Targeting Oral Pathogens with Salvia officinalis and Nigella sativa Supercritical CO2 Extracts: A Pharmacodynamic Approach and Three-Dimensional Checkerboard Synergy for Novel Dental Antimicrobials
Abstract
1. Introduction
2. Results
2.1. Chemical Characterization of Plant Samples
2.2. Antimicrobial Activity
2.2.1. Planktonic MIC and MBC Values
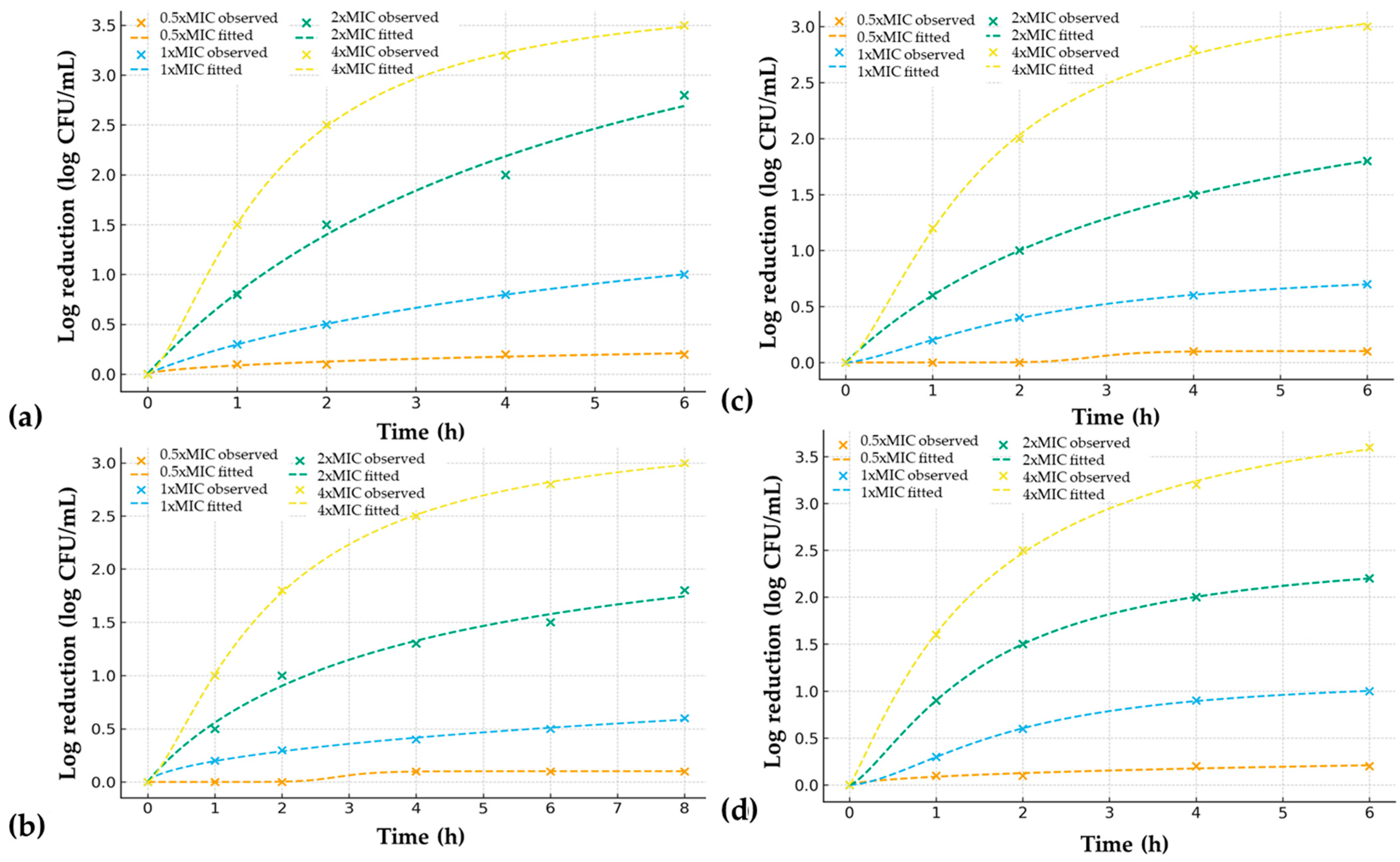
2.2.2. Time–Kill Kinetics
2.3. Biofilm Assays
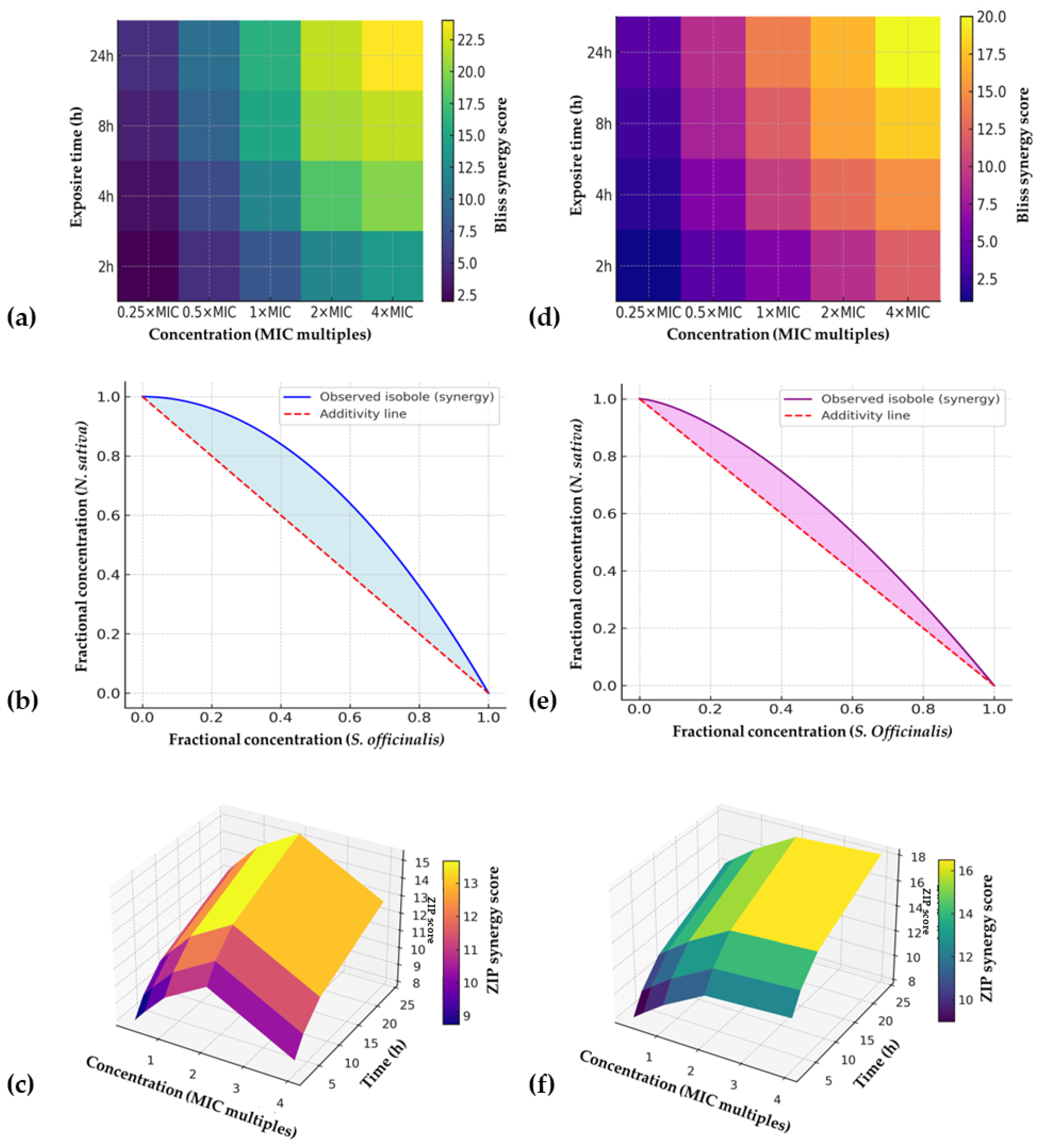
2.4. Three-Dimensional Checkerboard Synergy Optimization
2.5. Pharmacodynamic Synergy
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Microorganisms and Culture Conditions
4.3. Antimicrobial Susceptibility Testing
4.4. Time–Kill Kinetics and Post-Antibiotic Effect
4.5. Biofilm Inhibition and Eradication Assays
4.6. Three-Dimensional Checkerboard Synergy Optimization
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sabella, F.M.; de Feiria, S.N.; Ribeiro, A.D.; Theodoro, L.H.; Höfling, J.F.; Parisotto, T.M.; Duque, C. Exploring the interplay between oral diseases, microbiome, and chronic diseases driven by metabolic dysfunction in childhood. Front. Dent. Med. 2021, 17, 718441. [Google Scholar] [CrossRef]
- Sedghi, L.; DiMassa, V.; Harrington, A.; Lynch, S.V.; Kapila, Y.L. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontology 2021, 87, 107–131. [Google Scholar] [CrossRef]
- Alrashdan, M.S.; Leao, J.C.; McCullough, M.; Porter, S. The effects of antimicrobial mouthwashes on systemic disease: What is the evidence? Int. Dent. J. 2023, 1, S82–S88. [Google Scholar] [CrossRef]
- Boyd, N.K.; Teng, C.; Frei, C.R. Brief overview of approaches and challenges in new antibiotic development: A focus on drug repurposing. Front. Cell. Infect. Microbiol. 2021, 11, 684515. [Google Scholar] [CrossRef]
- Coates, A.; Hu, Y.; Bax, R.; Page, C. The future challenges facing the development of new antimicrobial drugs. Nat. Rev. Drug Discov. 2002, 1, 895–910. [Google Scholar] [CrossRef] [PubMed]
- Zouine, N.; El Ghachtouli, N.; El Abed, S.; Koraichi, S.I. A comprehensive review on medicinal plant extracts as antibacterial agents: Factors, mechanism insights and future prospects. Sci. Afr. 2024, 1, e02395. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological properties of Salvia officinalis and its components. J. Trad. Complem. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Mulat, M.; Banicod, R.J.; Tabassum, N.; Javaid, A.; Karthikeyan, A.; Jeong, G.J.; Kim, Y.M.; Jung, W.K.; Khan, F. Multiple Strategies for the Application of Medicinal Plant-Derived Bioactive Compounds in Controlling Microbial Biofilm and Virulence Properties. Antibiotics 2025, 14, 555. [Google Scholar] [CrossRef]
- Zeineldin, M.; Esmael, A.; Al-Hindi, R.R.; Alharbi, M.G.; Ashenafi Bekele, D.; Teklemariam, A.D. Beyond the risk of biofilms: An up-and-coming battleground of bacterial life and potential antibiofilm agents. Life 2023, 13, 503. [Google Scholar] [CrossRef]
- Majdalawieh, A.F.; Fayyad, M.W. Immunomodulatory and anti-inflammatory action of Nigella sativa and thymoquinone: A comprehensive review. Int. Immunopharmacol. 2015, 28, 295–304. [Google Scholar] [CrossRef]
- Sharma, S.; Mohler, J.; Mahajan, S.D.; Schwartz, S.A.; Bruggemann, L.; Aalinkeel, R. Microbial biofilm: A review on formation, infection, antibiotic resistance, control measures, and innovative treatment. Microorganisms 2023, 11, 1614. [Google Scholar] [CrossRef]
- Scoffone, V.C.; Barbieri, G.; Irudal, S.; Trespidi, G.; Buroni, S. New antimicrobial strategies to treat multi-drug resistant infections caused by gram-negatives in cystic fibrosis. Antibiotics 2024, 13, 71. [Google Scholar] [CrossRef]
- Huang, R.Y.; Pei, L.; Liu, Q.; Chen, S.; Dou, H.; Shu, G.; Yuan, Z.X.; Lin, J.; Peng, G.; Zhang, W.; et al. Isobologram analysis: A comprehensive review of methodology and current research. Fron. Pharm. 2019, 10, 1222. [Google Scholar] [CrossRef]
- Arzani, V.; Soleimani, M.; Fritsch, T.; Jacob, U.M.; Calabrese, V.; Arzani, A. Plant polyphenols, terpenes, and terpenoids in oral health. Open Med. 2025, 20, 20251183. [Google Scholar] [CrossRef] [PubMed]
- Touati, A.; Mairi, A.; Ibrahim, N.A.; Idres, T. Essential Oils for Biofilm Control: Mechanisms, Synergies, and Translational Challenges in the Era of Antimicrobial Resistance. Antibiotics 2025, 14, 503. [Google Scholar] [CrossRef]
- Onita, T.; Ishihara, N.; Yano, T. PK/PD-Guided Strategies for Appropriate Antibiotic Use in the Era of Antimicrobial Resistance. Antibiotics 2025, 14, 92. [Google Scholar] [CrossRef] [PubMed]
- Delamare, A.P.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial activity of the essential oils of Salvia officinalis L. and Salvia triloba L. cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Khan, A.; Ahmad, A.; Khan, L.A.; Padoa, C.J.; van Vuuren, S.; Manzoor, N. Effect of two monoterpene phenols on antioxidant defense system in Candida albicans. Microb. Pathog. 2015, 80, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Beheshti-Rouy, M.; Azarsina, M.; Rezaie-Soufi, L.; Alikhani, M.Y.; Roshanaie, G.; Komaki, S. The antibacterial effect of sage extract (Salvia officinalis) mouthwash against Streptococcus mutans in dental plaque: A randomized clinical trial. Iran. J. Microbiol. 2015, 7, 173. [Google Scholar]
- Ntondini, S.S.; Lenetha, G.; Dzogbewu, T.C. Antimicrobial Activity of Salvia officinalis against Streptococcus mutans Causing Dental Implant Failure: An in vitro Study. J. Int. Oral Health 2021, 13, 499–507. [Google Scholar] [CrossRef]
- Gloria-Garza, M.A.; Reyna-Martínez, G.R.; Jiménez-Salas, Z.; Campos-Góngora, E.; Kačániová, M.; Aguirre-Cavazos, D.E.; Bautista-Villarreal, M.; Leos-Rivas, C.; Elizondo-Luevano, J.H. Medicinal Plants Against Dental Caries: Research and Application of Their Antibacterial Properties. Plants 2025, 14, 1390. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Badger-Emeka, L.I.; Emeka, P.M.; Ibrahim, H.I. A molecular insight into the synergistic mechanism of Nigella sativa (black cumin) with β-lactam antibiotics against clinical isolates of methicillin-resistant Staphylococcus aureus. Appl. Sci. 2021, 11, 3206. [Google Scholar] [CrossRef]
- Jo, A.; Kim, H.E. Antibacterial Effects of Black Cumin Seed Oil on Oral Microcosm Biofilms. Microorganisms 2024, 12, 2098. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Candelario, L.; De la Rosa-García, S.; Zarza, E.; Guillen-Navarro, K.; Alvarez-Lemus, M.A.; Gómez-Cornelio, S. Sessile culture as a strategy to enhance the production of emulsifying exopolysaccharides by Glutamicibacter sp. XHA18. Int. Biodeterior. Biodegradation. 2026, 206, 106213. [Google Scholar] [CrossRef]
- Stipp, R.N.; Goncalves, R.B.; Höfling, J.F.; Smith, D.J.; Mattos-Graner, R.O. Transcriptional analysis of gtfB, gtfC, and gbpB and their putative response regulators in several isolates of Streptococcus mutans. Oral Microbiol. Immunol. 2008, 23, 466–473. [Google Scholar] [CrossRef]
- Kashi, M.; Varseh, M.; Hariri, Y.; Chegini, Z.; Shariati, A. Natural compounds: New therapeutic approach for inhibition of Streptococcus mutans and dental caries. Front. Pharmacol. 2025, 16, 1548117. [Google Scholar] [CrossRef] [PubMed]
- Nouri, N.; Mohammadi, S.R.; Beardsley, J.; Aslani, P.; Ghaffarifar, F.; Roudbary, M.; Rodrigues, C.F. Thymoquinone antifungal activity against Candida glabrata Oral isolates from patients in intensive care units-an in vitro study. Metabolites 2023, 13, 580. [Google Scholar] [CrossRef]
- Stein, C.; Makarewicz, O.; Bohnert, J.A.; Pfeifer, Y.; Kesselmeier, M.; Hagel, S.; Pletz, M.W. Three dimensional checkerboard synergy analysis of colistin, meropenem, tigecycline against multidrug-resistant clinical Klebsiella pneumonia isolates. PLoS ONE 2015, 10, e0126479. [Google Scholar] [CrossRef]
- Fratini, F.; Mancini, S.; Turchi, B.; Friscia, E.; Pistelli, L.; Giusti, G.; Cerri, D. A novel interpretation of the Fractional Inhibitory Concentration Index: The case Origanum vulgare L. and Leptospermum scoparium JR et G. Forst essential oils against Staphylococcus aureus strains. Microbiol. Res. 2017, 195, 11–17. [Google Scholar] [CrossRef]
- Repash, E.M.; Pensabene, K.M.; Palenchar, P.M.; Eggler, A.L. Solving the problem of assessing synergy and antagonism for non-traditional dosing curve compounds using the DE/ZI method: Application to Nrf2 activators. Front. Pharm. 2021, 12, 686201. [Google Scholar] [CrossRef]
- Dera, A.A.; Ahmad, I.; Rajagopalan, P.; Al Shahrani, M.; Saif, A.; Alshahrani, M.Y.; Alraey, Y.; Alamri, A.M.; Alasmari, S.; Makkawi, M.; et al. Synergistic efficacies of thymoquinone and standard antibiotics against multi-drug resistant isolates. Saudi Med. J. 2021, 42, 196. [Google Scholar] [CrossRef]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsakris, Z.; Rozos, G.; Tsigalou, C.; Bezirtzoglou, E. Interactions between medical plant-derived bioactive compounds: Focus on antimicrobial combination effects. Antibiotics 2022, 11, 1014. [Google Scholar] [CrossRef]
- Hannan, M.A.; Rahman, M.A.; Sohag, A.A.; Uddin, M.J.; Dash, R.; Sikder, M.H.; Rahman, M.S.; Timalsina, B.; Munni, Y.A.; Sarker, P.P.; et al. Black cumin (Nigella sativa L.): A comprehensive review on phytochemistry, health benefits, molecular pharmacology, and safety. Nutrients 2021, 13, 1784. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, H.A.; Rehman, A.; Karim, A.; Al-Asmari, F.; Cui, H.; Lin, L. A comprehensive insight into plant-derived extracts/bioactives: Exploring their antimicrobial mechanisms and potential for high-performance food applications. Food Biosci. 2024, 59, 104035. [Google Scholar] [CrossRef]
- Pereira, L.C.; Fátima, M.A.; Santos, V.V.; Brandão, C.M.; Alves, I.A.; Azeredo, F.J. Pharmacokinetic/pharmacodynamic modeling and application in antibacterial and antifungal pharmacotherapy: A narrative review. Antibiotics 2022, 11, 986. [Google Scholar] [CrossRef] [PubMed]
- Šovljanski, O.; Saveljić, A.; Aćimović, M.; Šeregelj, V.; Pezo, L.; Tomić, A.; Ćetković, G.; Tešević, V. Biological Profiling of Essential Oils and Hydrolates of Ocimum basilicum var. Genovese and var. Minimum Originated from Serbia. Processes 2022, 10, 1893. [Google Scholar]
- Zhang, L.; Xie, H.; Wang, Y.; Wang, H.; Hu, J.; Zhang, G. Pharmacodynamic parameters of pharmacokinetic/pharmacodynamic (PK/PD) integration models. Front. Vet. Sci. 2022, 9, 860472. [Google Scholar] [CrossRef]
- Sun, S.; Yu, Y.; Jo, Y.; Han, J.H.; Xue, Y.; Cho, M.; Bae, S.J.; Ryu, D.; Park, W.; Ha, K.T.; et al. Impact of extraction techniques on phytochemical composition and bioactivity of natural product mixtures. Front. Pharm. 2025, 16, 1615338. [Google Scholar] [CrossRef]
| Salvia officinalis | Nigella sativa | ||
|---|---|---|---|
| Compound | % | Compound | % |
| cis-Thujone | 19.9 | p-Cymene | 47.2 |
| Camphor | 15.8 | cis-4-methoxy Thujane | 7.9 |
| trans-Thujone | 13.3 | 2-butyl-2-Octenal | 6.0 |
| 1,8-Cineole | 11.3 | Longifolene | 5.4 |
| Camphene | 6.3 | γ-Terpinene | 4.7 |
| Salvia officinalis | Nigella sativa | Controls | ||||
|---|---|---|---|---|---|---|
| Microorganism | MIC (mg/L) | MBC/MFC (mg/L) | MIC (mg/L) | MBC/MFC (mg/L) | MIC (mg/L) | MBC/MFC (mg/L) |
| S. mutans | 256 | 512 | 512 | 1024 | 2 * | 4 * |
| 2 ** | 3 ** | |||||
| C. albicans | 512 | 1024 | 256 | 512 | 2 *** | 4 *** |
| 1 **** | 2 **** | |||||
| Salvia officinalis CO2 Extract | ||||||
| Microorganism | Time (h) | 0.5× MIC | 1× MIC | 2× MIC | 4× MIC | PAE (h) at 2× MIC |
| S. mutans | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.5 |
| 1 | 0.1 | 0.3 | 0.8 | 1.5 | ||
| 2 | 0.1 | 0.5 | 1.5 | 2.5 | ||
| 4 | 0.2 | 0.8 | 2.0 | 3.2 | ||
| 6 | 0.2 | 1.0 | 2.8 | 3.5 | ||
| C. albicans | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.5 |
| 1 | 0.0 | 0.2 | 0.5 | 1.0 | ||
| 2 | 0.0 | 0.3 | 1.0 | 1.8 | ||
| 4 | 0.1 | 0.4 | 1.3 | 2.5 | ||
| 6 | 0.1 | 0.5 | 1.5 | 2.8 | ||
| 8 | 0.1 | 0.6 | 1.8 | 3.0 | ||
| Nigella sativa CO2 Extract | ||||||
| Microorganism | Time (h) | 0.5× MIC | 1× MIC | 2× MIC | 4× MIC | PAE (h) at 2× MIC |
| S. mutans | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 |
| 1 | 0.0 | 0.2 | 0.6 | 1.2 | ||
| 2 | 0.0 | 0.4 | 1.0 | 2.0 | ||
| 4 | 0.1 | 0.6 | 1.5 | 2.8 | ||
| 6 | 0.1 | 0.7 | 1.8 | 3.0 | ||
| C. albicans | 0 | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 |
| 1 | 0.1 | 0.3 | 0.9 | 1.6 | ||
| 2 | 0.1 | 0.6 | 1.5 | 2.5 | ||
| 4 | 0.2 | 0.9 | 2.0 | 3.2 | ||
| 6 | 0.2 | 1.0 | 2.2 | 3.6 | ||
| Combination Extract Sample–Test Microorganism | MIC | Nmin | Nmax | t50 (h) | Hill | R2 | RMSE | MAE | SSres |
|---|---|---|---|---|---|---|---|---|---|
| S. officinalis–S. mutans | 0.5× | 0.63 | 0.00 | 18.14 | 0.63 | 0.946 | 0.017 | 0.015 | 0.002 |
| 1× | 2.35 | 0.00 | 8.32 | 0.91 | 1.000 | 0.002 | 0.002 | 0.000 | |
| 2× | 5.00 | 0.01 | 5.17 | 1.00 | 0.988 | 0.107 | 0.084 | 0.057 | |
| 4× | 3.86 | 0.00 | 1.35 | 1.50 | 1.000 | 0.015 | 0.012 | 0.001 | |
| S. officinalis–C. albicans | 0.5× | 0.10 | 0.00 | 2.86 | 10.00 | 0.999 | 0.002 | 0.001 | 0.000 |
| 1× | 1.67 | 0.00 | 20.00 | 0.68 | 0.996 | 0.013 | 0.011 | 0.001 | |
| 2× | 2.62 | 0.00 | 3.89 | 0.96 | 0.990 | 0.061 | 0.052 | 0.022 | |
| 4× | 3.37 | 0.00 | 1.85 | 1.38 | 1.000 | 0.016 | 0.014 | 0.001 | |
| N. sativa–S. mutans | 0.5× | 0.10 | 0.00 | 2.88 | 10.00 | 0.999 | 0.002 | 0.001 | 0.000 |
| 1× | 0.86 | 0.00 | 2.22 | 1.47 | 1.000 | 0.003 | 0.003 | 0.000 | |
| 2× | 3.00 | 0.00 | 4.00 | 1.00 | 1.000 | 0.000 | 0.000 | 0.000 | |
| 4× | 3.46 | 0.00 | 1.57 | 1.45 | 0.999 | 0.029 | 0.025 | 0.004 | |
| N. sativa–C. albicans | 0.5× | 0.63 | 0.00 | 18.14 | 0.63 | 0.946 | 0.017 | 0.015 | 0.002 |
| 1× | 1.15 | 0.00 | 1.88 | 1.67 | 1.000 | 0.004 | 0.003 | 0.000 | |
| 2× | 2.52 | 0.00 | 1.52 | 1.40 | 1.000 | 0.002 | 0.001 | 0.000 | |
| 4× | 4.33 | 0.00 | 1.57 | 1.16 | 1.000 | 0.025 | 0.021 | 0.003 |
| Microorganism | Agent | MBIC (mg/L) | MBEC (mg/L) | Biomass Inhibition (%) | EPS/Glucan Reduction (%) | Biofilm Eradication (%) | Metabolic Activity Reduction (%) |
|---|---|---|---|---|---|---|---|
| S. mutans | S. officinalis | 512 | 1024 | 72 ± 4 | 60 ± 5/55 ± 4 | 74 ± 3 | 65 ± 5 |
| Chlorhexidine | 4 | 8 | 92 ± 3 | 85 ± 4/83 ± 3 | 95 ± 2 | 94 ± 3 | |
| Amoxicillin | 3 | 7 | 94 ± 2 | 85 ± 3/84 ± 3 | 96 ± 2 | 94 ± 2 | |
| C. albicans | N. sativa | 512 | 1024 | 68 ± 5 | / * | 70 ± 5 | 71 ± 4 |
| Nystatin | 2 | 4 | 91 ± 4 | / | 94 ± 2 | 92 ± 3 | |
| Fluconazole | 1 | 2 | 89 ± 3 | / | 92 ± 2 | 91 ± 3 |
| Organism | Optimal Ratio (S. officinalis:N. sativa) | FICI | Bliss Excess (%) | ZIP Score | HSA Score | Loewe Interpretation |
|---|---|---|---|---|---|---|
| S. mutans | 70:30 | 0.31 ± 0.05 | 21.8 ± 2.7 | 15.2 ± 2.1 | +0.19 | Concave → synergy |
| C. albicans | 40:60 | 0.38 ± 0.06 | 16.4 ± 2.1 | 12.8 ± 1.9 | +0.14 | Concave → synergy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tucakov, L.; Tomić, A.; Šovljanski, O.; Aćimović, M.; Miljković, A. Targeting Oral Pathogens with Salvia officinalis and Nigella sativa Supercritical CO2 Extracts: A Pharmacodynamic Approach and Three-Dimensional Checkerboard Synergy for Novel Dental Antimicrobials. Antibiotics 2025, 14, 1100. https://doi.org/10.3390/antibiotics14111100
Tucakov L, Tomić A, Šovljanski O, Aćimović M, Miljković A. Targeting Oral Pathogens with Salvia officinalis and Nigella sativa Supercritical CO2 Extracts: A Pharmacodynamic Approach and Three-Dimensional Checkerboard Synergy for Novel Dental Antimicrobials. Antibiotics. 2025; 14(11):1100. https://doi.org/10.3390/antibiotics14111100
Chicago/Turabian StyleTucakov, Luka, Ana Tomić, Olja Šovljanski, Milica Aćimović, and Ana Miljković. 2025. "Targeting Oral Pathogens with Salvia officinalis and Nigella sativa Supercritical CO2 Extracts: A Pharmacodynamic Approach and Three-Dimensional Checkerboard Synergy for Novel Dental Antimicrobials" Antibiotics 14, no. 11: 1100. https://doi.org/10.3390/antibiotics14111100
APA StyleTucakov, L., Tomić, A., Šovljanski, O., Aćimović, M., & Miljković, A. (2025). Targeting Oral Pathogens with Salvia officinalis and Nigella sativa Supercritical CO2 Extracts: A Pharmacodynamic Approach and Three-Dimensional Checkerboard Synergy for Novel Dental Antimicrobials. Antibiotics, 14(11), 1100. https://doi.org/10.3390/antibiotics14111100









