Abstract
The growing prevalence of antimicrobial resistance has significantly compromised the efficacy of conventional antibiotic-based interventions in controlling Salmonella infections across human and veterinary settings. This growing challenge necessitates a strategic rethinking of pathogen control, prompting the integration of next-generation therapeutics capable of disrupting Salmonella pathogenesis through novel, antibiotic-sparing mechanisms. In this context, a diverse array of emerging alternatives, including bacteriophages, antimicrobial peptides, probiotics, prebiotics, short-chain fatty acids, nanoparticles, and host-directed immunomodulators, have gained prominence as a promising frontier in non-antibiotic therapeutics. These modalities offer targeted approaches to inhibit Salmonella colonization, virulence expression, and persistence, while minimizing collateral damage to the microbiota and avoiding the propagation of resistance genes. As Salmonella continues to pose a global threat to animal and public health, the development of scalable, resistance-conscious interventions remains a critical priority. Ongoing research efforts are increasingly focused on optimizing delivery systems, dosage strategies, and synergistic combinations to enhance the clinical and field applicability of these alternatives. By harnessing these innovative modalities, the future of Salmonella control may shift toward precision therapeutics that align with One Health principles and sustainable food safety goals.
1. Introduction
Salmonellosis is a globally significant zoonotic disease with substantial implications for public health, food safety, and veterinary medicine [1,2]. It is caused by Salmonella spp., a genus of Gram-negative, facultatively anaerobic, rod-shaped bacteria belonging to the Enterobacteriaceae family [3,4]. These pathogens are taxonomically diverse, encompassing over 2600 serotypes, and are broadly categorized into typhoidal and non-typhoidal serovars based on their host specificity and clinical manifestations [5]. Typhoidal strains, such as Salmonella Typhi and Salmonella Paratyphi, are human-restricted and responsible for invasive systemic infections, particularly in regions with inadequate sanitation infrastructure [6]. In contrast, non-typhoidal Salmonella (NTS) serovars include Salmonella Enteritidis, Salmonella Typhimurium, and Salmonella Heidelberg exhibits a broad host range and is predominantly associated with self-limiting gastroenteritis in humans, as well as subclinical or clinical infections in livestock and poultry [2,7,8].
NTS serovars are frequently transmitted through contaminated food products, especially those of animal origin, and are a leading cause of bacterial foodborne illness worldwide. Their persistence in agricultural environments, ability to colonize asymptomatic carriers, and resilience against standard sanitation practices pose ongoing challenges to containment [2,9]. In humans, salmonellosis typically presents as acute gastroenteritis characterized by diarrhea, fever, abdominal cramps, nausea, and vomiting, with symptoms appearing 6–72 h post-exposure and lasting 4–7 days [10]. While most cases are self-limiting, vulnerable populations such as infants, the elderly, and immunocompromised individuals may develop invasive infections, including bacteremia, meningitis, osteomyelitis, and septic arthritis [10,11]. Salmonella infections can affect a wide range of animals, including poultry (chickens, turkeys, ducks), cattle, pigs, sheep, goats, horses, reptiles (such as turtles and lizards), amphibians, rodents, and companion animals like dogs and cats [2]. In animals, clinical signs vary by species and age, with young poultry and livestock exhibiting anorexia, diarrhea, and systemic signs such as hepatosplenomegaly [2,12].
Numerous foodborne outbreaks have prompted large-scale recalls of contaminated products, including eggs, cucumbers, deli meats, and chocolate spreads [13,14,15,16]. Recent recalls linked to S. enteritidis and S. newport have affected multiple states and food categories, underscoring the need for robust surveillance and traceability systems [17,18].
The therapeutic landscape has grown increasingly complex due to the global rise of multidrug-resistant (MDR) Salmonella strains. Resistance to traditional antibiotics such as ampicillin, chloramphenicol, tetracyclines, and trimethoprim-sulfamethoxazole has been widely documented, particularly in serovars associated with livestock and poultry production [8,9]. These MDR strains often harbor mobile genetic elements, including plasmids, integrons, and transposons, which facilitate horizontal gene transfer and accelerate the dissemination of resistance determinants across bacterial populations [19,20]. Alarmingly, resistance to extended-spectrum β-lactams and fluoroquinolones has also emerged in animals and humans, narrowing therapeutic options and prompting the need for enhanced surveillance, stewardship, and alternative treatment strategies [21,22,23].
This review aims to critically evaluate emerging non-antibiotic strategies for the control of multidrug-resistant Salmonella, with a particular focus on interventions such as probiotics, prebiotics, organic acids, bacteriophages, essential oils, antimicrobial peptides, small molecules, quorum sensing inhibitors, and vaccines. By synthesizing current evidence on their mechanisms of action, efficacy, and applicability across human and veterinary settings, this article discusses their potential to reduce pathogen colonization, enhance host immune responses, and curb the spread of antimicrobial resistance. The overarching goal is to inform integrated, sustainable approaches to Salmonella management that align with One Health principles and address the limitations of conventional antibiotic therapies.
2. Antibiotic Alternatives
2.1. Small Molecules (SMs)
Small molecules are low molecular weight organic compounds that can easily diffuse across cell membranes and modulate biological processes [22,24,25]. They may be naturally occurring (like microbial metabolites or plant alkaloids) or synthetically produced, and are often used as pharmaceutical drugs, research tools and/or signaling agents [23,26,27]. SMs have demonstrated the ability to inhibit bacterial growth by targeting key molecular pathways and disrupting intracellular processes [28,29]. In the context of Salmonella infections, they offer a promising therapeutic strategy by attenuating bacterial growth and virulence while minimizing the emergence of antibiotic resistance. These compounds can interfere with quorum sensing, biofilm formation, and essential metabolic functions [2,22].
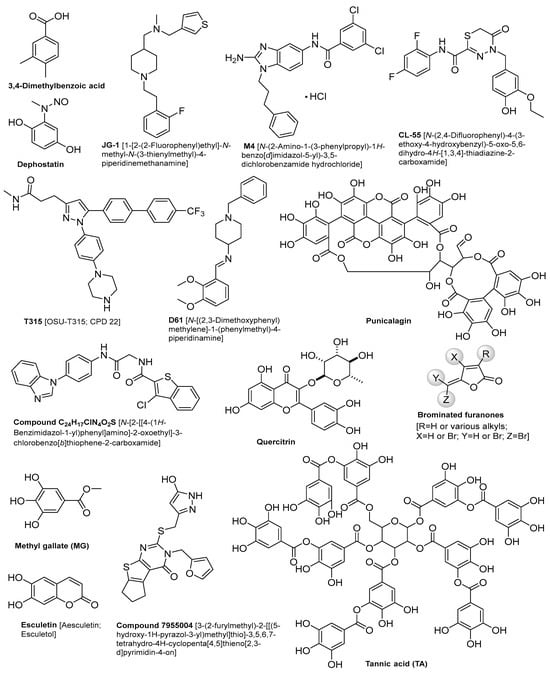
Several SMs have shown promise in combating Salmonella infections, as illustrated in Table 1. The chemical structures of these compounds are described in Figure 1. A 3,4-dimethylbenzoic acid, which is an aromatic compound extracted from the human fecal microbiome, has shown great interference with gene expression involved in Salmonella’s invasion of host cells [30]. A combination of JG-1 and M4 small molecules has been utilized to inhibit biofilm formation and disrupt pre-existing biofilm structures of S. typhi in a mouse model of chronic gallbladder Salmonella carriage [31]. This treatment successfully prevented bacterial dissemination to peripheral organs [31]. Furthermore, a novel CL-55[N-(2,4-difluorophenyl)-4-(3-ethoxy-4-hydroxybenzyl)-5-oxo-5,6-dihydro-4H-[1,3,4]-thiadiazine-2-carboxamide] SM has demonstrated a significant reduction in S. typhimurium counts within the spleen and peritoneal lavages of mice [32]. Remarkably, complete eradication of Salmonella was achieved within twelve days of treatment [32]. Moreover, dephostatin, a non-antibiotic SM, has attenuated S. typhimurium virulence in mouse models and in vitro. It also restores sensitivity to polymyxin antibiotics [33]. Similarly, the T315 compound, when used in combination with ciprofloxacin, has demonstrated effective inhibition of biofilm formation in S. typhimurium and S. typhi serovars. It was reported that the half-maximal effective concentrations (EC50) were 7.4 μM for S. typhimurium and 21.0 μM for S. typhi [34]. Furthermore, D61 SMs have reduced S. typhimurium load in primary mice and human macrophages with no effect on Salmonella growth in epithelial cells. It has also reduced Salmonella load in the livers and spleens of infected mice [35]. Likewise, a previous study on four novel SMs has shown a significant growth inhibition of different Salmonella enterica serotypes in chickens and tomato [36,37]. They reduced the intracellular survival of S. typhimurium in eukaryotic models with a significant reduction of S. typhimurium load and colonization in chicken ceca and systemic organs [36]. Quercitrin, a flavonoid with antioxidant properties, has demonstrated significant inhibition of S. typhimurium adhesion, invasion, and survival in HeLa cell lines, reducing bacterial presence by 70% [38]. Moreover, Jacob et al., 2015 reported that compound 7955004 induced a 55% reduction in S. typhimurium preformed biofilms, achieving complete clearance of planktonic S. typhimurium in vitro [39].
However, SMs continue to play a pivotal role in modern therapeutics, they have limitations. Challenges such as poor bioavailability, off-target effects, and the potential for resistance, particularly in antimicrobial applications can hinder their effectiveness [40]. Moreover, their relatively simple structures may limit their ability to engage complex or large biological targets, where biologics often excel [41,42]. Despite these constraints, ongoing advances in medicinal chemistry and drug delivery technologies are steadily expanding the therapeutic potential SMs.

Table 1.
Antimicrobial effect of SMs against different Salmonella spp. (for the chemical structures of the compounds, see Figure 1).
Table 1.
Antimicrobial effect of SMs against different Salmonella spp. (for the chemical structures of the compounds, see Figure 1).
| Small Molecules | Targeted Salmonella Serovars | Host | Observations | Refs. |
|---|---|---|---|---|
| 3,4-dimethylbenzoic acid | S. typhimurium | In vitro | Treatment with 3,4-dimethylbenzoic acid led to a ~94% reduction in hilA expression. Furthermore, when epithelial cells were exposed to Salmonella treated with the compound, invasion rates dropped significantly, with statistical significance reported as p < 0.001 across three biological replicates. In addition, the bioactive fraction responsible for repression had a molecular weight, and retained activity comparable to the full fecal extract, showing ~79% repression of hilA. | [30] |
| JG-1 and M4 | S. typhi and S. typhimurium | In vitro, Mice | Both compounds significantly reduced biofilm formation of S. typhi and S. typhimurium in vitro, with EC50 values of 38.9 μM (M4) and 53.6 μM (JG-1) for S. typhi. Additionally, M4 was more potent in dispersing pre-formed biofilms, with an EC50 of 46.4 μM, compared to 829 μM for JG-1. Also, in vivo treatment reduced bacterial burden in the gallbladder by 1–2 logs, and when co-administered with ciprofloxacin, the reduction reached 3–4.5 logs. | [31] |
| CL-55 (N-(2,4-difluorophenyl)-4-(3-ethoxy-4-hydroxybenzyl)-5-oxo-5,6-dihydro-4H-[1,3,4]-thiadiazine-2-carboxamide) | S. typhimurium | Mice | Mice treated with 10 mg/kg intraperitoneally for 4 days showed a 500-fold reduction in S. typhimurium counts in the spleen and peritoneal lavages. In addition, 12-day therapy led to the complete eradication of Salmonella from infected mice. Moreover, no detectable pathogen was found 4–6 weeks post-treatment, indicating durable clearance. | [43] |
| Dephostatin | S. typhimurium | In vitro, Mice | Dephostatin significantly reduced intracellular replication of S. typhimurium and restored susceptibility to colistin. Furthermore, mice infected with S. typhimurium and treated with Dephostatin and colistin showed prolonged survival compared to colistin alone, they also showed a >60% survival rate with combination therapy vs. 25% with colistin monotherapy. | [33] |
| T315 | S. typhi and S. typhimurium | In vitro | T315 compound, when used in combination with ciprofloxacin, has demonstrated effective inhibition of biofilm formation in S. typhimurium and S. typhi serovars. Not only that, the half-maximal effective concentrations also (EC50) were determined to be 7.4 μM for S. typhimurium and 21.0 μM for S. typhi. | [34] |
| D61 | S. typhimurium | In vitro, Mice | D61 significantly reduced S. typhimurium load in: RAW 264.7 macrophage-like cells (~20-fold reduction; IC50 = 1.3 μM), Primary mouse bone marrow-derived macrophages (~17-fold reduction; IC50 = 7.9 μM), and Primary human macrophages (~23-fold reduction). Also, D61 treatment led to a significant reduction in Salmonella burden in the spleen and liver of infected mice. | [35] |
| C24H17ClN4O2S | S. typhimurium | In vitro, chicken model | C24H17ClN4O2S inhibited the SPI-1 Type III Secretion System (T3SS) in S. typhimurium. In addition, it downregulated InvF, a key transcriptional regulator, and reduced effector proteins like SipA and SipC. | [36,44] |
| Quercitrin | S. typhimurium | In vitro, Mice | Quercitrin reduced S. typhimurium adhesion to HeLa cells in a dose-dependent manner, with no cytotoxicity observed up to 64 μg/mL. Moreover, in the mouse infection model, quercitrin treatment led to a ~1.5 log10 reduction in S. typhimurium counts in the cecal contents compared to untreated controls. | [38] |
| Compound 7955004 | S. typhimurium | In vitro | 7955004 induced a 55% reduction in preformed S. typhimurium biofilms. There was no toxicity observed in mammalian cells even at 30 μM, which is ~6× higher than EC50. Also, there was no significant change in OD600 of planktonic bacteria after 24 h exposure. | [39] |
2.2. Quorum Sensing Inhibitors (QSIs)
Quorum sensing is a bacterial communication system that enables populations of bacteria to coordinate gene expression and behavior based on their density [45,46]. During growth, individual bacterial cells release signaling molecules called autoinducers into their environment [47]. As the population increases, so does the concentration of these molecules. Once a threshold level is reached, the autoinducers bind to specific receptors, triggering changes in gene expression that lead to collective behaviors such as biofilm formation, virulence factor production, and bioluminescence [45,47,48,49]. Once the concentration of the signaling molecule surpasses a specific threshold, it triggers the expression of certain genes in microorganisms, thereby affecting the pathogenicity and physiological processes of microorganisms [50]. Most quorum sensing inhibitors (QSIs) can disrupt the interaction between receptor proteins and signaling molecules, inhibiting the synthesis of autoinducers, or facilitating the degradation of quorum sensing signal molecules [51,52]. S-Ribosylhomocysteinase (LuxS) and Autoinducer 2-binding protein LsrB are two proteins involved in the quorum sensing system of Salmonella, playing crucial roles in modulating bacterial biofilm formation and pathogenicity [53,54,55]
Quorum sensing inhibitors (QSIs) represent a promising approach for controlling Salmonella infections by targeting key communication pathways, as illustrated in Table 2. For instance, brominated furanones are well-known quorum sensing inhibitors in various bacterial species, particularly Pseudomonas aeruginosa. However, their mechanism of action in Salmonella enterica remains unclear. No evidence that furanones interfere with the currently known quorum sensing systems in Salmonella, such as SdiA and AI-2 [56]. Nonetheless, brominated furanones have been shown to reduce biofilm formation and motility in S. typhimurium, suggesting they may exert indirect regulatory effects on virulence-associated pathways [56]. Treatment of S. typhimurium with methyl gallate (MG) at 30 µg/mL resulted in downregulation of quorum sensing genes sdiA (52.8%), srgE (61.7%), and rck (22.2%). When combined with a sub-minimum inhibitory concentration (sub-MIC) of marbofloxacin (MRB), MG further suppressed the host cell signaling gene rac-1 by 71.9%, compared to 56.9% with MG alone. Additionally, MG significantly reduced the expression of virulence-associated genes: cheY (59.6%), ompD (60.2%), sipB (20.5%), lexA (31.4%), and ompF (16.2%) [57]. Additionally, tannic acid was reported to inhibit the swarming motility of S. typhi and S. Paratyphi at a minimum effective concentration of 400 μg/mL, without affecting bacterial growth [58]. The synthetic compound N-(3-oxo-octanoyl)-DL-homoserine lactone at 10 nM has also been reported to suppress sdiA expression and inhibit biofilm development in S. typhimurium [59]. Xanthone derivatives have reduced AI-2 production by 60–70% in S. typhimurium, in conjunction with inhibition of efflux pump activity [60]. Moreover, punicalagin at a concentration of 15.6 μg/mL has demonstrated repressive effects on motility (flhC) and quorum sensing-related genes (sdiA and srgE) in S. typhimurium [61].
However, QSIs hold promise as a novel therapeutic approach to combat Salmonella infections in humans and animals; there are challenges remaining. Achieving species-specific targeting without disrupting the normal microbiota remains a significant challenge. Moreover, delivering effectively within complex environments such as biofilms poses additional obstacles, particularly in terms of ensuring their bioavailability. Furthermore, the high costs and extended timelines associated with QSIs development limit their widespread application as therapeutic agents.

Table 2.
Antimicrobial effect of QSIs against different Salmonella spp. (For the chemical structures of the compounds, see Figure 1).
Table 2.
Antimicrobial effect of QSIs against different Salmonella spp. (For the chemical structures of the compounds, see Figure 1).
| QSIs | Targeted Salmonella Serovars | Host | Observations | Refs. |
|---|---|---|---|---|
| Brominated Furanones | S. typhimurium | In vitro | Brominated furanones do not act on known QS systems in Salmonella (e.g., SdiA, AI-2) but may exert indirect effects on biofilm and motility. | [56] |
| Punicalagin | S. typhimurium SL1344 | In vitro | Punicalagin, even at sub-inhibitory concentrations (1/16× and 1/32× MIC), significantly reduced Salmonella motility, including both swimming and swarming behaviors. This reduction was associated with the downregulation of key motility-related genes (fliA, fliY, fljB, flhC, and fimD). Additionally, punicalagin dose-dependently inhibited violacein production in Chromobacterium violaceum, indicating its quorum sensing inhibitory activity. In Salmonella, it suppressed the expression of QS-regulated genes (sdiA and srgE) and significantly reduced bacterial invasion of human colonic cells (p < 0.01) without affecting adhesion. | [61] |
| Methyl gallate (MG) | S. typhimurium | In vitro | Exposure of S. typhimurium to methyl gallate at a concentration of 30 µg/mL led to notable downregulation of quorum sensing genes, including sdiA (52.8%), srgE (61.7%), and rck (22.2%). When MG was combined with a sub-minimum inhibitory concentration of marbofloxacin, suppression of the host cell signaling gene rac-1 increased to 71.9%, compared to 56.9% with MG alone. Furthermore, MG significantly reduced the expression of key virulence-associated genes: cheY (59.6%), ompD (60.2%), sipB (20.5%), lexA (31.4%), and ompF (16.2%). | [62] |
| Tannic acid | S. typhi, S. Paratyphi | In vitro | Tannic acid exhibited strong quorum sensing inhibitory activity at a minimum effective concentration of 400 μg/mL. It reduced cell surface hydrophobicity by 38–43% and extracellular polymeric substance (EPS) production by 35–50%. TA significantly enhanced the susceptibility of S. typhi and S. Paratyphi to a range of antibiotics, including amikacin, ampicillin, ciprofloxacin, azithromycin, chloramphenicol, and gentamicin. Additionally, TA drastically inhibited swarming motility, a key quorum sensing-regulated phenotype, without affecting bacterial growth. | [58,63] |
| Star anise extract | S. typhimurium | Food matrix | Star anise extract inhibited violacein production by 89% in Chromobacterium violaceum, indicating strong interference with quorum sensing-regulated pigment synthesis. Although this biosensor assay does not directly evaluate quorum sensing in S. typhimurium, it suggests that the extract may possess broad-spectrum quorum sensing inhibitory activity. Supporting this, swarming motility in S. typhimurium was significantly reduced—by up to 95.9%—at higher extract concentrations, highlighting its potential to impair bacterial communication and motility. | [64] |
| Esculetin | S. typhimurium | Chicken | Esculetin demonstrated strong antimicrobial and quorum sensing inhibitory activity against S. typhimurium, with MIC of 500 μg/mL. It downregulated key genes involved in quorum sensing and biofilm regulation, including adrA, lsrB, luxS, and rpoS. Mechanistically, esculetin competes with the LsrB receptor, preventing AI-2 uptake and disrupting QS signaling. At 8× MIC, esculetin was able to kill 2 log CFU/mL of S. typhimurium within 30 min. | [53] |
2.3. Probiotics
Probiotics are live, non-pathogenic microorganisms that, when administered in adequate amounts, enhance gut health and confer a health benefit on the host [65,66,67]. The selection of probiotic strains for potential therapeutic use, particularly as antimicrobial alternatives, is guided by specific criteria. These criteria include the capacity to withstand the challenging conditions of the gastrointestinal tract, such as low pH and bile salts, the demonstrated safety for host consumption, the proven efficacy against targeted pathogenic bacteria, the ability to efficiently colonize the intestinal epithelium, and the maintenance of genotypic and phenotypic stability [29,68]. Probiotics could exert their beneficial effects through different mechanisms of action, as illustrated in Figure 2. They can adhere to the gut mucosa and exclude pathogenic bacteria from adhesion sites in the gut. They strengthen the gut lining and mucosal integrity, preventing harmful substances and toxins from invading the bloodstream. They maintain a healthy balance of beneficial gut microbiota, as well as increasing the bioavailability and absorption of micro- and macro-nutrients. Probiotics can also produce antimicrobial compounds, such as bacteriocins, which suppress the growth of pathogenic microorganisms. Additionally, probiotics interact with intestinal epithelial cells, reducing the production of anti-inflammatory cytokines and facilitating the recruitment of mononuclear cells and macrophages [69,70,71,72]. Probiotics can be categorized into three types: single-strain probiotics, which contain only one bacterial strain; multi-strain probiotics, which include multiple strains of the same species; and multi-species probiotics, which consist of strains from different bacterial species combined [73,74]. The combination of multiple groups of probiotics tends to have a synergistic effect on each other as well as broaden the spectrum of activity against different pathogens [75]. Numerous studies have shown that specific probiotic strains from the genera Lactobacillus, Bifidobacterium, Streptococcus, Pediococcus, Enterococcus, and Bacillus possess the ability to suppress the growth and virulence of various pathogens, including Salmonella, as illustrated in Table 3. Despite the promising value and potential benefits of probiotics for controlling and treating Salmonella infection, their clinical application still faces several challenges. These include the ability of probiotics to withstand bile salts and gastric juice in the gastrointestinal (GI) tract [76,77], the risk of acquiring resistance genes from pathogenic microorganisms [78,79], and the potential for certain probiotic strains to produce toxic substances that may induce alimentary infection and intoxication [80,81,82], as well as safety concerns for immunocompromised individuals and those with underlying health conditions.
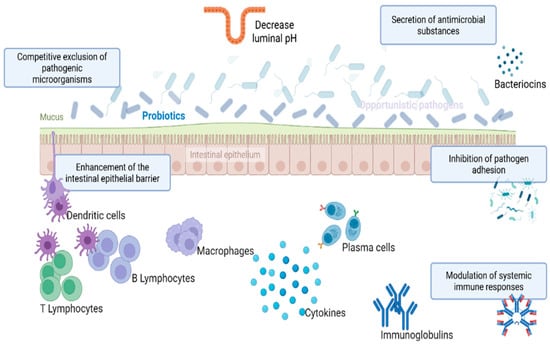
Figure 2.
Mechanism of action of probiotics. These include: (1) Competitive exclusion of pathogenic microorganisms via adhesion to epithelial surfaces and occupation of receptor sites; (2) Secretion of antimicrobial substances such as bacteriocins, organic acids, and hydrogen peroxide that inhibit pathogen growth; (3) Modulation of host immune responses, including enhancement of mucosal immunity, stimulation of secretory IgA production, and activation of innate immune cells; (4) Competition for nutrients and ecological niches, limiting the availability of resources for pathogenic species; (5) Strengthening of intestinal barrier integrity by promoting tight junction protein expression and reducing epithelial permeability.

Table 3.
Therapeutic and prophylactic effect of Probiotics against different Salmonella serovars in vitro and in different hosts.
2.4. Prebiotics
Prebiotics are non-digestible compounds that can promote the growth of beneficial bacteria. Prebiotics are often paired with probiotics to form synbiotics, a synergistic combination that significantly enhances therapeutic effectiveness against a range of pathogens [114]. There are different available prebiotics such as galacto-oligosaccharides (GOS), fructo-oligosaccharides (FOS), mannan-oligosaccharides (MOS), trans-galacto-oligosaccharides (TOS), xylo-oligosaccharides (XOS), arabinoxylo-oligosaccharides (AXOS), inulin, and lactulose [115,116]. These prebiotics are normally not digested by digestive enzymes, allowing them to stimulate the growth and activity of gut microbiota [72,117]. Prebiotics should have certain criteria to be effectively applied, such as resistance to the acidic environment of the gastrointestinal tract, susceptibility to fermentation by the gut microbiota, and the ability to selectively promote the activity and/or growth of beneficial bacterial populations [118]. The fermentation of prebiotics by gut microbiota leads to the production of short-chain fatty acids (SCFAs), including butyric acid, propionic acid, and lactic acid, which all play a crucial role in maintaining intestinal health and metabolic functions [115]. Short-chain fatty acids, produced through the fermentation of prebiotics by gut microbiota, can positively impact the host by stimulating the innate immune response and enhancing defense mechanisms against pathogenic microorganisms [119]. SCFAs can also influence the intestinal epithelial cell development [120]. Beyond their local effects within the gastrointestinal tract, the beneficial actions of prebiotics can extend to distant organs and systems, facilitated by the systemic bioavailability of their fermentation products, specifically SCFAs. Prebiotics have shown promising results in combating Salmonella infections in vitro and in vivo, particularly when combined with probiotics. It has been demonstrated that certain prebiotics inhibit the growth of Salmonella through direct or indirect stimulation of beneficial gut microbiota. Micciche et al., 2018 highlighted how oligosaccharides stimulate SCFA production, bolster microbial balance, and reduce pathogen load in poultry [121]. Additionally, Ismael et al., 2025 emphasized that prebiotics contribute to colonization resistance against drug-resistant Salmonella via microbial competition and immune modulation [122]. Khan et al., 2020 showed that compounds like inulin and galacto-oligosaccharides (GOS) enhance microbial diversity and strengthen mucosal barriers [65], while Deng and Wang reviewed how dietary fibers foster antimicrobial metabolite production and inhibit Salmonella through nutrient competition and quorum sensing disruption [123]. These beneficial bacteria can produce certain substances that suppress Salmonella growth and reduce its colonization in the gut [64]. Despite the beneficial effect of prebiotics to control Salmonella infection as illustrated in Table 4, there are some studies that have shown that certain prebiotics might even increase the severity of Salmonella infections under specific circumstances, potentially by impairing the gut barrier function or promoting the growth of certain pathogenic microorganisms [124]. For example, a study on mice fed diets with 10% FOS or XOS showed a significantly higher S. typhimurium count in the liver, spleen, and lymph nodes (p < 0.01) compared to controls [125].

Table 4.
Influence of various forms of prebiotics on different Salmonella serovars in different hosts.
2.5. Postbiotics
Postbiotics are bioactive compounds derived from microbial metabolism, including metabolites produced during fermentation, components released from inactivated (non-viable) microbial cells, and other microbial byproducts [134,135]. Unlike probiotics, which are live bacteria, postbiotics are beneficial byproducts of microbial activity. These compounds include short-chain fatty acids, enzymes, vitamins, and antimicrobial peptides, all of which contribute to gut health and overall well-being [136,137]. One of the most significant advantages of postbiotics is their role in immune system regulation. Certain postbiotics, such as butyrate, help stimulate the production of regulatory T cells, which control immune responses and reduce inflammation [138]. Additionally, postbiotics can enhance the gut barrier, preventing harmful bacteria from entering the bloodstream [139,140]. Since postbiotics are the final product of probiotic activity, they offer a safe and effective way to support gut health without the risks associated with live bacterial supplementation [137]. Importantly, postbiotics offer enhanced safety and stability, making them easier to handle and store compared to live probiotics [141]. Their inanimate nature eliminates concerns about viability, ensuring consistent effectiveness in various applications.
Postbiotics have demonstrated promising antimicrobial effects against Salmonella, particularly in applications related to food safety and gut health. Certain lactic acid bacteria-derived postbiotics, such as Lactobacillus, Bifidobacterium, and Pediococcus species, can inhibit the growth of Salmonella by producing a variety of bioactive compounds, such as SCFAs, bacteriocins and antimicrobial peptides [142,143,144]. These compounds help create an unfavorable environment for Salmonella, reducing its ability to colonize and cause infections. For example, Yin et al. found that weaned pigs fed on Lactobacillus-fermented feed have shown a significant reduction in S. typhimurium count by 50% in the spleens of pigs [145]. Additionally, Harris et al. investigated the effect of Saccharomyces cerevisiae fermentation products on the overall health performance of pre-weaned calves challenged with S. typhimurium [146]. They found that there was an improvement in fecal consistency and the immune status of the challenged calves [146]. Moreover, postbiotics have been explored as a natural intervention for controlling Salmonella contamination in poultry and eggs, offering an alternative to traditional disinfectants [147,148]. As research continues to validate their safety, stability, and efficacy, postbiotics stand poised to become a cornerstone of next-generation strategies for promoting gut health, enhancing immunity, and combating foodborne pathogens like Salmonella.
2.6. Antimicrobial Peptides (AMPs)
AMPs are emerging as a powerful alternative to conventional antibiotics, especially in the fight against MDR bacterial infections [149]. AMPs, typically ranging from 12 to 60 amino acids in length, are endogenous molecules present on mucosal surfaces and within tissues across diverse biological kingdoms, including humans, animals, and plants [150]. As integral components of the innate immune system, they exert broad-spectrum antimicrobial effects through multiple mechanisms of action [151,152].
AMPs have two primary mechanisms; (a) Membrane targeting mechanism through which AMPs may either align parallel to the cell membrane like a carpet (carpet-like model), or aggregate with each other and form channels that cause cytoplasmic leakage (barrel-stave model), or form a ring hole by vertically embedding in the bacterial cell membrane (toroidal pore model) [153,154,155]. (b) Non-membrane targeting mechanism through which AMPs target nucleic acid biosynthesis, protein biosynthesis, metabolic activities, nucleic acid replication and cell division [156,157]. There are many examples of AMPs that can ameliorate foodborne pathogens, including Salmonella. For example, AP2, an optimized version of native apidaecin (AP IB), has a protective effect against S. typhimurium infection in mice [158]. It ameliorated S. typhimurium infection by modulating the gut microbiota [158]. Another example is the 1018-K6 innate defense peptide that has been evaluated against S. enterica. It revealed a strong biofilm inhibition of several S. enterica strains using the sub-inhibitory concentrations [159]. Furthermore, antimicrobial peptides such as Kn2-5R-NH2, WK2, JH-3 and Bac7 (1–35) have been investigated a significant in vivo bactericidal effect against MDR-Salmonella strains [160,161,162,163]. Similarly, a preliminary in vivo study on WK2, a β-hairpin peptide, has demonstrated a significant reduction in S. typhimurium in the liver and spleen with inflammation relief and maintenance of intestinal mucosal integrity in mice [161]. Moreover, the thermostable modified cathelicidin-derived peptide P7 has demonstrated a significant antibacterial effect by reducing S. typhimurium viable cell counts to 103 and 104 CFU/mL within 2 to 4 h, respectively [164]. Mechanistically, P7 exerts its bactericidal effect by binding to the bacterial membrane, penetrating it, and accumulating within the cytoplasm. This interaction leads to membrane depolarization, permeabilization, and subsequent leakage of intracellular contents, culminating in rapid bacterial cell death [164]. Notably, a combination of a novel AMP derivative (A11) and nisin had a synergistic effect on drug-resistant S. typhimurium strains in vitro [165]. A11 destabilizes the bacterial membrane, increasing permeability, while Nisin binds to lipid II and forms pores, accelerating cell death. This dual action amplifies bacterial membrane damage, leading to a greater reduction in viable cell counts than either compound alone [165].
Another study on antimicrobial peptide HJH-3 has explored the significant antimicrobial effect against S. pullorum at higher peptide concentration [166]. The increased concentrations of HJH-3 led to a dose-dependent reduction in S. pullorum viability, indicating strong bactericidal activity [166]. Furthermore, a murine cathelicidin-related antimicrobial peptide (CRAMP) has increased macrophage expression and impaired S. typhimurium division and replication in vitro and in vivo by using an oxidase-dependent mechanism [167]. Additionally, the antimicrobial peptide Microcin J25 (MccJ25) has demonstrated significant efficacy in reducing Salmonella CVCC519 infection rates in broiler chickens. On day 42, challenged birds exhibited a 30% reduction in infection compared to the untreated control group [168]. Moreover, complete inhibition and elimination of S. typhimurium was observed in challenged mice treated with C-terminally hexahistidine-tagged A3-APO, loaded onto a gold nanoparticle DNA conjugate [169]. In addition, the antimicrobial peptide Css54 exhibits a potent growth inhibitory effect on S. typhimurium at a concentration of 6.25 μg/mL, with complete bactericidal activity achieved at 25 μg/mL [170].
Despite AMPs having proven efficacy in targeting bacterial pathogens, concerns remain regarding bacterial adaptation and resistance development. Some bacteria can modify their membrane composition or employ efflux pumps to counteract AMPs, reducing their efficacy over time. Additionally, certain peptides may inadvertently affect mammalian cells, leading to cytotoxic effects. So, optimizing AMP design through structural modifications and targeted delivery systems can help mitigate these challenges and enhance their therapeutic potential [153,154,155].
2.7. Essential Oils (EOs)
Essential oils consist of a complex mixture of volatile compounds that demonstrate considerable promise in biomedical applications. These compounds are derived from various plant parts, including leaves, flowers, stems, roots, bark, buds, and wood [171]. They exhibit potent antimicrobial properties and effectively suppress the growth of various bacteria, fungi, and viruses. Their efficacy is largely influenced by their chemical composition, the nature of their bioactive compounds, and the spatial orientation of their functional groups [172]. EOs can exhibit antimicrobial activity in its vapor phase without requiring direct contact with the pathogen [173].
EOs can be extracted using a range of techniques, such as steam distillation, solvent extraction, fermentation, and effleurage. The choice of method significantly affects the purity, chemical composition, and yield of the oil, thereby determining its suitability for specific plant materials and intended applications [174]. EOs demonstrates enhanced bioactivity when they exist in their active or oxygenated forms, as these molecular structures optimize their interactions with microbial cells, contributing to its antimicrobial, antioxidant, and therapeutic properties [175]. The antimicrobial activity of EOs is mediated through multiple mechanisms, including inhibition of biofilm formation, disruption of cell membrane integrity and permeability, interference with quorum-sensing pathways, suppression of bacterial protein synthesis, and impairment of cellular energy production [176,177,178]. These multifaceted actions contribute to their broad-spectrum antimicrobial potential [73,74]. Furthermore, the antimicrobial effectiveness of essential oils is influenced by the specific microbial species targeted, as different pathogens exhibit varying susceptibilities to the bioactive compounds within EOs [179,180]. Other factors, such as microbial cell structure, resistance mechanisms, and metabolic pathways, play a role in determining the extent of inhibition [181].
Essential oils are frequently utilized as natural preservatives in the food industry to inhibit the proliferation of foodborne pathogens, including E. coli, Salmonella, Listeria monocytogenes, and Campylobacter [178,182]. Their antimicrobial properties contribute to food safety by reducing contamination risks and extending the shelf life of products [183]. EOs derived from thyme, rosemary, oregano, clove, cinnamon, and curcuma are among the most widely recognized natural agents for combating antibiotic-resistant Salmonella and other pathogenic microorganisms [184]. Different studies evaluated the synergistic antimicrobial effect of combined EOs against Salmonella, as shown in Table 5.
While EOs have demonstrated significant antimicrobial activity against Salmonella spp., including S. typhimurium and S. enteritidis, their application requires careful consideration due to potential safety concerns. The high potency of EOs, particularly those from oregano, thyme, cinnamon, and clove, can lead to cytotoxic effects if not properly diluted, especially when used in food systems or biomedical formulations [185]. Additionally, variability in chemical composition due to plant origin, extraction method, and storage conditions can affect both efficacy and safety. Also, some EOs may also interact with host microbiota or immune responses, so their use in vulnerable populations such as young or immunocompromised hosts should be approached with caution.

Table 5.
Antimicrobial effect of essential oils against different Salmonella spp.
Table 5.
Antimicrobial effect of essential oils against different Salmonella spp.
| Essential Oils, EOs | Salmonella Serovars | Host | Observations | Ref. |
|---|---|---|---|---|
| EOs from leaves of Coriandrum sativum L. | S. typhi | In vitro | The oil exhibited strong antibacterial and antifungal activity against all tested strains, such as Staphylococcus aureus, Bacillus spp., E. coli, S. typhi, Klebsiella pneumoniae, Proteus mirabilis and Candida albicans, except Pseudomonas aeruginosa, which was resistant. Notably, a 65 × 102 μg concentration of EO resulted in a zone of inhibition (13.0 ± 1.4 mm) against S. typhi. | [186] |
| A blend of thyme EOs, savory, peppermint, and black pepper seeds | S. enteritidis | Broiler chickens | A microencapsulated blend of thyme essential oil (50%), savory (25%), peppermint (12.5%), and black pepper seeds (12.5%) caused a significant decrease in S. enteritidis population in broiler chickens. Specifically, the bacterial load in the ileum decreased by 2.1 log10 CFU/g, and in the cecum by 2.4 log10 CFU/g compared to the untreated infected group (p < 0.05). | [187] |
| EOs derived from the seeds of Foeniculum vulgar and Cuminum cyminum L. | S. typhimurium, E. coli | In vitro | F. vulgare and C. cyminum EOs induced zones of inhibition measuring 33 and 22 mm against S. typhimurium, respectively. In addition, the minimum inhibitory concentrations (MICs) were determined to be 0.031 mg/mL for F. vulgare and 0.125 mg/mL for C. cyminum. Furthermore, F. vulgare and C. cyminum EOs induced zones of inhibition measuring 28 and 17 mm against E. coli, respectively. In addition, the minimum inhibitory concentrations (MICs) were determined to be 0.062 mg/mL for F. vulgare and 0.250 mg/mL for C. cyminum | [188] |
| Carvacrol, eucalyptol, thymol and lemon EO blend | S. heidelberg | Broiler chickens | At a concentration of 0.05%, EOs significantly (p < 0.05) reduced S. heidelberg colonization in the crop of infected broilers. Additionally, lower concentrations (0.025% and 0.015%) showed no significant effect. However, EOs did not impact colonization in the ceca or fecal shedding, indicating their antimicrobial activity may be localized to specific regions of the gastrointestinal tract. | [189] |
| Zahter extract, zahter essential oil, laurel extract, and laurel essential oil | S. typhimurium | Chicken wings | The 0.4% laurel exhibited the strongest inhibitory effect against S. typhimurium, while the zahter showed comparatively lower antimicrobial activity. These findings suggest that laurel may possess superior bioactive compounds for bacterial suppression. | [190] |
| Satureja hortensis | S. enteritidis | In vitro | The disc diffusion assay showed that Salmonella had an average inhibition zone of 38 mm with a standard deviation of ±4 mm. in addition, both half and quarter concentrations of the MIC/2 and MIC/4 effectively suppressed biofilm formation by S. enteritidis. | [191] |
| Blend of cinnamaldehyde, thymol, citral, carvacrol, β-pinene and limonene | S. enteritidis | Chicken | Thymol, carvacrol, and cinnamaldehyde significantly inhibited S. enteritidis biofilm formation in a concentration-dependent manner. Thymol at MIC reduced biofilm formation as early as 12 h (p < 0.05), with stronger effects observed at 24 and 48 h. Carvacrol at MIC also showed consistent inhibition across all time points (p < 0.05), though lower concentrations (1/2 and 1/4 MIC) unexpectedly promoted biofilm formation at 12 and 24 h (p < 0.05). Cinnamaldehyde significantly reduced biofilm biomass at all concentrations after 24 and 48 h (p < 0.05). | [192] |
| EOs blend derived from Origanum vulgare, Thymus serpyllum, Thymus vulgaris, and Melaleuca alternifolia | 25 MDR Salmonella strains | In vitro | EOs of T. serpyllum and O. vulgare showed a significant antimicrobial activity against MDR-Salmonella strains. | [193] |
| Cinnamaldehyde, carvacrol, thymol, eugenol and citral | S. typhimurium, C. jejuni | In vitro | All tested compounds demonstrated strong bactericidal activity. The lowest concentration of trans-cinnamaldehyde (10 mM) significantly reduced (p ≤ 0.05) S. enteritidis populations by approximately 6.0 log10 CFU/mL after 8 h, and by more than 8.0 log10 CFU/mL after 24 h of incubation. At a concentration of 25 mM, trans-cinnamaldehyde eliminated detectable (p ≤ 0.05) S. enteritidis within 8 h. Furthermore, carvacrol and eugenol also significantly decreased (p ≤ 0.05) populations of S. enteritidis and C. jejuni to below 1.0 log10 CFU/mL at concentrations of 50 and 75 mM for carvacrol, and 20 and 30 mM for eugenol. | [194] |
2.8. Organic Acids (OAs)
Organic acids are carbon-based molecules composed of short- and medium-chain fatty acids. The chemical structure and acidic properties of OAs are essential for their antimicrobial and antifungal efficacy. OAs are categorized within the fatty acid classification and encompass a range of compounds, including acetic acid, lactic acid, formic acid, propionic acid, sorbic acid, butyric acid, benzoic acid, and citric acid [195]. These acids play essential roles in biochemical processes, food preservation, and antimicrobial applications. OAs can disrupt bacterial metabolic activities through lowering the intracellular pH, altering the bacterial cell membrane permeability, interfering with the bacterial energy production pathways, and interfering with bacterial DNA replication and transcription processes [73].
Studies have demonstrated that bacterial strains exhibit distinct sensitivity to various OAs, which can significantly influence their antimicrobial effectiveness. For instance, Salmonella enterica is particularly susceptible to formic and propionic acids, which disrupt membrane integrity and lower intracellular pH [196]. E. coli O157:H7 shows greater sensitivity to acetic acid than lactic acid, due to acetic acid’s superior ability to penetrate the cell membrane and acidify the cytoplasm [196]. Additionally, L. monocytogenes is effectively inhibited by lactic acid and citric acid, which interfere with its energy metabolism and membrane function [196]. Campylobacter jejuni is highly sensitive to medium-chain fatty acids like butyric and caprylic acids, which compromise membrane potential and ATP synthesis [197]. Meanwhile, Clostridium perfringens is notably affected by sorbic and benzoic acids, which hinder spore germination and vegetative growth [197]. These differences underscore the importance of selecting specific organic acids based on the target bacterial strain for optimal antimicrobial efficacy. Several studies have indicated that multi-chain fatty acids have more potent antimicrobial effects compared to short-chain fatty acids [198]. Furthermore, using organic acid blends provides a broader spectrum of activity than a single organic acid at a higher dose [199].
There are mixtures of different OAs that showed great efficacy in reducing the load of viable Salmonella bacterial cells in various animal species and food products [187]. For example, the organic acid blend, consisting of 0.024% tannic acid, 0.042% lactic acid, 0.048% butyric acid, and 0.048% acetic acid, demonstrated efficacy in reducing S. enteritidis levels by at least 1 log in broiler chicken (p = 0.05) [200]. This reduction suggests the potential of OAs as a microbial control strategy in poultry production. In addition, it had a significant effect in reducing S. enteritidis horizontal transmission among broiler chickens, whether administered solely or in combination with other probiotics (p = 0.05) [200]. Notably, pigs fed on OAs combined with Mannan-rich Copra Meal (OA/MCM) and OAs combined with Fermented Rye (OA/FR) diets exhibited significantly reduced S. typhimurium shedding post-infection (p < 0.05) [201]. Moreover, broiler chickens fed a blend of three different OAs (Sorbic acid, 25%; Thymol, 9.5%; Carvacrol, 2.5%) showed a reduction in S. heidelberg shedding and counts compared to the control birds [202]. Furthermore, administering esterified formic acid at 10 kg per 1000 L in water for five days before pig’s slaughter led to a 60% reduction in the proportion of Salmonella-shedding in pigs. Additionally, the odds of shedding Salmonella were 5.6 times higher in untreated pigs compared to those receiving treatment [203]. Another study comparing the efficacy of different OAs against S. enteritidis at the same concentration showed that capric acids and caprylic acids possessed bactericidal properties and eliminated S. enteritidis in vitro compared to other organic acids such as citric acid, fumaric acid, benzoic acid and acetic acid [204]. Moreover, a study reported a substantial reduction up to 2.5 log units in the bacterial counts of S. typhimurium, S. infantis, S. senftenberg, and S. putten when mash and rapeseed-based feed were supplemented with propionic acid, formic acid, and sodium format [205].
Despite their proven antimicrobial properties, the use of OAs in feed and water systems presents several limitations. One major challenge is their limited efficacy in the lower gastrointestinal tract, where Salmonella colonization typically occurs. This is due to the early absorption and metabolism of OAs in the upper digestive tract, which reduces their concentration at the site of infection. Additionally, high inclusion rates required for effective pathogen control can negatively impact feed palatability and animal performance and may even corrode feeding equipment. Moreover, OAs are primarily bacteriostatic, meaning they inhibit bacterial growth rather than eliminate pathogens, leaving room for recontamination during storage and transport. Finally, strain-specific resistance and the potential for microbial adaptation to acidic environments further complicate their long-term effectiveness.
2.9. Vaccines
Vaccines are biological preparations designed to stimulate the body’s immune system to recognize and combat specific infectious agents, such as viruses or bacteria [206]. They typically contain either attenuated (weakened) or inactivated forms of the pathogen, or purified components such as proteins, polysaccharides, or genetic material (e.g., mRNA or DNA) [207,208]. These elements serve as antigens that trigger an immune response, enabling the body to recognize and respond more effectively to future exposures to the actual pathogen [209,210]. Most vaccines do not completely prevent infection, but they are highly effective at reducing disease severity and complications. Additionally, they contribute to lowering the likelihood of disease transmission between hosts [211,212,213]. Their classification governs their mechanism of action. Live attenuated vaccines use a weakened form of a live virus or bacteria to stimulate the immune system and provide protection against a specific disease. They can stimulate humoral immunity and activate cytotoxic T-cells, resulting in long-lasting immunity [214,215]. Inactivated vaccines contain killed pathogens, so they cannot replicate or cause disease. They induce immunity without the risk of infection and may require multiple doses compared to live vaccines [216]. Subunit vaccines containing a specific part (proteins, polysaccharides, nucleic acids) of the pathogen, stimulating an immune response instead of the entire pathogen [217]. Additionally, conjugate vaccines are a combination of polysaccharide and a protein carrier. They combine the immunogenicity of protein carriers and the specificity of polysaccharides, providing effective and long-lasting protection [218]. Toxoid vaccines contain inactivated bacterial toxins that stimulate the immune response against specific diseases [219]. Outer Membrane Vesicle (OMV) vaccines consist of naturally released vesicular components derived from the bacterial outer membrane. These vesicles effectively stimulate an immune response while avoiding the induction of disease, making them a promising strategy for vaccine development [220]. Nucleic acid vaccines use genetic material (mRNA or DNA) to enhance cells to produce antigens and trigger the immune response [221]. Nanovaccines are a novel vaccine platform that utilizes nanoparticles (NPs) as adjuvants or delivery carriers. This approach facilitates precise targeting of infection sites while simultaneously enhancing systemic immune responses, improving vaccine efficacy and stability [222]. There are many examples of vaccines designed to target Salmonella in both humans and animals, as shown in Table 6. Despite the progressive development of vaccines targeting Salmonella. There are still different challenges include the extensive genetic diversity between Salmonella serovars [223], the potential for tolerance to toxoid vaccines, and the relatively low immunogenicity observed in outer membrane vesicle (OMV) vaccines [217,224].

Table 6.
Commercially available vaccines against Salmonellosis.
2.10. Bacteriophages
Bacteriophages are viruses that selectively infect and lyse bacterial cells, functioning as highly host-specific bacterial parasites. Due to their targeted antimicrobial activity, they are increasingly recognized as a promising alternative to conventional antimicrobials, contributing to enhanced food safety and security. This diversity in form and function underpins their versatility in biotechnology, diagnostics, and antimicrobial therapy. Bacteriophages can specifically infect and lyse bacterial cells through a highly targeted process, as illustrated in Figure 3. Upon encountering a susceptible host, the phage attaches to bacterial surface receptors using tail fibers or other specialized structures. It then injects its genetic material, either DNA or RNA into the bacterial cytoplasm, hijacking the host’s replication machinery to produce viral components [236]. In the lytic cycle, newly assembled phage particles accumulate until the host cell undergoes lysis, releasing progeny phages to infect neighboring bacteria. Alternatively, in the lysogenic cycle, the phage genome integrates into the host chromosome and replicates passively until triggered to enter the lytic phase [236,237]. For therapeutic purposes, only strictly lytic phages are considered suitable, as temperate phages capable of lysogeny may contribute to horizontal gene transfer and bacterial persistence, making them undesirable in clinical applications.
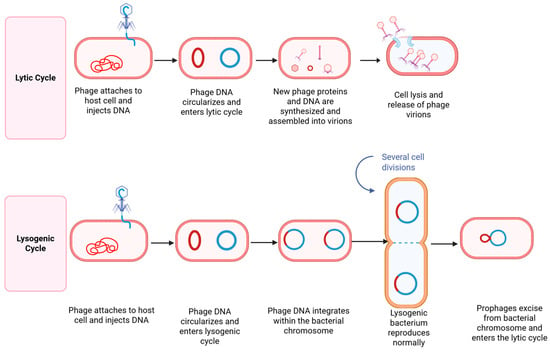
Figure 3.
Mechanism of action of bacteriophages via lytic and lysogenic cycles. Created with BioRender.com. In the lytic cycle, the phage attaches to the bacterial surface and injects its nucleic acid into the host. The viral genome commandeers the host’s replication machinery to synthesize phage components, assemble new virions, and produce lytic enzymes such as endolysins. These enzymes degrade the bacterial cell wall, resulting in cell lysis and the release of progeny phages to infect neighboring cells. In the lysogenic cycle, the phage genome integrates into the bacterial chromosome, forming a dormant prophage. This prophage replicates passively with the host genome during cell division.
Several factors influence the therapeutic efficacy of bacteriophages in treating bacterial infections. One key determinant is the phage host specificity, as phages must precisely match their bacterial targets to be effective. The timing and mode of administration, whether oral, topical, or intravenous, also impact outcomes, as does the multiplicity of infection (MOI), or the ratio of phages to bacteria. Environmental conditions such as pH, temperature, and the presence of biofilms can hinder phage access or activity. Additionally, immune system interactions may neutralize phages before they reach their targets, and bacterial resistance mechanisms can emerge, reducing long-term effectiveness. Other considerations include phage stability, distribution within the host, and the site of infection, all of which must be optimized for successful therapy [238,239]. Several studies identified bacteriophage cocktails as a promising tool against MDR pathogens, including Salmonella. A Salmonella-infected Galleria mellonella larva model was able to survive, and the Salmonella serovars including S. typhimurium, S. enteritidis, S. infantis, S. agona, S. derby, S. ohio, S. brandenburg and S. rissen were undetectable 24 h post administration of the three-phage cocktail [240]. Furthermore, the protective efficacy of Salmonella-specific bacteriophages and competitive exclusion products was assessed in chickens experimentally infected with S. typhimurium. Treatments with bacteriophages, competitive exclusion products, and their combination resulted in a marked reduction in S. typhimurium to undetectable levels in both the ileum and cecum, compared to untreated controls [241]. Similarly, an aerosol spray containing three distinct lytic bacteriophages, isolated from the sewage system of chicken flocks, demonstrated a significant reduction (72.7%) in the incidence of S. enteritidis (SE) infection within the challenged group compared to the control group [242]. Additionally, phage administration via drinking water or coarse spray effectively lowered intestinal S. enteritidis colonization, with statistically significant reductions (p < 0.05; p < 0.01, respectively) [242]. A higher Salmonella reduction was also observed after the spread of commercially available Salmonella bacteriophage on Salmonella-free skinless, boneless chicken meat inoculated with a cocktail of three Salmonella isolates [243]. Moreover, the bacteriophage cocktail SalmoFREE® has demonstrated effectiveness in significantly reducing the incidence of S. enteritidis in a commercial broiler farm. Importantly, this intervention did not negatively impact the production parameters of broiler chickens [244]. In experimentally contaminated feed, BAFASAL® reduced S. enteritidis counts by up to 2.5 log10 CFU/g under simulated crop conditions [245]. In in vivo trials, broiler chickens treated with BAFASAL® showed a significant reduction in S. enteritidis in the caeca, with bacterial counts dropping by approximately 1.5 to 2 log10 CFU/g compared to untreated controls [245]. Importantly, no signs of toxicity were observed, even at doses 100 times higher than the recommended level [245]. Additionally, A study investigating the survivability of microencapsulated and non-encapsulated Salmonella bacteriophages (BP FGS011) in the gastrointestinal tract found that microencapsulated phages exhibited enhanced resistance to harsh proventriculus-gizzard conditions in vitro compared to free phages. This suggests that microencapsulation may improve phage stability and effectiveness in targeting Salmonella within the digestive system [246]. Additionally, A study evaluated a six-phage cocktail for controlling the growth of Salmonella strains isolated from swine and poultry, tested in porcine (IPEC-1), avian (CHIC-8E11), and human (HT-29) epithelial cell cultures. Findings indicated that the optimized six-phage formulation demonstrated superior therapeutic potential when used as a prophylactic measure to reduce Salmonella colonization across porcine, human, and avian cell lines, outperforming coinfection and remedial treatment strategies [247]. Moreover, an in vitro study demonstrated reductions in S. enteritidis counts by 0.65, 1.49, and 0.58 log10 CFU/mL following a 3 h co-incubation with each of the three wild-type lytic bacteriophages (LBs) [248]. A significant reduction in the S. enteritidis count has also been observed after phage therapy in vivo [248]. Similarly, two Salmonella phages, S4lw and D5lw, demonstrated a 3.4–1.7 log10 reduction in S. typhimurium and S. enteritidis following 2–6 h of treatment [249]. Additionally, a survey assessing the sensitivity of 10 S. enteritidis and 10 S. typhimurium strains to a bacteriophage cocktail demonstrated a significant reduction in S. enteritidis and S. typhimurium colonization within the crop, liver, spleen, and caeca of broilers at 7, 14, and 21 days following bacteriophage application [250]. Moreover, Anisha et al. (2023) conducted an experimental trial to evaluate the efficacy of a bacteriophage cocktail delivered via feed in reducing S. typhimurium colonization in broiler chickens [166]. Birds treated with the phage cocktail exhibited a significant reduction in S. typhimurium levels compared to the challenged, untreated group (Salmonella was detected in fecal samples from the group receiving 105 PFU/day, while groups receiving 106 and 107 PFU/day showed residual counts of ~4 × 102 CFU/g, compared to ~3 × 104 CFU/g in untreated controls) [251]. Bacteriophages could be combined to make mixtures that can target most common infections. Bacteriophages also could be used alongside antibiotics, showing synergistic effects, especially against infections with antibiotic-resistant bacteria [252]. A significant inhibition of S. typhi isolated from stool samples of typhoid patients using a combination of ciprofloxacin and bacteriophages was reported. The Phage–antibiotic synergy was evident on agar plates, with a statistically significant enhancement of bacterial growth inhibition (p = 0.03) compared to either treatment [253]. Another study was done to evaluate phage-antibiotic combinations for the treatment of extended-spectrum β-lactamase-producing S. enteritidis. A synergistic effect was recorded at 0.25 × MIC of cefixime, gentamicin, ciprofloxacin, and aztreonam antibiotics in combination with phage at 104, 106 and 108 PFU/mL [254].
Despite the promising potential of bacteriophages as targeted therapeutics against MDR pathogens such as Salmonella, several challenges persist. Bacterial resistance to phages can emerge through mechanisms like receptor modification and CRISPR-Cas systems, compromising long-term efficacy. Moreover, the narrow host range of many phages limits their utility unless broad-spectrum cocktails are carefully formulated. Regulatory and safety concerns also pose hurdles, as standardized frameworks for phage therapy are still under development, and issues like endotoxin contamination and manufacturing consistency must be addressed. Therapeutic success is further influenced by delivery challenges, including phage survival in hostile environments such as acidic gastric conditions or biofilms, and their pharmacokinetics in vivo. Finally, the scarcity of large-scale clinical trials restricts the broader application of phages in both human and veterinary medicine, despite their demonstrated potential in preclinical studies [255,256,257,258].
2.11. Fecal Microbiota Transplantation (FMT)
Fecal microbiota transplantation (FMT) is a process of transferring fecal microorganisms from a healthy host into the intestinal tract of a diseased host [259]. This process can be done through multiple ways, such as colonoscopy, naso-jejunal tube, naso-duodenal tube, or retention enema [67]. FMT is considered a medicinal product in the United Kingdom, a biological product in North America, and a tissue product in different European regions [260]. The intestinal microbiota plays an important role in maintaining a healthy gut and protecting against microbial infections. The FMT approach aims to restore healthy and balanced gut microbiota after unintended disruption by any stressors like pathogenic infection, antibiotic treatment, or dietary change [261]. In November 2022, the FDA has formally approved the first microbiota-based therapy, Rebyota® (Ferring Pharmaceuticals), for preventing Clostridium difficile infection recurrence in adults [262,263]. The effect of FMT is multifactorial, involving the host, the microbiota, and the pathogen [264].
Several studies have been investigating the effect of FMT on the Salmonella pathogen in vitro and in vivo. For example, Wang et al., investigated the efficacy of FMT from specific pathogen-free (SPF) chickens against S. enteritidis infection in chicks. Their findings revealed that FMT successfully inhibited S. enteritidis colonization in the liver of challenged chicks. Additionally, FMT significantly increased the relative abundance of Parabacteroides and Bacteroides (p < 0.05), key bacterial genera associated with gut health [265]. The treatment also reduced IL-1β and IL-18 levels in serum, suggesting an anti-inflammatory effect. Notably, FMT improved liver inflammatory lesions, strengthened immune response, promoted weight gain, and enhanced overall resistance to SE infection [265]. Another study conducted by Soto et al. found that prolonged ertapenem administration followed by an encapsulated fecal transplant can cure immunocompromised individuals suffering from a GIT infection due to resistant S. infantis [264]. FMT is considered a safe approach, with minimal risks and complications; however, there are potential adverse outcomes, including diarrhea, gastrointestinal pain, constipation, and the transmission of pathogenic and antibiotic-resistant microorganisms [266].
2.12. Nanomaterials
Nanomaterials or nanoparticles are ultrafine particles that exhibit unique physical and chemical properties, strengthening their use in many biomedical and food quality industries [206]. There are different forms of nanomaterials, such as lipids, polymers, emulsions, proteins, nanobeads, inorganic nanomaterials, etc. [206]. These nanomaterials have been approved and successfully used for controlling infectious and non-infectious diseases. They have the capabilities to inhibit bacterial growth, reduce tissue inflammation, and boost the immune response [206].
Nanomaterials, such as silver nanoparticles (AgNPs), FeO nanoparticles, and chitosan, have shown a significant experimental effect on Salmonella infections in animals [267]. An experiment evaluating the antibacterial activity of silver nanoparticles against MDR-S. enteritidis showed that AgNPs have reduced the number of viable SE recovered from the challenged SE-infected mouse model [267]. It also noted that the average MIC and MBC of AgNPs were 0.085 ± 0.126 μg/mL and 0.508 ± 0.315 μg/mL, respectively [267]. Moreover, another research has demonstrated that Fe3O4 magnetic nanoparticles effectively inhibited the viability and invasion of intracellular S. enteritidis in chicken hepatocytes [268]. Fe3O4-NPs were administered orally at 50 mg/kg on days 2, 4, and 6 while S. enteritidis was inoculated on day 7. Fe3O4-NPs increased ROS production and antioxidant enzyme activity, resulting in a significant reduction of the invasion and colonization of S. enteritidis in chicken liver [268].
Additionally, an experimental study was done evaluating the effect of Nigella Sativa silver nanoparticles (NS AgNPs) on rats infected with Salmonella spp. [269]. It was indicated that NS AgNPs have reduced the severity of inflammatory responses caused by Salmonella. It also did not induce any significant changes in liver and kidney functions. Also, rats infected with Salmonella spp. and treated with NS AgNPs showed a marked decrease in colony-forming units compared to the untreated infected group [269]. Furthermore, Dolatyabi et al., proposed that orally administering S. enteritidis immunogenic outer membrane proteins and flagellin encapsulated in mannose chitosan nanoparticles (OMPs-FLA-mCS NPs) elicited cross-protective mucosal immune responses against S. typhimurium colonization in broilers [270]. It also significantly boosted IgA and IgY antibody secretion and resulted in a 0.8 log10 CFU reduction in S. typhimurium bacterial load within the cecal content [270]. Similarly, A study using Copper/Zinc-modified palygorskite (Cu/Zn-Pal) demonstrated that chickens infected with S. typhimurium and treated with Cu/Zn-Pal showed a significant reduction in intestinal colonization [271].
Nanomaterials have also been used as an adjuvant in Salmonella vaccines. Incorporating silicon dioxide, iron oxide, carboxymethyl chitosan, and iron oxide-chitosan nanomaterials in the vaccine has improved its efficacy against salmonellosis in chicken, as noted by Ibrahim et al. He found that the use of silicon dioxide SiNPs as a vaccine delivery system could enhance the immune response to Salmonella in chickens [272]. That study demonstrated that the use of adjuvanted vaccines with nanomaterials, particularly SiNPs, has significantly increased the protection rate from 67 to 93.3% when compared to the locally used vaccine, which had a protection rate of 83% [272].
Despite nanomaterials’ advantages, concerns remain regarding their long-term safety, potential cytotoxicity, and the possibility of developing bacterial resistance to nanomaterial-based therapies [273], comprehensive in vivo studies and rigorous toxicological assessments are essential to evaluate their biocompatibility, environmental impact, and therapeutic viability before widespread clinical application.
3. Potential of Genetic Engineering and CRISPR Technology
Genetic engineering and CRISPR technology hold significant potential for controlling Salmonella infections. CRISPR-Cas systems, which are adaptive immune systems found in many prokaryotes, can be engineered to target and edit nucleic acids with high precision [274,275]. This technology can be used to detect and control antibiotic-resistant Salmonella strains. It can be used to edit the Salmonella genome and potentially disable genes responsible for virulence or antibiotic resistance [276,277]. This approach can help in developing more effective treatments and preventive measures. In addition, CRISPR technology can be employed to combat antimicrobial resistance by re-sensitizing bacterial cells to antibiotics [278]. This can be particularly useful in treating infections caused by multidrug-resistant Salmonella. An interesting study was conducted through which the SpvB gene was deleted using CRISPR/Cas9 method from a virulent plasmid of S. gallinarum strain (SG18) [279]. The SpvB-deleted S. gallinarum strain, when tested for its virulence in broiler chickens, showed no clinical signs or mortality. It was also noted that S. gallinarum has lost its ability to invade from the intestine to the liver of broiler chickens [279]. However, despite its promise, the CRISPR-based approach faces several limitations and challenges. Of these challenges is the genetic diversity among circulating Salmonella strains, which makes it difficult to design universal CRISPR targets [280]. Editing each strain individually is impractical, especially in field conditions where multiple serovars may coexist. Additionally, efficient delivery of CRISPR components into bacterial populations remains a technical hurdle, particularly in vivo [281]. There are also concerns about off-target effects and the potential for horizontal gene transfer, which could inadvertently spread modified genetic material [282]. Regulatory and ethical considerations further complicate the deployment of gene-editing technologies in food animals. Therefore, while CRISPR offers exciting possibilities, its real-world application for controlling Salmonella infections requires careful evaluation and complementary strategies. Researchers are still exploring the use of Salmonella’s Endogenous CRISPR-Cas Systems to leverage the bacteria’s natural defense mechanisms to target and eliminate the pathogen [283,284].
4. Conclusions and Future Perspectives
Salmonella infections continue to pose a significant global health threat due to their zoonotic potential, environmental persistence, and increasing resistance to conventional antibiotics. The widespread misuse of antibiotics in both human and veterinary medicine has not only disrupted host microbiota but also accelerated the emergence of multidrug-resistant Salmonella strains, complicating treatment and control efforts. In light of these challenges, there is a growing shift toward alternative antimicrobial strategies that mitigate bacterial virulence without exerting the selective pressure typical of traditional antibiotics.
This review has explored a diverse array of promising alternatives—including probiotics, prebiotics, postbiotics, essential oils, organic acids, vaccines, small molecules, quorum sensing inhibitors, and fecal microbiota transplantation. These agents have demonstrated multifaceted mechanisms of action, such as inhibiting Salmonella colonization, biofilm formation, and toxin production, while also enhancing host immune responses. Importantly, several of these alternatives have shown synergistic effects when combined with conventional antibiotics, offering the potential to reduce antibiotic dosages and delay resistance development.
Despite encouraging progress, several challenges hinder the widespread adoption of these alternatives. These include variability in efficacy across Salmonella serovars and host species, lack of standardized formulations, safety concerns, and limited clinical validation. Regulatory barriers and gaps in mechanistic understanding further complicate their integration into routine practice.
Future research should prioritize well-controlled in vivo studies, mechanistic elucidation, and translational clinical trials to assess efficacy, safety, and long-term impacts on host health and microbial ecology. Moreover, integrating these strategies into food safety protocols, livestock management systems, and public health frameworks could significantly reduce the burden of MDR Salmonella. A multidisciplinary approach—bridging microbiology, pharmacology, veterinary science, and public health—is essential to fully harness the potential of these antimicrobial alternatives and advance toward sustainable, resistance-conscious disease control.
Author Contributions
Conceptualization, supervision, and funding, Y.A.H.; data curation, M.S. and Y.A.H.; original draft preparation, M.S., A.V., K.A.S. and Y.A.H.; Review and editing, M.S., A.V., K.A.S. and Y.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Center of Biomedical Research Excellence (COBRE) for Translational Chemical Biology (CTCB, NIH P20 GM130456), the National Center for Advancing Translational Sciences (KL2TR001996, UL1TR000117 and UL1TR001998), the National Institute of Food and Agriculture (USDA-NIFA 2022-09086 and 2023-38821-39584), and National Institutes of Health (R37 AI052218).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data was included in the published paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Helmy, Y.A.; El-Adawy, H.; Sanad, Y.M.; Ghanem, M. Editorial: Food safety and public health. Front. Microbiol. 2023, 14, 1169139. [Google Scholar] [CrossRef] [PubMed]
- Lamichhane, B.; Mawad, A.M.; Saleh, M.; Kelley, W.G.; Harrington, P.J.; Lovestad, C.W.; Amezcua, J.; Sarhan, M.M.; El Zowalaty, M.E.; Ramadan, H. Salmonellosis: An overview of epidemiology, pathogenesis, and innovative approaches to mitigate the antimicrobial resistant infections. Antibiotics 2024, 13, 76. [Google Scholar] [CrossRef]
- Oludairo, O.O.; Kwaga, J.K.; Kabir, J.; Abdu, P.A.; Gitanjali, A.; Perrets, A.; Cibin, V.; Lettini, A.; Aiyedun, J. A review on Salmonella characteristics, taxonomy, nomenclature with special reference to non-typhoidal and typhoidal salmonellosis. Zagazig Vet. J. 2022, 50, 161–176. [Google Scholar] [CrossRef]
- Helmy, Y.A.; El-Adawy, H.; Abdelwhab, E.M. A Comprehensive Review of Common Bacterial, Parasitic and Viral Zoonoses at the Human-Animal Interface in Egypt. Pathogens 2017, 6, 33. [Google Scholar] [CrossRef]
- Huang, C. Salmonella—Current Trends and Perspectives in Detection and Control: Current Trends and Perspectives in Detection and Control; IntechOpen: London, UK, 2024. [Google Scholar]
- Matrajt, G.; Lillis, L.; Meschke, J.S. Review of methods suitable for environmental surveillance of Salmonella Typhi and Paratyphi. Clin. Infect. Dis. 2020, 71, S79–S83. [Google Scholar] [CrossRef] [PubMed]
- Griffith, R.W.; Carlson, S.A.; Krull, A.C. Salmonellosis. In Diseases of Swine; Wiley: New York, NY, USA, 2019; pp. 912–925. [Google Scholar]
- Fahmy, N.A.; Karna, S.; Bhusal, A.; Kabir, A.; Erol, E.; Helmy, Y.A. Multidrug Resistance and Virulence Traits of Salmonella enterica Isolated from Cattle: Genotypic and Phenotypic Insights. Antibiotics 2025, 14, 689. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hossain, H.; Chowdhury, M.S.R.; Hossain, M.M.; Saleh, A.; Binsuwaidan, R.; Noreddin, A.; Helmy, Y.A.; El Zowalaty, M.E. Molecular Characterization of Multidrug-Resistant and Extended-Spectrum β-Lactamases-Producing Salmonella enterica Serovars Enteritidis and Typhimurium Isolated from Raw Meat in Retail Markets. Antibiotics 2024, 13, 586. [Google Scholar] [CrossRef]
- Bula-Rudas, F.J.; Rathore, M.H.; Maraqa, N.F. Salmonella infections in childhood. Adv. Pediatr. 2015, 62, 29–58. [Google Scholar] [CrossRef]
- Saggu, V.; Sajan, C.; Patel, K.; Mahant, M.; Besh, S. Salmonellosis: An Overview. Adv. Multidiscip. Res. Dev. 2023, 1, 129. [Google Scholar]
- He, Y.; Jia, Q.; Cai, K.; Xu, S.; Li, H.; Xie, Q.; Qiu, Y.; Zhang, L.; Jiao, X. The global, regional, and national burden of Invasive Non-typhoidal Salmonella (iNTS): An analysis from the Global Burden of Disease Study 1990–2021. PLoS Neglected Trop. Dis. 2025, 19, e0012960. [Google Scholar] [CrossRef]
- Bermudez-Aguirre, D.; Carter, J.; Niemira, B.A. An investigation about the historic global foodborne outbreaks of Salmonella spp. in eggs: From hatcheries to tables. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70202. [Google Scholar] [CrossRef] [PubMed]
- Seys, S.A.; Sampedro, F.; Hedberg, C.W. Assessment of meat and poultry product recalls due to Salmonella contamination: Product recovery and illness prevention. J. Food Prot. 2017, 80, 1288–1292. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Dewey-Mattia, D.; Subramhanya, S.; Cui, Z.; Griffin, P.M.; Lance, S.; Lanier, W.; Wise, M.E.; Crowe, S.J. Food recalls associated with foodborne disease outbreaks, United States, 2006–2016. Epidemiol. Infect. 2021, 149, e190. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, A.J.; Hargis, B.M.; Tsolis, R.M. Tracing the origins of Salmonella outbreaks. Science 2000, 287, 50–52. [Google Scholar] [CrossRef]
- Holst, M.M. Contributing Factors of Foodborne Illness Outbreaks—National Outbreak Reporting System, United States, 2014–2022. MMWR. Surveill. Summ. 2025, 74, 1–12. [Google Scholar] [CrossRef]
- Wang, J.; Sheng, H.; Xu, W.; Huang, J.; Meng, L.; Cao, C.; Zeng, J.; Meng, J.; Yang, B. Diversity of serotype, genotype, and antibiotic susceptibility of Salmonella prevalent in pickled ready-to-eat meat. Front. Microbiol. 2019, 10, 2577. [Google Scholar] [CrossRef]
- Helmy Yosra, A.; Kabir, A.; Saleh, M.; Kennedy Laura, A.; Burns, L.; Johnson, B. Draft genome sequence analysis of multidrug-resistant Salmonella enterica subsp. enterica serovar Mbandaka harboring colistin resistance gene mcr-9.1 isolated from foals in Kentucky, USA. Microbiol. Resour. Announc. 2024, 13, e00737-24. [Google Scholar] [CrossRef]
- Kabir, A.; Kelley, W.G.; Glover, C.; Erol, E.; Helmy, Y.A. Phenotypic and genotypic characterization of antimicrobial resistance and virulence profiles of Salmonella enterica serotypes isolated from necropsied horses in Kentucky. Microbiol. Spectr. 2025, 13, e0250124. [Google Scholar] [CrossRef]
- Kabir, A.; Lamichhane, B.; Habib, T.; Adams, A.; El-Sheikh Ali, H.; Slovis, N.M.; Troedsson, M.H.; Helmy, Y.A. Antimicrobial Resistance in Equines: A Growing Threat to Horse Health and Beyond—A Comprehensive Review. Antibiotics 2024, 13, 713. [Google Scholar] [CrossRef]
- Deblais, L.; Drozd, M.; Kumar, A.; Antwi, J.; Fuchs, J.; Khupse, R.; Helmy, Y.A.; Rajashekara, G. Identification of novel small molecule inhibitors of twin arginine translocation (Tat) pathway and their effect on the control of Campylobacter jejuni in chickens. Front. Microbiol. 2024, 15, 1342573. [Google Scholar] [CrossRef]
- Wilson, R.M.; Danishefsky, S.J. Small molecule natural products in the discovery of therapeutic agents: The synthesis connection. J. Org. Chem. 2006, 71, 8329–8351. [Google Scholar] [CrossRef] [PubMed]
- Vrisman, C.M.; Deblais, L.; Helmy, Y.A.; Johnson, R.; Rajashekara, G.; Miller, S.A. Discovery and characterization of low-molecular weight inhibitors of Erwinia tracheiphila. Phytopathology 2020, 110, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Deblais, L.; Rajashekara, G.; Miller, S.A.; Helmy, Y.A.; Zhang, H.; Wu, P.; Qiu, Y.; Xu, X. High-throughput screening reveals small molecule modulators inhibitory to Acidovorax citrulli. Plant Pathol. 2020, 69, 818–826. [Google Scholar] [CrossRef]
- Scott, D.E.; Bayly, A.R.; Abell, C.; Skidmore, J. Small molecules, big targets: Drug discovery faces the protein–protein interaction challenge. Nat. Rev. Drug Discov. 2016, 15, 533–550. [Google Scholar] [CrossRef]
- Tirkkonen, H.; Brown, K.V.; Niemczura, M.; Faudemer, Z.; Brown, C.; Ponomareva, L.V.; Helmy, Y.A.; Thorson, J.S.; Nybo, S.E.; Metsä-Ketelä, M.; et al. Engineering bioBricks for deoxysugar biosynthesis and generation of new tetracenomycins. ACS Omega 2023, 8, 21237–21253. [Google Scholar] [CrossRef]
- Li, Q.; Kang, C. Mechanisms of action for small molecules revealed by structural biology in drug discovery. Int. J. Mol. Sci. 2020, 21, 5262. [Google Scholar] [CrossRef]
- Kathayat, D.; Helmy Yosra, A.; Deblais, L.; Srivastava, V.; Closs, G.; Khupse, R.; Rajashekara, G. Novel Small Molecule Growth Inhibitor Affecting Bacterial Outer Membrane Reduces Extraintestinal Pathogenic Escherichia coli (ExPEC) Infection in Avian Model. Microbiol. Spectr. 2021, 9, e00006-21. [Google Scholar] [CrossRef]
- Peixoto, R.J.; Alves, E.S.; Wang, M.; Ferreira, R.B.; Granato, A.; Han, J.; Gill, H.; Jacobson, K.; Lobo, L.A.; Domingues, R.M. Repression of Salmonella host cell invasion by aromatic small molecules from the human fecal metabolome. Appl. Environ. Microbiol. 2017, 83, e01148-17. [Google Scholar] [CrossRef]
- Sandala, J.L.; Eichar, B.W.; Kuo, L.G.; Hahn, M.M.; Basak, A.K.; Huggins, W.M.; Woolard, K.; Melander, C.; Gunn, J.S. A dual-therapy approach for the treatment of biofilm-mediated Salmonella gallbladder carriage. PLoS Pathog. 2020, 16, e1009192. [Google Scholar] [CrossRef]
- Nesterenko, L.N.; Zigangirova, N.A.; Zayakin, E.S.; Luyksaar, S.I.; Kobets, N.V.; Balunets, D.V.; Shabalina, L.A.; Bolshakova, T.N.; Dobrynina, O.Y.; Gintsburg, A.L. A small-molecule compound belonging to a class of 2, 4-disubstituted 1, 3, 4-thiadiazine-5-ones suppresses Salmonella infection in vivo. J. Antibiot. 2016, 69, 422–427. [Google Scholar] [CrossRef]
- Tsai, C.N.; MacNair, C.R.; Cao, M.P.; Perry, J.N.; Magolan, J.; Brown, E.D.; Coombes, B.K. Targeting two-component systems uncovers a small-molecule inhibitor of Salmonella virulence. Cell Chem. Biol. 2020, 27, 793–805.e7. [Google Scholar] [CrossRef]
- Moshiri, J.; Kaur, D.; Hambira, C.M.; Sandala, J.L.; Koopman, J.A.; Fuchs, J.R.; Gunn, J.S. Identification of a small molecule anti-biofilm agent against Salmonella enterica. Front. Microbiol. 2018, 9, 2804. [Google Scholar] [CrossRef] [PubMed]
- Nagy, T.A.; Quintana, J.L.; Reens, A.L.; Crooks, A.L.; Detweiler, C.S. Autophagy induction by a small molecule inhibits Salmonella survival in macrophages and mice. Antimicrob. Agents Chemother. 2019, 63, 10-1128. [Google Scholar] [CrossRef] [PubMed]
- Deblais, L.; Helmy, Y.A.; Kathayat, D.; Huang, H.-c.; Miller, S.A.; Rajashekara, G. Novel imidazole and methoxybenzylamine growth inhibitors affecting Salmonella cell envelope integrity and its persistence in chickens. Sci. Rep. 2018, 8, 13381. [Google Scholar] [CrossRef] [PubMed]
- Deblais, L.; Vrisman, C.; Kathayat, D.; Helmy, Y.A.; Miller, S.A.; Rajashekara, G. Imidazole and Methoxybenzylamine Growth Inhibitors Reduce Salmonella Persistence in Tomato Plant Tissues. J. Food Prot. 2019, 82, 997–1006. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L.; Xu, J.; Liu, S.; Song, Z.; Chen, T.; Deng, X.; Wang, J.; Lv, Q. Quercitrin is a novel inhibitor of Salmonella enterica Serovar Typhimurium type III secretion system. Molecules 2023, 28, 5455. [Google Scholar] [CrossRef]
- Koopman, J.A.; Marshall, J.M.; Bhatiya, A.; Eguale, T.; Kwiek, J.J.; Gunn, J.S. Inhibition of Salmonella enterica biofilm formation using small-molecule adenosine mimetics. Antimicrob. Agents Chemother. 2015, 59, 76–84. [Google Scholar] [CrossRef]
- Wu, K.; Kwon, S.H.; Zhou, X.; Fuller, C.; Wang, X.; Vadgama, J.; Wu, Y. Overcoming Challenges in Small-Molecule Drug Bioavailability: A Review of Key Factors and Approaches. Int. J. Mol. Sci. 2024, 25, 13121. [Google Scholar] [CrossRef]
- Bedard, P.L.; Hyman, D.M.; Davids, M.S.; Siu, L.L. Small molecules, big impact: 20 years of targeted therapy in oncology. Lancet 2020, 395, 1078–1088. [Google Scholar] [CrossRef]
- Smith, A.J. New horizons in therapeutic antibody discovery: Opportunities and challenges versus small-molecule therapeutics. J. Biomol. Screen. 2015, 20, 437–453. [Google Scholar] [CrossRef]
- Zigangirova, N.A.; Nesterenko, L.N.; Sheremet, A.B.; Soloveva, A.V.; Luyksaar, S.I.; Zayakin, E.S.; Balunets, D.V.; Gintsburg, A.L. Fluorothiazinon, a small-molecular inhibitor of T3SS, suppresses Salmonella oral infection in mice. J. Antibiot. 2021, 74, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Boonyom, R.; Roytrakul, S.; Thinwang, P. A small molecule, C24H17ClN4O2S, inhibits the function of the type III secretion system in Salmonella typhimurium. J. Genet. Eng. Biotechnol. 2022, 20, 54. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef] [PubMed]
- Abisado, R.G.; Benomar, S.; Klaus, J.R.; Dandekar, A.A.; Chandler, J.R. Bacterial quorum sensing and microbial community interactions. MBio 2018, 9, 10-1128. [Google Scholar] [CrossRef]
- Hense, B.A.; Schuster, M. Core principles of bacterial autoinducer systems. Microbiol. Mol. Biol. Rev. 2015, 79, 153–169. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Kathayat, D.; Deblais, L.; Srivastava, V.; Closs, G., Jr.; Tokarski, R.J.; Ayinde, O.; Fuchs, J.R.; Rajashekara, G. Evaluation of novel quorum sensing inhibitors targeting auto-inducer 2 (AI-2) for the control of avian pathogenic Escherichia coli infections in chickens. Microbiol. Spectr. 2022, 10, e00286-22. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Deblais, L.; Kassem, I.I.; Kathayat, D.; Rajashekara, G. Novel small molecule modulators of quorum sensing in avian pathogenic Escherichia coli (APEC). Virulence 2018, 9, 1640–1657. [Google Scholar] [CrossRef]
- Mangwani, N.; Dash, H.R.; Chauhan, A.; Das, S. Bacterial quorum sensing: Functional features and potential applications in biotechnology. J. Mol. Microbiol. Biotechnol. 2012, 22, 215–227. [Google Scholar] [CrossRef]
- Kalia, V.C. Quorum sensing inhibitors: An overview. Biotechnol. Adv. 2013, 31, 224–245. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Kathayat, D.; Closs, G., Jr.; Galgozy, K.; Fuchs, J.R.; Rajashekara, G. Efficacy of quorum sensing and growth inhibitors alone and in combination against avian pathogenic Escherichia coli infection in chickens. Poult. Sci. 2023, 102, 102543. [Google Scholar] [CrossRef]
- Xu, Z.; Hu, Z.; Yu, Z.; Huang, L.; Yang, F.; Xie, Y. Virtual screening of quorum sensing inhibitors for Salmonella typhimurium and their application as preservatives in chicken breast. Food Biosci. 2023, 55, 102957. [Google Scholar] [CrossRef]
- Choi, J.; Shin, D.; Kim, M.; Park, J.; Lim, S.; Ryu, S. LsrR-mediated quorum sensing controls invasiveness of Salmonella typhimurium by regulating SPI-1 and flagella genes. PLoS ONE 2012, 7, e37059. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.S.; Thompson, J.A.; Xavier, K.B. AI-2-mediated signalling in bacteria. FEMS Microbiol. Rev. 2013, 37, 156–181. [Google Scholar] [CrossRef]
- Janssens, J.C.; Steenackers, H.; Robijns, S.; Gellens, E.; Levin, J.; Zhao, H.; Hermans, K.; De Coster, D.; Verhoeven, T.L.; Marchal, K. Brominated furanones inhibit biofilm formation by Salmonella enterica serovar Typhimurium. Appl. Environ. Microbiol. 2008, 74, 6639–6648. [Google Scholar] [CrossRef] [PubMed]
- Birhanu, B.T.; Park, N.-H.; Lee, S.-J.; Hossain, M.A.; Park, S.-C. Inhibition of Salmonella typhimurium adhesion, invasion, and intracellular survival via treatment with methyl gallate alone and in combination with marbofloxacin. Vet. Res. 2018, 49, 101. [Google Scholar] [CrossRef]
- Sivasankar, C.; Jha, N.K.; Ghosh, R.; Shetty, P.H. Anti quorum sensing and anti virulence activity of tannic acid and it’s potential to breach resistance in Salmonella enterica Typhi/Paratyphi A clinical isolates. Microb. Pathog. 2020, 138, 103813. [Google Scholar] [CrossRef]
- Styles, M.J.; Blackwell, H.E. Non-native autoinducer analogs capable of modulating the SdiA quorum sensing receptor in Salmonella enterica serovar Typhimurium. Beilstein J. Org. Chem. 2018, 14, 2651–2664. [Google Scholar] [CrossRef]
- Durães, F.; Resende, D.I.; Palmeira, A.; Szemerédi, N.; Pinto, M.M.; Spengler, G.; Sousa, E. Xanthones active against multidrug resistance and virulence mechanisms of bacteria. Antibiotics 2021, 10, 600. [Google Scholar] [CrossRef]
- Li, G.; Yan, C.; Xu, Y.; Feng, Y.; Wu, Q.; Lv, X.; Yang, B.; Wang, X.; Xia, X. Punicalagin inhibits Salmonella virulence factors and has anti-quorum-sensing potential. Appl. Environ. Microbiol. 2014, 80, 6204–6211. [Google Scholar] [CrossRef]
- Mechesso, A.F.; Yixian, Q.; Park, S.-C. Methyl gallate and tylosin synergistically reduce the membrane integrity and intracellular survival of Salmonella typhimurium. PLoS ONE 2019, 14, e0221386. [Google Scholar] [CrossRef]
- Shin, M.; Kim, K.; Shim, W.; Yang, J.W.; Lee, H. Tannic Acid as a Degradable Mucoadhesive Compound. ACS Biomater. Sci. Eng. 2016, 2, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.R.T.; Lou, Z.; Zhang, J.; Yu, F.; Timilsena, Y.P.; Zhang, C.; Zhang, Y.; Bakry, A.M. Star anise (Illicium verum Hook. f.) as quorum sensing and biofilm formation inhibitor on foodborne bacteria: Study in milk. J. Food Prot. 2017, 80, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Moore, R.J.; Stanley, D.; Chousalkar, K.K. The gut microbiota of laying hens and its manipulation with prebiotics and probiotics to enhance gut health and food safety. Appl. Environ. Microbiol. 2020, 86, e00600-20. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, B.; Delgado, S.; Blanco-Míguez, A.; Lourenço, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61, 1600240. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Taha-Abdelaziz, K.; Hawwas, H.A.E.-H.; Ghosh, S.; AlKafaas, S.S.; Moawad, M.M.; Saied, E.M.; Kassem, I.I.; Mawad, A.M. Antimicrobial resistance and recent alternatives to antibiotics for the control of bacterial pathogens with an emphasis on foodborne pathogens. Antibiotics 2023, 12, 274. [Google Scholar] [CrossRef]
- de Melo Pereira, G.V.; de Oliveira Coelho, B.; Júnior, A.I.M.; Thomaz-Soccol, V.; Soccol, C.R. How to select a probiotic? A review and update of methods and criteria. Biotechnol. Adv. 2018, 36, 2060–2076. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. Adv. Nutr. 2019, 10, S49–S66. [Google Scholar] [CrossRef]
- Abouelela, M.E.; Helmy, Y.A. Next-Generation Probiotics as Novel Therapeutics for Improving Human Health: Current Trends and Future Perspectives. Microorganisms 2024, 12, 430. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Kassem, I.I.; Rajashekara, G. Immuno-modulatory effect of probiotic E. coli Nissle 1917 in polarized human colonic cells against Campylobacter jejuni infection. Gut Microbes 2021, 13, 1857514. [Google Scholar] [CrossRef]
- Helmy Yosra, A.; Closs, G.; Jung, K.; Kathayat, D.; Vlasova, A.; Rajashekara, G. Effect of Probiotic E. coli Nissle 1917 Supplementation on the Growth Performance, Immune Responses, Intestinal Morphology, and Gut Microbes of Campylobacter jejuni Infected Chickens. Infect. Immun. 2022, 90, e00337-22. [Google Scholar] [CrossRef]
- McFarland, L.V. Efficacy of single-strain probiotics versus multi-strain mixtures: Systematic review of strain and disease specificity. Dig. Dis. Sci. 2021, 66, 694–704. [Google Scholar] [CrossRef]
- Ouwehand, A.C.; Invernici, M.M.; Furlaneto, F.A.; Messora, M.R. Effectiveness of multi-strain versus single-strain probiotics: Current status and recommendations for the future. J. Clin. Gastroenterol. 2018, 52, S35–S40. [Google Scholar] [CrossRef]
- Kwoji, I.D.; Aiyegoro, O.A.; Okpeku, M.; Adeleke, M.A. Multi-strain probiotics: Synergy among isolates enhances biological activities. Biology 2021, 10, 322. [Google Scholar] [CrossRef]
- Bezkorovainy, A. Probiotics: Determinants of survival and growth in the gut. Am. J. Clin. Nutr. 2001, 73, 399s–405s. [Google Scholar] [CrossRef] [PubMed]
- Stasiak-Różańska, L.; Berthold-Pluta, A.; Pluta, A.S.; Dasiewicz, K.; Garbowska, M. Effect of simulated gastrointestinal tract conditions on survivability of probiotic bacteria present in commercial preparations. Int. J. Environ. Res. Public Health 2021, 18, 1108. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Tomar, S.K.; Goswami, P.; Sangwan, V.; Singh, R. Antibiotic resistance among commercially available probiotics. Food Res. Int. 2014, 57, 176–195. [Google Scholar] [CrossRef]
- Zavišić, G.; Popović, M.; Stojkov, S.; Medić, D.; Gusman, V.; Jovanović Lješković, N.; Jovanović Galović, A. Antibiotic resistance and probiotics: Knowledge gaps, market overview and preliminary screening. Antibiotics 2023, 12, 1281. [Google Scholar] [CrossRef]
- Ripert, G.; Racedo, S.M.; Elie, A.-M.; Jacquot, C.; Bressollier, P.; Urdaci, M.C. Secreted compounds of the probiotic Bacillus clausii strain O/C inhibit the cytotoxic effects induced by Clostridium difficile and Bacillus cereus toxins. Antimicrob. Agents Chemother. 2016, 60, 3445–3454. [Google Scholar] [CrossRef]
- Zhu, K.; Hölzel, C.S.; Cui, Y.; Mayer, R.; Wang, Y.; Dietrich, R.; Didier, A.; Bassitta, R.; Märtlbauer, E.; Ding, S. Probiotic Bacillus cereus strains, a potential risk for public health in China. Front. Microbiol. 2016, 7, 718. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, S.; Ding, S.; Shen, J.; Zhu, K. Toxins and mobile antimicrobial resistance genes in Bacillus probiotics constitute a potential risk for One Health. J. Hazard. Mater. 2020, 382, 121266. [Google Scholar] [CrossRef]
- Tazehabadi, M.H.; Algburi, A.; Popov, I.V.; Ermakov, A.M.; Chistyakov, V.A.; Prazdnova, E.V.; Weeks, R.; Chikindas, M.L. Probiotic bacilli inhibit Salmonella biofilm formation without killing planktonic cells. Front. Microbiol. 2021, 12, 615328. [Google Scholar] [CrossRef] [PubMed]
- Cull, C.; Singu, V.K.; Cull, B.J.; Lechtenberg, K.F.; Amachawadi, R.G.; Schutz, J.S.; Bryan, K.A. Efficacy of Lactobacillus animalis and Propionibacterium freudenreichii-based feed additives in reducing Salmonella-associated health and performance effects in commercial beef calves. Antibiotics 2022, 11, 1328. [Google Scholar]
- Wang, L.; Wang, H.; Li, X.; Zhu, M.; Gao, D.; Hu, D.; Xiong, Z.; Li, X.; Qian, P. Bacillus velezensis HBXN2020 alleviates Salmonella typhimurium infection in mice by improving intestinal barrier integrity and reducing inflammation. eLife 2024, 13, RP93423. [Google Scholar] [CrossRef]
- Truusalu, K.; Naaber, P.; Kullisaar, T.; Tamm, H.; Mikelsaar, R.-H.; Zilmer, K.; Rehema, A.; Zilmer, M.; Mikelsaar, M. The influence of antibacterial and antioxidative probiotic lactobacilli on gut mucosa in a mouse model of Salmonella infection. Microb. Ecol. Health Dis. 2004, 16, 180–187. [Google Scholar]
- Junaid, M.; Lu, H.; Din, A.U.; Yu, B.; Liu, Y.; Li, Y.; Liu, K.; Yan, J.; Qi, Z. Deciphering microbiome, transcriptome, and metabolic interactions in the presence of probiotic Lactobacillus acidophilus against Salmonella typhimurium in a murine model. Antibiotics 2024, 13, 352. [Google Scholar] [CrossRef]
- Vinderola, G.; Matar, C.; Perdigón, G. Milk fermented by Lactobacillus helveticus R389 and its non-bacterial fraction confer enhanced protection against Salmonella enteritidis serovar Typhimurium infection in mice. Immunobiology 2007, 212, 107–118. [Google Scholar] [CrossRef]
- Okamoto, A.S.; Andreatti Filho, R.L.; Milbradt, E.L.; Moraes, A.C.I.; Vellano, I.H.B.; Guimarães-Okamoto, P.T.C. Bacterial communication between Lactobacillus spp. isolated from poultry in the inhibition of Salmonella Heidelberg—Proof of concept. Poult. Sci. 2018, 97, 2708–2712. [Google Scholar] [CrossRef]
- Hoepers, P.G.; Nunes, P.L.F.; Almeida-Souza, H.O.; Martins, M.M.; de Oliveira Carvalho, R.D.; Dreyer, C.T.; Aburjaile, F.F.; Sommerfeld, S.; Azevedo, V.; Fonseca, B.B. Harnessing probiotics capability to combat Salmonella Heidelberg and improve intestinal health in broilers. Poult. Sci. 2024, 103, 103739. [Google Scholar] [CrossRef]
- El-Sharkawy, H.; Tahoun, A.; Rizk, A.M.; Suzuki, T.; Elmonir, W.; Nassef, E.; Shukry, M.; Germoush, M.O.; Farrag, F.; Bin-Jumah, M. Evaluation of Bifidobacteria and Lactobacillus probiotics as alternative therapy for Salmonella typhimurium infection in broiler chickens. Animals 2020, 10, 1023. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, K.M.; Bhunia, A.K. Salmonella enterica serovar Typhimurium adhesion and cytotoxicity during epithelial cell stress is reduced by Lactobacillus rhamnosus GG. Gut Pathog. 2009, 1, 14. [Google Scholar] [CrossRef] [PubMed]
- De Keersmaecker, S.C.; Verhoeven, T.L.; Desair, J.; Marchal, K.; Vanderleyden, J.; Nagy, I. Strong antimicrobial activity of Lactobacillus rhamnosus GG against Salmonella typhimurium is due to accumulation of lactic acid. FEMS Microbiol. Lett. 2006, 259, 89–96. [Google Scholar] [CrossRef]
- Gill, H.S.; Shu, Q.; Lin, H.; Rutherfurd, K.J.; Cross, M.L. Protection against translocating Salmonella typhimurium infection in mice by feeding the immuno-enhancing probiotic Lactobacillus rhamnosus strain HN001. Med. Microbiol. Immunol. 2001, 190, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Shu, Q.; Lin, H.; Rutherfurd, K.J.; Fenwick, S.G.; Prasad, J.; Gopal, P.K.; Gill, H.S. Dietary Bifidobacterium lactis (HN019) enhances resistance to oral Salmonella typhimurium infection in mice. Microbiol. Immunol. 2000, 44, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.; Alinovi, C.; Couetil, L.; Glickman, L.; Wu, C. A randomized clinical trial using probiotics to prevent Salmonella faecal shedding in hospitalized horses. J. Equine Vet. Sci. 2004, 24, 242–247. [Google Scholar] [CrossRef]
- Tanner, S.A.; Chassard, C.; Rigozzi, E.; Lacroix, C.; Stevens, M.J. Bifidobacterium thermophilum RBL67 impacts on growth and virulence gene expression of Salmonella enterica subsp. enterica serovar Typhimurium. BMC Microbiol. 2016, 16, 46. [Google Scholar]
- Closs Jr, G.; Bhandari, M.; Helmy, Y.A.; Kathayat, D.; Lokesh, D.; Jung, K.; Suazo, I.D.; Srivastava, V.; Deblais, L.; Rajashekara, G. The probiotic Lacticaseibacillus rhamnosus GG supplementation reduces Salmonella load and modulates growth, intestinal morphology, gut microbiota, and immune responses in chickens. Infect. Immun. 2025, 93, e00420-24. [Google Scholar] [CrossRef]
- Sun, C.; Gao, X.; Sun, M.; Wang, Z.; Wang, Y.; Zhao, X.; Jia, F.; Zhang, T.; Ge, C.; Zhang, X. Protective effects of E. coli Nissle 1917 on chickens infected with Salmonella pullorum. Microb. Pathog. 2022, 172, 105768. [Google Scholar] [CrossRef]
- Rashid, M.; Narang, A.; Thakur, S.; Jain, S.K.; Kaur, S. Therapeutic and prophylactic effects of oral administration of probiotic Enterococcus faecium Smr18 in Salmonella enterica-infected mice. Gut Pathog. 2023, 15, 23. [Google Scholar] [CrossRef]
- Lan, D.; Xun, X.; Hu, Y.; Li, N.; Yang, C.; Jiang, X.; Liu, Y. Research on the Effect of Pediococcus pentosaceus on Salmonella enteritidis-Infected Chicken. BioMed Res. Int. 2020, 2020, 6416451. [Google Scholar] [CrossRef]
- Buddhasiri, S.; Sukjoi, C.; Kaewsakhorn, T.; Nambunmee, K.; Nakphaichit, M.; Nitisinprasert, S.; Thiennimitr, P. Anti-inflammatory effect of probiotic LimosiLactobacillus reuteri KUB-AC5 against Salmonella infection in a mouse colitis model. Front. Microbiol. 2021, 12, 716761. [Google Scholar]
- Silva, B.; Sandes, S.; Alvim, L.; Bomfim, M.; Nicoli, J.; Neumann, E.; Nunes, A. Selection of a candidate probiotic strain of Pediococcus pentosaceus from the faecal microbiota of horses by in vitro testing and health claims in a mouse model of Salmonella infection. J. Appl. Microbiol. 2017, 122, 225–238. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Zhen, W.; Guo, F.; Hu, Z.; Zhang, K.; Kong, L.; Guo, Y.; Wang, Z. Pretreatment with probiotics Enterococcus faecium NCIMB 11181 attenuated Salmonella typhimurium-induced gut injury through modulating intestinal microbiome and immune responses with barrier function in broiler chickens. J. Anim. Sci. Biotechnol. 2022, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- Mazkour, S.; Shekarforoush, S.S.; Basiri, S.; Nazifi, S.; Yektaseresht, A.; Honarmand, M. Effects of two probiotic spores of Bacillus species on hematological, biochemical, and inflammatory parameters in Salmonella typhimurium infected rats. Sci. Rep. 2020, 10, 8035. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.; Lei, J.; Liu, H.; Wang, D.; Liu, J.; Yang, F.; Chen, D. Bacillus pumilus SMU5927 protect mice from damage caused by Salmonella Enteritidis colonization. Life Sci. 2025, 361, 123291. [Google Scholar] [CrossRef]
- Zhang, R.; Li, Z.; Gu, X.; Zhao, J.; Guo, T.; Kong, J. Probiotic Bacillus subtilis LF11 protects intestinal epithelium against Salmonella infection. Front. Cell. Infect. Microbiol. 2022, 12, 837886. [Google Scholar] [CrossRef]
- Seo, H.-J.; Kang, S.-S. Inhibitory effect of bacteriocin produced by Pediococcus acidilactici on the biofilm formation of Salmonella typhimurium. Food Control 2020, 117, 107361. [Google Scholar] [CrossRef]
- Olsen, M.S.R.; Thøfner, I.; Sandvang, D.; Poulsen, L.L. Research Note: The effect of a probiotic E. faecium 669 mitigating Salmonella Enteritidis colonization of broiler chickens by improved gut integrity. Poult. Sci. 2022, 101, 102029. [Google Scholar] [CrossRef]
- Szabó, I.; Wieler, L.H.; Tedin, K.; Scharek-Tedin, L.; Taras, D.; Hensel, A.; Appel, B.; Nockler, K. Influence of a probiotic strain of Enterococcus faecium on Salmonella enterica serovar Typhimurium DT104 infection in a porcine animal infection model. Appl. Environ. Microbiol. 2009, 75, 2621–2628. [Google Scholar] [CrossRef]
- Siddique, A.; Azim, S.; Ali, A.; Adnan, F.; Arif, M.; Imran, M.; Ganda, E.; Rahman, A. Lactobacillus reuteri and Enterococcus faecium from poultry gut reduce mucin adhesion and biofilm formation of cephalosporin and fluoroquinolone-resistant Salmonella enterica. Animals 2021, 11, 3435. [Google Scholar] [CrossRef]
- Rodríguez-Sorrento, A.; Castillejos, L.; López-Colom, P.; Cifuentes-Orjuela, G.; Rodríguez-Palmero, M.; Moreno-Muñoz, J.A.; Martín-Orúe, S.M. Effects of Bifidobacterium longum subsp. infantis CECT 7210 and Lactobacillus rhamnosus HN001, combined or not with oligofructose-enriched inulin, on weaned pigs orally challenged with Salmonella typhimurium. Front. Microbiol. 2020, 11, 2012. [Google Scholar] [CrossRef]
- Bumbie, G.Z.; Abormegah, L.; Asiedu, P.; Oduro-Owusu, A.D.; Koranteng, A.A.-A.; Ansah, K.O.; Lamptey, V.K.; Chen, C.; Mohamed, T.M.; Tang, Z. Influence of Pediococcus pentosaceus GT001 on Performance, Meat Quality, Immune Function, Antioxidant and Cecum Microbial in Broiler Chickens Challenged by Salmonella typhimurium. Animals 2024, 14, 1676. [Google Scholar] [CrossRef] [PubMed]
- Khursheed, R.; Gulati, M.; Wadhwa, S.; Vishwas, S.; Sharma, D.S.; Corrie, L.; Alam, A.; Alnasser, S.M.; Alkhayl, F.F.A.; Parveen, Z. Multifaceted role of synbiotics as nutraceuticals, therapeutics and carrier for drug delivery. Chem. Biol. Interact. 2022, 368, 110223. [Google Scholar] [CrossRef] [PubMed]
- Davani-Davari, D.; Negahdaripour, M.; Karimzadeh, I.; Seifan, M.; Mohkam, M.; Masoumi, S.J.; Berenjian, A.; Ghasemi, Y. Prebiotics: Definition, types, sources, mechanisms, and clinical applications. Foods 2019, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- De Vrese, M.; Schrezenmeir. Probiotics, prebiotics, and synbiotics. Food Biotechnol. 2008, 111, 1–66. [Google Scholar]
- Markowiak, P.; Śliżewska, K. Effects of probiotics, prebiotics, and synbiotics on human health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Gibson, G.R.; Scott, K.P.; Rastall, R.A.; Tuohy, K.M.; Hotchkiss, A.; Dubert-Ferrandon, A.; Gareau, M.; Murphy, E.F.; Saulnier, D.; Loh, G. Dietary prebiotics: Current status and new definition. Food Sci. Technol. Bull. Funct. Foods 2010, 7, 1–19. [Google Scholar] [CrossRef]
- Clarke, T.B.; Davis, K.M.; Lysenko, E.S.; Zhou, A.Y.; Yu, Y.; Weiser, J.N. Recognition of peptidoglycan from the microbiota by Nod1 enhances systemic innate immunity. Nat. Med. 2010, 16, 228–231. [Google Scholar] [CrossRef]
- Hamer, H.M.; Jonkers, D.; Venema, K.; Vanhoutvin, S.; Troost, F.; Brummer, R.J. The role of butyrate on colonic function. Aliment. Pharmacol. Ther. 2008, 27, 104–119. [Google Scholar] [CrossRef]
- Micciche, A.C.; Foley, S.L.; Pavlidis, H.O.; McIntyre, D.R.; Ricke, S.C. A review of prebiotics against Salmonella in poultry: Current and future potential for microbiome research applications. Front. Vet. Sci. 2018, 5, 191. [Google Scholar] [CrossRef]
- Ismael, S.S.; Abdullah, B.H.; Abdulla, B.I. The Role of Probiotics and Prebiotics in Preventing Salmonellosis. In Holistic Health: Gut Microbiota and Holistic Health: The Role of Prebiotics and Probiotics; Unique Scientific Publishers: Faisalabad, Pakistan, 2025; pp. 155–160. [Google Scholar]
- Deng, L.; Wang, S. Colonization resistance: The role of gut microbiota in preventing Salmonella invasion and infection. Gut Microbes 2024, 16, 2424914. [Google Scholar] [CrossRef]
- ovee-Oudenhoven, I.; Ten Bruggencate, S.; Lettink-Wissink, M.; Van der Meer, R. Dietary fructo-oligosaccharides and lactulose inhibit intestinal colonisation but stimulate translocation of salmonella in rats. Gut 2003, 52, 1572–1578. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.; Heegaard, P.M.; Pedersen, A.L.; Andersen, J.B.; Sørensen, R.B.; Frøkiær, H.; Lahtinen, S.J.; Ouwehand, A.C.; Poulsen, M.; Licht, T.R. Some putative prebiotics increase the severity of Salmonella enterica serovar Typhimurium infection in mice. BMC Microbiol. 2009, 9, 245. [Google Scholar] [CrossRef] [PubMed]
- Donalson, L.; McReynolds, J.; Kim, W.; Chalova, V.; Woodward, C.; Kubena, L.; Nisbet, D.; Ricke, S. The influence of a fructooligosaccharide prebiotic combined with alfalfa molt diets on the gastrointestinal tract fermentation, Salmonella enteritidis infection, and intestinal shedding in laying hens. Poult. Sci. 2008, 87, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.L.; Keita, Å.V.; Parsons, B.N.; Prorok-Hamon, M.; Knight, P.; Winstanley, C.; Niamh, O.; Söderholm, J.D.; Rhodes, J.M.; Campbell, B.J. Soluble plantain fibre blocks adhesion and M-cell translocation of intestinal pathogens. J. Nutr. Biochem. 2013, 24, 97–103. [Google Scholar] [CrossRef]
- Badia, R.; Lizardo, R.; Martínez, P.; Brufau, J. Oligosaccharide structure determines prebiotic role of β-galactomannan against Salmonella enterica ser. Typhimurium in vitro. Gut Microbes 2013, 4, 72–75. [Google Scholar] [CrossRef]
- Letellier, A.; Messier, S.; Lessard, L.; Quessy, S. Assessment of various treatments to reduce carriage of Salmonella in swine. Can. J. Vet. Res. 2000, 64, 27. [Google Scholar]
- Andrés-Barranco, S.; Vico, J.P.; Grilló, M.J.; Mainar-Jaime, R.C. Reduction of subclinical Salmonella infection in fattening pigs after dietary supplementation with a ß-galactomannan oligosaccharide. J. Appl. Microbiol. 2015, 118, 284–294. [Google Scholar] [CrossRef]
- Ribeiro, A.M.L.; Vogt, L.K.; Canal, C.W.; Cardoso, M.d.I.; Labres, R.V.; Streck, A.F.; Bessa, M.C. Effects of prebiotics and probiotics on the colonization and immune response of broiler chickens challenged with Salmonella Enteritidis. Braz. J. Poult. Sci. 2007, 9, 193–200. [Google Scholar] [CrossRef]
- Pieper, R.; Bindelle, J.; Malik, G.; Marshall, J.; Rossnagel, B.G.; Leterme, P.; Van Kessel, A.G. Influence of different carbohydrate composition in barley varieties on Salmonella typhimurium var. Copenhagen colonisation in a “Trojan” challenge model in pigs. Arch. Anim. Nutr. 2012, 66, 163–179. [Google Scholar] [CrossRef]
- Babu, U.S.; Sommers, K.; Harrison, L.M.; Balan, K.V. Effects of fructooligosaccharide-inulin on Salmonella-killing and inflammatory gene expression in chicken macrophages. Vet. Immunol. Immunopathol. 2012, 149, 92–96. [Google Scholar] [CrossRef]
- Martyniak, A.; Medyńska-Przęczek, A.; Wędrychowicz, A.; Skoczeń, S.; Tomasik, P.J. Prebiotics, probiotics, synbiotics, paraprobiotics and postbiotic compounds in IBD. Biomolecules 2021, 11, 1903. [Google Scholar] [CrossRef] [PubMed]
- Vinderola, G.; Sanders, M.E.; Cunningham, M.; Hill, C. Frequently asked questions about the ISAPP postbiotic definition. Front. Microbiol. 2024, 14, 1324565. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, S.; Li, S.; Jiang, W.; Wang, J.; Xiao, J.; Chen, T.; Ma, J.; Khan, M.Z.; Wang, W. Unlocking the power of postbiotics: A revolutionary approach to nutrition for humans and animals. Cell Metab. 2024, 36, 725–744. [Google Scholar] [CrossRef] [PubMed]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A step beyond pre-and probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Russo, E.; Giudici, F.; Fiorindi, C.; Ficari, F.; Scaringi, S.; Amedei, A. Immunomodulating activity and therapeutic effects of short chain fatty acids and tryptophan post-biotics in inflammatory bowel disease. Front. Immunol. 2019, 10, 2754. [Google Scholar] [CrossRef]
- Gao, J.; Li, Y.; Wan, Y.; Hu, T.; Liu, L.; Yang, S.; Gong, Z.; Zeng, Q.; Wei, Y.; Yang, W. A novel postbiotic from Lactobacillus rhamnosus GG with a beneficial effect on intestinal barrier function. Front. Microbiol. 2019, 10, 477. [Google Scholar] [CrossRef]
- Moradi, M.; Kousheh, S.A.; Almasi, H.; Alizadeh, A.; Guimarães, J.T.; Yılmaz, N.; Lotfi, A. Postbiotics produced by lactic acid bacteria: The next frontier in food safety. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3390–3415. [Google Scholar] [CrossRef]
- Benkowski, A.A.; Schmitt, E.; Williams, E.; Copple, C.; Legan, J.D. Postbiotics: Considerations for Safety and Quality Management; International Association of Food Protection: Toronto, ON, Canada, 2023. [Google Scholar]
- Noori, S.M.A.; Behfar, A.; Saadat, A.; Ameri, A.; Yazdi, S.S.A.; Siahpoosh, A. Antimicrobial and antioxidant properties of natural postbiotics derived from five lactic acid bacteria. Jundishapur J. Nat. Pharm. Prod. 2023, 18, e130785. [Google Scholar] [CrossRef]
- Rosales Cavaglieri, L.A.; Isgro, M.C.; Aminahuel, C.; Parada, J.; Poloni, V.L.; Montenegro, M.A.; Alonso, V.; Falcone, R.D.; Cavaglieri, L.R. Exploring the potential of lactic acid bacteria to produce postbiotics with antimicrobial and antioxidant properties: Focus on the probiotic strain Pediococcus pentosaceus RC007 for industrial-scale production. Int. J. Food Sci. Technol. 2025, 60, vvae003. [Google Scholar] [CrossRef]
- Bueno, E.B.T.; Silva, K.d.O.; Mendes, M.E.F.; de Oliveira, L.B.; Menezes, F.P.d.; Imperador, A.C.; Correia, L.F.; Winkelstroter, L.K. Postbiotics Derived from Lactic Acid Bacteria Fermentation: Therapeutic Potential in the Treatment of Muscular Complications in Inflammatory Bowel Disease. Fermentation 2025, 11, 362. [Google Scholar] [CrossRef]
- Yin, F.; Farzan, A.; Wang, Q.; Yu, H.; Yin, Y.; Hou, Y.; Friendship, R.; Gong, J. Reduction of Salmonella enterica serovar Typhimurium DT104 infection in experimentally challenged weaned pigs fed a Lactobacillus-fermented feed. Foodborne Pathog. Dis. 2014, 11, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.; Liang, Y.; Sharon, K.; Sellers, M.; Yoon, I.; Scott, M.; Carroll, J.; Ballou, M. Influence of Saccharomyces cerevisiae fermentation products, SmartCare in milk replacer and Original XPC in calf starter, on the performance and health of preweaned Holstein calves challenged with Salmonella enterica serotype Typhimurium. J. Dairy Sci. 2017, 100, 7154–7164. [Google Scholar] [CrossRef] [PubMed]
- Reddyvari, R.; Amalaradjou, M.A. Postbiotic Wash Treatments: A Novel Post-Harvest Approach to Reduce Salmonella and Enhance Egg Safety. Food Control 2025, 176, 111398. [Google Scholar] [CrossRef]
- Ruvalcaba-Gómez, J.M.; Villagrán, Z.; Valdez-Alarcón, J.J.; Martínez-Núñez, M.; Gomez-Godínez, L.J.; Ruesga-Gutiérrez, E.; Anaya-Esparza, L.M.; Arteaga-Garibay, R.I.; Villarruel-López, A. Non-antibiotics strategies to control Salmonella infection in poultry. Animals 2022, 12, 102. [Google Scholar] [CrossRef]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.-M.; Bechinger, B.; Naas, T. Antimicrobial peptides: A potent alternative to antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef]
- Johnstone, K.F.; Herzberg, M.C. Antimicrobial peptides: Defending the mucosal epithelial barrier. Front. Oral Health 2022, 3, 958480. [Google Scholar] [CrossRef]
- Zhang, H.; Lv, J.; Ma, Z.; Ma, J.; Chen, J. Advances in antimicrobial peptides: Mechanisms, design innovations, and biomedical potential. Molecules 2025, 30, 1529. [Google Scholar] [CrossRef]
- Kathayat, D.; Closs, G., Jr.; Helmy, Y.A.; Deblais, L.; Srivastava, V.; Rajashekara, G. In vitro and in vivo evaluation of Lacticaseibacillus rhamnosus GG and Bifidobacterium lactis Bb12 against avian pathogenic Escherichia coli and identification of novel probiotic-derived bioactive peptides. Probiotics Antimicrob. Proteins 2022, 14, 1012–1028. [Google Scholar] [CrossRef]
- Malkoski, M.; Dashper, S.G.; O’Brien-Simpson, N.M.; Talbo, G.H.; Macris, M.; Cross, K.J.; Reynolds, E.C. Kappacin, a novel antibacterial peptide from bovine milk. Antimicrob. Agents Chemother. 2001, 45, 2309–2315. [Google Scholar] [CrossRef]
- Papo, N.; Shai, Y. Can we predict biological activity of antimicrobial peptides from their interactions with model phospholipid membranes? Peptides 2003, 24, 1693–1703. [Google Scholar] [CrossRef]
- Lohner, K.; Prossnigg, F. Biological activity and structural aspects of PGLa interaction with membrane mimetic systems. Biochim. Et Biophys. Acta (BBA)-Biomembr. 2009, 1788, 1656–1666. [Google Scholar] [CrossRef]
- He, S.-w.; Zhang, J.; Li, N.-q.; Zhou, S.; Yue, B.; Zhang, M. A TFPI-1 peptide that induces degradation of bacterial nucleic acids, and inhibits bacterial and viral infection in half-smooth tongue sole, Cynoglossus semilaevis. Fish Shellfish Immunol. 2017, 60, 466–473. [Google Scholar] [CrossRef]
- Le, C.-F.; Fang, C.-M.; Sekaran, S.D. Intracellular targeting mechanisms by antimicrobial peptides. Antimicrob. Agents Chemother. 2017, 61, 10-1128. [Google Scholar] [CrossRef]
- Li, L.; Mo, Q.; Wan, Y.; Zhou, Y.; Li, W.; Li, W. Antimicrobial peptide AP2 ameliorates Salmonella typhimurium infection by modulating gut microbiota. BMC Microbiol. 2025, 25, 64. [Google Scholar] [CrossRef] [PubMed]
- Festa, R.; Ambrosio, R.L.; Lamas, A.; Gratino, L.; Palmieri, G.; Franco, C.M.; Cepeda, A.; Anastasio, A. A study on the antimicrobial and antibiofilm peptide 1018-K6 as potential alternative to antibiotics against food-pathogen Salmonella enterica. Foods 2021, 10, 1372. [Google Scholar] [CrossRef] [PubMed]
- Benincasa, M.; Pelillo, C.; Zorzet, S.; Garrovo, C.; Biffi, S.; Gennaro, R.; Scocchi, M. The proline-rich peptide Bac7 (1-35) reduces mortality from Salmonella typhimurium in a mouse model of infection. BMC Microbiol. 2010, 10, 178. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhang, D.; Cheng, Z.; Niu, Y.; Kong, L.; Lu, Z.; Bie, X. Designed symmetrical β-hairpin peptides for treating multidrug-resistant Salmonella typhimurium infections. Eur. J. Med. Chem. 2022, 243, 114769. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, Q.; Dong, M.; Song, H.; Hang, B.; Sun, Y.; Zhang, H.; Hu, J. Evaluation of the efficacy of the antimicrobial peptide HJH-3 in chickens infected with Salmonella pullorum. Front. Microbiol. 2023, 14, 1102789. [Google Scholar] [CrossRef]
- Mangmee, S.; Reamtong, O.; Kalambaheti, T.; Roytrakul, S.; Sonthayanon, P. Antimicrobial peptide modifications against clinically isolated antibiotic-resistant Salmonella. Molecules 2021, 26, 4654. [Google Scholar] [CrossRef]
- Klubthawee, N.; Aunpad, R. A thermostable, modified cathelicidin-derived peptide with enhanced membrane-active activity against Salmonella enterica serovar Typhimurium. Front. Microbiol. 2021, 11, 592220. [Google Scholar] [CrossRef]
- Sengkhui, S.; Klubthawee, N.; Aunpad, R. A novel designed membrane-active peptide for the control of foodborne Salmonella enterica serovar Typhimurium. Sci. Rep. 2023, 13, 3507. [Google Scholar] [CrossRef]
- Wang, Q.; Xu, Y.; Hu, J. Intracellular mechanism of antimicrobial peptide HJH-3 against Salmonella pullorum. RSC Adv. 2022, 12, 14485–14491. [Google Scholar] [CrossRef]
- Rosenberger, C.M.; Gallo, R.L.; Finlay, B.B. Interplay between antibacterial effectors: A macrophage antimicrobial peptide impairs intracellular Salmonella replication. Proc. Natl. Acad. Sci. USA 2004, 101, 2422–2427. [Google Scholar] [CrossRef]
- Wang, G.; Song, Q.; Huang, S.; Wang, Y.; Cai, S.; Yu, H.; Ding, X.; Zeng, X.; Zhang, J. Effect of antimicrobial peptide microcin J25 on growth performance, immune regulation, and intestinal microbiota in broiler chickens challenged with Escherichia coli and Salmonella. Animals 2020, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Yeom, J.-H.; Lee, B.; Kim, D.; Lee, J.-k.; Kim, S.; Bae, J.; Park, Y.; Lee, K. Gold nanoparticle-DNA aptamer conjugate-assisted delivery of antimicrobial peptide effectively eliminates intracellular Salmonella enterica serovar Typhimurium. Biomaterials 2016, 104, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Tuxpan-Pérez, A.; Ibarra-Valencia, M.A.; Estrada, B.E.; Clement, H.; Corrales-García, L.L.; Espino-Solis, G.P.; Corzo, G. Antimicrobial and immunomodulatory effects of selected chemokine and antimicrobial peptide on cytokine profile during Salmonella typhimurium infection in mouse. Antibiotics 2022, 11, 607. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: An updated review. Evid. Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar]
- Inouye, S.; Abe, S.; Yamaguchi, H.; Asakura, M. Comparative study of antimicrobial and cytotoxic effects of selected essential oils by gaseous and solution contacts. Int. J. Aromather. 2003, 13, 33–41. [Google Scholar] [CrossRef]
- Aromatics, N.D. A Comprehensive Guide to Essential Oil Extraction Methods. Available online: https://www.newdirectionsaromatics.com/blog/articles/how-essential-oils-are-made.html#maceration (accessed on 7 September 2025).
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef]
- Bouyahya, A.; Abrini, J.; Dakka, N.; Bakri, Y. Essential oils of Origanum compactum increase membrane permeability, disturb cell membrane integrity, and suppress quorum-sensing phenotype in bacteria. J. Pharm. Anal. 2019, 9, 301–311. [Google Scholar] [CrossRef]
- Cáceres, M.; Hidalgo, W.; Stashenko, E.; Torres, R.; Ortiz, C. Essential oils of aromatic plants with antibacterial, anti-biofilm and anti-quorum sensing activities against pathogenic bacteria. Antibiotics 2020, 9, 147. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. The inhibitory effect of plant essential oils on foodborne pathogenic bacteria in food. Crit. Rev. Food Sci. Nutr. 2019, 59, 3281–3292. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Méndez-Vilas, A. Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Formatex Research Center: Badajoz, Spain, 2011; Volume 1. [Google Scholar]
- Al-Maqtari, Q.A.; Rehman, A.; Mahdi, A.A.; Al-Ansi, W.; Wei, M.; Yanyu, Z.; Phyo, H.M.; Galeboe, O.; Yao, W. Application of essential oils as preservatives in food systems: Challenges and future prospectives—A review. Phytochem. Rev. 2021, 21, 1209–1246. [Google Scholar] [CrossRef]
- O’Brien, T.F. Emergence, spread, and environmental effect of antimicrobial resistance: How use of an antimicrobial anywhere can increase resistance to any antimicrobial anywhere else. Clin. Infect. Dis. 2002, 34, S78–S84. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Meng, X.; Li, Y.; Zhao, C.-N.; Tang, G.-Y.; Li, H.-B. Antibacterial and antifungal activities of spices. Int. J. Mol. Sci. 2017, 18, 1283. [Google Scholar] [CrossRef] [PubMed]
- Vidaković Knežević, S.; Kocić-Tanackov, S.; Kravić, S.; Knežević, S.; Vranešević, J.; Savić Radovanović, R.; Karabasil, N. In vitro antibacterial activity of some essential oils against Salmonella Enteritidis and Salmonella typhimurium isolated from meat. J. Food Saf. Food Qual. 2021, 72, 4–11. [Google Scholar] [CrossRef]
- Matasyoh, J.; Maiyo, Z.; Ngure, R.; Chepkorir, R. Chemical composition and antimicrobial activity of the essential oil of Coriandrum sativum. Food Chem. 2009, 113, 526–529. [Google Scholar] [CrossRef]
- Moharreri, M.; Vakili, R.; Oskoueian, E. Evaluation of microencapsulated essential oils in broilers challenged with Salmonella enteritidis: A focus on the body’s antioxidant status, gut microbiology, and morphology. Arch. Razi Inst. 2022, 77, 629. [Google Scholar] [PubMed]
- Bisht, D.S.; Menon, K.; Singhal, M.K. Comparative antimicrobial activity of essential oils of Cuminum cyminum L. and Foeniculum vulgare Mill. seeds against Salmonella typhimurium and Escherichia coli. J. Essent. Oil Bear. Plants 2014, 17, 617–622. [Google Scholar] [CrossRef]
- Alali, W.; Hofacre, C.L.; Mathis, G.; Faltys, G. Effect of essential oil compound on shedding and colonization of Salmonella enterica serovar Heidelberg in broilers. Poult. Sci. 2013, 92, 836–841. [Google Scholar] [CrossRef]
- Yilmaz, E.A.; Yalçin, H.; Polat, Z. Antimicrobial effects of laurel extract, laurel essential oil, zahter extract, and zahter essential oil on chicken wings contaminated with Salmonella typhimurium. Vet. Med. Sci. 2024, 10, e1445. [Google Scholar] [CrossRef]
- Seyedtaghiya, M.H.; Fasaei, B.N.; Peighambari, S.M. Antimicrobial and antibiofilm effects of Satureja hortensis essential oil against Escherichia coli and Salmonella isolated from poultry. Iran. J. Microbiol. 2021, 13, 74. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Li, T.; Chen, J.; Ye, Y. Inhibition of Salmonella Enteritidis by essential oil components and the effect of storage on the quality of chicken. Foods 2023, 12, 2560. [Google Scholar] [CrossRef] [PubMed]
- Listorti, V.; Battistini, R.; Ercolini, C.; Tramuta, C.; Razzuoli, E.; Vencia, W.; Decastelli, L.; Gallina, S.; Masotti, C.; Serracca, L. In vitro susceptibility of multidrug resistant strains of Salmonella to essential oils. Nat. Prod. Commun. 2020, 15, 1934578X19878904. [Google Scholar] [CrossRef]
- Johny, A.K.; Darre, M.; Donoghue, A.; Donoghue, D.; Venkitanarayanan, K. Antibacterial effect of trans-cinnamaldehyde, eugenol, carvacrol, and thymol on Salmonella Enteritidis and Campylobacter jejuni in chicken cecal contents in vitro. J. Appl. Poult. Res. 2010, 19, 237–244. [Google Scholar] [CrossRef]
- Doores, S. Organic acids. In Food Science and Technology; Marcel Dekker: New York, NY, USA, 2005; Volume 145, p. 91. [Google Scholar]
- Coban, H.B. Organic acids as antimicrobial food agents: Applications and microbial productions. Bioprocess Biosyst. Eng. 2020, 43, 569–591. [Google Scholar] [CrossRef]
- Guan, N.; Liu, L. Microbial response to acid stress: Mechanisms and applications. Appl. Microbiol. Biotechnol. 2020, 104, 51–65. [Google Scholar] [CrossRef]
- Van Immerseel, F.; Russell, J.; Flythe, M.; Gantois, I.; Timbermont, L.; Pasmans, F.; Haesebrouck, F.; Ducatelle, R. The use of organic acids to combat Salmonella in poultry: A mechanistic explanation of the efficacy. Avian Pathol. 2006, 35, 182–188. [Google Scholar] [CrossRef]
- Polycarpo, G.V.; Andretta, I.; Kipper, M.; Cruz-Polycarpo, V.C.; Dadalt, J.C.; Rodrigues, P.H.M.; Albuquerque, R.d. Meta-analytic study of organic acids as an alternative performance-enhancing feed additive to antibiotics for broiler chickens. Poult. Sci. 2017, 96, 3645–3653. [Google Scholar] [CrossRef]
- Jarquin, R.; Nava, G.; Wolfenden, A.; Donoghue, A.; Hanning, I.; Higgins, S.; Hargis, B. The evaluation of organic acids and probiotic cultures to reduce Salmonella enteriditis horizontal transmission and crop infection in broiler chickens. Int. J. Poult. Sci. 2007, 6, 182–186. [Google Scholar] [CrossRef][Green Version]
- Fabà, L.; Litjens, R.; Allaart, J.; van den Hil, P.R. Feed additive blends fed to nursery pigs challenged with Salmonella. J. Anim. Sci. 2020, 98, skz382. [Google Scholar] [CrossRef] [PubMed]
- Sausen, L.; Mendes, S.; Refatti Sikorski, R.d.F. Use of an organic acid blend to control the spread of Salmonella Heidelberg and improve broiler performance. Med. Vet. (UFRPE) 2022, 16, 49–58. [Google Scholar] [CrossRef]
- Bernad-Roche, M.; Marín-Alcalá, C.M.; Vico, J.P.; Mainar-Jaime, R.C. Salmonella Control in Fattening Pigs through the Use of Esterified Formic Acid in Drinking Water Shortly before Slaughter. Animals 2023, 13, 2814. [Google Scholar] [CrossRef]
- Van Immerseel, F.; De Buck, J.; Boyen, F.; Bohez, L.; Pasmans, F.; Volf, J.; Sevcik, M.; Rychlik, I.; Haesebrouck, F.; Ducatelle, R. Medium-chain fatty acids decrease colonization and invasion through hilA suppression shortly after infection of chickens with Salmonella enterica serovar Enteritidis. Appl. Environ. Microbiol. 2004, 70, 3582–3587. [Google Scholar] [CrossRef]
- Koyuncu, S.; Andersson, M.G.; Löfström, C.; Skandamis, P.N.; Gounadaki, A.; Zentek, J.; Häggblom, P. Organic acids for control of Salmonella in different feed materials. BMC Vet. Res. 2013, 9, 1–9. [Google Scholar] [CrossRef]
- Saleh, M.; El-Moghazy, A.; Elgohary, A.H.; Saber, W.I.; Helmy, Y.A. Revolutionizing Nanovaccines: A New Era of Immunization. Vaccines 2025, 13, 126. [Google Scholar] [CrossRef]
- Kallerup, R.S.; Foged, C. Classification of vaccines. In Subunit Vaccine Delivery; Springer: New York, NY, USA, 2014; pp. 15–29. [Google Scholar]
- Plotkin, S.A.; Plotkin, S.L. The development of vaccines: How the past led to the future. Nat. Rev. Microbiol. 2011, 9, 889–893. [Google Scholar] [CrossRef]
- Delany, I.; Rappuoli, R.; De Gregorio, E. Vaccines for the 21st century. EMBO Mol. Med. 2014, 6, 708–720. [Google Scholar] [CrossRef]
- Helmy, Y.A.; Fawzy, M.; Elaswad, A.; Sobieh, A.; Kenney, S.P.; Shehata, A.A. The COVID-19 pandemic: A comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J. Clin. Med. 2020, 9, 1225. [Google Scholar] [CrossRef]
- Rappuoli, R.; Pizza, M.; Del Giudice, G.; De Gregorio, E. Vaccines, new opportunities for a new society. Proc. Natl. Acad. Sci. USA 2014, 111, 12288–12293. [Google Scholar] [CrossRef]
- Elaish, M.; Ngunjiri, J.M.; Ali, A.; Xia, M.; Ibrahim, M.; Jang, H.; Hiremath, J.; Dhakal, S.; Helmy, Y.A.; Jiang, X. Supplementation of inactivated influenza vaccine with norovirus P particle-M2e chimeric vaccine enhances protection against heterologous virus challenge in chickens. PLoS ONE 2017, 12, e0171174. [Google Scholar] [CrossRef]
- Fawzy, M.; Helmy, Y.A. The one health approach is necessary for the control of Rift Valley fever infections in Egypt: A comprehensive review. Viruses 2019, 11, 139. [Google Scholar] [CrossRef]
- Zepp, F. Principles of vaccination. In Vaccine Design: Methods and Protocols: Volume 1: Vaccines for Human Diseases; Springer: New York, NY, USA, 2016; pp. 57–84. [Google Scholar]
- Francis, M.J. Recent advances in vaccine technologies. Vet. Clin. North Am. Small Anim. Pract. 2017, 48, 231. [Google Scholar] [CrossRef]
- Bordin, A.I.; Cohen, N.D. Types of vaccines. In Equine Clinical Immunology; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 279–288. ISBN 9781119086512. [Google Scholar]
- Moyle, P.M.; Toth, I. Modern subunit vaccines: Development, components, and research opportunities. ChemMedChem 2013, 8, 360–376. [Google Scholar] [CrossRef]
- Bröker, M.; Berti, F.; Schneider, J.; Vojtek, I. Polysaccharide conjugate vaccine protein carriers as a “neglected valency”–potential and limitations. Vaccine 2017, 35, 3286–3294. [Google Scholar] [CrossRef] [PubMed]
- Angsantikul, P.; Fang, R.H.; Zhang, L. Toxoid vaccination against bacterial infection using cell membrane-coated nanoparticles. Bioconjugate Chem. 2017, 29, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; MacLennan, C.A. Outer membrane vesicle vaccines. Semin. Immunol. 2020, 50, 101433. [Google Scholar] [CrossRef] [PubMed]
- Robinson, H.L. Nucleic acid vaccines: An overview. Vaccine 1997, 15, 785–787. [Google Scholar]
- Gheibi Hayat, S.M.; Darroudi, M. Nanovaccine: A novel approach in immunization. J. Cell. Physiol. 2019, 234, 12530–12536. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100. [Google Scholar] [CrossRef]
- Tekle, Y.I.; Nielsen, K.M.; Liu, J.; Pettigrew, M.M.; Meyers, L.A.; Galvani, A.P.; Townsend, J.P. Controlling antimicrobial resistance through targeted, vaccine-induced replacement of strains. PLoS ONE 2012, 7, e50688. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.W.; Smith, F.; Zuidhof, S.; Foster, D.M. Characterization of the serologic response induced by vaccination of late-gestation cows with a Salmonella Dublin vaccine. J. Dairy Sci. 2015, 98, 2529–2532. [Google Scholar] [CrossRef] [PubMed]
- Edrington, T.S.; Arthur, T.M.; Loneragan, G.H.; Genovese, K.J.; Hanson, D.L.; Anderson, R.C.; Nisbet, D.J. Evaluation of two commercially-available Salmonella vaccines on Salmonella in the peripheral lymph nodes of experimentally-infected cattle. Ther. Adv. Vaccines Immunother. 2020, 8, 2515135520957760. [Google Scholar] [CrossRef] [PubMed]
- Muniz, E.C.; Verdi, R.; Leão, J.A.; Back, A.; Nascimento, V.P.d. Evaluation of the effectiveness and safety of a genetically modified live vaccine in broilers challenged with Salmonella Heidelberg. Avian Pathol. 2017, 46, 676–682. [Google Scholar] [CrossRef]
- Crouch, C.F.; Nell, T.; Reijnders, M.; Donkers, T.; Pugh, C.; Patel, A.; Davis, P.; van Hulten, M.C.; de Vries, S.P. Safety and efficacy of a novel inactivated trivalent Salmonella enterica vaccine in chickens. Vaccine 2020, 38, 6741–6750. [Google Scholar] [CrossRef]
- Huberman, Y.D.; Velilla, A.V.; Terzolo, H.R. Evaluation of different live Salmonella enteritidis vaccine schedules administered during layer hen rearing to reduce excretion, organ colonization, and egg contamination. Poult. Sci. 2019, 98, 2422–2431. [Google Scholar] [CrossRef]
- Renu, S.; Han, Y.; Dhakal, S.; Lakshmanappa, Y.S.; Ghimire, S.; Feliciano-Ruiz, N.; Senapati, S.; Narasimhan, B.; Selvaraj, R.; Renukaradhya, G.J. Chitosan-adjuvanted Salmonella subunit nanoparticle vaccine for poultry delivered through drinking water and feed. Carbohydr. Polym. 2020, 243, 116434. [Google Scholar] [CrossRef]
- Pavic, A.; Groves, P.J.; Cox, J.M. Utilization of a novel autologous killed tri-vaccine (serogroups B [Typhimurium], C [Mbandaka] and E [Orion]) for Salmonella control in commercial poultry breeders. Avian Pathol. 2010, 39, 31–39. [Google Scholar] [CrossRef]
- Wahid, R.; Simon, R.; Zafar, S.J.; Levine, M.M.; Sztein, M.B. Live oral typhoid vaccine Ty21a induces cross-reactive humoral immune responses against Salmonella enterica serovar Paratyphi A and S. paratyphi B in humans. Clin. Vaccine Immunol. 2012, 19, 825–834. [Google Scholar] [CrossRef]
- Levine, M.M.; Ferreccio, C.; Black, R.E.; Lagos, R.; Martin, O.S.; Blackwelder, W.C. Ty21a live oral typhoid vaccine and prevention of paratyphoid fever caused by Salmonella enterica Serovar Paratyphi B. Clin. Infect. Dis. 2007, 45, S24–S28. [Google Scholar] [CrossRef]
- Micoli, F.; Rondini, S.; Pisoni, I.; Giannelli, C.; Di Cioccio, V.; Costantino, P.; Saul, A.; Martin, L. Production of a conjugate vaccine for Salmonella enterica serovar Typhi from Citrobacter Vi. Vaccine 2012, 30, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Jossi, S.E.; Arcuri, M.; Alshayea, A.; Persaud, R.R.; Marcial-Juárez, E.; Palmieri, E.; Di Benedetto, R.; Pérez-Toledo, M.; Pillaye, J.; Channell, W.M. Vi polysaccharide and conjugated vaccines afford similar early, IgM or IgG-independent control of infection but boosting with conjugated Vi vaccines sustains the efficacy of immune responses. Front. Immunol. 2023, 14, 1139329. [Google Scholar] [CrossRef] [PubMed]
- Urban-Chmiel, R.; Pyzik, E. Selected Mechanisms of Action of Bacteriophages in Bacterial Infections in Animals. Viruses 2025, 17, 101. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Gaitero, M.; Seoane-Blanco, M.; van Raaij, M.J. Structure and function of bacteriophages. In Bacteriophages: Biology, Technology, Therapy; Springer: Cham, Switzerland, 2021; pp. 19–91. [Google Scholar]
- Ly-Chatain, M.H. The factors affecting effectiveness of treatment in phages therapy. Front. Microbiol. 2014, 5, 51. [Google Scholar] [CrossRef]
- Międzybrodzki, R.; Kasprzak, H.; Letkiewicz, S.; Rogóż, P.; Żaczek, M.; Thomas, J.; Górski, A. Pharmacokinetic and pharmacodynamic obstacles for phage therapy from the perspective of clinical practice. Clin. Infect. Dis. 2023, 77, S395–S400. [Google Scholar] [CrossRef]
- Nale, J.Y.; Vinner, G.K.; Lopez, V.C.; Thanki, A.M.; Phothaworn, P.; Thiennimitr, P.; Garcia, A.; AbuOun, M.; Anjum, M.F.; Korbsrisate, S. An optimized bacteriophage cocktail can effectively control Salmonella in vitro and in Galleria mellonella. Front. Microbiol. 2021, 11, 609955. [Google Scholar] [CrossRef]
- Toro, H.; Price, S.; McKee, S.; Hoerr, F.; Krehling, J.; Perdue, M.; Bauermeister, L. Use of bacteriophages in combination with competitive exclusion to reduce Salmonella from infected chickens. Avian Dis. 2005, 49, 118–124. [Google Scholar] [CrossRef]
- Borie, C.; Albala, I.; Sánchez, P.; Sánchez, M.; Ramírez, S.; Navarro, C.; Morales, M.; Retamales, J.; Robeson, J. Bacteriophage treatment reduces Salmonella colonization of infected chickens. Avian Dis. 2008, 52, 64–67. [Google Scholar] [CrossRef]
- Parveen, S.; Schwarz, J.; Hashem, F.; Vimini, B. Reduction of Salmonella in ground chicken using a bacteriophage. Poult. Sci. 2017, 96, 2845–2852. [Google Scholar] [CrossRef] [PubMed]
- Clavijo, V.; Baquero, D.; Hernandez, S.; Farfan, J.; Arias, J.; Arévalo, A.; Donado-Godoy, P.; Vives-Flores, M. Phage cocktail SalmoFREE® reduces Salmonella on a commercial broiler farm. Poult. Sci. 2019, 98, 5054–5063. [Google Scholar] [CrossRef] [PubMed]
- Wójcik, E.A.; Stańczyk, M.; Wojtasik, A.; Kowalska, J.D.; Nowakowska, M.; Łukasiak, M.; Bartnicka, M.; Kazimierczak, J.; Dastych, J. Comprehensive evaluation of the safety and efficacy of BAFASAL® bacteriophage preparation for the reduction of Salmonella in the food chain. Viruses 2020, 12, 742. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo-Rebenaque, L.; Malik, D.J.; Catalá-Gregori, P.; Marin, C.; Sevilla-Navarro, S. In vitro and in vivo gastrointestinal survival of non-encapsulated and microencapsulated Salmonella bacteriophages: Implications for bacteriophage therapy in poultry. Pharmaceuticals 2021, 14, 434. [Google Scholar] [CrossRef]
- Nale, J.Y.; Ahmed, B.; Haigh, R.; Shan, J.; Phothaworn, P.; Thiennimitr, P.; Garcia, A.; AbuOun, M.; Anjum, M.F.; Korbsrisate, S. Activity of a bacteriophage cocktail to control Salmonella growth ex vivo in Avian, Porcine, and human epithelial cell cultures. Phage 2023, 4, 11–25. [Google Scholar] [CrossRef]
- Vaz, C.S.L.; Voss-Rech, D.; Alves, L.; Coldebella, A.; Brentano, L.; Trevisol, I.M. Effect of time of therapy with wild-type lytic bacteriophages on the reduction of Salmonella Enteritidis in broiler chickens. Vet. Microbiol. 2020, 240, 108527. [Google Scholar] [CrossRef]
- Zhang, Y.; Chu, M.; Liao, Y.-T.; Salvador, A.; Wu, V.C. Characterization of two novel Salmonella phages having biocontrol potential against Salmonella spp. in gastrointestinal conditions. Sci. Rep. 2024, 14, 12294. [Google Scholar] [CrossRef]
- Pourabadeh, A.H.; Madani, S.A.; Dorostkar, R.; Rezaeian, M.; Esmaeili, H.; Bolandian, M.; Salavati, A.; Hashemian, S.M.M.; Aghahasani, A. Evaluation of the in vitro and in vivo efficiency of in-feed bacteriophage cocktail application to control Salmonella typhimurium and Salmonella Enteritidis infection in broiler chicks. Avian Pathol. 2024, 53, 174–181. [Google Scholar] [CrossRef]
- Thanki, A.M.; Hooton, S.; Whenham, N.; Salter, M.G.; Bedford, M.R.; O’Neill, H.V.M.; Clokie, M.R. A bacteriophage cocktail delivered in feed significantly reduced Salmonella colonization in challenged broiler chickens. Emerg. Microbes Infect. 2023, 12, 2217947. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Wang, Z.; Wei, J.; Hu, T.; Si, J.; Tao, G.; Zhang, L.; Xie, L.; Abdalla, A.E. A combination therapy of Phages and Antibiotics: Two is better than one. Int. J. Biol. Sci. 2021, 17, 3573. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Aslam, M.A.; Kanwar, R.; Mehmood, Z.; Arshad, M.I.; Hussain, S. Phage-antibiotic synergism against Salmonella typhi isolated from stool samples of typhoid patients. Ir. J. Med. Sci. (1971) 2024, 193, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, M.G.; Al-Hindi, R.R.; Alotibi, I.A.; Azhari, S.A.; Farsi, R.M.; Teklemariam, A.D. Evaluation of phage—Antibiotic combinations in the treatment of extended-spectrum β-lactamase-producing Salmonella enteritidis strain PT1. Heliyon 2023, 9, e13077. [Google Scholar] [CrossRef] [PubMed]
- Callaway, T.R.; Edrington, T.S.; Brabban, A.D.; Anderson, R.C.; Rossman, M.L.; Engler, M.J.; Carr, M.A.; Genovese, K.J.; Keen, J.E.; Looper, M.L. Bacteriophage isolated from feedlot cattle can reduce Escherichia coli O157: H7 populations in ruminant gastrointestinal tracts. Foodborne Pathog. Dis. 2008, 5, 183–191. [Google Scholar] [CrossRef]
- Segundo-Arizmendi, N.; Arellano-Maciel, D.; Rivera-Ramírez, A.; Piña-González, A.M.; López-Leal, G.; Hernández-Baltazar, E. Bacteriophages: A Challenge for Antimicrobial Therapy. Microorganisms 2025, 13, 100. [Google Scholar] [CrossRef]
- Thanki, A.M.; Hooton, S.P.; Thiennimitr, P.; Nale, J.Y. The role of bacteriophages in Salmonella diversity, pathogenicity and control. Front. Microbiol. 2025, 16, 1591189. [Google Scholar] [CrossRef]
- Esteves, N.C.; Bigham, D.N.; Scharf, B.E. Phages on filaments: A genetic screen elucidates the complex interactions between Salmonella enterica flagellin and bacteriophage Chi. PLoS Pathog. 2023, 19, e1011537. [Google Scholar] [CrossRef]
- Wang, J.-W.; Kuo, C.-H.; Kuo, F.-C.; Wang, Y.-K.; Hsu, W.-H.; Yu, F.-J.; Hu, H.-M.; Hsu, P.-I.; Wang, J.-Y.; Wu, D.-C. Fecal microbiota transplantation: Review and update. J. Formos. Med. Assoc. 2019, 118, S23–S31. [Google Scholar] [CrossRef]
- Merrick, B.; Allen, L.; Zain, N.M.M.; Forbes, B.; Shawcross, D.L.; Goldenberg, S.D. Regulation, risk and safety of faecal microbiota transplant. Infect. Prev. Pract. 2020, 2, 100069. [Google Scholar] [CrossRef]
- Ademe, M. Benefits of fecal microbiota transplantation: A comprehensive review. J. Infect. Dev. Ctries. 2020, 14, 1074–1080. [Google Scholar] [CrossRef]
- Nicco, C.; Paule, A.; Konturek, P.; Edeas, M. From donor to patient: Collection, preparation and cryopreservation of fecal samples for fecal microbiota transplantation. Diseases 2020, 8, 9. [Google Scholar] [CrossRef]
- Wang, Y.; Hunt, A.; Danziger, L.; Drwiega, E.N. A comparison of currently available and investigational fecal microbiota transplant products for recurrent clostridioides difficile infection. Antibiotics 2024, 13, 436. [Google Scholar] [CrossRef]
- Torres Soto, M.; Hammond, S.; Elshaboury, R.H.; Johnson, J.; Hohmann, E.L. Recurrent relatively resistant Salmonella infantis infection in 2 immunocompromised hosts cleared with prolonged antibiotics and fecal microbiota transplantation. Open Forum. Infect. Dis. 2019, 6, ofy334. [Google Scholar] [CrossRef]
- Wang, X.; Wu, X.; Cong, X.; Ren, J.; Li, J.; Zhu, J.; Dai, M.; Hrabchenko, N.; Du, Y.; Qi, J. The functional role of fecal microbiota transplantation on Salmonella Enteritidis infection in chicks. Vet. Microbiol. 2022, 269, 109449. [Google Scholar] [CrossRef] [PubMed]
- Baxter, M.; Colville, A. Adverse events in faecal microbiota transplant: A review of the literature. J. Hosp. Infect. 2016, 92, 117–127. [Google Scholar] [CrossRef]
- Farouk, M.M.; El-Molla, A.; Salib, F.A.; Soliman, Y.A.; Shaalan, M. The role of silver nanoparticles in a treatment approach for multidrug-resistant Salmonella species isolates. Int. J. Nanomed. 2020, 15, 6993–7011. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Xiao, Y.; Zhang, S.; Wu, S.; Gao, L.; Shi, S. Fe3O4 nanoparticles attenuated Salmonella infection in chicken liver through reactive oxygen and autophagy via PI3K/Akt/mTOR signaling. Front. Physiol. 2020, 10, 1580. [Google Scholar] [CrossRef] [PubMed]
- Abdalhamed, A.M.; Zeedan, G.S.; Dorgham, S.M.; Ghazy, A.A. In vivo experimentally study the effect of Nigella Sativa silver nanoparticles for treatment of Salmonella species causing diarrhea in ruminants. Microb. Pathog. 2023, 180, 106133. [Google Scholar] [CrossRef]
- Dolatyabi, S.; Renu, S.; Schrock, J.; Renukaradhya, G.J. Chitosan-nanoparticle-based oral Salmonella enteritidis subunit vaccine elicits cross-protection against Salmonella typhimurium in broilers. Poult. Sci. 2024, 103, 103569. [Google Scholar] [CrossRef]
- Zhang, C.; Yao, D.; Su, Z.; Chen, H.; Hao, P.; Liao, Y.; Guo, Y.; Yang, D. Copper/Zinc-modified palygorskite protects against Salmonella typhimurium infection and modulates the intestinal microbiota in chickens. Front. Microbiol. 2021, 12, 739348. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Mohammed, G.M.; Sayed, R.H.; Elshoky, H.A.; Elzorkany, H.E.; Elsaady, S.A. Efficacy improvement of tri-serotypes vaccine for Salmonella using nanomaterial-based adjuvant in chicken. Beni-Suef Univ. J. Basic Appl. Sci. 2024, 13, 18. [Google Scholar] [CrossRef]
- Abdallah, O.M.; Shebl, H.R.; Abdelsalam, E.; Mehrez, S.I. The impact and safety of encapsulated nanomaterials as a new alternative against carbapenem resistant bacteria. a systematic review. World J. Microbiol. Biotechnol. 2024, 40, 72. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Robledo, J.E.; Barrera, M.C.; Tobón, G.J. CRISPR/Cas: From adaptive immune system in prokaryotes to therapeutic weapon against immune-related diseases: CRISPR/Cas9 offers a simple and inexpensive method for disease modeling, genetic screening, and potentially for disease therapy. Int. Rev. Immunol. 2020, 39, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Marraffini, L.A. CRISPR-Cas systems: Prokaryotes upgrade to adaptive immunity. Mol. Cell 2014, 54, 234–244. [Google Scholar] [CrossRef]
- Balcha, F.B.; Neja, S.A. CRISPR-Cas9 mediated phage therapy as an alternative to antibiotics. Anim. Dis. 2023, 3, 4. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Al Fares, M.A.; Almaghaslah, M.; Alpakistany, T.; Al Kaabi, N.A.; Alshamrani, S.A.; Alshehri, A.A.; Almazni, I.A.; Saif, A.; Hakami, A.R. Application of CRISPR-cas system to mitigate superbug infections. Microorganisms 2023, 11, 2404. [Google Scholar] [CrossRef]
- Hwang, I.Y.; Koh, E.; Kim, H.R.; Yew, W.S.; Chang, M.W. Reprogrammable microbial cell-based therapeutics against antibiotic-resistant bacteria. Drug Resist. Updates 2016, 27, 59–71. [Google Scholar] [CrossRef]
- Basit, A.; Tahir, H.; Haider, Z.; Tariq, H.; Ullah, A.; Rehman, S.U. CRISPR/Cas9-based deletion of SpvB gene from Salmonella Gallinarum leads to loss of virulence in chicken. Front. Bioeng. Biotechnol. 2022, 10, 885227. [Google Scholar] [CrossRef]
- Bai, G.; You, L.; Long, L.; Wang, D.; Wang, M.; Wang, J.; Li, J.; Wei, X.; Li, S. The CRISPR genotypes and genetic diversity of different serogroups of nontyphoidal Salmonella in Guizhou Province, 2013–2018. PLoS ONE 2022, 17, e0278321. [Google Scholar] [CrossRef]
- Wei, T.; Cheng, Q.; Farbiak, L.; Anderson, D.G.; Langer, R.; Siegwart, D.J. Delivery of tissue-targeted scalpels: Opportunities and challenges for in vivo CRISPR/Cas-based genome editing. Acs Nano 2020, 14, 9243–9262. [Google Scholar] [CrossRef]
- Modrzejewski, D.; Hartung, F.; Lehnert, H.; Sprink, T.; Kohl, C.; Keilwagen, J.; Wilhelm, R. Which factors affect the occurrence of off-target effects caused by the use of CRISPR/Cas: A systematic review in plants. Front. Plant Sci. 2020, 11, 574959. [Google Scholar] [CrossRef]
- Wu, Y.; Battalapalli, D.; Hakeem, M.J.; Selamneni, V.; Zhang, P.; Draz, M.S.; Ruan, Z. Engineered CRISPR-Cas systems for the detection and control of antibiotic-resistant infections. J. Nanobiotechnol. 2021, 19, 401. [Google Scholar] [CrossRef]
- Wu, Q.; Cui, L.; Liu, Y.; Li, R.; Dai, M.; Xia, Z.; Wu, M. CRISPR-Cas systems target endogenous genes to impact bacterial physiology and alter mammalian immune responses. Mol. Biomed. 2022, 3, 22. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).