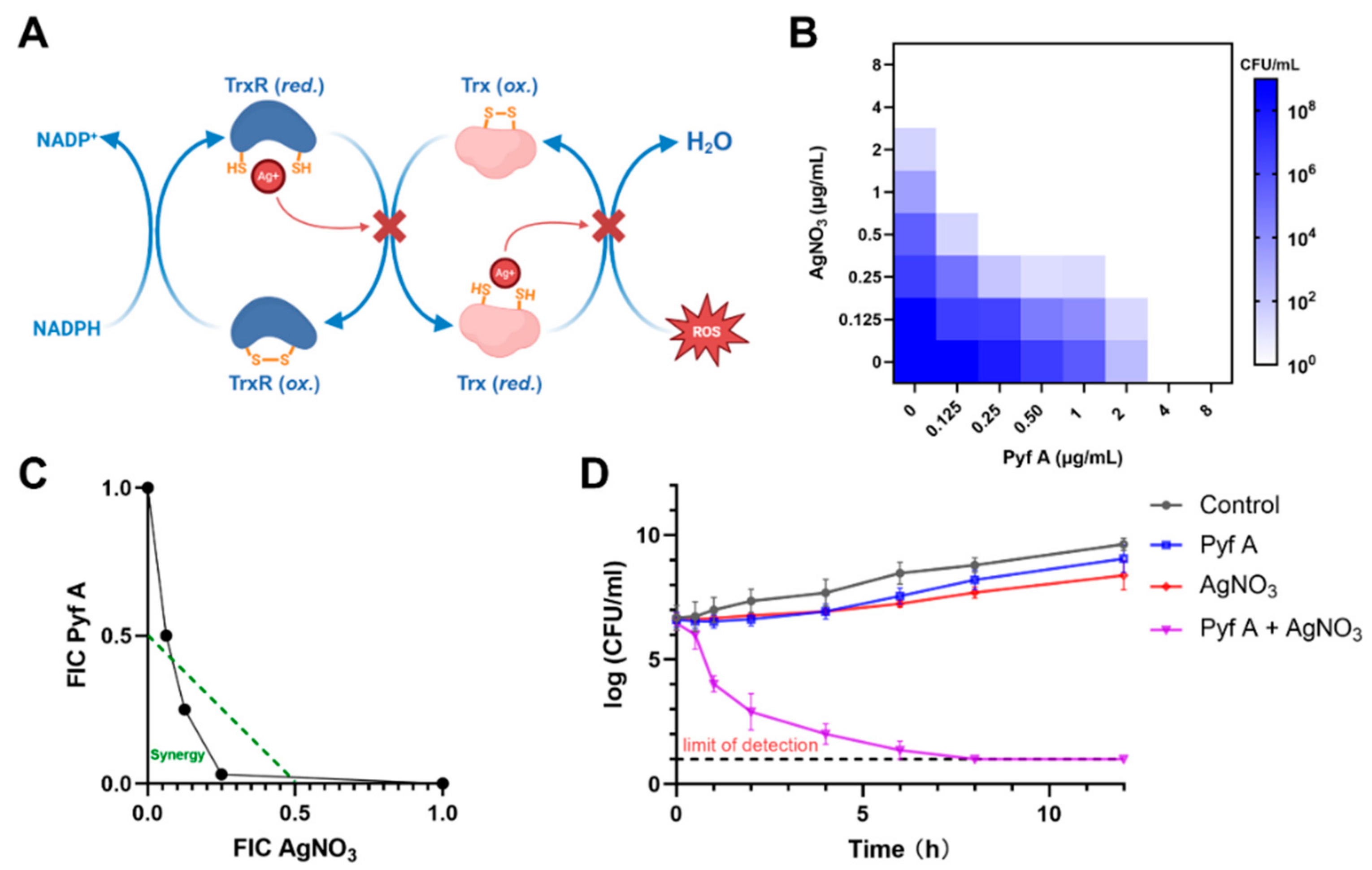
2.5. Pyf A and AgNO3 Synergistically Induce ROS and Bactericidal Activity
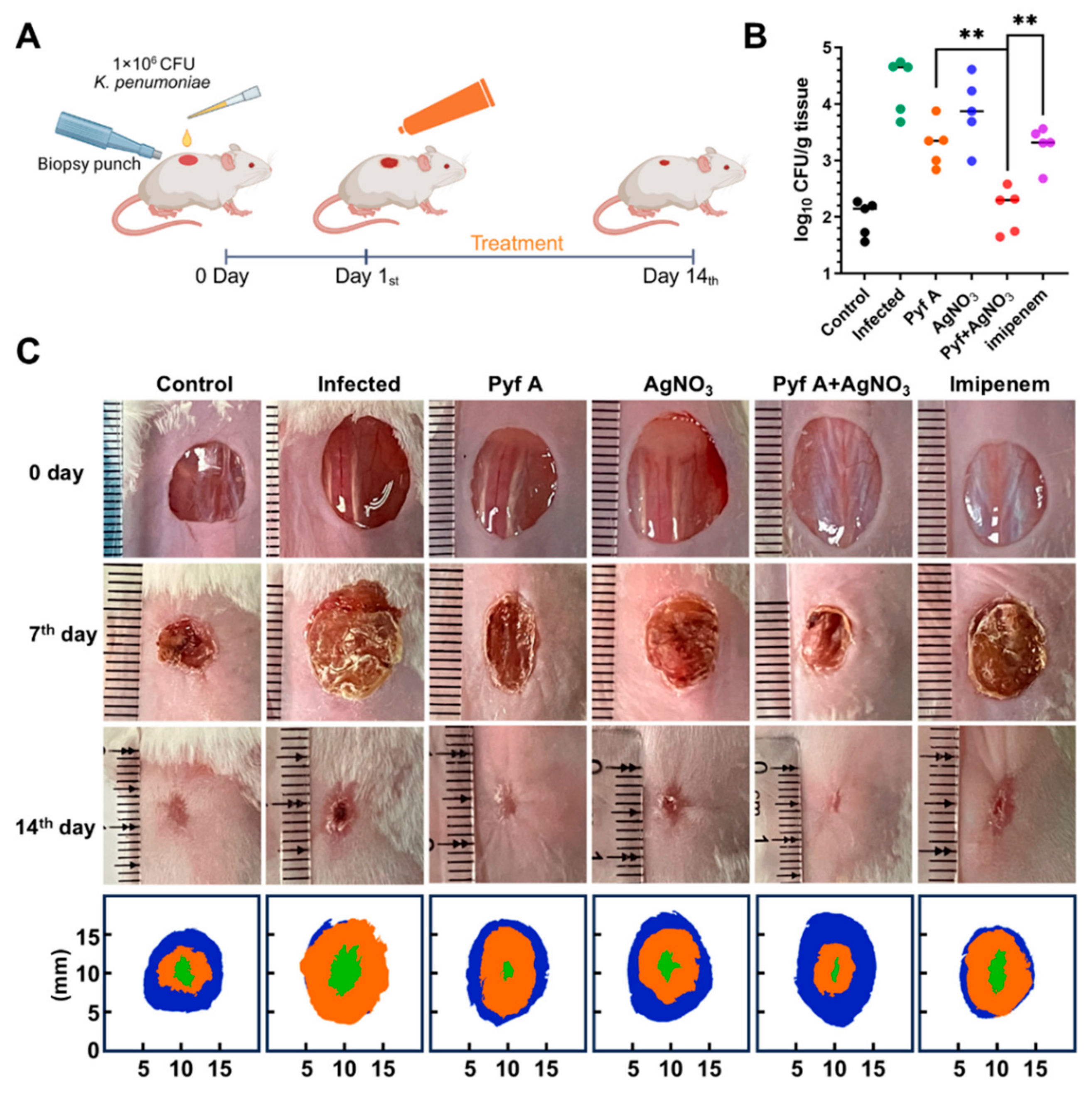
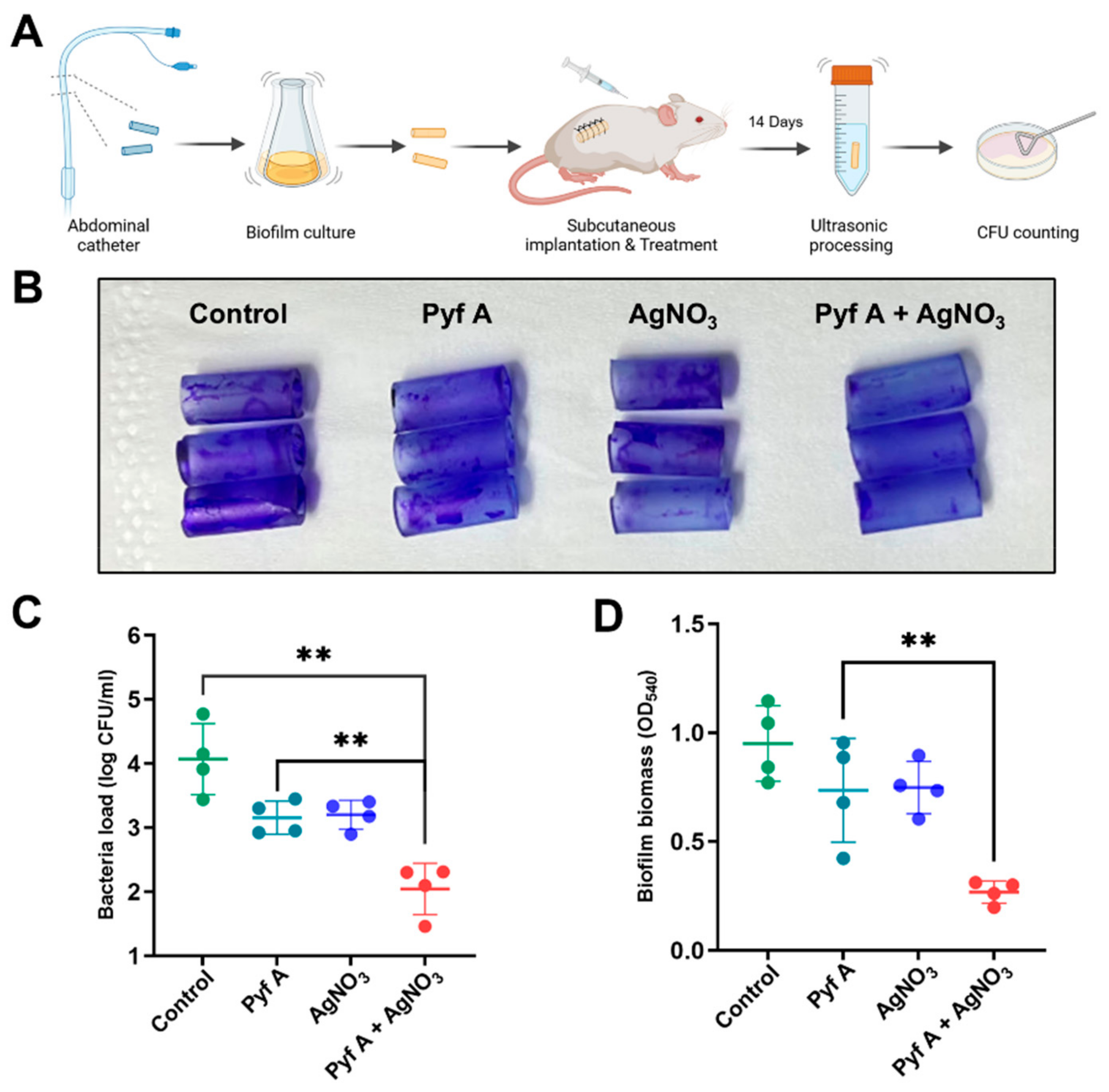
2.6. The Combination of Pyf A and AgNO3 Is Effective in Mouse Models
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Chemicals and Reagents
4.3. Antimicrobial Susceptibility Testing and MIC Determination
4.4. Time-Kill Assay
4.5. Biofilm Inhibition and Pre-Formed Biofilm Clearance Assay
4.6. Confocal Laser Scanning Microscopy
4.7. Scanning Electron Microscopy
4.8. PI Uptake Assay
4.9. ROS Measurement
4.10. GSH/GSSG Quantification
4.11. TrxR Activity Assay
4.12. Drug Combination and Checkboard Assay
4.13. In Vivo Models
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qiao, W.; Wang, L.; Luo, Y.; Yang, T. Synthetic approaches and therapeutic applications of FDA-approved antibacterial agents: A comprehensive review from 2003 to 2023. Eur. J. Med. Chem. 2025, 285, 117267–117291. [Google Scholar] [CrossRef] [PubMed]
- Kinch, M.S.; Patridge, E.; Plummer, M.; Hoyer, D. An analysis of FDA-approved drugs for infectious disease: Antibacterial agents. Drug Discov. Today 2014, 19, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- WHO Bacterial Priority Pathogens List, 2024: Bacterial Pathogens of Public Health Importance to Guide Research, Development and Strategies to Prevent and Control Antimicrobial Resistance. 2024. Available online: https://www.who.int/publications/i/item/9789240093461 (accessed on 17 May 2025).
- Antimicrobial Resistance, Hypervirulent Klebsiella pneumoniae—Global Situation. 2024. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2024-DON527 (accessed on 17 May 2025).
- Cai, W.; Kang, J.; Ma, Y.; Yin, D.; Song, Y.; Liu, Y.; Duan, J. Molecular epidemiology of carbapenem resistant Klebsiella pneumoniae in northern China: Clinical characteristics, antimicrobial resistance, virulence and geographic distribution. Infect. Drug Resist. 2023, 16, 7289–7304. [Google Scholar] [CrossRef]
- Jacob, C. A scent of therapy: Pharmacological implications of natural products containing redox-active sulfur atoms. Nat. Prod. Rep. 2006, 23, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.X.; Yun, B.S.; Ma, M.; Basu, H.S.; Church, D.R.; Ingenhorst, G.; Huang, Y.; Yang, D.; Lohman, J.R.; Tang, G.L.; et al. Leinamycin E1 acting as an anticancer prodrug activated by reactive oxygen species. Proc. Natl. Acad Sci. USA 2015, 7, 8278–8283. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Xu, Z.; Guo, Z.; Ma, H.; Yang, D.; Zhou, H.; Gansemans, Y.; Zhu, X.; Huang, Y.; Zhao, L.X.; et al. Discovery of the leinamycin family of natural products by mining actinobacterial genomes. Proc. Natl. Acad. Sci. USA 2017, 26, E11131–E11140. [Google Scholar] [CrossRef]
- Huber, E.M. Epipolythiodioxopiperazine-based natural products: Building blocks, biosynthesis and biological activities. Chembiochem 2022, 23, e202200341. [Google Scholar] [CrossRef]
- Pozzetti, L.; Asquith, C.R.M. Pentathiepins are an understudied molecular prism of biological activities. Arch. Pharm. 2024, 12, e2400646. [Google Scholar] [CrossRef]
- Li, B.; Wever, W.J.; Walsh, C.T.; Bowers, A.A. Dithiolopyrrolones: Biosynthesis, synthesis, and activity of a unique class of disulfide-containing antibiotics. Nat. Prod. Rep. 2014, 31, 905–923. [Google Scholar] [CrossRef]
- Qin, Z.; Huang, S.; Yu, Y.; Deng, H. Dithiolopyrrolone natural products: Isolation, synthesis and biosynthesis. Mar. Drugs 2013, 11, 3970–3997. [Google Scholar] [CrossRef]
- Jimenez, A.; Tipper, D.J.; Davies, J. Mode of action of thiolutin, an inhibitor of macromolecular synthesis in Saccharomyces cerevisiae. Antimicrob. Agents Chemother. 1973, 3, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Tipper, D.J. Inhibition of yeast ribonucleic acid polymerases by thiolutin. J. Bacteriol. 1973, 116, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Arora, P.; Malik, I.; Laperuta, A.J.; Pavlovic, E.M.; Ugochukwu, S.; Naik, M.; Kaplan, C.D. Thiolutin has complex effects in vivo but is a direct inhibitor of RNA polymerase II in vitro. Nucleic Acids Res. 2024, 52, 2546–2564. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.N.; Shiver, A.L.; Wever, W.J.; Razvi, S.Z.A.; Traxler, M.F.; Li, B. Role for dithiolopyrrolones in disrupting bacterial metal homeostasis. Proc. Natl. Acad. Sci. USA 2017, 114, 2717–2722. [Google Scholar] [CrossRef]
- Albini, F.; Bormann, S.; Gerschel, P.; Ludwig, V.A.; Neumann, W. Dithiolopyrrolones are prochelators that are activated by glutathione. Chem. Eur. J. 2023, 29, e202202567. [Google Scholar] [CrossRef]
- Lauinger, L.; Li, J.; Shostak, A.; Cemel, I.A.; Ha, N.; Zhang, Y.; Merkl, P.E.; Obermeyer, S.; Stankovic-Valentin, N.; Schafmeier, T. Thiolutin is a zinc chelator that inhibits the Rpn11 and other JAMM metalloproteases. Nat. Chem. Biol. 2017, 13, 709–714. [Google Scholar] [CrossRef]
- Jing, C.; Li, X.; Zhou, M.; Zhang, S.; Lai, Q.; Liu, D.; Ye, B.; Li, L.; Wu, Y.; Li, H. The PSMD14 inhibitor Thiolutin as a novel therapeutic approach for esophageal squamous cell carcinoma through facilitating SNAIL degradation. Theranostics 2021, 11, 5847–5862. [Google Scholar] [CrossRef]
- Zhou, W.; Liang, H.; Qin, X.; Cao, D.; Zhu, X.; Ju, J.; Shen, B.; Duan, Y.; Huang, Y. The isolation of pyrroloformamide congeners and characterization of their biosynthetic gene cluster. J. Nat. Prod. 2020, 83, 202–209. [Google Scholar] [CrossRef]
- Von Daehne, W.; Godtfredsen, W.O.; Tybring, L.; Schaumburg, K. New antibiotics containing the 1,2-dithiolo[4,3-b] pyrrole ring system. J. Antibiot. 1969, 22, 233–236. [Google Scholar] [CrossRef]
- Jensen, B. On the crystal structure of 5-oxo-6-N-methylformylamino-4,5-dihydro-1,2-dithiolo[4,3-b] pyrrole. J. Antibiot. 1969, 22, 231–232. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, D.J.; Belenky, P.A.; Yang, J.H.; MacDonald, I.C.; Martell, J.D.; Takahashi, N.; Chan, C.T.Y.; Lobritz, M.A.; Braff, D.; Schwarz, E.G. Antibiotics induce redox-related physiological alterations as part of their lethality. Proc. Natl. Acad. Sci. USA 2014, 111, 2100–2109. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Zhang, X.; Zhang, S.; Niu, P.; Gao, H. Photothermal theranostics with glutathione depletion and enhanced reactive oxygen species generation for efficient antibacterial treatment. RSC Adv. 2023, 13, 22863–22874. [Google Scholar] [CrossRef]
- Steinbuch, K.B.; Fridman, M. Mechanisms of resistance to membrane-disrupting antibiotics in Gram-positive and Gram-negative bacteria. Med. Chem. Comm. 2016, 7, 86–102. [Google Scholar] [CrossRef]
- Prinz, W.A.; Åslund, F.; Holmgren, A.; Beckwith, J. The role of the thioredoxin and glutaredoxin pathways in reducing protein disulfide bonds in the Escherichia coli cytoplasm. J. Biol. Chem. 1997, 272, 15661–15667. [Google Scholar] [CrossRef]
- Liao, X.; Yang, F.; Li, H.; So, P.-K.; Yao, Z.; Xia, W.; Sun, H. Targeting the thioredoxin reductase–thioredoxin system from Staphylococcus aureus by silver ions. Inorg. Chem. 2017, 56, 14823–14830. [Google Scholar] [CrossRef] [PubMed]
- Gruhlke, M.C.; Slusarenko, A.J. The biology of reactive sulfur species (RSS). Plant Physiol. Biochem. 2012, 59, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Toledano, M.B.; Kumar, C.; Le Moan, N.; Spector, D.; Tacnet, F. The systems biology of thiol redox system in Escherichia coli and yeast: Differential functions in oxidative stress, iron metabolism and DNA synthesis. FEBS Lett. 2007, 581, 3598–3607. [Google Scholar] [CrossRef]
- Chai, Y.C.; Mieyal, J.J. Glutathione and glutaredoxin—Key players in cellular redox homeostasis and signaling. Antioxidants 2023, 12, 1553. [Google Scholar] [CrossRef]
- Liu, H.; Fan, J.; Zhang, P.; Hu, Y.; Liu, X.; Li, S.-M.; Yin, W.-B. New insights into the disulfide bond formation enzymes in epidithiodiketopiperazine alkaloids. Chem. Sci. 2021, 12, 4132–4138. [Google Scholar] [CrossRef]
- Kilgore, H.R.; Olsson, C.R.; D’Angelo, K.A.; Movassaghi, M.; Raines, R.T. n→π interactions modulate the disulfide reduction potential of epidithiodiketopiperazines. J. Am. Chem. Soc. 2020, 142, 15107–15115. [Google Scholar] [CrossRef] [PubMed]
- Steiner, O.M.; Johnson, R.A.; Chen, X.; Simke, W.C.; Li, B. Activation of dithiolopyrrolone antibiotics by cellular reductants. Biochemistry 2025, 64, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.N.; Chen, X.; Falco, J.A.; Bak, D.W.; Weerapana, E.; Li, B. Chemoproteomics reveals disruption of metal homeostasis and metalloproteins by the antibiotic holomycin. ACS Chem. Biol. 2023, 18, 1909–1914. [Google Scholar] [CrossRef]
- Chan, A.N.; Wever, W.J.; Massolo, E.; Allen, S.E.; Li, B. Reducing holomycin thiosulfonate to its disulfide with thiols. Chem. Res. Toxicol. 2019, 32, 400–404. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Lu, J.; Wang, J.; Ren, X.; Zhang, L.; Gao, Y.; Rottenberg, M.E.; Holmgren, A. Synergistic antibacterial effect of silver and ebselen against multidrug-resistant Gram-negative bacterial infections. EMBO Mol. Med. 2017, 9, 1165–1178. [Google Scholar] [CrossRef]
- Bouhrour, N.; Nibbering, P.H.; Bendali, F. Medical Device-Associated Biofilm Infections and Multidrug-Resistant Pathogens. Pathogens 2024, 13, 393–444. [Google Scholar]
- Penesyan, A.; Nagy, S.S.; Kjelleberg, S.; Gillings, M.R.; Paulsen, I.T. Rapid microevolution of biofilm cells in response to antibiotics. NPJ Biofilms Microbiomes 2019, 5, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Budia-Silva, M.; Kostyanev, T.; Ayala-Montaño, S.; Bravo-Ferrer Acosta, J.; Garcia-Castillo, M.; Cantón, R.; Goossens, H.; Rodriguez-Baño, J.; Grundmann, H.; Reuter, S. International and regional spread of carbapenem-resistant Klebsiella pneumoniae in Europe. Nat. Commun. 2024, 15, 5092–5102. [Google Scholar] [CrossRef]
- Jiang, J.; Long, T.; Porter, A.; Lovey, A.; Lee, A.; Jacob, J.T.; Arias, C.; Bonomo, R.; Kalayjian, R.; Zhao, Y. Carbapenem-resistant, virulence plasmid–harboring Klebsiella pneumoniae, United States. Emerg. Infect. Dis. 2025, 31, 761–771. [Google Scholar] [CrossRef]
- CLSI M07-A10; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2015.
- EUCAST. The European Committee on Antimicrobial Susceptibility Testing (EUCAST) Broth Microdilution Reference Method for MIC Determination (ISO-20776-1): Standard Protocol and Guidance. Available online: https://www.eucast.org (accessed on 19 June 2025).
- Schön, T.; Werngren, J.; Machado, D.; Borroni, E.; Wijkander, M.; Lina, G.; Mouton, J.; Matuschek, E.; Kahlmeter, G.; Giske, C.; et al. Multicentre testing of the EUCAST broth microdilution reference method for MIC determination on Mycobacterium tuberculosis. Clin. Microbiol. Infect. 2021, 27, 288. [Google Scholar] [CrossRef]
- Jenkins, S.G.; Schuetz, A.N. Current concepts in laboratory testing to guide antimicrobial therapy. Mayo Clin. Proc. 2012, 87, 290–308. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, K.A.; Imtiaz, M.; Parvez, A.; Rai, P.K.; Jaremko, M.; Emwas, A.-H.; Bholay, A.D.; Fatmi, M.Q. In vitro and in silico approaches for the evaluation of antimicrobial activity, time-kill Kinetics, and anti-biofilm potential of thymoquinone (2-methyl-5-propan-2-ylcyclohexa-2,5-diene-1,4-dione) against selected human pathogens. Antibiotics 2022, 11, 79. [Google Scholar] [CrossRef] [PubMed]
- Peeters, E.; Nelis, H.J.; Coenye, T. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J. Microbiol. Methods 2008, 72, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Schlafer, S.; Meyer, R.L. Confocal microscopy imaging of the biofilm matrix. J. Microbiol. Methods 2017, 138, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Priester, J.H.; Horst, A.M.; Van de Werfhorst, L.C.; Saleta, J.L.; Mertes, L.A.K.; Holden, P.A. Enhanced visualization of microbial biofilms by staining and environmental SEM. J. Microbiol. Methods 2007, 68, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Berney, M.; Hammes, F.; Bosshard, F.; Weilenmann, H.U.; Egli, T. Assessment and interpretation of bacterial viability by using the LIVE/DEAD BacLight Kit in combination with flow cytometry. Appl. Environ. Microbiol. 2007, 73, 3283–3290. [Google Scholar] [CrossRef]
- Koon, M.A.; Almohammed Ali, K.; Speaker, R.M.; McGrath, J.P.; Linton, E.W.; Steinhilb, M.L. Preparation of prokaryotic and eukaryotic organisms using chemical drying for morphological analysis in scanning electron microscopy (SEM). J. Vis. Exp. 2019, 143, e58761. [Google Scholar]
- Ma, B.; Fang, C.; Zhang, J.; Wang, M.; Luo, X.; Hou, Z. Contemporaneous measurement of outer and inner membrane permeability in gram-negative bacteria. Bio Protoc. 2020, 5, e3548. [Google Scholar] [CrossRef]
- Foss, M.H.; Eun, Y.J.; Grove, C.I.; Pauw, D.A.; Sorto, N.A.; Rensvold, J.W.; Pagliarini, D.J.; Shaw, J.T.; Weibel, D.B. Inhibitors of bacterial tubulin target bacterial membranes in vivo. MedChem. Comm. 2013, 4, 112–119. [Google Scholar] [CrossRef]
- Wang, H.; Joseph, J.A. Quantifying cellular oxidative stress by dichlorofluorescein assay using microplate reader. Free Radic. Biol Med. 1999, 27, 612–616. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for quantitative determination of glutathione and glutathione disulfide levels using enzymatic recycling method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, A.; Bjornstedt, M. Thioredoxin and thioredoxin reductase. Methods Enzymol. 1995, 252, 199–208. [Google Scholar]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother 2003, 52, 1. [Google Scholar] [CrossRef] [PubMed]
- Youn, C.; Archer, N.K.; Miller, L.S. Research techniques made simple: Mouse bacterial skin infection models for immunity research. J. Investig. Dermatol. 2020, 140, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Hurlow, J.; Bowler, P.G. Clinical experience with wound biofilm and evidence-based strategies for prevention and management. Wounds Int. 2009, 1, 17–21. [Google Scholar]
- Xu, L.C.; Wo, Y.; Meyerhoff, M.E.; Siedlecki, C.A. In vivo biofilm formation on indwelling catheters and efficacy of antimicrobial coatings. Biomaterials 2012, 33, 6594–6602. [Google Scholar]
- Kadurugamuwa, J.L.; Sin, L.; Albert, E.; Yu, J.; Francis, K.; DeBoer, M.; Rubin, M.; Bellinger-Kawahara, C.; Parr, T.R., Jr.; Contag, P.R. Direct continuous method for monitoring biofilm infection in a mouse model. Infect Immun. 2003, 71, 882–890. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, E.; Tan, Q.; Yi, X.; Yao, J.; Duan, Y.; Huang, Y. Dual Redox Targeting by Pyrroloformamide A and Silver Ions Enhances Antibacterial and Anti-Biofilm Activity Against Carbapenem-Resistant Klebsiella pneumoniae. Antibiotics 2025, 14, 640. https://doi.org/10.3390/antibiotics14070640
Bai E, Tan Q, Yi X, Yao J, Duan Y, Huang Y. Dual Redox Targeting by Pyrroloformamide A and Silver Ions Enhances Antibacterial and Anti-Biofilm Activity Against Carbapenem-Resistant Klebsiella pneumoniae. Antibiotics. 2025; 14(7):640. https://doi.org/10.3390/antibiotics14070640
Chicago/Turabian StyleBai, Enhe, Qingwen Tan, Xiong Yi, Jianghui Yao, Yanwen Duan, and Yong Huang. 2025. "Dual Redox Targeting by Pyrroloformamide A and Silver Ions Enhances Antibacterial and Anti-Biofilm Activity Against Carbapenem-Resistant Klebsiella pneumoniae" Antibiotics 14, no. 7: 640. https://doi.org/10.3390/antibiotics14070640
APA StyleBai, E., Tan, Q., Yi, X., Yao, J., Duan, Y., & Huang, Y. (2025). Dual Redox Targeting by Pyrroloformamide A and Silver Ions Enhances Antibacterial and Anti-Biofilm Activity Against Carbapenem-Resistant Klebsiella pneumoniae. Antibiotics, 14(7), 640. https://doi.org/10.3390/antibiotics14070640







