Abstract
Background: The increasing attention on extended-spectrum beta-lactamase (ESBL)-producing Escherichia coli strains isolated from poultry flocks stems from concerns about their virulence potential and zoonotic risk. Of particular significance is the identification of extraintestinal pathogenic E. coli (ExPEC) pathotypes in poultry, as these strains pose not only animal health concerns but also serious threats to food safety and public health. Mapping the genetic background of pathogenicity and antimicrobial resistance is essential for risk assessment and the development of effective control strategies. Methods: A total of 87 E. coli isolates were isolated from tracheal and cloacal swab samples collected from healthy chickens between 2022 and 2023. Whole-genome sequencing was performed using Illumina and MGI next-generation sequencing platforms. Bioinformatic analyses were conducted to identify virulence-associated genes and pathotype markers using multiple reference databases, including VirulenceFinder. The frequency of virulence genes was summarized both in tabular form and visualized through graphical representations. Results: A substantial proportion of the isolates harbored virulence genes linked to various ExPEC pathotypes, particularly uropathogenic E. coli (UPEC), avian pathogenic E. coli (APEC), and neonatal meningitis-causing E. coli (NMEC). The most frequently detected colonization factors included members of the fim, pap, ecp, and fae gene families. Among fitness-related genes, iron acquisition systems—ent, chu, iro, iuc, fep, and ybt—were especially prevalent. Classic UPEC-associated genes such as pap and fimH, along with the APEC-related iutA and vat, were found at high frequencies. Four isolates exhibited a virulence gene profile characteristic of the NMEC pathotype (ibeA, kpsD/M/T, fimH). In contrast, hallmark genes of enteric pathotypes were absent from all isolates. Conclusions: The predominance of extraintestinal virulence factors in the examined poultry-derived E. coli strains underscores their zoonotic potential. The complete absence of enteric pathotype markers indicates that the studied poultry populations primarily harbor ExPEC-like strains. These findings highlight the critical need for ongoing genomic surveillance and targeted preventive strategies within poultry production systems.
1. Introduction
Escherichia coli is a rod-shaped, Gram-negative bacterium belonging to the family Enterobacteriaceae [1]. While commonly present as a commensal in the gastrointestinal tract, even non-pathogenic strains may cause disease in hosts with compromised barriers or weakened immunity [2,3].
Certain E. coli strains are capable of causing both intestinal and extraintestinal diseases in humans and animals alike [4]. Pathogenic E. coli are classified into pathotypes based on their virulence traits, most of which are encoded by mobile genetic elements (MGEs) such as plasmids and transposons. These elements facilitate horizontal gene transfer, enabling diverse combinations of virulence factors to arise. Once integrated into the bacterial genome, these virulence-associated regions may become stable features of the organism [5].
Pathotypes are broadly grouped based on clinical manifestations. The first group encompasses diarrheagenic E. coli (DEC), including six main categories: enteropathogenic (EPEC), enterohemorrhagic (EHEC), enterotoxigenic (ETEC), enteroaggregative (EAEC), enteroinvasive (EIEC), and diffusely adherent E. coli (DAEC). The second group includes extraintestinal (ExPEC) strains causing urinary tract infections (primarily uropathogenic E. coli, UPEC), while the third group comprises strains associated with neonatal meningitis and sepsis (NMEC). A distinct category is avian pathogenic E. coli (APEC), which can lead to respiratory disease, pericarditis, and septicemia in poultry [5,6,7].
Many of these pathotypes are of serious public health concern due to their foodborne transmission potential and history of causing large-scale outbreaks in both developing and developed regions [8,9]. The associated virulence genes encode functions such as adhesion, invasion, iron acquisition, motility, and toxin production. These factors are typically classified into colonization, fitness, toxin, and effector modules [10]. The emergence of novel pathotypes can trigger deadly outbreaks, highlighting the need for effective surveillance systems. Given that food, water, animals, and humans all serve as possible vectors for enteric E. coli, broad-based monitoring is essential [8,11].
High-throughput sequencing technologies are increasingly used as a powerful surveillance tool, supporting not only outbreak tracing but also the development of new therapeutic strategies and the design of more effective vaccines [12]. Alongside molecular monitoring, strict adherence to biosecurity protocols [13] and the growing application of antibiotic alternatives—such as plant extracts and essential oils [14,15,16,17,18], antimicrobial peptides [19], probiotics, and other biologics [20,21,22]—play a critical role in reducing antimicrobial use. This, in turn, decreases the selective pressure on microbial populations and helps preserve the long-term efficacy of antibiotics in treating E. coli infections within the framework of the One Health concept.
In Hungary, E. coli infections are a leading cause of economic losses in poultry production, particularly due to colibacillosis caused by APEC strains. Several reports have documented high isolation rates of APEC strains from broilers and layers, with prevalence estimates ranging from 30% to 70%, depending on farm conditions and biosecurity practices [23,24]. These strains not only impact animal health and productivity but also pose a zoonotic risk via the food chain.
Despite the growing interest in antimicrobial resistance and zoonotic potential of E. coli, there is limited published data on the virulence profiles and pathotype distribution of ESBL-producing strains circulating in Hungarian poultry. This study represents the first in-depth analysis of virulence gene repertoires in poultry-derived, ESBL-producing E. coli strains from Hungary, focusing on the coexistence of multiple pathotype-associated markers, particularly the overlap between ExPEC and other virulence traits in multidrug-resistant isolates. This study was designed to help address this knowledge gap by characterizing their virulence gene content and pathotype affiliations.
Domestic chickens (Gallus gallus domesticus) represent one of the most widely consumed sources of animal protein globally and play a central role in the epidemiology of zoonotic pathogens, including E. coli. Their intensive production and close interaction with humans via the food chain make them a critical target for One Health-oriented surveillance efforts [25,26]. Furthermore, poultry farms have been identified as major reservoirs for pathogenic and multidrug-resistant E. coli strains, highlighting the need for regular monitoring. While several studies from other European countries (e.g., Poland, Germany, Italy) have reported on virulence traits in poultry-associated E. coli, comparative data from Central and Eastern Europe remain sparse [27,28,29]. Moreover, contrasting these findings with recent datasets from Asia may help reveal regional variations in virulence gene distribution and provide a broader epidemiological context.
In this study, we aimed to characterize the virulence gene repertoire of E. coli strains isolated from chickens and evaluate their potential pathotypes in light of One Health priorities.
2. Results
2.1. Identified Virulence Gene Repertoire
A total of 87 E. coli isolates isolated from chickens, which had been confirmed as extended-spectrum beta-lactamase (ESBL) producers by both phenotypic and genotypic screening, were subjected to genomic characterization to investigate their virulence gene repertoire. The analysis of colonization-associated virulence factors (Table 1) revealed the presence of thirty-nine distinct genes, of which 26 (66.7%) were associated with UPEC, 11 (28.2%) with ETEC, and 2 (5.1%) with ExPEC-related traits. Among the 87 ESBL-producing E. coli isolates, UPEC-associated genes were present in 58 strains (66.7%), ETEC-characteristic genes in 63 strains (72.4%), and ExPEC-related colonization factors in 49 strains (56.3%). Representative UPEC markers such as fimH, papG, and yagZ/ecpA were frequently detected, alongside ETEC-associated fimbrial genes including faeC–faeJ and members of the csg family. ExPEC-related colonization factors, such as fdeC and ompA, were also identified in a considerable proportion of isolates (Supplementary Materials, Additional data (chickens)).

Table 1.
Identified virulence factor genes of Escherichia coli pathotypes originating from domestic chickens, considering their colonization ability.
The complete presence of the fim and pap gene families—particularly the co-occurrence of fimH, papG, and ompA—is strongly indicative of the presence of UPEC-like pathotypes, even in poultry-derived strains, suggesting potential zoonotic relevance. Additionally, the full representation of the E. coli common pilus (ECP) operon (yagV–ykgK) further supports the substantial colonization capacity of these isolates, especially in relation to UPEC and broader ExPEC lineages.
As shown in Table 2, a diverse range of genes related to siderophore systems and heme utilization were identified among the poultry-derived E. coli strains. The chu gene cluster (chuA, chuS, chuT, chuU, chuV, chuW, chuX, chuY) was detected in 22 out of 87 isolates (25.3%), suggesting a functional capability for iron acquisition directly from heme. Similarly, the shu gene system (shuA, shuS, shuT, shuX, shuY) was identified in 19 isolates (21.8%), indicating potential redundancy in heme-based iron uptake mechanisms.

Table 2.
Identified virulence factor genes of Escherichia coli pathotypes originating from domestic chickens, in terms of fitness.
Among the siderophore-associated genes, components from multiple systems were identified. The classical enterobactin biosynthetic and transport machinery was nearly fully represented (entA–F, entS, fepA–G, fes), alongside genes associated with the salmochelin (iroB–E, iroN), yersiniabactin (irp1, irp2, ybtA, ybtE, ybtP–X), and aerobactin (iucA–D, iutA) pathways. While many of these genes are generally classified as ExPEC-associated virulence factors, several—particularly iroN, fyuA, and iutA—are pathotype-specific markers commonly linked to APEC and UPEC strains.
Siderophore systems were widely distributed among the isolates. A total of 9 isolates (14.3%) harbored components of all four major siderophore systems (enterobactin, aerobactin, salmochelin, and yersiniabactin), while 7 isolates (11.1%) carried three, 25 isolates (39.7%) carried two, and 19 isolates (30.2%) carried one. This cumulative presence of multiple iron uptake systems highlights the exceptional adaptability of these strains to iron-limited environments and suggests increased virulence potential.
In the analysis of E. coli isolates derived from domestic chickens, several toxin-associated genes were identified (Table 3). The aslA gene, encoding a protease–adhesin with hemagglutinin activity, was the most prevalent, found in 23 out of 87 isolates (36.5%). The vat gene—a vacuolating autotransporter toxin typically associated with APEC—was present in 3 isolates (4.8%), while pic and stcE, both encoding proteins with proteolytic and immune-modulating functions, were each detected in 1 isolate (1.6%).

Table 3.
Identified virulence factor genes of Escherichia coli pathotypes originating from domestic chickens, in light of toxins and effectors.
Among the effector proteins, only espY4, a gene commonly linked to EPEC and EHEC strains, was detected, occurring in 10 isolates (15.9%). Other T3SS-related genes, including espL, espR, espX, and espY, were not detected in any of the isolates. The presence of espY4 may suggest horizontal gene transfer or a mosaic virulence gene architecture.
2.2. Pathotypes
UPEC-associated virulence factors were identified in 58 out of 87 E. coli isolates originating from poultry, corresponding to a prevalence of 66.7%. This indicates that two-thirds of the strains harbored at least one gene typically linked to UPEC pathotypes (Figure 1). The most frequently detected gene was fimH, present in all but one of these strains (n = 57). Genes belonging to the kps family were found in 36 isolates, with kpsM being the most prevalent (n = 17), followed by kpsD (n = 13) and kpsT (n = 6). Members of the pap gene family were present in 32 strains, with papX being the most common (n = 4), while the remaining pap genes occurred in 2 to 3 strains each. The siderophore-associated genes iroN (n = 21) and iutA (n = 18), both linked to UPEC pathogenesis, were also frequently identified. In contrast, sfaX was detected in only a single isolate.
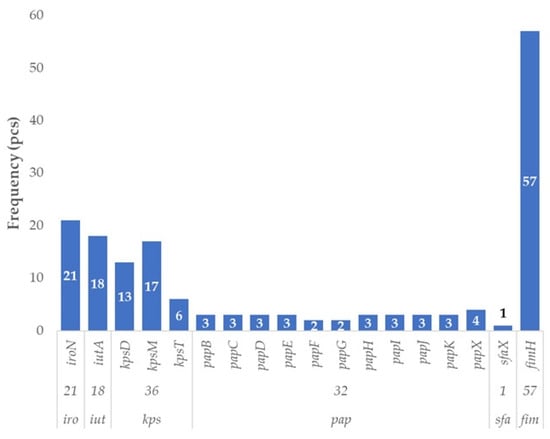
Figure 1.
Prevalence of virulence factor genes characteristic of uropathogenic strains in Escherichia coli strains (UPEC) isolated from domestic chickens (n = 87). Among the 87 poultry-derived E. coli isolates, the fimH gene—encoding a type 1 fimbrial adhesin—was the most frequently detected UPEC-associated gene (57/87; 65.5%). Capsule synthesis genes (kpsD, kpsM, kpsT) were present in 36 isolates (41.4%), while iron acquisition genes such as iroN and iutA were found in 24.1% and 20.7% of strains, respectively. Although pap operon genes (e.g., papC, papG) and sfaX were less frequent, their presence indicates mosaic extraintestinal (ExPEC) virulence profiles. The overall distribution highlights the high prevalence of colonization and immune-evasion factors typical of ExPEC pathotypes in poultry strains, supporting their potential zoonotic relevance.
Among the virulence factors associated with the ETEC pathotype (Figure 2), sixty-three poultry-derived isolates harbored genes belonging to this group. The curli fimbriae biosynthesis and regulatory genes csgB and csgD were identified in all sixty-three strains, while csgF and csgG were detected in sixty-one and sixty isolates, respectively. These genes are responsible for the assembly and secretion of surface fimbriae that contribute to bacterial adhesion.
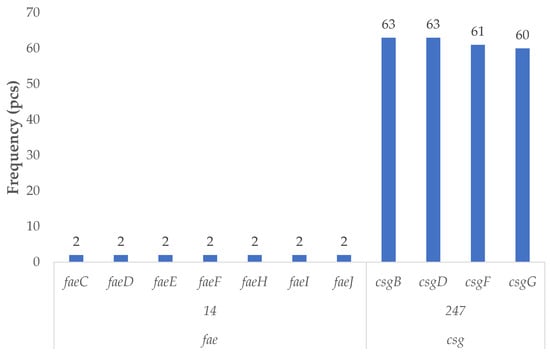
Figure 2.
Prevalence of virulence factor genes characteristic of enterotoxigenic strains in Escherichia coli strains isolated from domestic chickens (n = 87). Curli fimbriae genes (csgB, csgD, csgF, csgG) were highly prevalent, occurring in more than 68% of isolates, highlighting strong colonization potential. Specifically, csgB and csgD were each detected in 63 strains (72.4%), followed closely by csgF (70.1%) and csgG (69.0%). These genes are involved in biofilm formation and epithelial adhesion. In contrast, the complete fae operon (F4/K88 fimbriae) was identified in only two isolates (2.3%), suggesting a rare but notable presence of classic enterotoxigenic (ETEC) adhesins. These findings suggest that while full enterotoxigenic profiles are uncommon, poultry E. coli may carry colonization-associated genes with pathogenic relevance and zoonotic implications.
In addition, genes involved in the biosynthesis of F4 (K88) fimbriae—a hallmark of classical ETEC strains—faeC, faeD, faeE, faeF, faeH, faeI, and faeJ were detected in both of the identified F4-positive isolates. The complete presence of this operon confirms their classification as F4-positive ETEC strains.
Virulence genes associated with the APEC pathotype were identified in thirty isolates. The iutA gene, encoding the receptor for the siderophore aerobactin, was present in eighteen strains. The vat gene, which encodes a vacuolating autotransporter toxin, was detected in three isolates. The iroN gene, part of the salmochelin iron acquisition system, was the most frequently identified, occurring in twenty-one strains. Additionally, the fyuA gene—encoding the receptor for the siderophore yersiniabactin—was found in thirteen isolates (Figure 3).
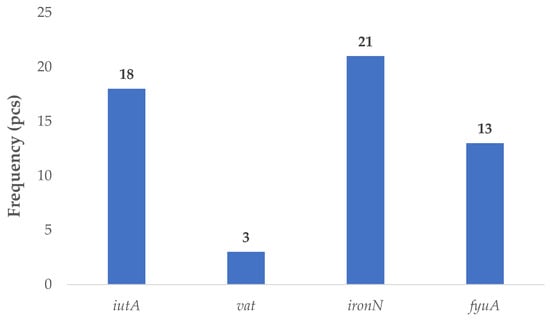
Figure 3.
Prevalence of virulence factor genes characteristic of avian pathogenic (APEC) strains in Escherichia coli strains isolated from domestic chickens (n = 87). The salmochelin receptor gene iroN was the most frequently detected APEC-associated virulence gene, found in 21 isolates (24.1%), followed by iutA (20.7%), encoding the aerobactin receptor. The fyuA gene, which encodes the yersiniabactin receptor, was present in 13 strains (14.9%). The vacuolating autotransporter toxin gene vat appeared infrequently, identified in only 3 strains (3.4%). These findings suggest a heterogeneous distribution of APEC-associated virulence factors in the studied poultry population, with iron acquisition systems being particularly prominent. The data underscore the potential for extraintestinal disease development in avian hosts and raise concerns over zoonotic risk through shared virulence profiles with human extraintestinal (ExPEC) strains.
Among the poultry-derived E. coli strains analyzed, four isolates exhibited a gene combination characteristic of the neonatal NMEC pathotype. All four strains harbored the ibeA gene, a key invasion factor required for translocation across the blood–brain barrier. In addition, all four isolates carried the fimH gene, which is critical for epithelial adhesion. The capsule biosynthesis genes kpsD, kpsM, and kpsT were also identified and found more broadly across thirteen, seventeen, and six isolates, respectively.
To assess potential spatial differences in the distribution of virulence-associated E. coli pathotypes, we compared their prevalence across Hungary’s seven administrative regions. The most common pathotypes were ETEC and UPEC, particularly in Észak-Magyarország and Dél-Dunántúl. A chi-squared test revealed no statistically significant association between region and pathotype distribution (χ2 = 5.52, df = 20, p = 0.999), suggesting a broadly uniform geographical spread of virulence traits among ESBL-producing strains (Figure 4).
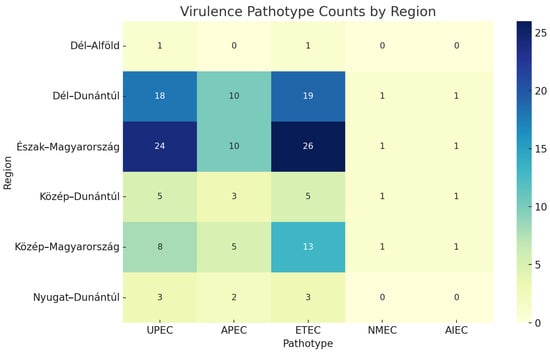
Figure 4.
Distribution of five virulence-associated Escherichia coli pathotypes (UPEC—uropathogenic Escherichia coli, APEC—avian pathogenic Escherichia coli, ETEC—enterotoxigenic Escherichia coli, NMEC—neonatal meningitis-causing Escherichia coli, AIEC—adherent-invasive Escherichia coli) across poultry farms grouped by Hungary’s seven administrative regions. No statistically significant regional differences were observed (χ2 = 5.52, p = 0.999). Heatmap colors indicate the absolute number of isolates per category.
3. Discussion
A total of 87 E. coli isolates were isolated from clinically healthy domestic chickens (Gallus gallus domesticus) reared in large-scale commercial broiler, layer, and breeder farms across Hungary. Following phenotypic pre-screening for multidrug resistance; results presented in a separate manuscript under review, we performed next-generation sequencing to elucidate the virulence gene repertoire of the isolates.
A substantial proportion of these poultry-derived E. coli isolates harbored colonization-related virulence factors typically associated with ExPEC E. coli pathotypes, particularly UPEC E. coli. Notably, the co-occurrence of genes belonging to the fim and pap operons, ompA, and the E. coli ECP operon suggests a high colonization potential within this collection. The fimH gene, in particular, merits attention due to its critical role in adhesion during urinary tract infections; previous studies have shown its presence in over 90% of UPEC isolates [30]. Members of the pap operon, especially papG and papC, are also instrumental in epithelial cell adherence and the development of ascending infections—an established role for P-fimbriae in ExPEC pathogenesis [31]. Interestingly, the presence of fae and csg gene families in these strains echoes traits seen in ETEC E. coli, reinforcing the notion of zoonotic potential. Previous studies have reported the presence of ETEC factors in poultry-derived E. coli strains; however, the complete fimbrial operon is rarely detected [32]. The detection of such elements raises public health concerns, particularly due to their frequent association with MGEs, which could facilitate interspecies gene transfer to human pathogens. This potential for genetic exchange may have implications for food safety and antimicrobial resistance dissemination, underscoring the importance of continuous genomic monitoring of virulence-rich poultry isolates [33].
Iron acquisition is a critical determinant of E. coli pathogenicity, enabling survival, colonization, and infection establishment within iron-restricted host environments—conditions shaped by nutritional immunity [34]. The detection of multiple siderophore systems in our isolates suggests robust adaptation to iron-limiting niches. The identification of both the chu and shu operons—heme utilization systems associated with ExPEC—points to functional redundancy in iron scavenging. Moreover, the diversity of siderophore systems identified, including enterobactin (ent/fep/fes), salmochelin (iro), yersiniabactin (irp/ybt), and aerobactin (iuc/iut), indicates high virulence potential and reinforces their zoonotic relevance [34]. Among these, fyuA, iroN, and iutA were frequently detected and are known markers of UPEC and APEC pathotypes, further suggesting that such strains may circulate within the poultry population. This extensive virulence arsenal in poultry-derived E. coli isolates supports concerns that such strains could act as potential reservoirs of virulence determinants relevant to public health, rather than directly confirming a zoonotic threat [35,36].
Comparable findings have been reported across Central and Eastern Europe, though the specific virulence gene combinations observed in this study appear distinct. For instance, Lithuanian and Polish poultry isolates showed ESBL-producing E. coli prevalence rates between 45% and 60%, often associated with APEC-type virulence markers rather than UPEC-related profiles [27]. In contrast, our Hungarian isolates—derived exclusively from healthy birds—displayed mixed virulence signatures that included ExPEC- and UPEC-characteristic genes, highlighting potential host adaptation and cross-pathotype evolution. Similar One Health surveillance work from Germany and Italy has also emphasized the increasing overlap between APEC and human ExPEC gene pools [28]. Collectively, these studies support a regional trend toward convergence of poultry and human-associated E. coli virulence determinants, suggesting ongoing zoonotic risk in European poultry production systems.
Bacterial toxins and effector proteins also play a pivotal role in modulating host cellular responses, immune evasion, and infection success. The detection of aslA, pic, and stcE genes in our study is consistent with ExPEC-specific pathogenic mechanisms. Notably, aslA has been implicated in blood–brain barrier translocation in both in vitro and in vivo models [37]. The Pic protease contributes to mucosal colonization through mucin layer degradation, a well-documented mechanism in enteroaggregative E. coli [38].
The presence of type III secretion system (T3SS) effectors—particularly those belonging to the esp gene family—is of special interest. These effectors are capable of modulating host signaling pathways to enhance bacterial persistence and replication. While the specific role of espY4 in avian strains has not yet been characterized, its presence, previously documented in EPEC and EHEC strains, suggests novel effector activities with possible zoonotic implications [39,40], especially in strains exhibiting mosaic virulence gene content.
The detection of UPEC-associated virulence genes in poultry-derived E. coli isolates is particularly striking, as it highlights the avian population as a potential zoonotic reservoir. The near-universal detection of fimH—encoding the adhesin subunit of type 1 fimbriae—corroborates its well-established role in urinary tract epithelial adhesion, with a prevalence of up to 92.8% in clinical isolates [30]. Genes of the kps cluster, involved in capsule biosynthesis, provide protection against host immune responses and have been consistently observed in ExPEC strains [41]. The presence of iroN and iutA further supports the notion that these strains are well-equipped to survive in iron-depleted environments [42]. Although pap genes were less frequently identified—consistent with their predominance in human UPEC strains—their occurrence in avian isolates suggests zoonotic potential [43]. The detection of a single sfaX-positive isolate underscores the mosaic nature of ExPEC-associated virulence traits in poultry. Collectively, these findings point to the significant public health relevance of poultry E. coli as potential reservoirs of UPEC virulence determinants.
The detection of adhesion-associated virulence genes typical of the ETEC pathotype in poultry-derived isolates also raises potential zoonotic concerns [44]. The widespread presence of csg genes responsible for curli fimbriae biosynthesis (csgB and csgD were found in 100% of isolates) indicates robust colonization capabilities, particularly in facilitating adhesion to the intestinal epithelium [45]. Notably, two F4-positive (K88) strains were identified, both carrying the complete fae operon, responsible for the synthesis of K88 fimbriae—a hallmark of both human and animal ETEC strains [46]. While rare, this finding has significant implications: it suggests that poultry may serve as a reservoir not only for ExPEC but also for ETEC pathotypes. These strains possess considerable genetic potential for adhesion and colonization, posing risks to both animal and human hosts. Potential explanations include horizontal gene transfer (HGT) from pigs to poultry via MGEs such as plasmids or transposons, as well as cross-contamination in integrated animal husbandry systems.
The presence of APEC-associated virulence factors (iroN, iutA, vat, and fyuA) in 30 poultry-derived E. coli isolates further supports the notion that these strains can cause extraintestinal infections (e.g., colibacillosis) in avian hosts, raising additional zoonotic concerns [47]. The iroN, encoding a key component of the salmochelin siderophore system, was the most frequently detected gene and plays a central role in both APEC and UPEC pathogenesis, especially in invasive phenotypes [48]. The iutA gene, encoding the aerobactin receptor, was present in approximately 60% of isolates, consistent with previous reports identifying iutA as a major APEC marker [49]. Although vat was less common, its contribution to cellular damage underscores its relevance in disease pathology. The presence of fyuA, encoding a yersiniabactin receptor, reflects the diversity of iron acquisition systems—critical determinants of bacterial survival and virulence [50]. Altogether, these findings reinforce the view that poultry flocks may act as reservoirs for APEC strains, posing not only animal health risks but also a tangible zoonotic threat.
The detection of an NMEC-like gene profile (ibeA, fimH, kpsD/M/T) in four poultry isolates was unexpected, as these genes are typically associated with neonatal meningitis in humans [51]. The ibeA gene is particularly noteworthy due to its established role in promoting translocation across the blood–brain barrier via macrophage-mediated mechanisms [52]. The fimH, crucial for adhesion and colonization, was also present, along with the kps gene cluster (kpsD, kpsM, kpsT) required for the synthesis of the K1 capsule, a recognized NMEC virulence determinant [47]. Although NMEC strains are rarely documented in poultry, their detection here raises the possibility of zoonotic transmission. These findings underscore the need for comprehensive bacteriological surveillance and resistance monitoring in poultry production systems, aligned with the One Health framework.
Globally, reports from Asia indicate high prevalence and diverse virulence/resistance profiles in poultry-derived ESBL-producing E. coli. For example, a recent Malaysian study found 84.5% of chicken isolates positive for at least one ESBL gene [53], while in Taiwan, ESBL-producing poultry E. coli carried ExPEC-associated sequence types such as ST69 and ST617 [36]. These findings contrast with our results in Hungary, where, although the isolates were derived from healthy poultry, a high proportion harbored virulence factors typical of UPEC and APEC, rather than purely enteric pathotypes. This suggests regional variation in virulence gene distribution and highlights the importance of country-specific surveillance studies, particularly within the One Health framework.
In contrast, no key virulence genes associated with classical enteric pathotypes such as EHEC/STEC, EPEC, or DAEC were detected in any of the sequenced strains. Specifically, hallmark genes such as stx1, stx2, eae, tir, bfp (EHEC/EPEC), or afa/dra (DAEC) were entirely absent from the dataset. These results confirm that the analyzed poultry-derived E. coli isolates do not belong to enteric pathotypes but rather exhibit characteristics typical of extraintestinal strains [54].
Despite the numerical differences observed in pathotype counting across regions, no significant spatial clustering was detected. This uniform distribution might reflect the widespread movement of poultry stocks, shared hatchery sources, or similar selective pressures in farming practices across regions. The apparent dominance of ETEC and UPEC types aligns with their known prevalence in extraintestinal infections in both animals and humans.
In summary, a considerable proportion of the poultry-derived E. coli isolates in this study harbored virulence factors indicative of ExPEC potential—particularly genetic signatures characteristic of UPEC, APEC, and NMEC. In contrast, no markers associated with enteric pathotypes (EHEC/STEC, EPEC, DAEC) were detected, suggesting that current E. coli populations in poultry farming are predominantly extraintestinal in nature. These findings raise important public and animal health concerns, especially regarding zoonotic transmission and food chain safety, as supported by genomic studies highlighting overlaps between avian and human ExPEC strains, including the spread of high-risk clones like ST131 [55].
4. Materials and Methods
4.1. Sampling and Identification of Escherichia coli Strains
During the 2022–2023 period, biological samples were obtained from asymptomatic domestic chickens (Gallus gallus domesticus) housed on commercial poultry farms across Hungary. Farms were enrolled in the study on a voluntary basis and were selected to ensure geographic representativeness. A total of 23 sampling locations were visited, spanning all seven administrative regions of Hungary, with a minimum of three farms included per region to ensure adequate coverage. In most regions, exactly three farms were sampled, while in the Közép-Dunántúl region, five farms were included to enhance representativeness.
At each site, 15 tracheal and 15 cloacal swabs were collected per flock, based on practical considerations and to provide a representative sample of the microbial population. Sampling was performed as part of routine diagnostic monitoring by the attending veterinary officers. The swabs were collected using aluminum-shafted, non-charcoal Amies transport swabs (Biolab Zrt., Budapest, Hungary), following standard veterinary protocols.
The animals sampled were selected randomly from clinically healthy individuals within each flock, without prior knowledge of health status or antimicrobial exposure. No formal sample size calculation was performed, as the study was designed to be observational and exploratory in nature, aiming to detect the diversity of virulence factors present in poultry-derived E. coli strains on a national scale.
Samples were streaked onto ChromoBio® Coliform agar (Biolab Zrt., Budapest, Hungary) to select presumptive E. coli colonies. Subsequent subculturing was performed on tryptone soya agar and incubated at 41 °C for 18–24 h. Identification of isolates was carried out by matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS; Flextra-LAB Ltd., Budapest, Hungary) using Biotyper software v12.0 (Bruker Daltonics, Bremen, Germany) [56]. All confirmed strains were cryopreserved at −80 °C using Microbank™ storage systems (Pro-Lab Diagnostics, Richmond Hill, ON, Canada).
Phenotypic screening for antimicrobial resistance, including minimum inhibitory concentrations (MICs) testing and ESBL detection, was performed as part of the initial laboratory workflow. However, detailed results and interpretation of resistance profiles are beyond the scope of this study and are presented in a separate manuscript under review.
4.2. Next-Generation Sequencing
Genomic DNA was extracted from E. coli isolates using the Zymo Quick-DNA Fungal/Bacterial Miniprep Kit (Zymo Research, Irvine, CA, USA). Mechanical lysis was performed with a Qiagen TissueLyzer LT (Qiagen, Hilden, Germany). Extracted DNA was stored at −20 °C prior to sequencing. Whole-genome sequencing was carried out on the Illumina NextSeq 500 platform (Illumina, San Diego, CA, USA), generating paired-end reads using sequencing-by-synthesis chemistry.
DNA libraries were prepared using the Vazyme TruePrep DNA Library Prep Kit V2 (Vazyme, Nanjing, China) and Nextera XT Index Kits (Illumina, San Diego, CA, USA), followed by purification and quantification with Qubit dsDNA HS assays (Thermo Fisher Scientific, Waltham, MA, USA). For additional sequencing, libraries were adapted for the MGI DNBSEQ-G400RS platform using the MGIEasy Universal Library Conversion Kit and HotMPS High-Throughput chemistry (MGI Tech, Shenzhen, China). Circularization, DNB preparation, and quantification steps followed the manufacturer’s instructions.
4.3. Bioinformatic Analysis
Raw sequencing reads were evaluated using FastQC v0.11.9, Fastp v0.23.2-3, and Bloocoo v1.0.7 to assess base quality, adapter content, and sequencing depth. Low-quality reads were removed using TrimGalore v0.6.6.
De novo assembly was performed using MEGAHIT v1.2.9 and SPAdes v4.0.0 and merged with GAM-NGS v1.1b to improve assembly quality. The resulting contigs were assessed using QUAST v5.2 and BUSCO v5. Genome characteristics (e.g., k-mer distribution, genome size) were analyzed via GenomeScope v2.2.
Gene prediction was performed with Prodigal v2.6.3. Antimicrobial resistance genes (ARGs) were identified using RGI v5.1.0 and ABRicate against the CARD database, applying a ≥90% identity and coverage threshold.
MGEs were screened using MobileElementFinder v1.0.3. Putative plasmid-originated contigs and prophages were identified using PlasFlow v1.1 and VirSorter v2.2.2, respectively.
Taxonomic confirmation and completeness assessment were performed using CheckM v1.2.2 and Kraken v1.1.1. Chromosomal mutations were detected with ResFinder v4.1, and genome-wide SNPs were identified using Snippy v4.6.0. Serotype and virulence gene profiling were conducted using Ectyper v1.0 and VirulenceFinder v2.0, respectively.
Average Nucleotide Identity (ANI) was calculated with ANI v2.0, using E. coli SYNB8802 (RefSeq: GCF_020995495.1) as reference.
To explore potential geographical patterns in the distribution of virulence-associated E. coli pathotypes, each isolate was assigned to one of Hungary’s seven administrative regions, based on the location of the poultry farm where the sample originated. Pathotypes were classified as UPEC, APEC, ETEC, NMEC, or adherent-invasive E. coli (AIEC), according to the presence of specific virulence-associated genes identified through whole-genome sequencing.
The total number of isolates in each pathotype category was aggregated by region, and a contingency table was constructed to examine their spatial distribution. A chi-squared test of independence was applied to assess whether the distribution of pathotypes differed significantly among regions. Given the low counts observed for some categories (e.g., NMEC and AIEC), assumptions of the chi-squared test were verified, and Fisher’s exact test was considered as a more conservative alternative when appropriate.
All statistical analyses were carried out in R (version 4.3.1) using the base stats package and gmodels. Heatmaps visualizing the frequency of each pathotype by region were created using the ggplot2 v3.5.0 and reshape2 v1.4.4 packages. A p-value below 0.05 was considered statistically significant.
5. Conclusions
Comprehensive virulence profiling of the poultry-derived E. coli isolates revealed a clear predominance of genetic traits associated with ExPEC, particularly those linked to UPEC, APEC, and NMEC pathotypes. The complete absence of hallmark genes characteristic of enteric pathotypes (EHEC/STEC, EPEC, DAEC) further confirms that these isolates are primarily equipped to cause extraintestinal infections. This observation carries significant implications for zoonotic transmission and public health.
Our findings highlight that E. coli strains circulating in poultry flocks may represent a substantial risk not only to animal health but also to food chain integrity, especially when coupled with multidrug resistance phenotypes. Such dual pathogenic and antimicrobial resistance potential reinforces the urgency of implementing effective monitoring systems.
These findings provide actionable insights for One Health-oriented surveillance strategies by identifying specific virulence patterns circulating in poultry populations. The observed co-occurrence of ExPEC-, APEC-, and ETEC-associated genes in ESBL-producing E. coli underscores the necessity of genomic monitoring beyond resistance profiles alone. Such detailed virulome data could inform targeted vaccine development, particularly by prioritizing conserved colonization and iron acquisition factors like fimH, iroN, and iutA. In addition, the apparent regional similarities and differences, when compared to European and Asian datasets, highlight the need for harmonized surveillance protocols across borders. From a policy perspective, these insights support the rationale for reducing antimicrobial use in poultry farming to mitigate the zoonotic spillover of high-risk E. coli strains.
To mitigate these risks, sustained epidemiological surveillance, the routine application of high-throughput genomic screening, and the development of targeted intervention strategies are imperative—guided by the principles of the One Health approach. Future research should focus on characterizing potential transitions between pathotypes and identifying novel virulence gene combinations, with a view toward refining risk assessment and informing evidence-based control strategies.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/antibiotics14111083/s1, Additional data (chickens).
Author Contributions
Conceptualization, Á.K. and Á.J.; methodology, Á.K. and K.B.; software, E.K.; validation, Á.J.; formal analysis, E.K. and K.B.; investigation, G.T. and Á.S.; resources, Á.K.; data curation, Á.K.; writing—original draft preparation, Á.K.; writing—review and editing, Á.K.; visualization, Á.K.; supervision, Á.J.; project administration, G.T.; funding acquisition, Á.J. All authors have read and agreed to the published version of the manuscript.
Funding
Project no. 2024-2.1.1-EKÖP-2024-00018 has been implemented with the support provided by the Ministry of Culture and Innovation of Hungary from the National Research, Development and Innovation Fund, financed under the 2024-2.1.1-EKÖP funding scheme. Project no. RRF-2.3.1-21-2022-00001 was implemented with the support provided by the Recovery and Resilience Facility (RRF), financed under the National Recovery Fund budget estimate, RRF-2.3.1-21 funding scheme.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The sequencing files are available at the https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1332072, accessed on 20 September 2025.
Acknowledgments
We would like to thank all the laboratory technical assistants, without whom this research would not have been possible.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Escobar-Páramo, P.; Giudicelli, C.; Parsot, C.; Denamur, E. The Evolutionary History of Shigella and Enteroinvasive Escherichia coli Revised. J. Mol. Evol. 2003, 57, 140–148. [Google Scholar] [CrossRef]
- Tenaillon, O.; Skurnik, D.; Picard, B.; Denamur, E. The Population Genetics of Commensal Escherichia coli. Nat. Rev. Microbiol. 2010, 8, 207–217. [Google Scholar] [CrossRef]
- Sadi, M.; Akkou, M.; Martínez-Álvarez, S.; Carvalho, I.; Fernández-Fernández, R.; Abdullahi, I.N.; Hakem, A.; Menoueri, M.-N.; Torres, C. Antimicrobial Resistance of Escherichia coli Involved in Algerian Bovine Carriage, Esbl Detection, Integron Characterization and Genetic Lineages. Kafkas Univ. Vet. Fak. Derg. 2024, 30, 191–199. [Google Scholar] [CrossRef]
- Nash, J.H.; Villegas, A.; Kropinski, A.M.; Aguilar-Valenzuela, R.; Konczy, P.; Mascarenhas, M.; Ziebell, K.; Torres, A.G.; Karmali, M.A.; Coombes, B.K. Genome Sequence of Adherent-Invasive Escherichia coli and Comparative Genomic Analysis with Other E. coli Pathotypes. BMC Genom. 2010, 11, 667. [Google Scholar] [CrossRef] [PubMed]
- Kaper, J.B.; Nataro, J.P.; Mobley, H.L.T. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2004, 2, 123–140. [Google Scholar] [CrossRef]
- Rahman, A.; Chowdhury, R.S.; Hossain, H.; Elsaid, F.G.; Almutairi, L.A.; Begum, R.; Sabrin, M.S.; Akanda, R.; Hossain, M.; Islam, R.; et al. Identification of Virulence Genes and Multidrug Resistance in Shiga-Toxin Producing Escherichia coli (STEC) from Migratory and Captive Wild Birds. Pak. Vet. J. 2024, 44, 1120–1130. [Google Scholar] [CrossRef]
- Mohamed, A.H.; Gaber, E.B.; Saber, A.S.; Eman, M. Study on Enterotoxigenic Escherichia coli Producing Extended Spectrum Beta Lactamase (ESBL) from Chicken Meat and Its Products. Int. J. Vet. Sci. 2023, 12, 652–658. [Google Scholar]
- Yang, S.-C.; Lin, C.-H.; Aljuffali, I.A.; Fang, J.-Y. Current Pathogenic Escherichia coli Foodborne Outbreak Cases and Therapy Development. Arch. Microbiol. 2017, 199, 811–825. [Google Scholar] [CrossRef] [PubMed]
- Alegbeleye, O.O.; Sant’Ana, A.S. Pathogen Subtyping Tools for Risk Assessment and Management of Produce-Borne Outbreaks. Curr. Opin. Food Sci. 2020, 32, 83–89. [Google Scholar] [CrossRef]
- Mainil, J. Escherichia coli Virulence Factors. Vet. Immunol. Immunopathol. 2013, 152, 2–12. [Google Scholar] [CrossRef]
- Riaz, A.; Shahzad, M.; Aslam, R.; Irtash, M.; Kashif, M.; Ali, A.; Mahmood Sajid, S.; Ahsan, A.; Hussain, M.; Usman, M.; et al. Investigations into the Role of Zoo Animals in Transmitting the Extended Spectrum Beta Lactamases (ESBL) E. coli in the Environment. Int. J. Vet. Sci. 2023, 12, 832–837. [Google Scholar] [CrossRef]
- Bogaerts, B.; Nouws, S.; Verhaegen, B.; Denayer, S.; Van Braekel, J.; Winand, R.; Fu, Q.; Crombé, F.; Piérard, D.; Marchal, K.; et al. Validation Strategy of a Bioinformatics Whole Genome Sequencing Workflow for Shiga Toxin-Producing Escherichia coli Using a Reference Collection Extensively Characterized with Conventional Methods. Microb. Genom. 2021, 7, 531. [Google Scholar] [CrossRef]
- Farkas, M.; Könyves, L.; Csorba, S.; Farkas, Z.; Józwiák, Á.; Süth, M.; Kovács, L. Biosecurity Situation of Large-Scale Poultry Farms in Hungary According to the Databases of National Food Chain Safety Office Centre for Disease Control and Biosecurity Audit System of Poultry Product Board of Hungary in the Period of 2021–2022. Magy. Állatorvosok Lapja 2024, 146, 723–742. [Google Scholar] [CrossRef]
- Olasz, Á.; Jerzsele, Á.; Balta, L.; Dobra, P.F.; Kerek, Á. In vivo Efficacy of Different Extracts of Propolis in Broiler Salmonellosis. Magy. Állatorvosok Lapja 2023, 145, 461–475. [Google Scholar] [CrossRef]
- Kerek, Á.; Csanády, P.; Jerzsele, Á. Antibacterial Efficiency of Propolis—Part 1. Magy. Állatorvosok Lapja 2022, 144, 285–298. [Google Scholar]
- Kerek, Á.; Csanády, P.; Jerzsele, Á. Antiprotozoal and Antifungal Efficiency of Propolis—Part 2. Magy. Állatorvosok Lapja 2022, 144, 691–704. [Google Scholar]
- Jerzsele, Á.; Somogyi, Z.; Szalai, M.; Kovács, D. Effects of Fermented Wheat Germ Extract on Artificial Salmonella Typhimurium Infection in Broiler Chickens. Magy. Állatorvosok Lapja 2020, 142, 77–85. [Google Scholar]
- Kovács, L.; Nagy, D.; Könyves, L.; Jerzsele, Á.; Kerek, Á. Antimicrobial Properties of Essential Oils—Animal Health Aspects. Magy. Állatorvosok Lapja 2023, 145, 497–510. [Google Scholar] [CrossRef]
- Sebők, C.; Márton, R.A.; Meckei, M.; Neogrády, Z.; Mátis, G. Antimicrobial Peptides as New Tools to Combat Infectious Diseases. Magy. Állatorvosok Lapja 2024, 146, 181–191. [Google Scholar] [CrossRef]
- Essősy, M.; Fodor, I.; Ihnáth, Z.; Karancsi, Z.; Kovács, D.; Szalai, K.V.; Szentmiklósi, D.; Jerzsele, Á. The Possibilities of Antibiotic-Free Broiler-Hen Fattening, with Special Reference to the Use of Pre- and Probiotics. Magy. Állatorvosok Lapja 2020, 142, 397–407. [Google Scholar]
- Hetényi, N.; Bersényi, A.; Hullár, I. Physiological Effects of Medium-Chain Fatty Acids and Triglycerides, and Their Potential Use in Poultry and Swine Nutrition: A Literature Review. Magy. Állatorvosok Lapja 2024, 146, 651–659. [Google Scholar] [CrossRef]
- Jócsák, G.; Schilling-Tóth, B.; Bartha, T.; Tóth, I.; Ondrašovičová, S.; Kiss, D.S. Metal Nanoparticles—Immersion in the „tiny” World of Medicine. Magy. Állatorvosok Lapja 2025, 147, 115–127. [Google Scholar] [CrossRef]
- Szmolka, A.; Nagy, B. Multidrug Resistant Commensal Escherichia coli in Animals and Its Impact for Public Health. Front. Microbiol. 2013, 4, 258. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2023 Zoonoses Report. EFSA J. 2024, 22, e9106. [Google Scholar] [CrossRef]
- Radhouani, H.; Silva, N.; Poeta, P.; Torres, C.; Correia, S.; Igrejas, G. Potential Impact of Antimicrobial Resistance in Wildlife, Environment and Human Health. Front. Microbiol. 2014, 5, 23. [Google Scholar] [CrossRef]
- Nhung, N.T.; Chansiripornchai, N.; Carrique-Mas, J.J. Antimicrobial Resistance in Bacterial Poultry Pathogens: A Review. Front. Vet. Sci. 2017, 4, 126. [Google Scholar] [CrossRef]
- Wilczyński, J.; Stępień-Pyśniak, D.; Wystalska, D.; Wernicki, A. Molecular and Serological Characteristics of Avian Pathogenic Escherichia coli Isolated from Various Clinical Cases of Poultry Colibacillosis in Poland. Animals 2022, 12, 1090. [Google Scholar] [CrossRef] [PubMed]
- Cordoni, G.; Woodward, M.J.; Wu, H.; Alanazi, M.; Wallis, T.; La Ragione, R.M. Comparative Genomics of European Avian Pathogenic E. coli (APEC). BMC Genom. 2016, 17, 960. [Google Scholar] [CrossRef] [PubMed]
- Apostolakos, I.; Laconi, A.; Mughini-Gras, L.; Yapicier, Ö.Ş.; Piccirillo, A. Occurrence of Colibacillosis in Broilers and Its Relationship with Avian Pathogenic Escherichia coli (APEC) Population Structure and Molecular Characteristics. Front. Vet. Sci. 2021, 8, 737720. [Google Scholar] [CrossRef]
- Hojati, Z.; Zamanzad, B.; Hashemzadeh, M.; Molaie, R.; Gholipour, A. The FimH Gene in Uropathogenic Escherichia coli Strains Isolated From Patients with Urinary Tract Infection. Jundishapur J. Microbiol. 2015, 8, e17520. [Google Scholar] [CrossRef]
- Lane, M.C.; Mobley, H.L.T. Role of P-Fimbrial-Mediated Adherence in Pyelonephritis and Persistence of Uropathogenic Escherichia coli (UPEC) in the Mammalian Kidney. Kidney Int. 2007, 72, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Obeng, A.S.; Rickard, H.; Ndi, O.; Sexton, M.; Barton, M. Antibiotic Resistance, Phylogenetic Grouping and Virulence Potential of Escherichia coli Isolated from the Faeces of Intensively Farmed and Free Range Poultry. Vet. Microbiol. 2012, 154, 305–315. [Google Scholar] [CrossRef]
- Hu, J.; Afayibo, D.J.A.; Zhang, B.; Zhu, H.; Yao, L.; Guo, W.; Wang, X.; Wang, Z.; Wang, D.; Peng, H.; et al. Characteristics, Pathogenic Mechanism, Zoonotic Potential, Drug Resistance, and Prevention of Avian Pathogenic Escherichia coli (APEC). Front. Microbiol. 2022, 13, 1049391. [Google Scholar] [CrossRef]
- Kuznetsova, M.V.; Mihailovskaya, V.S.; Selivanova, P.A.; Kochergina, D.A.; Remezovskaya, N.B.; Starčič Erjavec, M. Siderophore Production, Diversity of Siderophore Receptors and Associations with Virulence-Associated Genes, Phylogroups and Bacteriocin Production in Escherichia coli Strains Isolated from Humans, Animals and Organic Fertilizers. Microbiol. Res. 2025, 16, 50. [Google Scholar] [CrossRef]
- Mitchell, N.M.; Johnson, J.R.; Johnston, B.; Curtiss, R.; Mellata, M. Zoonotic Potential of Escherichia coli Isolates from Retail Chicken Meat Products and Eggs. Appl. Environ. Microbiol. 2015, 81, 1177–1187. [Google Scholar] [CrossRef]
- Kuan, N.-L.; Chen, Y.-P.; Shien, J.-H.; Yeh, K.-S. Characteristics of the Extended-Spectrum-β-Lactamase-Producing Escherichia coli Isolated from Diseased Livestock and Poultry in Taiwan. Sci. Rep. 2024, 14, 29459. [Google Scholar] [CrossRef]
- Hoffman, J.A.; Badger, J.L.; Zhang, Y.; Huang, S.H.; Kim, K.S. Escherichia coli K1 aslA Contributes to Invasion of Brain Microvascular Endothelial Cells in vitro and in vivo. Infect. Immun. 2000, 68, 5062–5067. [Google Scholar] [CrossRef] [PubMed]
- Harrington, S.; Sheikh, J.; Henderson, I.; Ruiz-Perez, F.; Cohen, P.; Nataro, J. The Pic Protease of Enteroaggregative Escherichia coli Promotes Intestinal Colonization and Growth in the Presence of Mucin. Infect. Immun. 2009, 77, 2465–2473. [Google Scholar] [CrossRef]
- Larzábal, M.; Marques Da Silva, W.; Riviere, N.A.; Cataldi, Á.A. Novel Effector Protein EspY3 of Type III Secretion System from Enterohemorrhagic Escherichia coli Is Localized in Actin Pedestals. Microorganisms 2018, 6, 112. [Google Scholar] [CrossRef]
- Mei, X.; Zhonghong, L.; Ping, Z.; Weiqiang, L. Genomic Characteristics of ETT2 Gene Clusters in Avian Pathogenic Escherichia coli Identified by Whole-Genome Sequencing. Pak. Vet. J. 2024, 44, 833–839. [Google Scholar] [CrossRef]
- Azimzadeh, P.N.; Birchenough, G.M.; Gualbuerto, N.C.; Pinkner, J.S.; Tamadonfar, K.O.; Beatty, W.; Hannan, T.J.; Dodson, K.W.; Ibarra, E.C.; Kim, S.; et al. Mechanisms of Uropathogenic E. coli Mucosal Association in the Gastrointestinal Tract. Sci. Adv. 2025, 11, eadp7066. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, P.; Dhakal, S.; Dozois, C.M. The Diversity of Escherichia coli Pathotypes and Vaccination Strategies against This Versatile Bacterial Pathogen. Microorganisms 2023, 11, 344. [Google Scholar] [CrossRef]
- Golpasand, T.; Keshvari, M.; Behzadi, P. Distribution of Chaperone-Usher Fimbriae and Curli Fimbriae among Uropathogenic Escherichia coli. BMC Microbiol. 2024, 24, 344. [Google Scholar] [CrossRef]
- Dubreuil, J.D.; Isaacson, R.E.; Schifferli, D.M. Animal Enterotoxigenic Escherichia coli. EcoSal Plus 2016, 7, 10-1128. [Google Scholar] [CrossRef]
- Luna-Pineda, V.M.; Moreno-Fierros, L.; Cázares-Domínguez, V.; Ilhuicatzi-Alvarado, D.; Ochoa, S.A.; Cruz-Córdova, A.; Valencia-Mayoral, P.; Rodríguez-Leviz, A.; Xicohtencatl-Cortes, J. Curli of Uropathogenic Escherichia coli Enhance Urinary Tract Colonization as a Fitness Factor. Front. Microbiol. 2019, 10, 2063. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Song, Y.; Zou, Y.; Yang, Y.; Zhu, G. F4+ Enterotoxigenic Escherichia coli (ETEC) Adhesion Mediated by the Major Fimbrial Subunit FaeG. J. Basic. Microbiol. 2015, 55, 1118–1124. [Google Scholar] [CrossRef]
- Kathayat, D.; Lokesh, D.; Ranjit, S.; Rajashekara, G. Avian Pathogenic Escherichia coli (APEC): An Overview of Virulence and Pathogenesis Factors, Zoonotic Potential, and Control Strategies. Pathogens 2021, 10, 467. [Google Scholar] [CrossRef]
- Galván, H.E.D.; López, H.M.; Ramos, E.M.; Blandón, J.A.Y.; Nieto, O.C.; Torres, K.N.Q.; Tirado, L.C.E.; Julio, R.G.V. Evaluation of Virulence Factors in Clinical Isolates of Pathogenic E. coli in Avian Samples in Caloto, Colombia. Rev. Colomb. Biotecnol. 2023, 25, 33–49. [Google Scholar] [CrossRef]
- Johnson, T.J.; Wannemuehler, Y.; Doetkott, C.; Johnson, S.J.; Rosenberger, S.C.; Nolan, L.K. Identification of Minimal Predictors of Avian Pathogenic Escherichia coli Virulence for Use as a Rapid Diagnostic Tool. J. Clin. Microbiol. 2008, 46, 3987–3996. [Google Scholar] [CrossRef]
- Cunha, M.P.V.; Saidenberg, A.B.; Moreno, A.M.; Ferreira, A.J.P.; Vieira, M.A.M.; Gomes, T.A.T.; Knöbl, T. Pandemic Extra-Intestinal Pathogenic Escherichia coli (ExPEC) Clonal Group O6-B2-ST73 as a Cause of Avian Colibacillosis in Brazil. PLoS ONE 2017, 12, e0178970. [Google Scholar] [CrossRef] [PubMed]
- Ewers, C.; Li, G.; Wilking, H.; Kießling, S.; Alt, K.; Antáo, E.-M.; Laturnus, C.; Diehl, I.; Glodde, S.; Homeier, T.; et al. Avian Pathogenic, Uropathogenic, and Newborn Meningitis-Causing Escherichia coli: How Closely Related Are They? Int. J. Med. Microbiol. 2007, 297, 163–176. [Google Scholar] [CrossRef]
- Johnson, T.J.; Wannemuehler, Y.; Johnson, S.J.; Stell, A.L.; Doetkott, C.; Johnson, J.R.; Kim, K.S.; Spanjaard, L.; Nolan, L.K. Comparison of Extraintestinal Pathogenic Escherichia coli Strains from Human and Avian Sources Reveals a Mixed Subset Representing Potential Zoonotic Pathogens. Appl. Environ. Microbiol. 2008, 74, 7043–7050. [Google Scholar] [CrossRef]
- Lemlem, M.; Aklilu, E.; Mohammed, M.; Kamaruzzaman, F.; Zakaria, Z.; Harun, A.; Devan, S.S. Molecular Detection and Antimicrobial Resistance Profiles of Extended-Spectrum Beta-Lactamase (ESBL) Producing Escherichia coli in Broiler Chicken Farms in Malaysia. PLoS ONE 2023, 18, e0285743. [Google Scholar] [CrossRef]
- Vodnala, S.K.; Lundbäck, T.; Sjöberg, B.; Svensson, R.; Rottenberg, M.E.; Hammarström, L.G.J. In vitro and in vivo Activities of 2-Aminopyrazines and 2-Aminopyridines in Experimental Models of Human African Trypanosomiasis. Antimicrob. Agents Chemother. 2013, 57, 1012–1018. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Chanoine, M.-H.; Bertrand, X.; Madec, J.-Y. Escherichia coli ST131, an Intriguing Clonal Group. Clin. Microbiol. Rev. 2014, 27, 543–574. [Google Scholar] [CrossRef] [PubMed]
- Dingle, T.C.; Butler-Wu, S.M. Maldi-Tof Mass Spectrometry for Microorganism Identification. Clin. Lab. Med. 2013, 33, 589–609. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).