Early Administration of Rifampicin Does Not Induce Increased Resistance in Septic Two-Stage Revision Knee and Hip Arthroplasty
Abstract
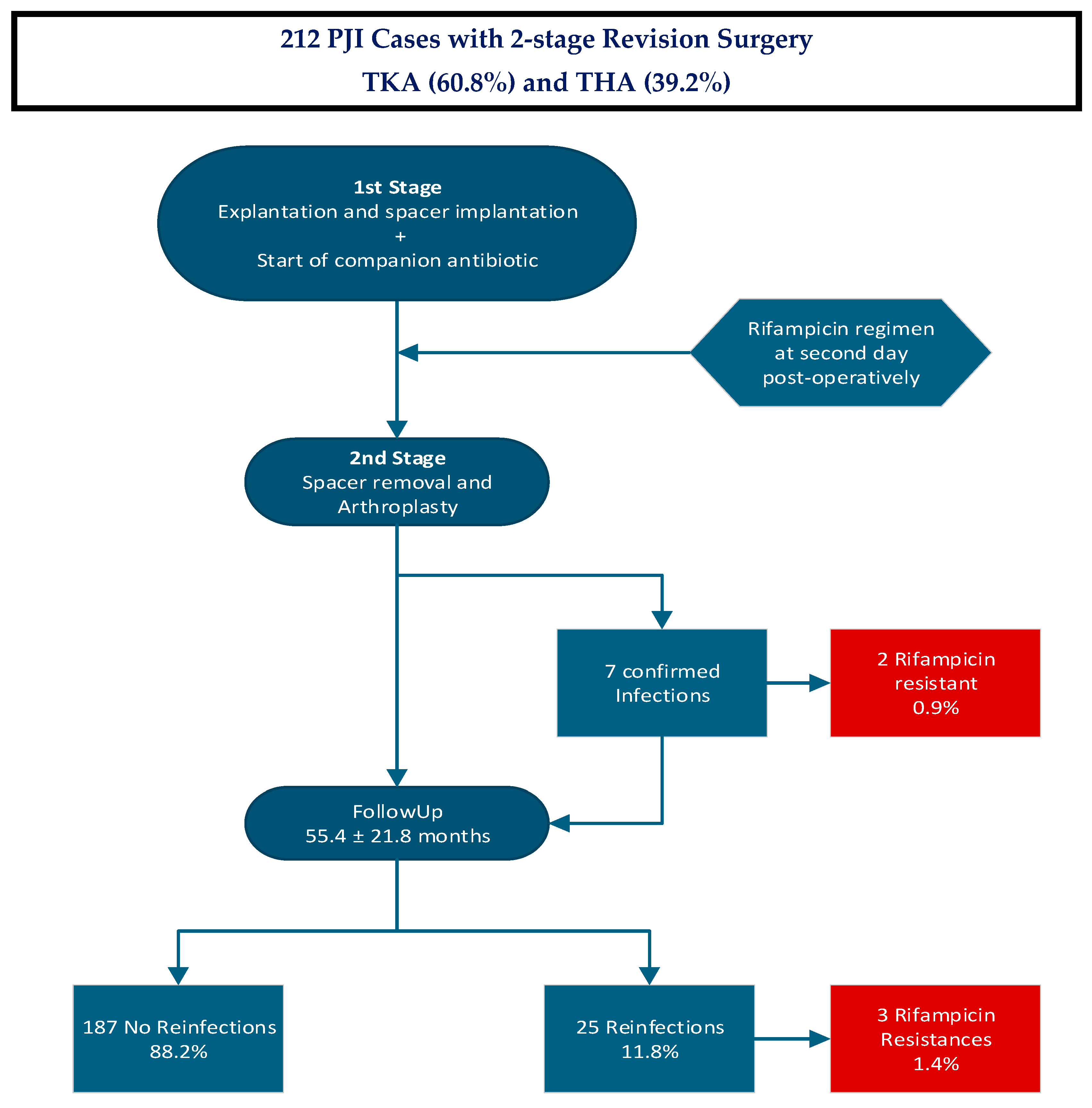
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patient Characteristics
4.2. Characteristics of PJI
4.3. Treatment Protocol
4.4. Tests at Second Stage Revision Surgery
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASA | American Society of Anesthesiologists |
| BMI | Body Mass Index |
| CCI | Charlson Comorbidity Index |
| DTT | Difficult to Treat |
| ETT | Easy to Treat |
| ICM | International Consensus on Musculoskeletal Infection |
| MRS | Methicillin-resistant staphylococci |
| MRSA | methicillin-resistant staphylococcus aureus |
| MRSE | methicillin-resistant staphylococcus epidermidis |
| MSIS | Musculoskeletal Infection Society |
| PJI | Periprosthetic Joint Infection |
| THA | Total Hip Arthroplasty |
| TKA | Total Knee Arthroplasty |
References
- Nelson, S.B.; Pinkney, J.A.; Chen, A.F.; Tande, A.J. Periprosthetic Joint Infection: Current Clinical Challenges. Clin. Infect. Dis. 2023, 77, e34–e45. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, B.H.; Berg, R.A.; Daley, J.A.; Fritz, J.; Bhave, A.; Mont, M.A. Periprosthetic joint infection. Lancet 2016, 387, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Blersch, B.P.; Sax, F.H.; Mederake, M.; Benda, S.; Schuster, P.; Fink, B. Effect of Multiantibiotic-Loaded Bone Cement on the Treatment of Periprosthetic Joint Infections of Hip and Knee Arthroplasties-A Single-Center Retrospective Study. Antibiotics 2024, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Fink, B. Revision of late periprosthetic infections of total hip endoprostheses: Pros and cons of different concepts. Int. J. Med. Sci. 2009, 6, 287–295. [Google Scholar] [CrossRef]
- Goud, A.L.; Harlianto, N.I.; Ezzafzafi, S.; Veltman, E.S.; Bekkers, J.E.J.; Van der Wal, B.C.H. Reinfection rates after one- and two-stage revision surgery for hip and knee arthroplasty: A systematic review and meta-analysis. Arch. Orthop. Trauma Surg. 2021, 143, 829–838. [Google Scholar] [CrossRef]
- Svensson, K.; Rolfson, O.; Kärrholm, J.; Mohaddes, M. Similar risk of re-revision in patients after one-or two-stage surgical revision of infected total hip arthroplasty: An analysis of revisions in the Swedish Hip Arthroplasty Register 1979–2015. J. Clin. Med. 2019, 8, 485. [Google Scholar] [CrossRef]
- Steadman, W.; Chapman, P.R.; Schuetz, M.; Schmutz, B.; Trampuz, A.; Tetsworth, K. Local Antibiotic Delivery Options in Prosthetic Joint Infection. Antibiotics 2023, 12, 752. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- Pupaibool, J. The Role of Rifampin in Prosthetic Joint Infections: Efficacy, Challenges, and Clinical Evidence. Antibiotics 2024, 13, 1223. [Google Scholar] [CrossRef]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Executive Summary: Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, 1–10. [Google Scholar] [CrossRef]
- Lazarinis, S.; Hailer, N.P.; Jarhult, J.D.; Bruggemann, A. Incidence of Rifampicin Resistance in Periprosthetic Joint Infection: A Single-Centre Cohort Study on 238 Patients. Antibiotics 2023, 12, 1499. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, E.; Bramer, W.; Anas, A.A. Clinical outcomes of rifampicin combination therapy in implant-associated infections due to staphylococci and streptococci: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2024, 63, 107015. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Sendi, P. Role of Rifampin against Staphylococcal Biofilm Infections In Vitro, in Animal Models, and in Orthopedic-Device-Related Infections. Antimicrob. Agents Chemother. 2019, 63, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Beldman, M.; Lowik, C.; Soriano, A.; Albiach, L.; Zijlstra, W.P.; Knobben, B.A.S.; Jutte, P.; Sousa, R.; Carvalho, A.; Goswami, K.; et al. If, When, and How to Use Rifampin in Acute Staphylococcal Periprosthetic Joint Infections, a Multicentre Observational Study. Clin. Infect. Dis. 2021, 73, 1634–1641. [Google Scholar] [CrossRef]
- Karlsen, Ø.E.; Borgen, P.; Bragnes, B.; Figved, W.; Grogaard, B.; Rydinge, J.; Sandberg, L.; Snorrason, F.; Wangen, H.; Witsoe, E.; et al. Rifampin combination therapy in staphylococcal prosthetic joint infections: A randomized controlled trial. J. Orthop. Surg. Res. 2020, 15, 365. [Google Scholar] [CrossRef]
- Kramer, T.S.; Soriano, A.; Tedeschi, S.; Chen, A.F.; Tattevin, P.; Senneville, E.; Gomez-Junyent, J.; Birlutiu, V.; Petersdorf, S.; De Brito, V.D.; et al. Should We Use Rifampicin in Periprosthetic Joint Infections Caused by Staphylococci When the Implant Has Been Exchanged? A Multicenter Observational Cohort Study. Open Forum Infect. Dis. 2023, 10, ofad491. [Google Scholar] [CrossRef]
- Tshefu, K.; Zimmerli, W.; Waldvogel, F.A. Short-term administration of rifampin in the prevention or eradication of infection due to foreign bodies. Rev. Infect. Dis. 1983, 5 (Suppl. 3), S474–S480. [Google Scholar] [CrossRef]
- Darwich, A.; Dally, F.J.; Bdeir, M.; Kehr, K.; Miethke, T.; Hetjens, S.; Gravius, S.; Assaf, E.; Mohs, E. Delayed Rifampin Administration in the Antibiotic Treatment of Periprosthetic Joint Infections Significantly Reduces the Emergence of Rifampin Resistance. Antibiotics 2021, 10, 1139. [Google Scholar] [CrossRef]
- Blersch, B.P.; Sax, F.H.; Schuster, P.; Fink, B. Predictive Factors for Risk of Reinfection in Septic Two-Stage Revision of Total Hip and Knee Arthroplasties. Antibiotics 2025, 14, 167. [Google Scholar] [CrossRef]
- Krizsan, G.; Sallai, I.; Veres, D.S.; Prinz, G.; Szeker, D.; Skaliczki, G. Rifampicin resistance and risk factors associated with significantly lower recovery rates after two-stage revision in patients with prosthetic joint infection. J. Glob. Antimicrob. Resist. 2022, 30, 231–236. [Google Scholar] [CrossRef]
- Nguyen, S.; Robineau, O.; Titecat, M.; Blondiaux, N.; Valette, M.; Loiez, C.; Beltrand, E.; Migaud, H.; Senneville, E. Influence of daily dosage and frequency of administration of rifampicin-levofloxacin therapy on tolerance and effectiveness in 154 patients treated for prosthetic joint infections. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Zmistowski, B.; Berbari, E.F.; Bauer, T.W.; Springer, B.D.; Della Valle, C.J.; Garvin, K.L.; Mont, M.A.; Wongworawat, M.D.; Zalavras, C.G. New definition for periprosthetic joint infection: From the Workgroup of the Musculoskeletal Infection Society. Clin. Orthop. Relat. Res. 2011, 469, 2992–2994. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, J.; Tan, T.L.; Goswami, K.; Higuera, C.; Della Valle, C.; Chen, A.F.; Shohat, N. The 2018 Definition of Periprosthetic Hip and Knee Infection: An Evidence-Based and Validated Criteria. J. Arthroplast. 2018, 33, 1309–1314.e2. [Google Scholar] [CrossRef] [PubMed]
- Krenn, V.; Morawietz, L.; Perino, G.; Kienapfel, H.; Ascherl, R.; Hassenpflug, G.J.; Thomsen, M.; Thomas, P.; Huber, M.; Kendoff, D.; et al. Revised histopathological consensus classification of joint implant related pathology. Pathol. Res. Pract. 2014, 210, 779–786. [Google Scholar] [CrossRef]
- Krenn, V.; Otto, M.; Morawietz, L.; Hopf, T.; Jakobs, M.; Klauser, W.; Schwantes, B.; Gehrke, T. Histopathologic diagnostics in endoprosthetics: Periprosthetic neosynovialitis, hypersensitivity reaction, and arthrofibrosis. Orthopade 2009, 38, 520–530. [Google Scholar] [CrossRef]
- Müller, M.; Morawietz, L.; Hasart, O.; Strube, P.; Perka, C.; Tohtz, S. Histopathological diagnosis of periprosthetic joint infection following total hip arthroplasty: Use of a standardized classification system of the periprosthetic interface membrane. Orthopäde 2009, 38, 1087–1096. [Google Scholar] [CrossRef]
- Teves, J.; Holc, F.; Castro Lalin, A.; Garcia-Mansilla, A.; Vildoza, S.R.B.; Carbo, L.; Costantini, J. Are frailty scores superior to the ASA score in predicting complications, hospital stay, and readmissions in total knee replacement? A comparative study between octogenarian and septuagenarian patients. Rev. Esp. Cir. Ortop. Traumatol. 2024, 68, 128–133. [Google Scholar] [CrossRef]
- Quach, L.H.; Jayamaha, S.; Whitehouse, S.L.; Crawford, R.; Pulle, C.R.; Bell, J.J. Comparison of the Charlson Comorbidity Index with the ASA score for predicting 12-month mortality in acute hip fracture. Injury 2020, 51, 1004–1010. [Google Scholar] [CrossRef]
- Glasheen, W.P.; Cordier, T.; Gumpina, R.; Haugh, G.; Davis, J.; Renda, A. Charlson Comorbidity Index: ICD-9 Update and ICD-10 Translation. Am. Health Drug Benefits 2019, 12, 188–197. [Google Scholar]
- Fink, B.; Grossmann, A.; Fuerst, M.; Schafer, P.; Frommelt, L. Two-stage cementless revision of infected hip endoprostheses. Clin. Orthop. Relat. Res. 2009, 467, 1848–1858. [Google Scholar] [CrossRef]
- Fink, B.; Oremek, D. The Transfemoral Approach for Removal of Well-Fixed Femoral Stems in 2-Stage Septic Hip Revision. J. Arthroplast. 2016, 31, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Schafer, P.; Fink, B.; Sandow, D.; Margull, A.; Berger, I.; Frommelt, L. Prolonged bacterial culture to identify late periprosthetic joint infection: A promising strategy. Clin. Infect. Dis. 2008, 47, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Faschingbauer, M.; Bieger, R.; Kappe, T.; Weiner, C.; Freitag, T.; Reichel, H. Difficult to treat: Are there organism-dependent differences and overall risk factors in success rates for two-stage knee revision? Arch. Orthop. Trauma Surg. 2020, 140, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Ledezma, C.; Higuera, C.A.; Parvizi, J. Success after treatment of periprosthetic joint infection: A Delphi-based international multidisciplinary consensus. Clin. Orthop. Relat. Res. 2013, 471, 2374–2382. [Google Scholar] [CrossRef]
| Sum | Age | BMI | Diabetes | Rheum. Disorders | ASA Risk Classification | ||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | M (SD) | M (SD) | N (%) | N (%) | ASA1 | ASA2 | ASA3 | ASA4 | |
| TKA | 83 (39.2%) | 70.29 (11.41) | 32.22 (7.75) | 20 (24.1%) | 0 (0.0%) | 4 (4.8%) | 34 (41.0%) | 42 (50.6%) | 3 (3.6%) |
| THA | 129 (60.8%) | 68.50 (11.32) | 29.28 (6.12) | 24 (18.6%) | 10 (7.8%) | 1 (0.8%) | 60 (46.5%) | 65 (50.4%) | 3 (2.3%) |
| Total | 212 (100%) | 69.20 (11.36) | 30.43 (6.94) | 44 (20.8%) | 10 (4.7%) | 5 (2.4%) | 94 (44.3%) | 107 (50.5%) | 6 (2.8%) |
| Antibiotic One | Antibiotic Two | Antibiotic Three | Number |
|---|---|---|---|
| Vancomycin | Rifampicin | 58 | |
| Flucloxacillin | Rifampicin | 46 | |
| Ampicillin/Sulbactam | Rifampicin | 43 | |
| Cefuroxim | Rifampicin | 12 | |
| Levofloxacin | Rifampicin | 11 | |
| Imipenem | Rifampicin | 10 | |
| Penicillin G | Rifampicin | 9 | |
| Daptomycin | Rifampicin | 7 | |
| Amoxicillin/Clavulanacid | Rifampicin | 4 | |
| Ampicillin | Rifampicin | 3 | |
| Vancomycin | Meropenem | Rifampicin | 3 |
| Imipenem/cilastatin | Rifampicin | 2 | |
| Vancomycin | Imipenem | Rifampicin | 2 |
| Flucloxacillin | Levofloxacin | Rifampicin | 1 |
| Flucloxacillin | Meropenem | Rifampicin | 1 |
| Antibiotic One | Antibiotic Two | Antibiotic Three | Number |
|---|---|---|---|
| Levofloxacin | Rifampicin | 141 | |
| Amoxicillin/Clavulanacid | Rifampicin | 31 | |
| Cotrimoxazol | Rifampicin | 13 | |
| Linezolid | Rifampicin | 9 | |
| Clindamycin | Rifampicin | 7 | |
| Ampicillin/Sulbactam | Rifampicin | 4 | |
| Moxifloxacin | Rifampicin | 3 | |
| Clarythromycin | Levofloxacin | Rifampicin | 2 |
| Amoxicillin/Clavulanacid | Levofloxacin | Rifampicin | 1 |
| Cotrimoxazol | Levofloxacin | Rifampicin | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grünwald, L.; Blersch, B.P.; Fink, B. Early Administration of Rifampicin Does Not Induce Increased Resistance in Septic Two-Stage Revision Knee and Hip Arthroplasty. Antibiotics 2025, 14, 610. https://doi.org/10.3390/antibiotics14060610
Grünwald L, Blersch BP, Fink B. Early Administration of Rifampicin Does Not Induce Increased Resistance in Septic Two-Stage Revision Knee and Hip Arthroplasty. Antibiotics. 2025; 14(6):610. https://doi.org/10.3390/antibiotics14060610
Chicago/Turabian StyleGrünwald, Leonard, Benedikt Paul Blersch, and Bernd Fink. 2025. "Early Administration of Rifampicin Does Not Induce Increased Resistance in Septic Two-Stage Revision Knee and Hip Arthroplasty" Antibiotics 14, no. 6: 610. https://doi.org/10.3390/antibiotics14060610
APA StyleGrünwald, L., Blersch, B. P., & Fink, B. (2025). Early Administration of Rifampicin Does Not Induce Increased Resistance in Septic Two-Stage Revision Knee and Hip Arthroplasty. Antibiotics, 14(6), 610. https://doi.org/10.3390/antibiotics14060610





