Abstract
Background: Clove (Syzygium aromaticum) essential oil is widely recognized for its potent antimicrobial properties, making it a valuable natural preservative in food products, particularly in meat and meat derivatives, where it helps extend shelf life and enhance food safety. Methods: This systematic review aims to evaluate the application of clove essential oil in meat and meat products, following the PRISMA 2020 methodology, to analyze its antimicrobial efficacy and its impact on the preservation of these products. The information search was carried out in the PubMed, ScienceDirect, SCOPUS, and Web of Science databases and included research articles in English published between 1999 and 2024, and 37 studies were confirmed as eligible. Results: Due to the heterogeneity of methodologies and concentrations evaluated, a narrative analysis was chosen, organizing the studies into three categories according to the application of the essential oil: direct addition, use in edible films and coatings, and encapsulation. The analysis included the main components of the essential oil, the activity analysis method, a concentration evaluation, storage conditions, the activities obtained, and a sensory evaluation. However, variability in methodologies and concentrations made direct comparison between studies difficult. Conclusions: Overall, this review confirms the effectiveness of clove essential oil in preserving meat and meat products but highlights the need to standardize its concentration and application conditions to optimize its use in the food industry.
1. Introduction
The meat industry, as an integral part of the global food chain, constantly faces the challenge of ensuring the quality and safety of its meat products [1]. In a context in which the demand for healthier and more sustainable foods is constantly increasing, the search for natural solutions has become a promising alternative to reducing dependence on chemical additives in the production and preservation of meat and meat products. Thus, among the natural ingredients that have attracted the attention of researchers and professionals in the meat industry are compounds of plant origin [2,3].
Syzygium aromaticum, commonly known as clove, is a very interesting plant species with enormous potential as a food preservative and is a rich source of antioxidant compounds [4]. Phytochemical analysis of various types of extract has revealed the presence of different chemical groups such as phenolic compounds, sesquiterpenes, and monoterpenes. Furthermore, eugenol, eugenol acetate, and caryophyllene have been reported as the main compounds present in its essential oil (EO) [5,6]. The presence of these molecules in the species Syzygium aromaticum strongly supports its wide range of biological activities reported in the literature [7].
Essential oils (EOs) are highly concentrated and volatile compounds extracted from various plant parts [7]. These compounds, characterized by their aromatic properties, have become the subject of interdisciplinary research ranging from organic chemistry and botany to medicine and psychology [8]. Among EOs, clove essential oil (CEO), derived from the dried buds of the clove tree (Syzygium aromaticum), stands out for its distinctive warm and spicy aroma. Furthermore, this oil is characterized by its richness in bioactive compounds, mainly with antimicrobial and antioxidant properties, which have been well documented [5]. The literature shows several studies on the effects of CEO on the microbiological, sensory, and nutritional quality of meat and meat products [9].
The results obtained by several authors have revealed that EOs present promising antimicrobial properties against a wide range of pathogenic and spoilage microorganisms, including bacteria, fungi, and yeasts [10]. Indeed, several studies have suggested that the active components of EO, such as eugenol, have the ability to damage the cell membranes of microorganisms, inhibiting their growth and survival [11]. These findings suggest that CEO could be an effective conservation barrier to improve the quality and microbiological safety of meat and meat products, reducing the need for synthetic chemical additives that often raise concerns about antimicrobial resistance.
Finally, this systematic review aims to compile and analyze the available scientific evidence on the application of CEO in meat and meat products, with the aim of providing a comprehensive view of its effectiveness and possible applications in a meat production context, thus producing healthier and safer foods, which is in line with the demands of current consumers [12].
2. Selected Studies
This systematic review was carried out following the PRISMA reporting guideline in accordance with the PRISMA 2020 statement [13]. Searches of the PubMed, ScienceDirect, Scopus, and Web of Science databases yielded 994 records. Following the removal of duplicates, 621 studies were screened by title and abstract, which yielded 113 studies for retrieval for full-text analysis. Among the excluded reports are those that only report the biological activity of the CEO (e.g., [14]); these were excluded since the EO is not tested in meat or meat products. Reports that publish the use of EOs as food supplements for slaughter animals (e.g., [15]) will also be excluded, and these reports were excluded since EO was not used directly in meat or meat products. All these reports were retrieved, and 37 were included in the final review following full-text assessments. A PRISMA flowchart [13] depicting this process is displayed in Figure 1. A total of 37 individual journal article reports were included and are available in Supplementary Information Table S1 [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52].
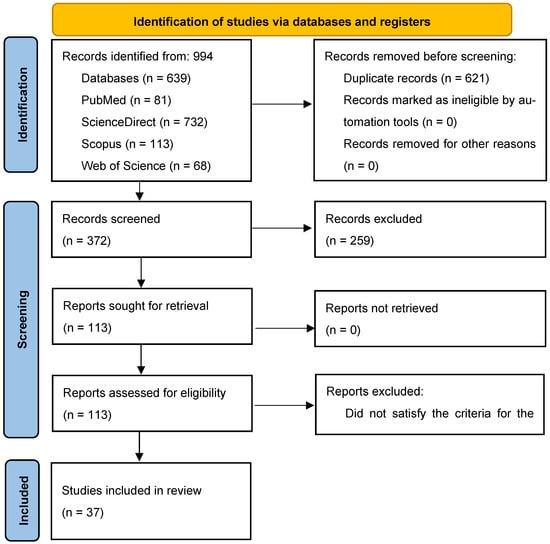
Figure 1.
PRISMA flowchart of the study selection procedure from the reviewed article.
The findings are presented according to the method of incorporation of the CEO into the food matrix, that is, through direct addition, via edible films or coatings, and as encapsulated pills. In each incorporation method section, the results of the different biological activities tested are detailed.
3. Incorporation Matrix of Clove Essential Oil in Meat and Meat Products
The most common meat matrix for CEO testing is beef (16 studies). Figure 2 shows the different matrices used to test the EO of cloves and the number of studies carried out in each one. In total, 44 studies are shown, this is because in some articles, more than one matrix is used.
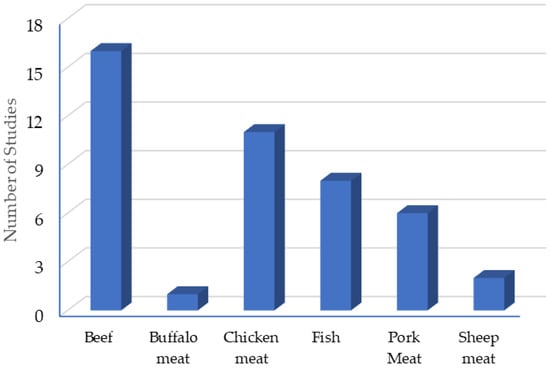
Figure 2.
Clove essential oil application matrices.
An analysis of the presentation of the different products shows that 7 of the 37 studies were conducted on sausages (Figure 3). Of the different presentations, the hamburger, mortadella, and sausages are considered meat products, since their composition contains other ingredients besides meat.
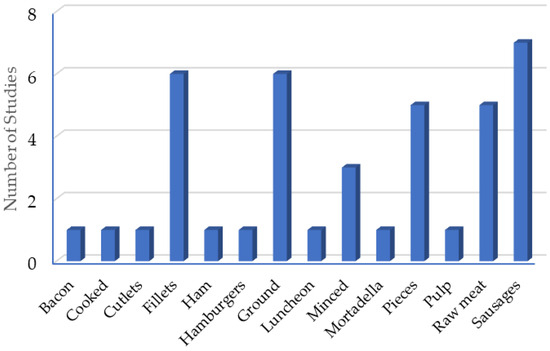
Figure 3.
Presentation of the matrices for the application of clove essential oil.
4. Direct Addition of Clove Essential Oil into Meat and Meat Products
Direct addition refers to the incorporation of clove EO directly into meats and meat products. Once the EO was incorporated, antimicrobial and antifungal activity was determined. Additionally, some studies provided information on sensory evaluations.
4.1. Antibacterial Activity of Direct Addition
Meat is a food with high nutritional value, as it provides essential proteins, vitamins, and minerals that are required in a balanced diet. However, its preservation methods are somewhat limited when it comes to marketing it and maintaining its nutritional quality and freshness; due to its structure, it is an ideal food for the proliferation of pathogenic microorganisms. In turn, meat products have similar limitations, since meat is the main ingredient, so processing, storage, and marketing meat products must be carried out in the best way to reduce damage. The implementation of CEO arises from the proposal to implement natural preservatives due to its strong antimicrobial and antifungal activity. Table 1 shows the antibacterial activity obtained through the direct addition of CEO to meat and meat products, where the information is found in a summarized and synthesized form. The main bacteria studied were Listeria monocytogenes, Escherichia coli, Staphylococcus aureus, and Bacillus cereus.

Table 1.
Antibacterial activity obtained through the direct addition of clove essential oil to meat and meat products.
In general, all the articles showed that the concentration of EO directly influences the inhibition of microbial growth. It was observed that in the articles where EO concentrations greater than 1% were used, the reduction in bacterial load was greater. However, as mentioned in the sensory evaluation, increasing the content had a negative impact on the taste of the samples. In turn, in most of the studies, promising results were obtained on the antimicrobial activity in vitro (commonly dilution in broth) and in vivo (meat), given that in all the samples with treatments, they had lower values in their counts in relation to the control samples. Authors such as Sharma et al. [44] attribute the reduction in these microorganisms to the changes in the environment that the oil exerted on the matrix.
According to the research by Nunes Barbosa et al. [33], Hernández-Ochoa et al. [26], Guran et al. [24], and Khaleque et al. [29], EO showed a greater inhibitory effect on Gram-positive bacteria compared to Gram-negative bacteria. The authors explain that this is due to the presence and direct interaction with the peptidoglycan layer present in Gram-positive bacteria. The membrane of Gram-negative bacteria contains lipopolysaccharides, which prevent the passage of hydrophobic molecules, creating a barrier between these microorganisms and the EO, thus obtaining favorable results of the inhibitory effect on bacteria such as L. monocytogenes, S. aureus, and B. cereus.
On the other hand, some authors such as Tajik et al. [48] sought to work with oils and extracts to increase their effectiveness; however, the result was not as expected. Based on their research, it was observed that the combination of CEO and grape (Vitis vinifera) extract generated an additive effect, so the authors recommend evaluating the synergistic effect before applying their mixture. The antimicrobial effect is greater in in vitro analyses because there are no other compounds that can intervene or influence the result, while in vivo analyses showed that there are intrinsic factors such as pH variations and macromolecules in contact, among others [44].
Clove essential oil has shown variable antimicrobial efficacy when directly applied to meat and meat products. In some studies, CEO exhibited strong antimicrobial activity against specific pathogens, whereas in others, no significant inhibition was observed. For instance, in ground beef, a 10% CEO concentration completely inactivated Listeria monocytogenes within three days, while a 5% concentration was ineffective throughout the storage period [29]. However, in cured ham, the addition of 10% CEO did not inhibit L. monocytogenes [20]. These discrepancies may be attributed to differences in the food matrix, CEO concentration, and potential protective effects of food components on microbial survival. In poultry products, CEO combined with irradiation achieved complete pathogen inactivation in minced chicken meat, whereas CEO alone reduced microbial counts in a dose-dependent manner [22]. Similarly, in chicken sausages, the CEO treatment maintained microbial counts below permissible limits; however, it was not the most effective method compared to alternative treatments [43]. Regarding beef burgers, CEO provided moderate control over aerobic and psychrophilic microorganisms, though microbial counts gradually increased over prolonged storage at −18 °C [16]. In buffalo meat burgers, CEO exhibited limited efficacy against L. monocytogenes, and its combination with grape seed extract did not produce a significant synergistic effect [48]. Another crucial aspect is the sensory acceptability of CEO-treated products. In several studies, high CEO concentrations, such as 10% in ground beef, were deemed unacceptable due to their strong flavor, particularly in East Asian consumer groups [29]. In beef hamburgers, CEO at 250 mg/kg was well accepted, while 500 mg/kg imparted an overpowering clove flavor, leading to lower sensory scores [16]. In pork meat and bacon, CEO combined with cinnamon oil showed no significant differences compared to commercial curing salts, indicating strong antimicrobial potential without compromising the sensory quality [46].
4.2. Antifungal Activity of Direct Addition
Molds and yeasts are microorganisms that can cause food degradation and spoilage, which can result in noticeable sensory changes such as bad odors, unpleasant flavors, and changes in texture. In addition, some molds and yeasts can produce toxic compounds called mycotoxins, which are dangerous to human health if ingested in significant quantities [53]. Meat is a highly perishable food due to its high water activity and favorable pH, making it an optimal medium for the proliferation of pathogenic microorganisms that cause spoilage. Habashy et al. [25] argue that the presence of mold in foods such as meat or in a meat product is due to the hygienic conditions that existed during the handling, processing, and storage process, as well as the quality of the spices that are added to the product.
Authors such as Hu et al. [54] evaluated the antifungal activity of EOs against fungi; the study showed that, although this EO has a strong and moderate activity against these microorganisms, the concentration is a key factor for their inhibition. Rana et al. [55] states that CEO presented greater antifungal activity against the genera Aspergillus and Mucor spp. and that eugenol is a powerful antifungal agent, so it is an inhibitor against all strains of fungi. However, Marchese et al. [56] establish that the effectiveness of the EO is due to the synergistic effect that occurs between eugenol and compounds of lesser proportion. Similarly, Cai et al. [57] mention that this substance causes great cellular damage in the morphology of fungi, this being its main antifungal characteristic. Table 2 shows the antifungal activity obtained via the direct addition of CEO to meat and meat products.

Table 2.
Antifungal activity obtained via the direct addition of clove essential oil to meat and meat products.
The antifungal activity of CEO has been evaluated in various meat products through direct application, showing variable results depending on the concentration applied and the type of product. In a study with several meat products (raw meat, fresh minced meat, ham slices, beef hamburgers, and sausages), the efficacy of CEO at concentrations between 0.5 and 1% (v/w) was evaluated against various fungal genera, including Aspergillus, Penicillium, Cladosporium, and others. The highest total mold count (2.85 CFU/g) was recorded in sausages, while the lowest appeared in raw meat. Regarding species distribution, Aspergillus predominated in all products (between 41.7% and 49%), followed by Penicillium (19–25.5%) and Cladosporium (6.3–11.9%). The 1% CEO concentration proved to be the most effective, causing significant inhibition of fungal growth. However, the treated products were considered unappetizing from a sensory perspective [25]. In the case of chicken sausages, CEO was evaluated at a concentration of 0.25% during storage at 25 °C for 15 days. The results indicated that CEO produced lower yeast and mold counts compared to control samples and other EOs evaluated. Nevertheless, from the fifth day onwards, all samples showed an unattractive appearance, unpleasant flavors, a loss of texture, and reduced juiciness, which limits its practical application from a sensory perspective [43]. The activity of CEO was also evaluated against yeasts in sheep meat at a concentration of 0.25% (v/w) during storage at 4 °C for 9 days. In this case, the inhibitory effect of CEO against yeast growth was very weak, with no significant difference compared to control samples on the third day. At the end of the testing period, samples treated with CEO obtained good scores for color and overall acceptability but low scores for odor [18]. In bonito burgers, CEO was evaluated at a concentration of 2.65 ppm during storage at 4 °C for 16 days. Initial counts of molds and yeasts were 1.3 log CFU/g, increasing to 3.40 log CFU/g in samples with CEO, indicating that it was not effective in inhibiting the growth of these microorganisms. In the sensory evaluation, no significant differences were observed in color, appearance, odor, and texture, but samples with CEO received low scores for flavor and overall acceptability [24].
The limitations in the analysis of the antimicrobial and antifungal efficacy of CEO in meat and meat products stem from the variability in the results obtained, making it difficult to generalize its effectiveness. First, the discrepancies in antimicrobial effects observed across different studies can be attributed to the influence of the food matrix, the concentration used, and the potential protective effects of food components on microbial survival. For example, while a 10% CEO concentration achieved complete inactivation of L. monocytogenes in ground beef [29], the same concentration showed no inhibitory effect in cured ham [20], suggesting that food composition may affect the bioavailability and efficacy of CEO. Additionally, combining CEO with other treatments, such as irradiation in poultry products, has been shown to enhance its antimicrobial action; however, this introduces variability in results and makes it difficult to attribute the effect exclusively to CEO. In terms of antifungal activity, studies show that CEO efficacy depends on the type of meat and the concentration applied, but in some cases, even at effective concentrations, a negative impact on sensory acceptability has been reported, limiting its commercial applicability.
5. Edible Films and Coatings Containing Clove Essential Oil
One of the main limitations in using EOs as additives is their strong aroma and prominent flavor [32]. As evidenced in the previous section, the direct application of CEO negatively influenced some attributes related to the sensory perception evaluated in the different matrices. Likewise, clove is a photosensitive and thermolabile substance, since it can easily decompose under normal environmental conditions [58]. In view of this problem, some authors investigated its EO, but through the implementation of edible films and coatings. In turn, numerous studies indicate that CEO has high antimicrobial and antifungal power (Table 1 and Table 2). Therefore, several authors proposed incorporating this additive into active packaging in such a way as to minimize the adverse effects on its direct addition, thus increasing its activity and effectiveness against deterioration reactions and microbiological growth. The main objective of this barrier technology is to minimize contact between the product matrix and its environment, thereby inhibiting bacterial growth and slowing down oxidative and other degradation reactions. Regarding the quantification of studies, a total of 37 articles that analyzed the EO through this form of application were obtained, of which 16 studies investigated antimicrobial activity and only 1 investigated the antifungal activity. Some of them studied two activities.
5.1. Antibacterial Activity in Edible Films and Coatings
Food is wasted every day worldwide. One of the main causes is the deterioration of its surface, which is caused by microbiological agents. The meat of an animal is highly perishable since it has a high water activity and a pH that is favorable for the development of microorganisms, so it is prone to microbiological alterations from the moment it is slaughtered. Although refrigeration temperatures are always used, from processing to the supply chain, this method has certain limitations on the shelf life of the food. It is necessary to complement it with other methods that allow the shelf life of the product to be increased, such as active films and edible coatings. Likewise, meat products are susceptible to microbiological contamination at any stage of processing and distribution. Microbial growth can cause damage to consumer health. Table 3 presents the results of research that has been carried out on the antimicrobial activity of films and edible coatings containing CEO in meat and meat products.

Table 3.
Antibacterial activity of clove essential oil incorporated into edible films and coatings for meat and meat products.
The results of the research showed that regardless of the food matrix and the biodegradable compounds of the films and coatings, the incorporation of CEO further improved the inhibition compared to the coated samples that did not have this bioactive compound and the control samples. In the case of beef, the research by Stoleru et al. [47] showed that the coating with polylactic acid and chitosan had a greater inhibitory effect on Salmonella Typhimurium, E. coli, and L. monocytogenes. On the other hand, Saricaoglu and Turhan [41] carried out their research on a fermented meat product, where they evaluated the S. aureus count for 45 days. The result showed minimal growth up to 30 days; however, there was no presence of coliforms in the fermented sausage. Likewise, Radha et al. [34] revealed inhibition levels of 0.65 log CFU/g and 0.57 log CFU/g for lactic acid bacteria and Pseudomonas spp.
Regarding pork, Jun et al. [30] obtained favorable results on the inhibition of S. aureus thanks to the mechanism of action of eugenol against this bacterium. According to the research by Xu et al. [59], the majority of compounds of CEO affect the cell walls of the bacteria, leading to a loss of intracellular substances and causing the death of the microorganism. In turn, Roy et al. [39] showed total inhibition against L. monocytogenes and slightly less in E. coli. Curiously, their result contradicts the findings by Stoleru et al. [47], where the antimicrobial activity was greater in E. coli than in L. monocytogenes.
Regarding chicken meat, the work by Shukla et al. [45] showed that the inhibition of a chitosan coating with CEO was greater in Gram-negative bacteria (coliforms), keeping their count below the limit (2 log CFU/g); In contrast, in Gram-positive bacteria (S. aureus), it crossed the limit; however, the film with the highest concentration exceeded day 35. Another article that focused on chicken meat was that by Hosseini et al. [28], where a reduction in psychrotropic bacteria, enterobacteria, and Pseudomonas was evident, although the film could not reduce lactic acid bacteria. However, the results presented by Requena et al. [37] revealed promising effects against Listeria innocua and E. coli in the in vitro analysis, but in the in vivo analysis, it was lower. The authors assume that this decrease is due to contact with the chemical composition of the food matrix used. In turn, Fernández-Pan et al. [21] demonstrated that mesophilic groups had lower growth than psychrophilic bacteria.
Regarding fish, Nisar et al. [32] found that an edible pectin coating with CEO at a higher concentration was more potent in reducing the growth of psychrophilic bacteria, reducing 2.3 log CFU/g. Some articles such as those by Viera et al. [51] specify that the antimicrobial activity of CEO is due to its majority compounds, mainly eugenol. Likewise, the research revealed that the oil had a strong effect against L. monocytogenes and a moderate effect against E. coli, S. aureus, and Salmonella enterica serovar Enteritidis. However, it was not efficient in inhibiting Pseudomonas aeruginosa. In contrast, Salgado et al. [40] showed greater inhibition against Photobacterium phosphoreum (Gram-negative) and Brochothrix thermosphacta (Gram-positive). Unlike the direct incorporation of oil into the matrix, films and coatings allowed for the growth of Gram-positive and Gram-negative bacteria to be inhibited. Therefore, an increase in the efficiency of the oil in terms of antimicrobial activity is shown.
Clove essential oil has proven to be an effective alternative for improving the preservation of meat and fishery products through its incorporation into edible films and coatings. In pork meat, studies indicate that CEO exhibits strong antibacterial activity against S. aureus, with an MIC of 1 mg/mL and an MBC of 2 mg/mL. A decrease in bacterial ATP content and an increase in APK enzyme activity were observed, suggesting damage to the microbial cell wall without affecting the sensory quality of the product [30]. In another study on pork belly, CEO at a concentration of 0.75% (w/v) completely inhibited L. monocytogenes and significantly reduced E. coli counts [39]. In beef products, CEO, whose main component is eugenol (85.7%), exhibited inhibitory effects against L. monocytogenes, S. Typhimurium, and E. coli, with the highest efficacy against S. Typhimurium [47]. In raw beef, CEO at a 3% concentration significantly reduced the growth rate of bacteria such as Pseudomonas spp., enterobacteria, and lactic acid bacteria [34]. In beef sucuk (fermented sausage), CEO delayed the growth of psychrotrophic bacteria and S. aureus, maintaining good microbiological quality for up to 45 days of storage [41]. In poultry products, the incorporation of CEO into edible coatings for chicken breast resulted in a reduction in mesophilic aerobic bacteria and Pseudomonas spp. during refrigerated storage for 8 days [21]. However, in another study, no antilisterial activity or total inhibition of E. coli was observed in PHBV-CEO coatings stored at 4 °C, although a slight reduction in bacterial counts was noted at 10 °C [37]. In chicken burgers, a 1% CEO concentration maintained coliform counts below the permissible microbiological limit for 35 days of storage, although it was ineffective against S. aureus [45].
In fishery products, CEO has shown variable antimicrobial activity depending on the food matrix and bacterial species. In tambaqui (Colossoma macropomum), CEO at concentrations of 0.08% and 0.16% exhibited moderate effects against E. coli, S. enteritidis, and S. aureus but was highly effective against L. monocytogenes [50]. In frozen tambaqui filets, CEO prevented bacterial growth for up to 120 days [51]. In rainbow trout (Oncorhynchus mykiss), CEO coatings inhibited the proliferation of various pathogenic bacteria, with Shewanella putrefaciens being the most sensitive and Aeromonas hydrophila being the most resistant [17]. In sardine burgers and cod filets, CEO exhibited strong activity against P. phosphoreum and B. thermosphacta but lower efficacy against Pseudomonas spp., Citrobacter freundii, and Listeria spp. [23,40]. From a sensory perspective, most studies report that CEO does not negatively affect the organoleptic quality of meat and fishery products at low concentrations. However, at higher concentrations, alterations in flavor and aroma have been observed. For example, in chicken burger coatings, the best acceptance was achieved with 0.5% CEO [45], while in beef filets, panelists detected sensory changes after 12 days of storage [34].
The activity of CEO in edible coatings presents inconsistencies, with some studies demonstrating significant bacterial population reductions while others report no notable inhibition, particularly against Listeria monocytogenes and Escherichia coli in certain coating materials. In fishery products, the variability in bacterial species’ response to CEO, with microorganisms such as Shewanella putrefaciens showing higher sensitivity while Aeromonas hydrophila is more resistant [17], suggests that effectiveness depends on the specific microbiota of the treated product. From a sensory perspective, although CEO at low concentrations generally does not affect organoleptic quality, at higher concentrations, its strong flavor and aroma have been rejected by consumers, particularly in products such as ground meat and hamburgers.
5.2. Antifungal Activity in Edible Films and Coatings
Molds and yeasts can also negatively affect the quality and visual appearance of meat products. These microorganisms can cause changes in the texture, appearance, and color of meat, resulting in a decrease in the quality perceived by consumers. In addition, the presence of molds and yeasts can contribute to product degradation over time, reducing its shelf life and affecting the consumer experience. Antifungal activity was analyzed in sardine (Sardina pilchardus) presented as fish patties. The microorganisms tested were Debaryomyces hansenii, Aspergillus niger, and Penicillium expansum. Sardine filets were obtained from a local store (Madrid, Spain). The meat was ground and mixed with salt. The mixture was divided into portions and pressed into disks. The patties were placed on acrylic plates containing the films and were stored. The concentration tested was 0.75 mL/g, and once treated, the samples were stored at 2 ± 1 °C for 13 days. The highest percentage of inhibition was in the yeast D. hansenii with 60.74 ± 9.90%. The authors showed that there was an antifungal effect due to the eugenol present in CEO [40]. However, unlike yeasts, molds such as P. expansum are more difficult to inactivate, because they are very resistant organisms despite changes in their environment [55].
6. Encapsulated Clove Essential Oil
Applications of CEO are limited due to its low solubility (hydrophobicity), high volatility, and instability to parameters such as light, temperature, and air (oxygen) [60]. In addition, it has limitations in terms of taste and smell, even when added directly to edible films and coatings as mentioned in the previous sections. Faced with this challenge, some authors, including Wang et al. [60], Gasti et al. [61], Radünz et al. [35], Wang et al. [52], and Rajaei et al. [36] have explored alternatives such as the encapsulation of bioactive compounds. This proposal seeks to provide greater stability for the bioactive compounds as well as a controlled release, thus allowing us to work with solutions or incorporate them directly into the meat. However, the main challenge is to find the ideal coating material to increase the biological activity of the EO.
6.1. Antimicrobial Activity of Encapsulated CEO
Clove essential oil has strong antimicrobial activity due to many of its compounds. However, its use is limited due to volatility and instability under environmental conditions. In addition, to completely inhibit the growth of microorganisms, it is necessary to increase its concentration, which generates alterations in the organoleptic perception of the food. For this reason, the microencapsulation of the oil is a novel alternative. The results of antibacterial activity obtained with the encapsulated CEO in meat and meat products are shown in Table 4. The analysis of the antimicrobial activity of the encapsulated CEO was analyzed using two methods: disk diffusion and minimum inhibitory concentration and minimum bactericidal concentration.

Table 4.
Antibacterial and antifungal activity obtained from the incorporation of encapsulated clove essential oil in meat and meat products.
The susceptibility of the bacteria can be determined based on the size and presence of the inhibition zone. For this purpose, Arora and Kaur [62] establish that if the diameter of the zone is greater than 1.2 cm, satisfactory inhibition is achieved. According to the results of the work by Radünz et al. [35], promising results were obtained regarding the inhibition of pathogens that can be transmitted by food. The authors mention that the CEO showed an inhibitory effect on all the bacteria evaluated, and the authors assume that this antimicrobial effect is due to the lipophilic characteristics of the EO, since it causes an alteration in the permeability of the lipids of the cell membrane of the bacteria.
In turn, the EO microcapsules showed a minimum inhibitory effect on all bacteria, and the concentration of 0.304 mg/mL completely inhibited the growth of pathogens, regardless of the characteristics of their membranes. The authors assume that the action of eugenol caused a rupture of the cytoplasmic membrane in bacteria, which led to cell death, since it increased membrane permeability, causing ion extravasation (electrolyte leakage) and a loss of intracellular proteins. Likewise, the minimum bactericidal concentration was the same as the minimum inhibitory concentration. From the point of view of the mechanism of action, phenolic compounds, mainly eugenol, act by transporting protons through lipid bilayers, which causes a loss of proton motive force [36].
On the other hand, Rajaei et al. investigated the antimicrobial effect for S. enterica serovar Enteritidis, and they showed that there was an increase in the inhibitory effect of the free EO and the microcapsules, since the values of the minimum inhibitory concentration and the minimum bactericidal concentration of the free particles were 100 mg/mL and 200 mg/mL, respectively. In contrast, the encapsulated molecules presented lower values, specifically 5 mg/mL and 10 mg/mL. This finding indicated that there was greater interaction between the surface of the microcapsules and the cell wall of the membranes, which caused bacterial death [36].
Radünz et al. [35] made a comparison between commercial nitrite (preservative) and encapsulated CEO molecules to determine the inhibitory effect of both additives, where it was found that, regardless of the concentration, the EO was more effective in inhibiting the microbial growth of S. aureus than commercial nitrite. However, the authors discard the idea of completely replacing nitrite, since although this additive generates potentially carcinogenic compounds, such as nitrosamines, it also favors the formation of sensory attributes characteristic of meat products, such as aroma and color, so they recommend a combination of both additives [63].
Likewise, the results of the research by Rajaei et al. [36] revealed promising results from the first day, regardless of whether CEO was encapsulated or existed as free-form molecules: CEO reduced 3 log CFU/g of the Salmonella enteritidis serotype Enteritidis population. Likewise, it was found that the encapsulated particles of the EO were more effective against the free oil, since a lower concentration was needed, but it presented a greater effect throughout the test period. The authors, through in vitro and in vivo analyses, assume that the encapsulated bioactive compounds can increase their dispersion in the environment, which increases their adsorption mechanism. However, both treatments were better compared to the control samples.
6.2. Antifungal Activity of Encapsulated CEO
While it is true that edible films and coatings are an option to increase the shelf life of meat in general, bioactive compounds have limitations due to their volatility as well as a declining effect, since they are depleted over time, so their efficiency is not constant. For this reason, encapsulation is an alternative to protect these molecules from parameters such as light or heat, and in turn, it is possible to use them in matrices in which a thermal treatment was applied, maintaining their biological activity [52].
Mold spores are difficult to eliminate since they can withstand high temperatures or extreme pH (acids and bases) and still reproduce. Although preservatives allow us to reduce fungal growth, there are certain problems when they are subjected to high-temperature treatments, thus losing their effectiveness due to their high volatility [64]. The research by Wang et al. [52] focused on evaluating the antifungal effect of encapsulated CEO at both room temperature and cooking temperature, as well as determining the effective fungicidal concentration in both heat treatments. The result of their study revealed that as the days passed, the level of mold began to grow in the cooked samples that had low concentrations or did not contain encapsulated molecules on their surface, while the samples with higher concentrations of the EO reported a significant reduction in mold levels. Thus, it was found that the minimum effective concentration for fish was 0.07% to completely inhibit the growth of molds and spores, while for chicken and pork, it was 0.06%. Beef reported better results since its minimum effective concentration was 0.05%.
In the case of solutions with microcapsules treated at high temperatures, a decrease in the antifungal effect was evident. However, the study found that after severe thermal processing, microencapsulation provided thermal resistance to the particles, so the minimum effective concentration increased to 0.08% in each treated sample. The authors assumed that a large number of encapsulated particles maintained their antifungal activity, since the coating material, β-cyclodextrin, served as a heat-tolerant protective layer [52].
The incorporation of encapsulated CEO into meat and meat products has shown promising antimicrobial activity through different application methods, including direct addition and edible films/coatings. The antibacterial efficacy of CEO has been demonstrated in ground meat products, specifically hamburgers. When tested against common foodborne pathogens, CEO particles at concentrations of 3.04 and 0.304 mg/mL showed significant inhibition zones for S. aureus (2.83 cm), E. coli (2.81 cm), L. monocytogenes (2.47 cm), and S. Typhimurium (2.22 cm). Notably, these concentrations were effective at inhibiting S. aureus growth compared to commercial nitrite, demonstrating the potential of CEO as a natural preservative alternative [35]. The application of CEO through edible films and coatings has also shown effectiveness in preserving meat quality and safety. In a study with beef chops, researchers evaluated its efficacy against S. enterica serotype Enteritidis at concentrations of 5 and 10 μg/mL during storage at 4 °C for 12 days. Surface treatment with 100 mg free CEO, 1 mg CS-MA nanogel, and 1 and 2 mg CS-MA nanogel-encapsulated CEO resulted in significant reductions in Salmonella populations. The study revealed that the encapsulated CEO coating was more effective compared to its free counterpart in reducing the population of S. enterica serotype Enteritidis in both in vitro and in vivo assays, highlighting the benefit of encapsulation for enhanced antibacterial activity [36]. The antifungal activity of CEO was evaluated in various meat products including fish, chicken, pork, and beef. Different concentrations ranging from 0.01% to 0.1% (w/w) were tested at 37 °C for 4 days. The results revealed the presence of mold in treatments without CEO and at low concentrations. However, effective fungicidal concentrations were found to be ≤0.07% for fish, ≤0.06% for chicken and pork, and ≤0.05% for beef, demonstrating slightly different thresholds of efficacy depending on the meat type. In thermally treated coatings, the effective concentration was found to be ≤0.08% across all samples, suggesting that thermal treatment may slightly reduce the antifungal efficacy of CEO [52].
Clove essential oil encapsulation has shown promising potential by improving its stability and prolonging its antimicrobial effect; however, its effectiveness depends on the encapsulation method and interaction with the food matrix, which can influence its release and final activity. Overall, these limitations indicate that while CEO presents significant potential as a natural preservative, its application requires standardization in terms of concentration, application method, and combination with other treatments to optimize its effectiveness without compromising the sensory quality of the final product.
7. Research Strategy
This systematic review study aimed to evaluate the application of CEO in meat and meat products, following the guidelines of the PRISMA 2020 methodology [13]. The review was carried out following a series of methodical steps inspired by the Cochrane systematic review framework, with necessary modifications to address the specific focus of this study.
7.1. Information Sources
The literature search was conducted using several academic databases: ScienceDirect, SCOPUS, Web of Science (WOS), and PubMed. These databases were selected due to their comprehensive collections of scientific articles and their accessibility.
7.2. Search Strategy
To capture all relevant studies on the application of CEO in meat and meat products, a detailed search strategy was developed. The search terms used were “clove” OR “Syzygium aromaticum” AND “essential oil” AND “antimicrobial” OR “antibacterial” OR “antifungal” combined with several meat-related terms, such as “meat”, “fish”, “chicken”, and “meat products”. Boolean operators (AND, OR, and NOT), phrase search, truncation and wildcard (“*”), and field code functions were used to refine the search. The search terms may appear in the title, abstract, or keywords. The search was carried out in the databases of PubMed, SCOPUS, ScienceDirect, or Web of Science. Table 5 shows the additional information such as the literature type, language, and chronology entered according to the criteria of the researchers. This strategy allowed us to identify relevant studies.

Table 5.
Keywords used for the process of finding the relevant literature.
7.3. Eligibility Criteria
The inclusion criteria for this review were clearly defined to ensure the relevance and quality of the selected studies. Studies that focused on the application of CEO in meat and meat products intended for human consumption were included. Research involving the use of CEO alone or in combination with other EOs was also considered. Studies that were not research articles, such as books, conference reports, editorials, etc., were excluded. Exclusion criteria included studies focusing solely on extracts other than EOs, research on animal feed supplements, and theses and dissertations unless enough articles were available. These criteria ensure that only the most relevant and high-quality studies were included in this review.
7.4. Study Selection
The study selection process was carried out in several stages to ensure rigor and precision. Initially, a selection was made based on the titles and abstracts of the articles identified in the search. Studies that met the inclusion criteria were selected for a full-text review. To avoid the inclusion of duplicates, reference management tools, specifically the Mendeley reference manager, were used to identify and eliminate duplicate records. Study eligibility was assessed independently by two reviewers, who reviewed the full articles. Any discrepancies between reviewers were resolved through discussion and consensus, thus ensuring the inclusion of the most relevant studies.
7.5. Data Extraction
Data extraction was carried out using a standardized form designed to systematically collect relevant information from each study. Eligible articles were selected by removing duplicates and excluding the articles that did not meet the inclusion and exclusion criteria. Extracted data included study characteristics such as author and year of publication. Specific information was also collected on the type of meat used and the applied concentration of the EO. This systematic approach to data extraction ensures that relevant information is collected consistently and can be adequately compared across studies.
7.6. Quality Assessment
Two reviewers conducted the quality assessment of all selected articles, and any differences were resolved through discussion. The quality analysis was based on the experimental design, methodology, application mechanisms, and results obtained.
7.7. Synthesis Methods
Initially, the number of products and presentations of the meat and meat product matrices was analyzed. Due to the nature of the results, statistical analysis was not possible; therefore, a narrative synthesis was planned. The studies were divided into three groups based on the application of the EO: direct addition, in the form of edible films and coatings, and encapsulated. Within these subgroups, the information was summarized in tables, which provide information regarding the matrix (product and presentation), main EO compounds, activity analysis method, concentration evaluated, storage conditions, results, and sensory evaluation.
7.8. Limitation
Our review used the PRISMA guidelines to identify as many relevant studies as possible. The search was limited to four databases recognized for their quality and contribution to research to ensure the rigor and quality of the articles included in our evaluation. The variability in methodologies and concentrations used presented a limitation, and this added to the nature of the results obtained and did not allow for the realization of a meta-analysis.
8. Recent Trends and Regulatory Aspects
Finally, aggregate data on the antimicrobial efficacy of CEO in meat and meat products are robust and project a potential use of the EO or its components as a natural preservative. Antimicrobial activity depends on its concentration, the food matrix, and storage conditions. Its practical application is limited by its negative effects on the sensory characteristics of products, especially flavor and odor, which affect overall consumer acceptability. Therefore, its impact on sensory properties must be carefully considered to achieve a balance between food safety and consumer acceptability. Studies with edible films and coatings, as well as studies with microencapsulated EO, are also encouraging.
Concerning regulatory aspects, the Food and Drug Administration (United States) categorizes EOs as “Generally Recognised As Safe” or “GRAS”, and CEO has been included in the group of raw EOs permitted for use in foods. Furthermore, considering the Code of Federal Regulations (CFRs), there is extensive regulation based on the definition of EOs as food additives or food contact surfaces. Finally, labeling rules have developed over time and are mandatory when it is clear that the presence of EOs has a direct impact in relation to the residual technical effects on the food placed on the market [65]. European regulations also regulate materials that come into direct contact with food (Regulation (EC) No 1935/2004) by controlling the safety and compliance of materials that come into contact with food. This regulation therefore applies to active packaging containing EOs. In parallel, Regulation (EU) No. 10/2011 was drafted with the aim of controlling nanotechnological derivatives, and it is the task of the European Food Safety Authority (EFSA) to subject foods, packaging, or additives to the relevant controls [66]. A further level of regulation is provided by two other bodies, WHO (World Health Organization) and FAO (Food and Agriculture Organization). Both institutions have created a joint committee of experts (JECFA—Expert Committee on Food Additives) that has the mandate to assess the safety of food additives, which include herbal medicines and EOs [67].
In light of these findings, the following new research trends can be proposed: (I) Further studies should focus on the action of EO blends, as well as those containing the EO of CEO, with a pleasant sensory profile that have previously tested on a consumer panel. (II) The efficacy of treatments with the volatile organic compounds (VOCs) of CEO should be considered for further research. (III) Further studies should be carried out on the effect of the main components of CEO as isolated compounds, testing again the organoleptic characteristics and palatability of processed food products.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antibiotics14050494/s1, Table S1: Reference list of all papers included in the review.
Author Contributions
Conceptualization, E.V., M.R. and X.J.-F.; methodology, G.L.-M.; validation, X.J.-F. and M.A.M.; resources, E.V.; data curation, G.L.-M., X.J.-F. and M.A.M.; writing—original draft preparation, G.L.-M., X.J.-F., M.R. and M.A.M.; writing—review and editing, E.V. and M.R.; visualization, X.J.-F. and M.A.M.; supervision, E.V.; project administration, E.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors acknowledge the support of the technical staff of the Universidad Técnica Particular de Loja.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| APC | Aerobic plate count |
| CEO | Clove essential oil |
| CFU | Colony–forming unit |
| CS-MA | Chitosan–myristic acid |
| D10 | Radiation dose required to reduce 90% of the population |
| EO | Essential oil |
| EOs | Essential oils |
| G-Ch-Cl | Gelatine–chitosan–clove essential oil |
| G-Cl | Gelatinclove–clove essential oil |
| Gy | Gray |
| MBC | Minimum bactericidal concentration |
| MIC | Minimum inhibitory concentration |
| PC | Psychrophilic count |
| PHBV | Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) |
| PPC | Psychrotrophic plate count |
| TMAB | Total mesophilic aerobic bacteria |
| TPC | Total plate count |
| VRE | Vancomycin-resistant enterococci |
References
- Smaoui, S.; Ben Hlima, H.; Tavares, L.; Ennouri, K.; Ben Braiek, O.; Mellouli, L.; Abdelkafi, S.; Mousavi Khaneghah, A. Application of essential oils in meat packaging: A systemic review of recent literature. Food Control 2022, 132, 108566. [Google Scholar] [CrossRef]
- Li, P.; Zhang, Y.; Huang, Y.; Chen, L. Activity and mechanism of macroporous carbon/nano-TiO2 composite photocatalyst for treatment of cyanide wastewater. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130728. [Google Scholar] [CrossRef]
- Olvera-Aguirre, G.; Piñeiro-Vázquez, Á.T.; Sanginés-García, J.R.; Sánchez Zárate, A.; Ochoa-Flores, A.A.; Segura-Campos, M.R.; Vargas-Bello-Pérez, E.; Chay-Canul, A.J. Using plant-based compounds as preservatives for meat products: A review. Heliyon 2023, 9, e17071. [Google Scholar] [CrossRef] [PubMed]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents. J. Agric. Food. Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- El Faqer, O.; Bendiar, S.; Rais, S.; Elkoraichi, I.; Dakir, M.; Elouaddari, A.; Amrani, A.E.; Oudghiri, M.; Mtairag, E.M. Phytochemical characterization and immunomodulatory effects of aqueous, ethanolic extracts and essential oil of Syzygium aromaticum L. on human neutrophils. Sci. Afr. 2022, 18, e01395. [Google Scholar] [CrossRef]
- da Silva, B.D.; do Rosário, D.K.A.; Weitz, D.A.; Conte-Junior, C.A. Essential oil nanoemulsions: Properties, development, and application in meat and meat products. Trends Food Sci. Technol. 2022, 121, 1–13. [Google Scholar] [CrossRef]
- Falleh, H.; Ben Jemaa, M.; Saada, M.; Ksouri, R. Essential oils: A promising eco-friendly food preservative. Food Chem. 2020, 330, 127268. [Google Scholar] [CrossRef]
- Valderrama, F.; Ruiz, F. An optimal control approach to steam distillation of essential oils from aromatic plants. Comput. Chem. Eng. 2018, 117, 25–31. [Google Scholar] [CrossRef]
- Gülçin, İ.; Elmastaş, M.; Aboul-Enein, H.Y. Antioxidant activity of clove oil—A powerful antioxidant source. Arab. J. Chem. 2012, 5, 489–499. [Google Scholar] [CrossRef]
- Pateiro, M.; Munekata, P.E.S.; Sant’Ana, A.S.; Domínguez, R.; Rodríguez-Lázaro, D.; Lorenzo, J.M. Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. Int. J. Food Microbiol. 2021, 337, 108966. [Google Scholar] [CrossRef]
- Hyldgaard, M.; Mygind, T.; Meyer, R. Essential Oils in Food Preservation: Mode of Action, Synergies, and Interactions with Food Matrix Components. Front. Microbiol. 2012, 3, 12. [Google Scholar] [CrossRef]
- Rout, S.; Tambe, S.; Deshmukh, R.K.; Mali, S.; Cruz, J.; Srivastav, P.P.; Amin, P.D.; Gaikwad, K.K.; Andrade, E.H.d.A.; Oliveira, M.S.d. Recent trends in the application of essential oils: The next generation of food preservation and food packaging. Trends Food Sci. Technol. 2022, 129, 421–439. [Google Scholar] [CrossRef]
- Matthew, J.P.; Joanne, E.M.; Patrick, M.B.; Isabelle, B.; Tammy, C.H.; Cynthia, D.M.; Larissa, S.; Jennifer, M.T.; Elie, A.A.; Sue, E.B.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Selles, S.M.A.; Kouidri, M.; Belhamiti, B.T.; Ait Amrane, A. Chemical composition, in-vitro antibacterial and antioxidant activities of Syzygium aromaticum essential oil. J. Food Meas. Charact. 2020, 14, 2352–2358. [Google Scholar] [CrossRef]
- Santos, M.V.d.O.; Nascimento, L.E.; Praxedes, É.A.; Borges, A.A.; Silva, A.R.; Bertini, L.M.; Pereira, A.F. Syzygium aromaticum essential oil supplementation during in vitro bovine oocyte maturation improves parthenogenetic embryonic development. Theriogenology 2019, 128, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, M.E.; Morsy, N.F.S. Keeping Quality of Frozen Beef Patties by Marjoram and Clove Essential Oils. J. Food Process. Preserv. 2015, 39, 956–965. [Google Scholar] [CrossRef]
- Albertos, I.; Rico, D.; Diez, A.M.; González-Arnáiz, L.; García-Casas, M.J.; Jaime, I. Effect of edible chitosan/clove oil films and high-pressure processing on the microbiological shelf life of trout fillets. J. Sci. Food Agric. 2015, 95, 2858–2865. [Google Scholar] [CrossRef]
- Aliakbarlu, J.; Khalili Sadaghiani, S. Effect of Avishane Shirazi (Zataria Multiflora) and Clove (Syzygium Aromaticum) Essential Oils on Microbiological, Chemical and Sensory Properties of Ground Sheep Meat During Refrigerated Storage. J. Food Qual. 2015, 38, 240–247. [Google Scholar] [CrossRef]
- De Oliveira, T.L.C.; Cardoso, M.G.; Soares, R.A.; Ramos, E.M.; Piccoli, R.H.; Tebaldi, V.M.R. Inhibitory activity of Syzygium aromaticum and Cymbopogon citratus (DC.) Stapf. essential oils against Listeria monocytogenes inoculated in bovine ground meat. Braz. J. Microbiol. 2013, 44, 357–365. [Google Scholar] [CrossRef]
- dos Santos, L.R.; Alía, A.; Martin, I.; Gottardo, F.M.; Rodrigues, L.B.; Borges, K.A.; Furian, T.Q.; Córdoba, J.J. Antimicrobial activity of essential oils and natural plant extracts against in a dry-cured ham-based model. J. Sci. Food Agric. 2022, 102, 1729–1735. [Google Scholar] [CrossRef]
- Fernández-Pan, I.; Mendoza, M.; Maté, J.I. Whey protein isolate edible films with essential oils incorporated to improve the microbial quality of poultry. J. Sci. Food Agric. 2013, 93, 2986–2994. [Google Scholar] [CrossRef] [PubMed]
- Gibriel, A.Y.; Ali, H.G.M.; Abdeldaiem, M.H. Antibacterial Activity of Clove (Syzigium aromaticum L.) Essential Oil and Gamma Irradiation against Some Food-Borne Pathogens in Minced Chicken Meat. Arab J. Nucl. Sci. Appl. 2017, 50, 179–193. [Google Scholar]
- Gómez-Estaca, J.; López de Lacey, A.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Montero, P. Biodegradable gelatin–chitosan films incorporated with essential oils as antimicrobial agents for fish preservation. Food Microbiol. 2010, 27, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Guran, H.S.; Oksuztepe, G.; Coban, O.E.; Incili, G.K. Influence of different essential oils on refrigerated fish patties produced from bonito fish (Sarda sarda Bloch, 1793). Czech J. Food Sci. 2015, 33, 37–44. [Google Scholar] [CrossRef]
- Habashy, A.H.A.; Darwish, W.S.; Hussein, M.A.; El-Dien, W.M.S. Prevalence of different mould genera in meat and meat products with some reduction trials using essential oils. Adv. Anim. Vet. Sci 2019, 7, 79–85. [Google Scholar]
- Hernández-Ochoa, L.; Aguirre-Prieto, Y.B.; Nevárez-Moorillón, G.V.; Gutierrez-Mendez, N.; Salas-Muñoz, E. Use of essential oils and extracts from spices in meat protection. J. Food Sci. Technol. 2014, 51, 957–963. [Google Scholar] [CrossRef][Green Version]
- Hetta, H.F.; Meshaal, A.K.; Algammal, A.M.; Yahia, R.; Makharita, R.R.; Marraiki, N.; Shah, M.A.; Hassan, H.A.M.; Batiha, G.E.S. In-vitro Antimicrobial Activity of Essential Oils and Spices Powder of some Medicinal Plants Against Bacillus Species Isolated from Raw and Processed Meat. Infect. Drug Resist. 2020, 13, 4367–4378. [Google Scholar] [CrossRef]
- Hosseini, M.; Jamshidi, A.; Raeisi, M.; Azizzadeh, M. Effect of sodium alginate coating containing clove (Syzygium Aromaticum) and lemon verbena (Aloysia Citriodora) essential oils and different packaging treatments on shelf life extension of refrigerated chicken breast. J. Food Process. Preserv. 2021, 45, e14946. [Google Scholar] [CrossRef]
- Khaleque, M.A.; Keya, C.A.; Hasan, K.N.; Hoque, M.M.; Inatsu, Y.; Bari, M.L. Use of cloves and cinnamon essential oil to inactivate Listeria monocytogenes in ground beef at freezing and refrigeration temperatures. LWT 2016, 74, 219–223. [Google Scholar] [CrossRef]
- Li, J.; Li, C.; Shi, C.; Aliakbarlu, J.; Cui, H.; Lin, L. Antibacterial mechanisms of clove essential oil against Staphylococcus aureus and its application in pork. Int. J. Food Microbiol. 2022, 380, 109864. [Google Scholar] [CrossRef]
- Martins, H.H.d.A.; Simões, L.A.; Isidoro, S.R.; Nascimento, S.d.S.; Alcântara, J.P.; Ramos, E.M.; Piccoli, R.H. Preservative of Essential Oil Blends: Control of Clostridium perfringens Type a in Mortadella. Braz. Arch. Biol. Technol. 2021, 64, e21200106. [Google Scholar] [CrossRef]
- Nisar, T.; Yang, X.; Alim, A.; Iqbal, M.; Wang, Z.-C.; Guo, Y. Physicochemical responses and microbiological changes of bream (Megalobrama ambycephala) to pectin based coatings enriched with clove essential oil during refrigeration. Int. J. Biol. Macromol. 2019, 124, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Nunes Barbosa, L.; Mores Rall, V.L.; Henrique Fernandes, A.A.; Ikeda Ushimaru, P.; da Silva Probst, I.; Fernandes, A. Essential Oils Against Foodborne Pathogens and Spoilage Bacteria in Minced Meat. Foodborne Pathog. Dis. 2009, 6, 725–728. [Google Scholar] [CrossRef]
- Radha krishnan, K.; Babuskin, S.; Rakhavan, K.R.; Tharavin, R.; Azhagu Saravana Babu, P.; Sivarajan, M.; Sukumar, M. Potential application of corn starch edible films with spice essential oils for the shelf life extension of red meat. J. Appl. Microbiol. 2015, 119, 1613–1623. [Google Scholar] [CrossRef]
- Radünz, M.; da Trindade, M.L.M.; Camargo, T.M.; Radünz, A.L.; Borges, C.D.; Gandra, E.A.; Helbig, E. Antimicrobial and antioxidant activity of unencapsulated and encapsulated clove (Syzygium aromaticum, L.) essential oil. Food Chem. 2019, 276, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Rajaei, A.; Hadian, M.; Mohsenifar, A.; Rahmani-Cherati, T.; Tabatabaei, M. A coating based on clove essential oils encapsulated by chitosan-myristic acid nanogel efficiently enhanced the shelf-life of beef cutlets. Food Packag. Shelf Life 2017, 14, 137–145. [Google Scholar] [CrossRef]
- Requena, R.; Vargas, M.; Chiralt, A. Eugenol and carvacrol migration from PHBV films and antibacterial action in different food matrices. Food Chem. 2019, 277, 38–45. [Google Scholar] [CrossRef]
- Rounds, L.; Havens, C.M.; Feinstein, Y.; Friedman, M.; Ravishankar, S. Plant Extracts, Spices, and Essential Oils Inactivate Escherichia coli O157:H7 and Reduce Formation of Potentially Carcinogenic Heterocyclic Amines in Cooked Beef Patties. J. Agric. Food. Chem. 2012, 60, 3792–3799. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Rhim, J.-W. Gelatin/agar-based multifunctional film integrated with copper-doped zinc oxide nanoparticles and clove essential oil Pickering emulsion for enhancing the shelf life of pork meat. Food Res. Int. 2022, 160, 111690. [Google Scholar] [CrossRef]
- Salgado, P.R.; López-Caballero, M.E.; Gómez-Guillén, M.C.; Mauri, A.N.; Montero, M.P. Sunflower protein films incorporated with clove essential oil have potential application for the preservation of fish patties. Food Hydrocoll. 2013, 33, 74–84. [Google Scholar] [CrossRef]
- Saricaoglu, F.T.; Turhan, S. Performance of mechanically deboned chicken meat protein coatings containing thyme or clove essential oil for storage quality improvement of beef sucuks. Meat Sci. 2019, 158, 107912. [Google Scholar] [CrossRef]
- Selim, S. Antimicrobial activity of essential oils against vancomycin-resistant enterococci (VRE) and escherichia coli O157: H7 in feta soft cheese and minced beef meat. Braz. J. Microbiol. 2011, 42, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Mendiratta, S.K.; Agarwal, R.K.; Kumar, S.; Soni, A. Evaluation of anti-oxidant and anti-microbial activity of various essential oils in fresh chicken sausages. J. Food Sci. Technol. 2017, 54, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Mendiratta, S.K.; Agrawal, R.K.; Gurunathan, K.; Kumar, S.; Singh, T.P. Use of various essential oils as bio preservatives and their effect on the quality of vacuum packaged fresh chicken sausages under frozen conditions. LWT-Food Sci. Technol. 2017, 81, 118–127. [Google Scholar] [CrossRef]
- Shukla, V.; Mendiratta, S.K.; Zende, R.J.; Agrawal, R.K.; Kumar Jaiswal, R. Effects of chitosan coating enriched with Syzygium aromaticum essential oil on quality and shelf-life of chicken patties. J. Food Process. Preserv. 2020, 44, e14870. [Google Scholar] [CrossRef]
- Sirena, J.T.; Magro, J.D.; Junges, A.; Steffens, C.; Cansian, R.L.; Paroul, N. Characterization of free and encapsulated cinnamon and clove essential oils for enhancing fresh sausage quality: A natural substitute for synthetic preservatives. Food Biosci. 2024, 61, 104649. [Google Scholar] [CrossRef]
- Stoleru, E.; Vasile, C.; Irimia, A.; Brebu, M. Towards a Bioactive Food Packaging: Poly(Lactic Acid) Surface Functionalized by Chitosan Coating Embedding Clove and Argan Oils. Molecules 2021, 26, 4500. [Google Scholar] [CrossRef]
- Tajik, H.; Farhangfar, A.; Moradi, M.; Razavi Rohani, S.M. Effectiveness of Clove Essential Oil and Grape Seed Extract Combination on Microbial and Lipid Oxidation Characteristics of Raw Buffalo Patty During Storage at Abuse Refrigeration Temperature. J. Food Process. Preserv. 2014, 38, 31–38. [Google Scholar] [CrossRef]
- Turgis, M.; Han, J.; Millette, M.; Salmieri, S.; Borsa, J.; Lacroix, M. Effect of selected antimicrobial compounds on the radiosensitization of Salmonella Typhi in ground beef. Lett. Appl. Microbiol. 2009, 48, 657–662. [Google Scholar] [CrossRef]
- Vieira, B.B.; Carvalho, E.A.d.; Bispo, A.S.d.R.; Ferreira, M.A.; Evangelista-Barreto, N.S. Efficiency of chitosan synergism with clove essential oil in the coating of intentionally contaminated Tambaqui fillets. Semin. Ciências Agrárias 2020, 41, 2793–2802. [Google Scholar] [CrossRef]
- Vieira, B.B.; Mafra, J.F.; Bispo, A.S.d.R.; Ferreira, M.A.; Silva, F.d.L.; Rodrigues, A.V.N.; Evangelista-Barreto, N.S. Combination of chitosan coating and clove essential oil reduces lipid oxidation and microbial growth in frozen stored tambaqui (Colossoma macropomum) fillets. LWT 2019, 116, 108546. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Jia, J.-X.; Tian, Y.-Q.; Shu, X.; Ren, X.-J.; Guan, Y.; Yan, Z.-Y. Antifungal effects of clove oil microcapsule on meat products. LWT 2018, 89, 604–609. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Dominguez, R.; Pateiro, M.; Saraiva, J.A.; Franco, D. Chapter 3-Main Groups of Microorganisms of Relevance for Food Safety and Stability: General Aspects and Overall Description. In Innovative Technologies for Food Preservation; Barba, F.J., Sant’Ana, A.S., Orlien, V., Koubaa, M., Eds.; Academic Press: Cambridge, MA, USA, 2018; pp. 53–107. [Google Scholar]
- Hu, F.; Tu, X.-F.; Thakur, K.; Hu, F.; Li, X.-L.; Zhang, Y.-S.; Zhang, J.-G.; Wei, Z.-J. Comparison of antifungal activity of essential oils from different plants against three fungi. Food Chem. Toxicol. 2019, 134, 110821. [Google Scholar] [CrossRef] [PubMed]
- Rana, I.S.; Rana, A.S.; Rajak, R.C. Evaluation of antifungal activity in essential oil of the Syzygium aromaticum (L.) by extraction, purification and analysis of its main component eugenol. Braz. J. Microbiol. 2011, 42, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Marchese, A.; Barbieri, R.; Coppo, E.; Orhan, I.E.; Daglia, M.; Nabavi, S.F.; Izadi, M.; Abdollahi, M.; Nabavi, S.M.; Ajami, M. Antimicrobial activity of eugenol and essential oils containing eugenol: A mechanistic viewpoint. Crit. Rev. Microbiol. 2017, 43, 668–689. [Google Scholar] [CrossRef]
- Cai, R.; Hu, M.; Zhang, Y.; Niu, C.; Yue, T.; Yuan, Y.; Wang, Z. Antifungal activity and mechanism of citral, limonene and eugenol against Zygosaccharomyces rouxii. LWT 2019, 106, 50–56. [Google Scholar] [CrossRef]
- El-Saber Batiha, G.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef]
- Xu, J.-G.; Liu, T.; Hu, Q.-P.; Cao, X.-M. Chemical Composition, Antibacterial Properties and Mechanism of Action of Essential Oil from Clove Buds against Staphylococcus aureus. Molecules 2016, 21, 1194. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Y.T.; Xue, W.Y.; Wang, L.; Li, R.; Jiang, Z.T.; Tang, S.H.; Tan, J. Enhanced preservation effects of clove (Syzygium aromaticum) essential oil on the processing of Chinese bacon (preserved meat products) by beta cyclodextrin metal organic frameworks (β-CD-MOFs). Meat Sci. 2023, 195, 108998. [Google Scholar] [CrossRef]
- Gasti, T.; Dixit, S.; Hiremani, V.D.; Chougale, R.B.; Masti, S.P.; Vootla, S.K.; Mudigoudra, B.S. Chitosan/pullulan based films incorporated with clove essential oil loaded chitosan-ZnO hybrid nanoparticles for active food packaging. Carbohydr. Polym. 2022, 277, 118866. [Google Scholar] [CrossRef]
- Arora, D.S.; Kaur, J. Antimicrobial activity of spices. Int. J. Antimicrob. Agents 1999, 12, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Pérez Álvarez, J.Á.; Fernández-López, J.; Sayas Barberá, M.E. Industrializacion de Productos de Origen Animal, 3rd ed.; Universidad Miguel Hernandez: Elche, Spain, 2001. [Google Scholar]
- Dijksterhuis, J. The fungal spore and food spoilage. Curr. Opin. Food Sci. 2017, 17, 68–74. [Google Scholar] [CrossRef]
- Jackson-Davis, A.; White, S.; Kassama, L.S.; Coleman, S.; Shaw, A.; Mendonca, A.; Cooper, B.; Thomas-Popo, E.; Gordon, K.; London, L. A Review of Regulatory Standards and Advances in Essential Oils as Antimicrobials in Foods. J. Food Prot. 2023, 86, 100025. [Google Scholar] [CrossRef] [PubMed]
- De Farias, P.M.; De Sousa, R.V.; Maniglia, B.C.; Pascall, M.; Matthes, J.; Sadzik, A.; Schmid, M.; Fai, A.E.C. Biobased Food Packaging Systems Functionalized with Essential Oil via Pickering Emulsion: Advantages, Challenges, and Current Applications. ACS Omega 2025, 10, 4173–4186. [Google Scholar] [CrossRef]
- Osaili, T.M.; Dhanasekaran, D.K.; Zeb, F.; Faris, M.E.; Naja, F.; Radwan, H.; Cheikh Ismail, L.; Hasan, H.; Hashim, M.; Obaid, R.S. A Status Review on Health-Promoting Properties and Global Regulation of Essential Oils. Molecules 2023, 28, 1809. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).