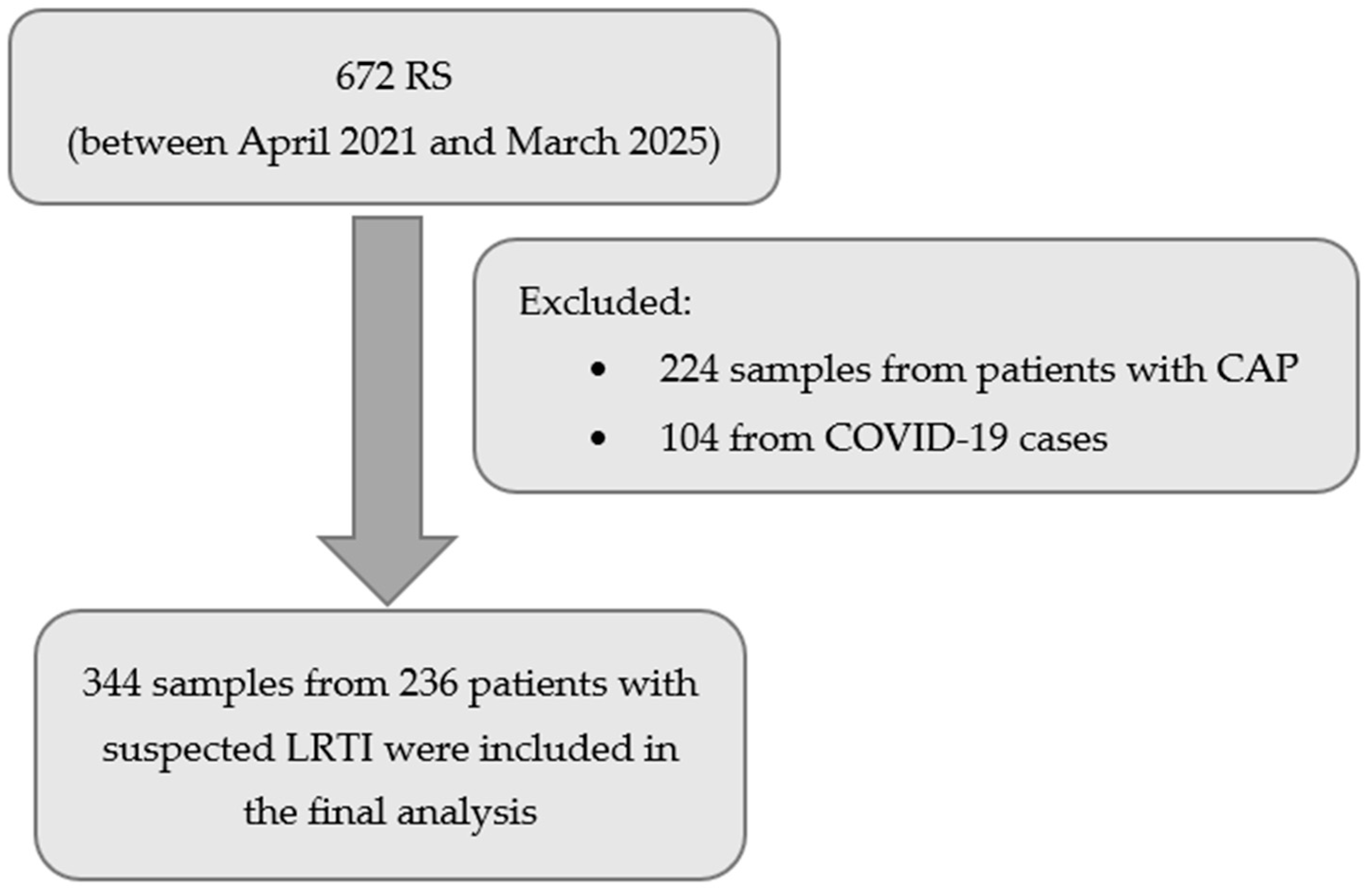
In this single-centre, real-world evaluation of the BioFire
® FilmArray
® Pneumonia (FA-PNEU
®) panel for suspected nosocomial lower respiratory tract infections (LRTI) in critically ill patients, we observed that access to rapid multiplex PCR results had a substantial impact on antimicrobial prescribing, with changes or decisions directly attributable to the assay in 57.6% of cases. This aligns with prior studies reporting rapid molecular diagnostics as powerful antimicrobial stewardship tools, capable of supporting earlier escalation, targeted therapy, or de-escalation in high-risk ICU populations [
11,
12,
13].
3.1. Diagnostic Performance and Concordance with Culture
FA-PNEU® demonstrated high sensitivity (93.4%) and an excellent negative predictive value (NPV, 97.9%), making it particularly useful for ruling out bacterial pathogens in suspected LRTI. However, specificity was moderate (65.0%) and the positive predictive value (PPV) was low (36.5%). The low PPV, largely driven by prior antibiotic therapy in 85.8% of false positives, is consistent with previous observations that molecular methods may detect non-viable bacterial DNA in patients already receiving antibiotics.
From a clinical perspective, interpreting these apparent false positives requires integrating microbiological findings with the patient’s trajectory and inflammatory response. In pretreated patients, FA-PNEU® detections may represent residual DNA from effectively treated pathogens or colonization rather than ongoing infection. Distinguishing between these scenarios is essential to avoid unnecessary escalation. A practical framework involves combining FA-PNEU® results with quantitative bacterial load, radiographic evolution, and biomarkers such as C-reactive protein or procalcitonin. When bacterial load is low and inflammatory markers are falling, results should be interpreted as colonization or microbiological residue rather than active infection. Conversely, high bacterial load or persistently elevated biomarkers despite prior therapy should prompt reassessment for treatment failure or superinfection. Embedding such an interpretative algorithm into stewardship discussions ensures FA-PNEU® is used to refine, rather than expand, antimicrobial coverage in complex ICU cases.
Lee et al. reported that the BioFire
® FilmArray
® Pneumonia Panel had strong concordance for common Gram-negative pathogens but frequently detected additional organisms in patients with prior antibiotic exposure, many of which were of uncertain clinical relevance [
14]. Similarly, Yoo et al. observed excellent NPV for Gram-negative pathogens but noted that polymicrobial detections were more frequent compared to culture, raising questions about interpretation in pre-treated patients [
15].
The clinical utility of multiplex PCR testing is therefore highly dependent on pretest probability. In patients with low suspicion of LRTI, the high NPV of FA-PNEU® allows clinicians to confidently rule out bacterial pneumonia and redirect diagnostic efforts toward alternative sources. In contrast, in patients with a high pretest probability, a positive FA-PNEU® result may support rapid, targeted antimicrobial therapy, while a negative result should prompt clinicians to consider pathogens not represented in the panel.
Concordance analysis revealed complete agreement with conventional culture in 57.0% of cases. FA-PNEU
® detected additional organisms more often than culture (104 vs. 23), consistent with findings from Buchan et al., who reported higher pathogen detection with FA-PNEU
® (90% vs. 66% for culture) in hospitalized pneumonia patients, though often at lower bacterial loads [
16]. Murphy et al. also confirmed the analytical performance of FA-PNEU
® across multiple centers and highlighted the importance of bacterial load thresholds in interpreting results [
10]. The added detection of viral pathogens by FA-PNEU
® further increases its clinical relevance, enabling adjunctive antiviral therapy or avoiding unnecessary antibacterial use.
The INHALE WP3 programme has provided further insight into the clinical application of FA-PNEU
®. The published trial protocol [
17] outlined a pragmatic, multicenter RCT comparing PCR-guided versus standard care in ICU patients with HAP/VAP. A subsequent cost-effectiveness analysis reported reduced ICU costs (£33,149 vs. £40,951; –£7802 difference) and stewardship benefits with FA-PNEU
®, but no improvement in clinical cure rates [
18]. This reinforces the recurring theme that the strongest contribution of FA-PNEU
® lies in optimizing early prescribing and antimicrobial stewardship, while its direct effect on hard clinical outcomes remains uncertain.
3.2. Organism and Resistance Detection
The organism detection profile differed notably between FA-PNEU
® and conventional culture. Some Gram-negative bacteria, such as
Haemophilus influenzae, were more frequently identified by FA-PNEU
®, whereas others, including
Serratia marcescens, were more commonly detected in culture. This discrepancy likely reflects differences in assay design (i.e., specific genetic targets included in the multiplex panel), analytical sensitivity thresholds, and pre-analytical factors such as sample quality and handling. Importantly, FA-PNEU
® is limited by its closed target list: clinically relevant pathogens such as
Aspergillus spp. and
Stenotrophomonas maltophilia are not represented in the panel. This limitation may reduce its standalone utility in immunocompromised populations or in patients at risk of infections caused by atypical or emerging pathogens [
19]. Our previous studies have repeatedly emphasized this gap, particularly in the setting of ventilator-associated pneumonia (VAP), where broad microbiological coverage and careful integration with clinical context are essential for accurate diagnosis and antimicrobial stewardship [
20,
21].
With regard to resistance genes, FA-PNEU
® detected a greater number of carbapenemase-producing organisms than standard culture, especially
blaVIM positive isolates, underscoring its potential value for infection control and early containment strategies. This rapid recognition of multidrug-resistant Gram-negative organisms is particularly relevant in the ICU, where timely initiation of appropriate therapy strongly influences outcomes. However, it is critical to recognize that carbapenem resistance in
Pseudomonas aeruginosa often results from mechanisms outside the scope of the panel (e.g., efflux pump upregulation, porin loss), meaning that phenotypic susceptibility testing remains indispensable. In our own work, we have shown that reliance on molecular platforms alone may over- or under-estimate resistance profiles, and that a hybrid approach combining rapid diagnostics with confirmatory phenotypic testing best supports clinical decision-making and stewardship goals [
22,
23].
Taken together, while FA-PNEU® offers an important step forward in rapid pathogen identification and resistance gene detection, its integration into clinical care must be guided by an understanding of its limitations, complemented by culture, and interpreted within the broader clinical context—an approach consistently advocated in our research on ICU pneumonia diagnostics and management.
3.3. Impact on Antimicrobial Management
FA-PNEU® results prompted a wide range of antibiotic decisions. In patients without prior antibiotic exposure, initiation or withholding of therapy was fully concordant with panel findings, suggesting strong clinician confidence in negative results. In contrast, among patients already on antibiotics, adherence to panel guidance was lower (69.6% in those with therapy changes, 30.4% in those without). This discrepancy likely reflects both the difficulty of de-escalating or discontinuing antimicrobials in hemodynamically unstable patients and the integration of other diagnostic information, such as imaging or inflammatory biomarkers, into clinical reasoning.
Notably, 17.7% of antibiotic changes were triggered by suspicion of an alternative infectious source following a negative FA-PNEU® result, illustrating the assay’s indirect diagnostic value. This “rule-out” function has been less frequently emphasised in prior evaluations, but is particularly relevant in critically ill patients, where diagnostic uncertainty is common. Similar findings have been reported in other cohorts.
Collectively, these studies suggest that while FA-PNEU
® can directly shape initial prescribing, its greatest added value may lie in its ability to provide rapid reassurance in cases where pneumonia is not supported, thereby redirecting attention toward alternative infectious or non-infectious diagnoses. This aligns with stewardship principles recently reinforced in ICU pneumonia management guidelines [
24].
3.4. Cost-Effectiveness Evidence from Randomised Data
Our findings regarding antibiotic stewardship and rapid decision-making are supported by evidence from the INHALE WP3 pragmatic multi-centre RCT, which compared BioFire
® FilmArray
® Pneumonia Panel-guided therapy with standard care in ICU patients with HAP or VAP [
17]. In this trial, PCR-guided therapy achieved superior antibiotic stewardship outcomes at 24 h and was associated with lower mean ICU costs (£33,149 vs. £40,951; −£7802 difference) [
18], despite the cost of the assay. Importantly, while the intervention was cost-effective for stewardship outcomes, it did not demonstrate superiority for clinical cure at 14 days, with fewer cases classified as clinically cured in the PCR arm [
25,
26]. This reflects a potential trade-off also observed in our real-world setting: FA-PNEU
® supports earlier, more precise stewardship decisions, but its effect on hard clinical outcomes may be blunted unless results are embedded within clear clinical pathways.
The INHALE data align with findings from other evaluations of syndromic PCR in lower respiratory tract infections. For example, Timbrook et al. [
11] reported that rapid multiplex PCR testing reduced time to appropriate therapy and enabled de-escalation, though stewardship impact varied by setting and clinician adoption. Similarly, Buchan et al. found that BioFire
® FilmArray
® Pneumonia Panel improved pathogen detection compared to culture and influenced antibiotic prescribing, particularly by reducing unnecessary broad-spectrum coverage [
16].
These converging data reinforce the concept that the principal, consistent benefit of syndromic PCR platforms in ICU pneumonia is optimization of early antimicrobial prescribing. By rapidly ruling in or out common pathogens and key resistance genes, these assays can reduce unnecessary exposure to carbapenems and other broad-spectrum agents, thereby lowering costs and potentially limiting resistance selection pressure. However, translating these microbiological and stewardship gains into improved clinical outcomes such as mortality, length of stay, or cure rates appears to require additional steps—specifically, the integration of diagnostics into multidisciplinary stewardship programmes, structured decision algorithms, and ongoing clinician training. This has been highlighted in pneumonia guideline commentaries; which emphasize that molecular diagnostics should be embedded within stewardship frameworks rather than used in isolation [
27].
3.5. Strengths and Limitations
Strengths of this study include its real-world setting, sizeable sample over a four-year period, and integration of microbiological and clinical decision-making data. This provides an authentic picture of how FA-PNEU® is used outside a research protocol and how it influences antimicrobial stewardship.
Limitations include its single-centre design, which may limit generalisability, and the observational nature of the study, which precludes definitive attribution of patient outcomes to FA-PNEU® use. The absence of standardised protocols for interpreting FA-PNEU® results also means that management decisions were subject to individual clinician judgement. Furthermore, the study did not assess cost-effectiveness or long-term outcomes such as antibiotic days, resistance emergence, or mortality.