New Curcumin Analogue (PAC) Inhibits Candida albicans Virulence, Restricts Its Adhesion Potential, and Relieves Oral Epithelial Cell Inflammation and Defense Mechanisms
Abstract
1. Introduction
2. Results
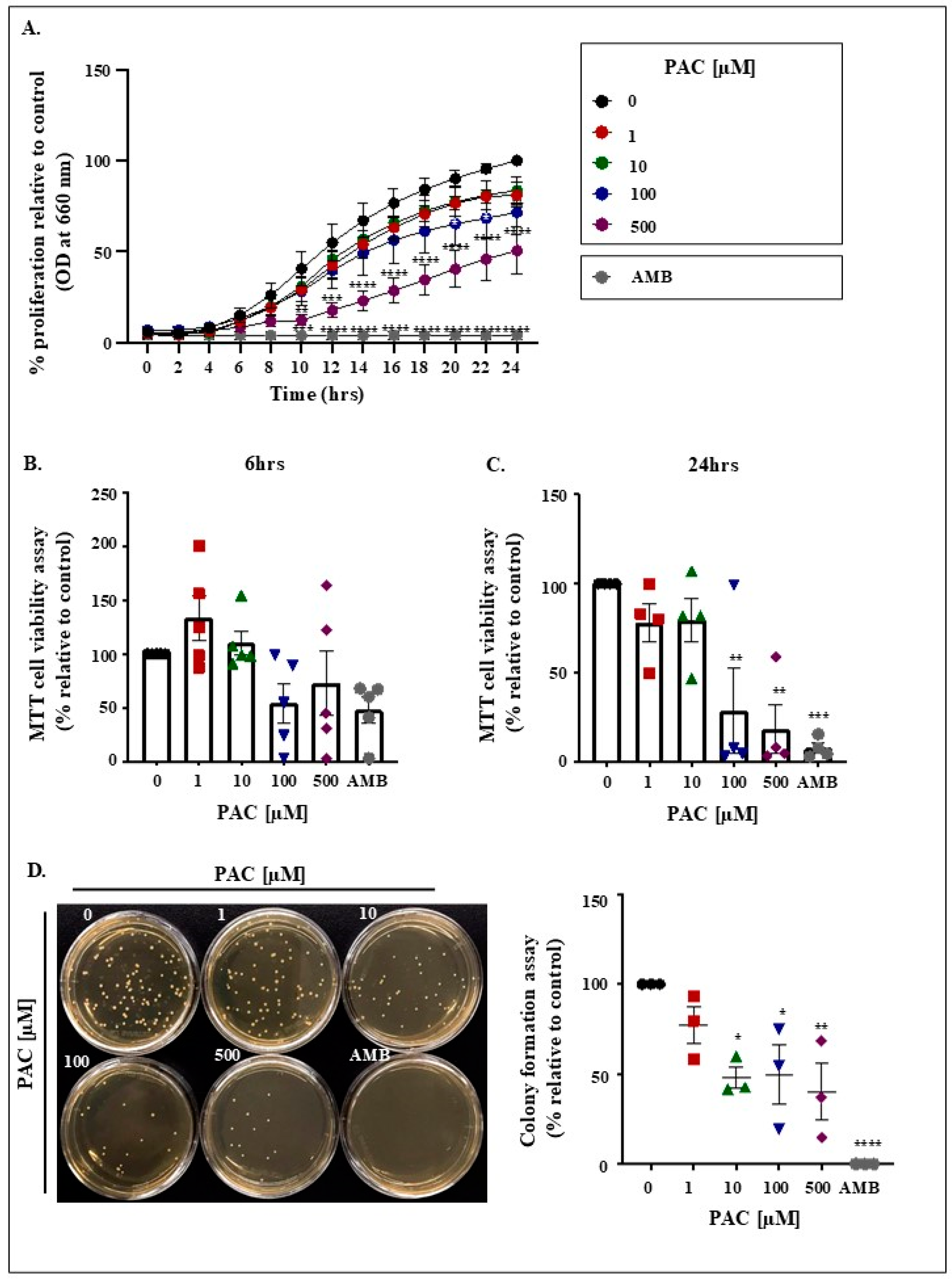
2.1. PAC Impairs Viability Together with Restricting Candida Growth and Colony Formation
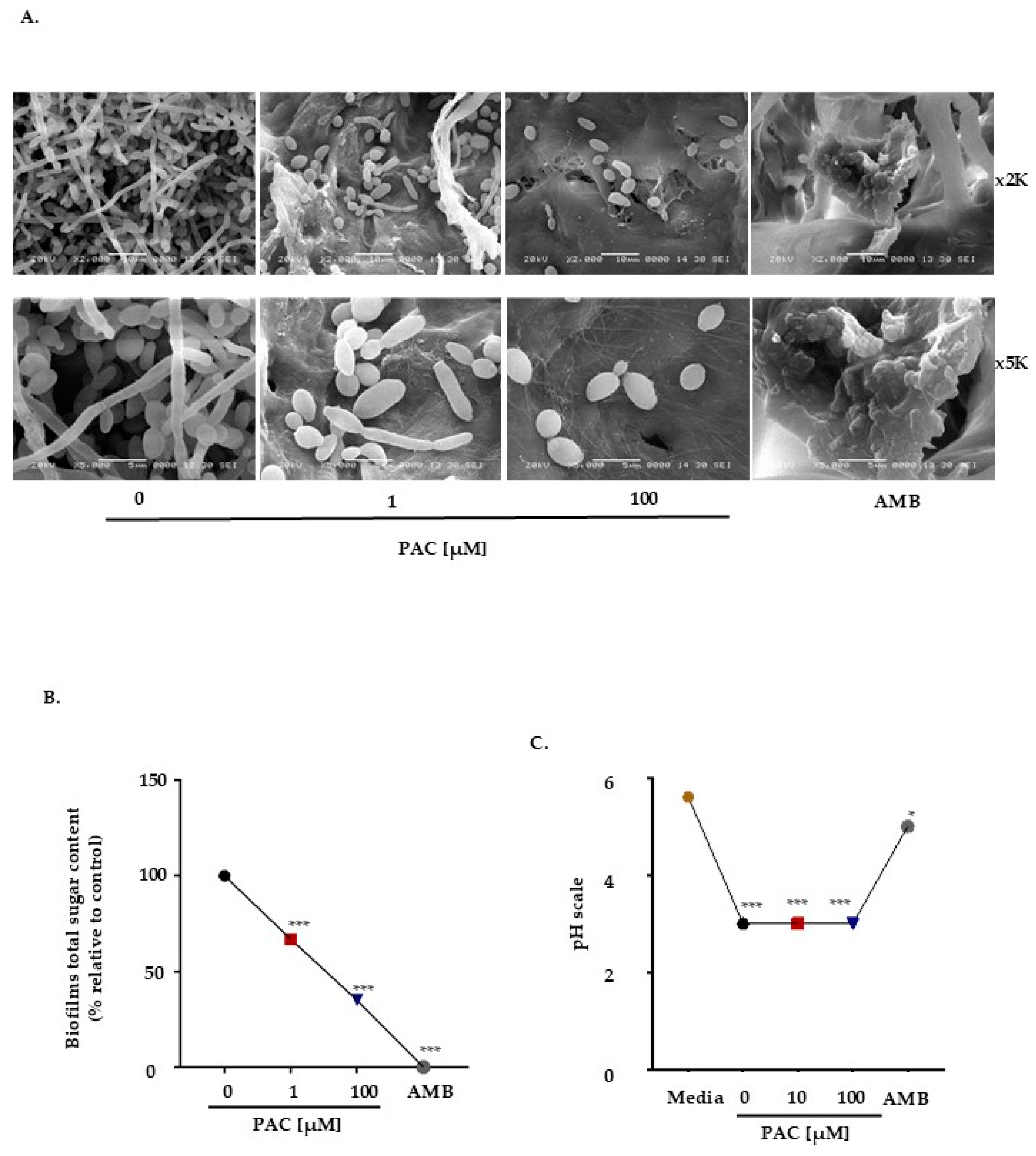
2.2. PAC Compromises Biofilm Formation and Modifies Matrix Composition
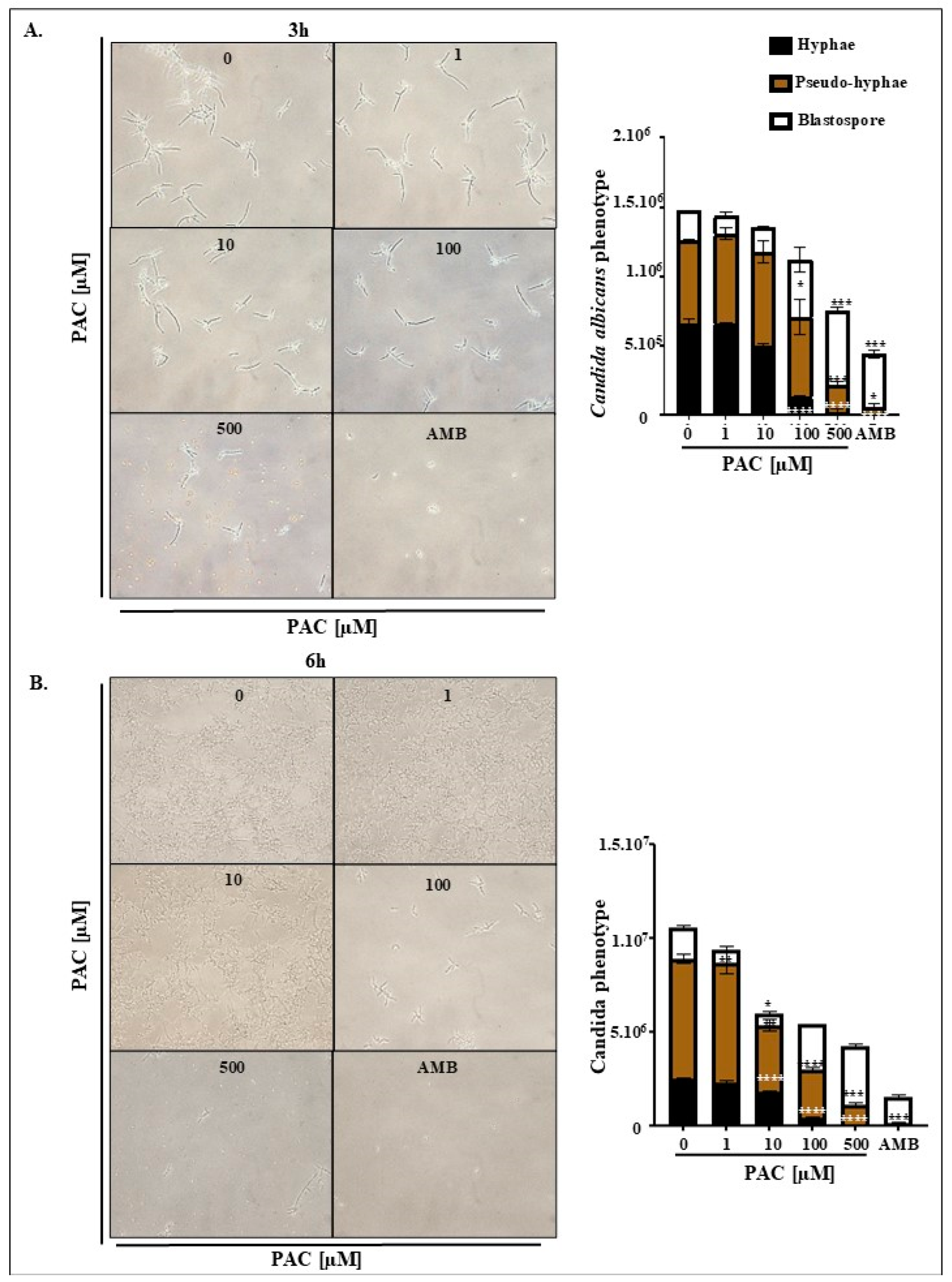
2.3. PAC Ensures C. albicans Conversion to a Less Virulent Phenotype
2.4. PAC Alters Pathogen Adhesion Potential
2.5. PAC Controls C. albicans Growth Through Necrotic and ROS Pathways
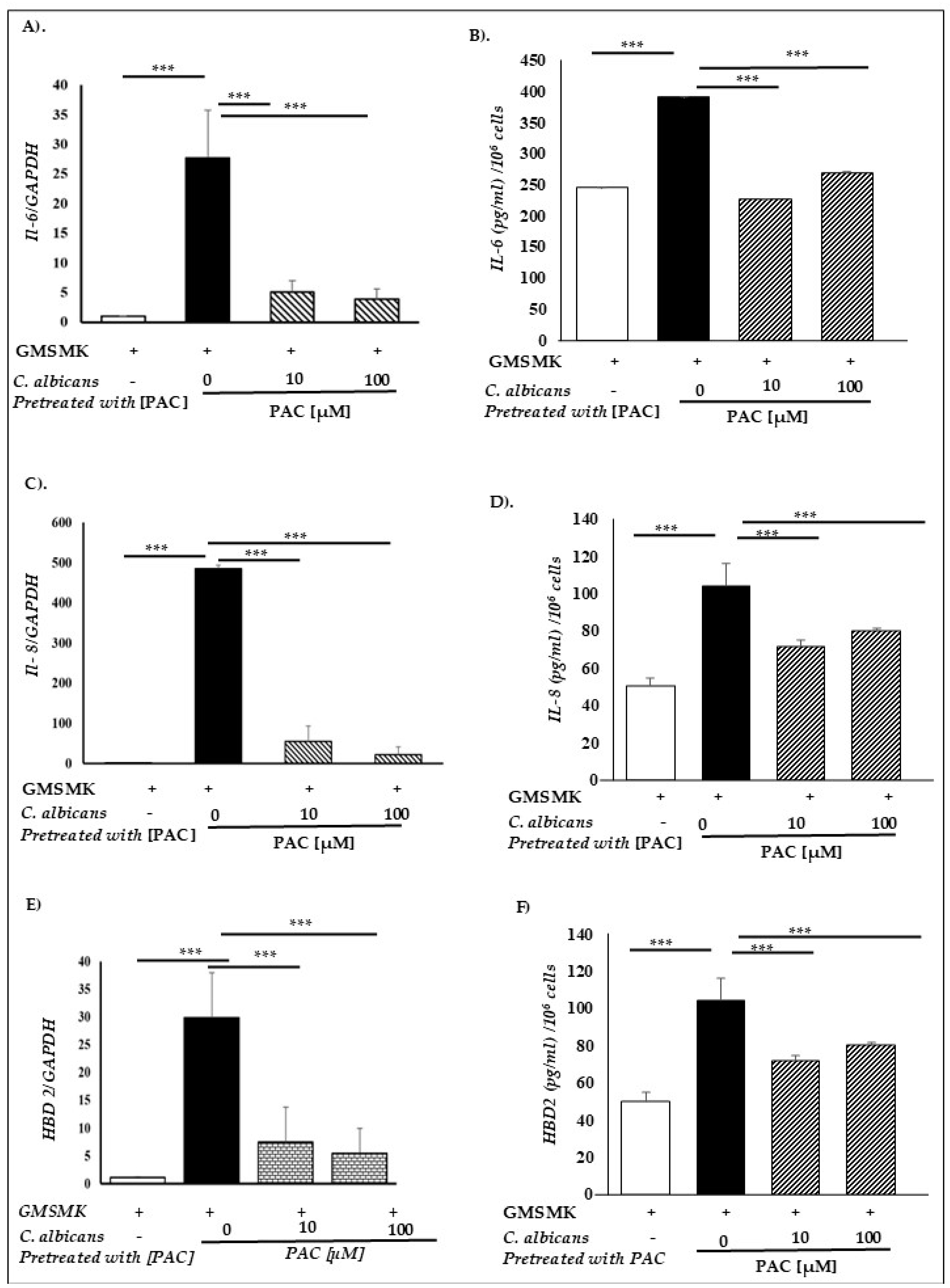
2.6. PAC Relieves Gingival Epithelial Cells’ Defense Mechanisms
3. Discussion
4. Materials and Methods
4.1. Candida Strain and Epithelial Cell Culture
4.2. C. albicans Growth Assay
4.3. MTT Viability and Proliferation Assay
4.4. Colony Formation
4.5. Biofilm Formation by Transmission Electron Microscopy
4.6. Assessment of Morphological Transition
4.7. pH Measurement
4.8. Measurement of Biofilm Matrix Composition: Total Sugars
4.9. Quantitative Real-Time RT-PCR
4.10. Adhesion to Host Cells
4.11. Cell Viability Analysis by Propidium Iodide (PI) Staining
4.12. Reactive Oxygen Species (ROS) Analysis by Flow Cytometry
4.13. ELISA Assay
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PAC | 3,5-Bis(4-hydroxy-3-methoxybenzylidene)-N-methyl-4-piperidone |
| C. albicans | Candida albicans |
| SAPs | Secreted aspartyl proteases |
| HWP1 | Hyphal wall protein 1 |
| EAP1 | Epithelial adhesin protein 1 |
| RT-PCR | Reverse transcription polymerase chain reaction |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| p38 | p38 mitogen-activated protein kinases |
| JNK | c-Jun N-terminal kinase |
| IL-6, IL-8, IL-1β | Interleukin-6, Interleukin-8, Interleukin-1 beta |
| hBD | Human beta-defensin |
| AMB | Amphotericin B |
| RPMI | Roswell Park Memorial Institute medium |
| DMSO | Dimethyl sulfoxide |
| FBS | Fetal bovine serum |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| SEM | Scanning electron microscopy |
| XTT | 2,3-Bis(2-methoxy-4-nitro-5-sulfophenyl)-5-[carbonyl(phenylamino)]-2H-tetrazolium hydroxide |
| PBS | Phosphate-buffered saline |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | Fetal bovine serum |
| TLR4 | Toll-like receptor 4 |
| CD284 | Cluster of differentiation 284 (TLR4 marker) |
| FITC | Fluorescein isothiocyanate |
| RNA | Acide ribonucléique |
| DNA | Acide désoxyribonucléique |
| cDNA | ADN complémentaire |
| PCR | Polymerase chain reaction |
| Ct | Cycle threshold |
| bp | Base pair |
References
- Escapa, I.F.; Chen, T.; Huang, Y.; Gajare, P.; Dewhirst, F.E.; Lemon, K.P. New Insights into Human Nostril Microbiome from the Expanded Human Oral Microbiome Database (eHOMD): A Resource for the Microbiome of the Human Aerodigestive Tract. mSystems 2018, 3, e00187-18. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The oral microbiota: Dynamic communities and host interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.A.O.; Williams, D.W. Diagnosis and management of oral candidosis. Br. Dent. J. 2017, 223, 675–681. [Google Scholar] [CrossRef]
- Zdanavičienė, E.; Sakalauskienė, J.; Gleiznys, A.; Gleiznys, D.; Žilinskas, J. Host responses to Candida albicans. A review. Stomatologija 2017, 19, 109–123. [Google Scholar]
- Ouchi, C.; Hasebe, A.; Sakata, K.-I.; Sato, J.; Yamazaki, Y.; Ohga, N.; Kitagawa, Y. Genotypes and virulence-related activities of Candida albicans derived from oral cavity of patients in Hokkaido. Arch. Oral Biol. 2023, 157, 105827. [Google Scholar] [CrossRef] [PubMed]
- Mane, A.; Gaikwad, S.; Bembalkar, S.; Risbud, A. Increased expression of virulence attributes in oral Candida albicans isolates from human immunodeficiency virus-positive individuals. J. Med Microbiol. 2012, 61, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Soysa, N.S.; Samaranayake, L.P.; Ellepola, A.N.B. Diabetes mellitus as a contributory factor in oral candidosis. Diabet. Med. 2005, 23, 455–459. [Google Scholar] [CrossRef]
- Suryana, K.; Suharsono, H.; Antara, I.G.P.J. Factors Associated with Oral Candidiasis in People Living with HIV/AIDS: A Case Control Study. HIV/AIDS-Res. Palliat. Care 2020, 12, 33–39. [Google Scholar] [CrossRef]
- Tsui, C.; Kong, E.F.; Jabra-Rizk, M.A. Pathogenesis of Candida albicans biofilm. Pathog. Dis. 2016, 74, ftw018. [Google Scholar] [CrossRef]
- Li, L.; Wei, M.-P.; Yu, H.; Xie, Y.-F.; Guo, Y.-H.; Cheng, Y.-L.; Yao, W.-R. Antifungal activity of Sapindus saponins against Candida albicans: Interruption of biofilm formation. J. Herb. Med. 2023, 42, 100776. [Google Scholar] [CrossRef]
- Meylani, V.; Putra, R.R.; Miftahussurur, M.; Sukardiman, S.; Hermanto, F.E.; Abdullah, A. Molecular docking analysis of Cinnamomum zeylanicum phytochemicals against Secreted Aspartyl Proteinase 4–6 of Candida albicans as anti-candidiasis oral. Results Chem. 2022, 5, 100721. [Google Scholar] [CrossRef]
- Telles, D.R.; Karki, N.; Marshall, M.W. Oral Fungal Infections. Dent. Clin. N. Am. 2017, 61, 319–349. [Google Scholar] [CrossRef] [PubMed]
- Holmstrup, P.; Axéll, T. Classification and clinical manifestations of oral yeast infections. Acta Odontol. Scand. 1990, 48, 57–59. [Google Scholar] [CrossRef]
- Agrawal, A.; Singh, A.; Verma, R.; Murari, A. Oral candidiasis: An overview. J. Oral Maxillofac. Pathol. 2014, 18, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Pouso, A.I.; Pérez-Jardón, A.; Caponio, V.C.A.; Spirito, F.; Chamorro-Petronacci, C.M.; Álvarez-Calderón-Iglesias, Ó.; Gándara-Vila, P.; Muzio, L.L.; Pérez-Sayáns, M. Oral Chronic Hyperplastic Candidiasis and Its Potential Risk of Malignant Transformation: A Systematic Review and Prevalence Meta-Analysis. J. Fungi 2022, 8, 1093. [Google Scholar] [CrossRef]
- Vadovics, M.; Ho, J.; Igaz, N.; Alföldi, R.; Rakk, D.; Veres, É.; Szücs, B.; Horváth, M.; Tóth, R.; Szücs, A.; et al. Candida albicans Enhances the Progression of Oral Squamous Cell Carcinoma In Vitro and In Vivo. mBio 2022, 13, e0314421. [Google Scholar] [CrossRef]
- Frohner, I.E.; Bourgeois, C.; Yatsyk, K.; Majer, O.; Kuchler, K. Candida albicans cell surface superoxide dismutases degrade host-derived reactive oxygen species to escape innate immune surveillance. Mol. Microbiol. 2008, 71, 240–252. [Google Scholar] [CrossRef]
- Carlisle, P.L.; Banerjee, M.; Lazzell, A.; Monteagudo, C.; López-Ribot, J.L.; Kadosh, D. Expression levels of a filament-specific transcriptional regulator are sufficient to determine Candida albicans morphology and virulence. Proc. Natl. Acad. Sci. USA 2009, 106, 599–604. [Google Scholar] [CrossRef]
- Tronchin, G.; Pihet, M.; Lopes-Bezerra, L.M.; Bouchara, J.-P. Adherence mechanisms in human pathogenic fungi. Med. Mycol. 2008, 46, 749–772. [Google Scholar] [CrossRef]
- Phan, Q.T.; Myers, C.L.; Fu, Y.; Sheppard, D.C.; Yeaman, M.R.; Welch, W.H.; Ibrahim, A.S.; Edwards, J.E., Jr.; Filler, S.G. Als3 Is a Candida albicans Invasin That Binds to Cadherins and Induces Endocytosis by Host Cells. PLoS Biol. 2007, 5, e64. [Google Scholar] [CrossRef]
- Hube, B.; Naglik, J. Candida albicans proteinases: Resolving the mystery of a gene family. Microbiology 2001, 147, 1997–2005. [Google Scholar] [CrossRef] [PubMed]
- Naglik, J.R.; Challacombe, S.J.; Hube, B. Candida albicans Secreted Aspartyl Proteinases in Virulence and Pathogenesis. Microbiol. Mol. Biol. Rev. 2003, 67, 400–428. [Google Scholar] [CrossRef] [PubMed]
- Schaller, M.; Borelli, C.; Korting, H.C.; Hube, B. Hydrolytic enzymes as virulence factors of Candida albicans. Mycoses 2005, 48, 365–377. [Google Scholar] [CrossRef] [PubMed]
- White, T.C.; Agabian, N. Candida albicans secreted aspartyl proteinases: Isoenzyme pattern is determined by cell type, and levels are determined by environmental factors. J. Bacteriol. 1995, 177, 5215–5221. [Google Scholar] [CrossRef]
- White, T.C.; Miyasaki, S.H.; Agabian, N. Three distinct secreted aspartyl proteinases in Candida albicans. J. Bacteriol. 1993, 175, 6126–6133. [Google Scholar] [CrossRef][Green Version]
- Albrecht, A.; Felk, A.; Pichova, I.; Naglik, J.R.; Schaller, M.; de Groot, P.; MacCallum, D.; Odds, F.C.; Schäfer, W.; Klis, F.; et al. Glycosylphosphatidylinositol-anchored Proteases of Candida albicans Target Proteins Necessary for Both Cellular Processes and Host-Pathogen Interactions. J. Biol. Chem. 2006, 281, 688–694. [Google Scholar] [CrossRef]
- Li, F.; Palecek, S.P. EAP1, a Candida albicans Gene Involved in Binding Human Epithelial Cells. Eukaryot. Cell 2003, 2, 1266–1273. [Google Scholar] [CrossRef]
- Sundstrom, P. Adhesion in Candida spp. Cell. Microbiol. 2002, 4, 461–469. [Google Scholar] [CrossRef]
- Nobile, C.J.; Nett, J.E.; Andes, D.R.; Mitchell, A.P. Function of Candida albicans Adhesin Hwp1 in Biofilm Formation. Eukaryot. Cell 2006, 5, 1604–1610. [Google Scholar] [CrossRef]
- Di Cosola, M.; Cazzolla, A.P.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Santacroce, L. Candida albicans and Oral Carcinogenesis. A Brief Review. J. Fungi 2021, 7, 476. [Google Scholar] [CrossRef]
- Wang, X.; Wu, S.; Wu, W.; Zhang, W.; Li, L.; Liu, Q.; Yan, Z. Candida albicans Promotes Oral Cancer via IL-17A/IL-17RA-Macrophage Axis. mBio 2023, 14, e0044723. [Google Scholar] [CrossRef]
- Semlali, A.; Leung, K.; Curt, S.; Rouabhia, M. Antimicrobial decapeptide KSL-W attenuates Candida albicans virulence by modulating its effects on Toll-like receptor, human β-defensin, and cytokine expression by engineered human oral mucosa. Peptides 2011, 32, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Krauthauser, C.; Maduskuie, V.; Fawcett, P.T.; Olson, J.M.; A Rajasekaran, S. Curcumin-induced HDAC inhibition and attenuation of medulloblastoma growth in vitro and in vivo. BMC Cancer 2011, 11, 144. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.J.; Bisht, S.; Bar, E.E.; Maitra, A.; Eberhart, C.G. A polymeric nanoparticle formulation of curcumin inhibits growth, clonogenicity and stem-like fraction in malignant brain tumors. Cancer Biol. Ther. 2011, 11, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Abuarqoub, H.; Green, C.J.; Foresti, R.; Motterlini, R. Curcumin reduces cold storage-induced damage in human cardiac myoblasts. Exp. Mol. Med. 2007, 39, 139–148. [Google Scholar] [CrossRef]
- Adams, B.K.; Ferstl, E.M.; Davis, M.C.; Herold, M.; Kurtkaya, S.; Camalier, R.F.; Hollingshead, M.G.; Kaur, G.; Sausville, E.A.; Rickles, F.R.; et al. Synthesis and biological evaluation of novel curcumin analogs as anti-cancer and anti-angiogenesis agents. Bioorg. Med. Chem. 2004, 12, 3871–3883. [Google Scholar] [CrossRef]
- Egan, M.E.; Pearson, M.; Weiner, S.A.; Rajendran, V.; Rubin, D.; Glöckner-Pagel, J.; Canny, S.; Du, K.; Lukacs, G.L.; Caplan, M.J. Curcumin, a Major Constituent of Turmeric, Corrects Cystic Fibrosis Defects. Science 2004, 304, 600–602. [Google Scholar] [CrossRef]
- FitzGerald, G.A.; Patrono, C. The Coxibs, Selective Inhibitors of Cyclooxygenase-2. N. Engl. J. Med. 2001, 345, 433–442. [Google Scholar] [CrossRef]
- Smith, W.L.; Marnett, L.J.; DeWitt, D.L. Prostaglandin and thromboxane biosynthesis. Pharmacol. Ther. 1991, 49, 153–179. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Ray, B.; Bisht, S.; Maitra, A.; Maitra, A.; Lahiri, D.K. Neuroprotective and Neurorescue Effects of a Novel Polymeric Nanoparticle Formulation of Curcumin (NanoCurcTM) in the Neuronal Cell Culture and Animal Model: Implications for Alzheimer’s disease. J. Alzheimers Dis. 2011, 23, 61–77. [Google Scholar] [CrossRef] [PubMed]
- Mythri, R.B.; Harish, G.; Dubey, S.K.; Misra, K.; Bharath, M.M.S. Glutamoyl diester of the dietary polyphenol curcumin offers improved protection against peroxynitrite-mediated nitrosative stress and damage of brain mitochondria in vitro: Implications for Parkinson’s disease. Mol. Cell. Biochem. 2010, 347, 135–143. [Google Scholar] [CrossRef]
- Tuncer, E.; Unver-Saraydin, S.; Tepe, B.; Karadayi, S.; Ozer, H.; Karadayi, K.; Inan, D.; Elagoz, S.; Polat, Z.; Duman, M.; et al. Antitumor effects of Origanum acutidens extracts on human breast cancer. J. BUON 2013, 18, 77–85. [Google Scholar]
- Semlali, A.; Contant, C.; Al-Otaibi, B.; Al-Jammaz, I.; Chandad, F. The curcumin analog (PAC) suppressed cell survival and induced apoptosis and autophagy in oral cancer cells. Sci. Rep. 2021, 11, 11701. [Google Scholar] [CrossRef]
- Semlali, A.; Beji, S.; Ajala, I.; Al-Zharani, M.; Rouabhia, M. Synergistic Effects of New Curcumin Analog (PAC) and Cisplatin on Oral Cancer Therapy. Curr. Issues Mol. Biol. 2023, 45, 5018–5035. [Google Scholar] [CrossRef] [PubMed]
- Youssef, K.M.; El-Sherbeny, M.A.; El-Shafie, F.S.; Farag, H.A.; Al-Deeb, O.A.; Awadalla, S.A.A. Synthesis of Curcumin Analogues as Potential Antioxidant, Cancer Chemopreventive Agents. Arch. Pharm. 2004, 337, 42–54. [Google Scholar] [CrossRef]
- Al-Hujaily, E.M.; Mohamed, A.G.; Al-Sharif, I.; Youssef, K.M.; Manogaran, P.S.; Al-Otaibi, B.; Al-Haza’a, A.; Al-Jammaz, I.; Al-Hussein, K.; Aboussekhra, A. PAC, a novel curcumin analogue, has anti-breast cancer properties with higher efficiency on ER-negative cells. Breast Cancer Res. Treat. 2010, 128, 97–107. [Google Scholar] [CrossRef]
- Al-Mohanna, M.; Alraouji, N.N.; Alhabardi, S.A.; Al-Mohanna, F.; Al-Otaibi, B.; Al-Jammaz, I.; Aboussekhra, A. The curcumin analogue PAC has potent anti-anaplastic thyroid cancer effects. Sci. Rep. 2023, 13, 4217. [Google Scholar] [CrossRef] [PubMed]
- Almalki, E.; Al-Amri, A.; Alrashed, R.; Al-Zharani, M.; Semlali, A. The Curcumin Analog PAC Is a Potential Solution for the Treatment of Triple-Negative Breast Cancer by Modulating the Gene Expression of DNA Repair Pathways. Int. J. Mol. Sci. 2023, 24, 9649. [Google Scholar] [CrossRef]
- Sazdova, I.; Keremidarska-Markova, M.; Dimitrova, D.; Mitrokhin, V.; Kamkin, A.; Hadzi-Petrushev, N.; Bogdanov, J.; Schubert, R.; Gagov, H.; Avtanski, D.; et al. Anticarcinogenic Potency of EF24: An Overview of Its Pharmacokinetics, Efficacy, Mechanism of Action, and Nanoformulation for Drug Delivery. Cancers 2023, 15, 5478. [Google Scholar] [CrossRef]
- He, Y.; Li, W.; Hu, G.; Sun, H.; Kong, Q. Bioactivities of EF24, a Novel Curcumin Analog: A Review. Front. Oncol. 2018, 8, 614. [Google Scholar] [CrossRef]
- Romo, J.A.; Pierce, C.G.; Chaturvedi, A.K.; Lazzell, A.L.; McHardy, S.F.; Saville, S.P.; Lopez-Ribot, J.L. Development of Anti-Virulence Approaches for Candidiasis via a Novel Series of Small-Molecule Inhibitors of Candida albicans Filamentation. mBio 2017, 8, e01991-17. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.-R.; Bao, M.-Y.; Yang, Y.; Wang, T.-M.; Wang, C.-Z. Targeting Virulence Factors of Candida albicans with Natural Products. Foods 2022, 11, 2951. [Google Scholar] [CrossRef]
- Jurėnas, D.; Cascales, E. T6SS: Killing two bugs with one stone. Trends Microbiol. 2022, 30, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Jurėnas, D.; Fraikin, N.; Goormaghtigh, F.; Van Melderen, L. Biology and evolution of bacterial toxin–antitoxin systems. Nat. Rev. Microbiol. 2022, 20, 335–350. [Google Scholar] [CrossRef] [PubMed]
- Jurėnas, D.; Garcia-Pino, A.; Van Melderen, L. Novel toxins from type II toxin-antitoxin systems with acetyltransferase activity. Plasmid 2017, 93, 30–35. [Google Scholar] [CrossRef]
- Jurėnas, D.; Journet, L. Activity, delivery, and diversity of Type VI secretion effectors. Mol. Microbiol. 2020, 115, 383–394. [Google Scholar] [CrossRef]
- Fox, E.P.; Bui, C.K.; Nett, J.E.; Hartooni, N.; Mui, M.C.; Andes, D.R.; Nobile, C.J.; Johnson, A.D. An expanded regulatory network temporally controls Candida albicans biofilm formation. Mol. Microbiol. 2015, 96, 1226–1239. [Google Scholar] [CrossRef]
- Schaller, M.; Korting, H.C.; Schäfer, W.; Bastert, J.; Chen, W.; Hube, B. Secreted aspartic proteinase (Sap) activity contributes to tissue damage in a model of human oral candidosis. Mol. Microbiol. 1999, 34, 169–180. [Google Scholar] [CrossRef]
- Chen, E.; Benso, B.; Seleem, D.; Ferreira, L.E.N.; Pasetto, S.; Pardi, V.; Murata, R.M. Fungal-Host Interaction: Curcumin Modulates Proteolytic Enzyme Activity of Candida albicans and Inflammatory Host Response In Vitro. Int. J. Dent. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Hajifathali, S.; Lesan, S.; Lotfali, E.; Salimi-Sabour, E.; Khatibi, M. Investigation of the antifungal effects of curcumin against nystatin-resistant Candida albicans. Dent. Res. J. 2023, 20, 50. [Google Scholar] [CrossRef]
- Shahzad, M.; Millhouse, E.; Culshaw, S.; Edwards, C.A.; Ramage, G.; Combet, E. Selected dietary (poly)phenols inhibit periodontal pathogen growth and biofilm formation. Food Funct. 2014, 6, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Li, H.; Jia, J.; He, M. Anti-inflammatory effect of curcumin on mast cell-mediated allergic responses in ovalbumin-induced allergic rhinitis mouse. Cell. Immunol. 2015, 298, 88–95. [Google Scholar] [CrossRef]
- Seleem, D.; Chen, E.; Benso, B.; Pardi, V.; Murata, R.M. In vitro evaluation of antifungal activity of monolaurin against Candida albicans biofilms. PeerJ 2016, 4, e2148. [Google Scholar] [CrossRef] [PubMed]
- Fusco, A.; Savio, V.; Donniacuo, M.; Perfetto, B.; Donnarumma, G. Antimicrobial Peptides Human Beta-Defensin-2 and -3 Protect the Gut During Candida albicans Infections Enhancing the Intestinal Barrier Integrity: In Vitro Study. Front. Cell. Infect. Microbiol. 2021, 11, 666900. [Google Scholar] [CrossRef]
- Alanazi, H.; Semlali, A.; Perraud, L.; Chmielewski, W.; Zakrzewski, A.; Rouabhia, M. Cigarette Smoke-Exposed Candida albicans Increased Chitin Production and Modulated Human Fibroblast Cell Responses. BioMed Res. Int. 2014, 2014, 963156. [Google Scholar] [CrossRef]
- Lermann, U.; Morschhäuser, J. Secreted aspartic proteases are not required for invasion of reconstituted human epithelia by Candida albicans. Microbiology 2008, 154, 3281–3295. [Google Scholar] [CrossRef]
- Ramage, G.; Vandewalle, K.; Wickes, B.L.; López-Ribot, J.L. Characteristics of biofilm formation by Candida albicans. Rev. Iberoam. De Micol. 2001, 18, 163–170. [Google Scholar]
- Tomalka, J.; Azodi, E.; Narra, H.P.; Patel, K.; O’neill, S.; Cardwell, C.; A Hall, B.; Wilson, J.M.; Hise, A.G. β-Defensin 1 Plays a Role in Acute Mucosal Defense against Candida albicans. J. Immunol. 2015, 194, 1788–1795. [Google Scholar] [CrossRef]
- Dale, B.A.; Fredericks, L.P. Antimicrobial peptides in the oral environment: Expression and function in health and disease. Curr. Issues Mol. Biol. 2005, 7, 119–134. [Google Scholar] [CrossRef]
- Vylkova, S.; Nayyar, N.; Li, W.; Edgerton, M. Human β-Defensins Kill Candida albicans in an Energy-Dependent and Salt-Sensitive Manner without Causing Membrane Disruption. Antimicrob. Agents Chemother. 2007, 51, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Jayatilake, J.A.; Samaranayake, L.P.; Jin, L. Hyphal Invasion of Candida albicans Inhibits the Expression of Human β-Defensins in Experimental Oral Candidiasis. J. Investig. Dermatol. 2006, 126, 2049–2056. [Google Scholar] [CrossRef]
- Kumari, A.; Dash, D.; Singh, R. Curcumin inhibits lipopolysaccharide (LPS)-induced endotoxemia and airway inflammation through modulation of sequential release of inflammatory mediators (TNF-α and TGF-β1) in murine model. Inflammopharmacology 2017, 25, 329–341. [Google Scholar] [CrossRef]
- Sadeghi, A.; Rostamirad, A.; Seyyedebrahimi, S.; Meshkani, R. Curcumin ameliorates palmitate-induced inflammation in skeletal muscle cells by regulating JNK/NF-kB pathway and ROS production. Inflammopharmacology 2018, 26, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Sordillo, P.P.; Helson, L. Curcumin suppression of cytokine release and cytokine storm. A potential therapy for patients with Ebola and other severe viral infections. In Vivo 2015, 29, 1–4. [Google Scholar] [PubMed]
- Dinarello, C.A. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef]
- Sikora, E.; Scapagnini, G.; Barbagallo, M. Curcumin, inflammation, ageing and age-related diseases. Immun. Ageing 2010, 7, 1. [Google Scholar] [CrossRef]
- Meng, Z.; Yan, C.; Deng, Q.; Gao, D.-F.; Niu, X.-L. Curcumin inhibits LPS-induced inflammation in rat vascular smooth muscle cells in vitro via ROS-relative TLR4-MAPK/NF-κB pathways. Acta Pharmacol. Sin. 2013, 34, 901–911. [Google Scholar] [CrossRef]
- Li, W.; Yin, Y.; Li, T.; Wang, Y.; Shi, W. Licochalcone A Protects Vaginal Epithelial Cells Against Candida albicans Infection Via the TLR4/NF-κB Signaling Pathway. J. Microbiol. 2024, 62, 525–533. [Google Scholar] [CrossRef]
- Weindl, G.; Naglik, J.R.; Kaesler, S.; Biedermann, T.; Hube, B.; Korting, H.C.; Schaller, M. Human epithelial cells establish direct antifungal defense through TLR4-mediated signaling. J. Clin. Investig. 2007, 117, 3664–3672. [Google Scholar] [CrossRef]
- Moyes, D.L.; Runglall, M.; Murciano, C.; Shen, C.; Nayar, D.; Thavaraj, S.; Kohli, A.; Islam, A.; Mora-Montes, H.; Challacombe, S.J.; et al. A Biphasic Innate Immune MAPK Response Discriminates between the Yeast and Hyphal Forms of Candida albicans in Epithelial Cells. Cell Host Microbe 2010, 8, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Gow, N.A.R.; Joosten, L.A.B.; Verschueren, I.; van der Meer, J.W.M.; Kullberg, B.J. Variable recognition of Candida albicans strains by TLR4 and lectin recognition receptors. Med. Mycol. 2010, 48, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Dumitru, R.; Hornby, J.M.; Nickerson, K.W. Defined Anaerobic Growth Medium for Studying Candida albicans Basic Biology and Resistance to Eight Antifungal Drugs. Antimicrob. Agents Chemother. 2004, 48, 2350–2354. [Google Scholar] [CrossRef] [PubMed]
- Theberge, S.; Semlali, A.; Alamri, A.; Leung, K.P.; Rouabhia, M. C. albicans growth, transition, biofilm formation, and gene expression modulation by antimicrobial decapeptide KSL-W. BMC Microbiol. 2013, 13, 246. [Google Scholar] [CrossRef]
- Alanazi, H.; Semlali, A.; Chmielewski, W.; Rouabhia, M. E-Cigarettes Increase Candida albicans Growth and Modulate its Interaction with Gingival Epithelial Cells. Int. J. Environ. Res. Public Health 2019, 16, 294. [Google Scholar] [CrossRef]
- Pemmaraju, S.C.; Pruthi, P.A.; Prasad, R.; Pruthi, V. Modulation of Candida albicans Biofilm by Different Carbon Sources. Mycopathologia 2016, 181, 341–352. [Google Scholar] [CrossRef]
- Semlali, A.; Killer, K.; Alanazi, H.; Chmielewski, W.; Rouabhia, M. Cigarette smoke condensate increases C. albicans adhesion, growth, biofilm formation, and EAP1, HWP1 and SAP2 gene expression. BMC Microbiol. 2014, 14, 61. [Google Scholar] [CrossRef]


| Gene for Candida | Primer Sequence (5′-3′) | Amp Size (bp) |
|---|---|---|
| HWP1 | Forward-GACCGTCTACCTGTGGGACAGT Reverse-GCTCAACTTATTGCTATCGCTTATTACA | 67 |
| EAP1 | Forward-CTGCTCACTCAACTTCAATTGTCG Reverse -GAACACATCCACCTTCGGGA | 51 |
| SAP1 | Forward-TTTCATCGCTCTTGCTATTGCTT Reverse -TGACATCAAAGTCTAAAGTGACAAAACC | 86 |
| SAP2 | Forward-TCCTGATGTTAATGTTGATTGTCAAG Reverse -TGGATCATATGTCCCCTTTTGTT | 82 |
| SAP3 | Forward-GGACCAGTAACATTTTTATGAGTTTTGAT Reverse -TGCTACTCCAACAACTTTCAACAAT | 87 |
| SAP4 | Forward-AGATATTGAGCCCACAGAAATTCC Reverse -CAATTTAACTGCAACAGGTCCTCTT | 81 |
| SAP5 | Forward-CATTGTGCAAAGTAACTGCAACAG Reverse -CAGAATTTCCCGTCGATGAGA | 77 |
| SAP6 | Forward-CCTTTATGAGCACTAGTAGACCAAACG Reverse -TTACGCAAAAGGTAACTTGTATCAAGA | 101 |
| SAP7 | Forward: 5′-GAAATGCAAAGAGTATTAGAGTTATTAC Reverse: GAATGATTTGGTTTACATCATCTTCAACTG | 196 |
| SAP8 | Forward: TCCCTGAAGACATTGATAAAAGAGC-3′ Reverse: AGAATCAACCACCCATAAATCAGAA-5′ | 278 |
| SAP9 | Forward-ATTTACTCCACAGTTTATCACTGAAGGT Reverse -CCACAAGAACCACCCTCAGTT | 86 |
| SAP10 | Forward -CCCGGTATCCAATAGAATCGAA Reverse-TCAGTGAATGTGACGAATTTGAAGA | 78 |
| ACT1 | Forward-GCTGGTAGAGACTTGACCAACCA-3′ Reverse: GACAATTTCTCTTTCAGCACTAGTAGTGA | 87 |
| GMSM-K Genes | Primer Sequence (5′-3′) | Size (bp) |
|---|---|---|
| IL-1β | Forward: CTGTCCTGCGTGTTGAAAGA Reverse: TTGGGTAATTTTTGGGATCTACA | 69 |
| IL-6 | Forward: TCTCCACAAGCGCCTTCG Reverse: CTCAGGGCTGAGATGCCG | 203 |
| IL-8 | Forward: TTGGCAGCCTTCCTGATT Reverse: AACTTCTCCACAACCCTCTG | 248 |
| hBD-1 | Forward: GCCTCTCCCCAGTTCCTGAA Reverse: GCAGAGAGTAAACAGCAGAAGGTA | 82 |
| hBD-2 | Forward: TGTGGTCTCCCTGGAACAAAAT Reverse: GTCGCACGTCTCTGATGAGG | 105 |
| hBD-3 | Forward: CTTCTGTTTGCTTTGCTCTTCCT Reverse: CTGTTCCTCCTTTGGAAGGCA | 138 |
| hBD-4 | Forward: CACTCTACCAACACGCACCTAG Reverse: CGCAACTGGAACCACACACT | 133 |
| GAPDH | Forward: GGTATCGTCGAAGGACTCATGAC Reverse: ATGCCAGTGAGCTTCCCGTTCAGC | 180 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mezni, G.; Issa, H.; Dahdah, M.; Poulin, A.; Daïch, A.; Alamri, A.; Rouabhia, M.; Semlali, A. New Curcumin Analogue (PAC) Inhibits Candida albicans Virulence, Restricts Its Adhesion Potential, and Relieves Oral Epithelial Cell Inflammation and Defense Mechanisms. Antibiotics 2025, 14, 495. https://doi.org/10.3390/antibiotics14050495
Mezni G, Issa H, Dahdah M, Poulin A, Daïch A, Alamri A, Rouabhia M, Semlali A. New Curcumin Analogue (PAC) Inhibits Candida albicans Virulence, Restricts Its Adhesion Potential, and Relieves Oral Epithelial Cell Inflammation and Defense Mechanisms. Antibiotics. 2025; 14(5):495. https://doi.org/10.3390/antibiotics14050495
Chicago/Turabian StyleMezni, Ghazoua, Hawraa Issa, Manal Dahdah, Anaïs Poulin, Adam Daïch, Abdulaziz Alamri, Mahmoud Rouabhia, and Abdelhabib Semlali. 2025. "New Curcumin Analogue (PAC) Inhibits Candida albicans Virulence, Restricts Its Adhesion Potential, and Relieves Oral Epithelial Cell Inflammation and Defense Mechanisms" Antibiotics 14, no. 5: 495. https://doi.org/10.3390/antibiotics14050495
APA StyleMezni, G., Issa, H., Dahdah, M., Poulin, A., Daïch, A., Alamri, A., Rouabhia, M., & Semlali, A. (2025). New Curcumin Analogue (PAC) Inhibits Candida albicans Virulence, Restricts Its Adhesion Potential, and Relieves Oral Epithelial Cell Inflammation and Defense Mechanisms. Antibiotics, 14(5), 495. https://doi.org/10.3390/antibiotics14050495








