Comparing the Outcomes of Cefoperazone/Sulbactam-Based and Non-Cefoperazone/Sulbactam-Based Therapeutic Regimens in Patients with Multiresistant Acinetobacter baumannii Infections—A Meta-Analysis
Abstract
1. Introduction
2. Relevant Sections
2.1. Data Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Definition
2.5. Quality Assessment
2.6. Statistical Analysis
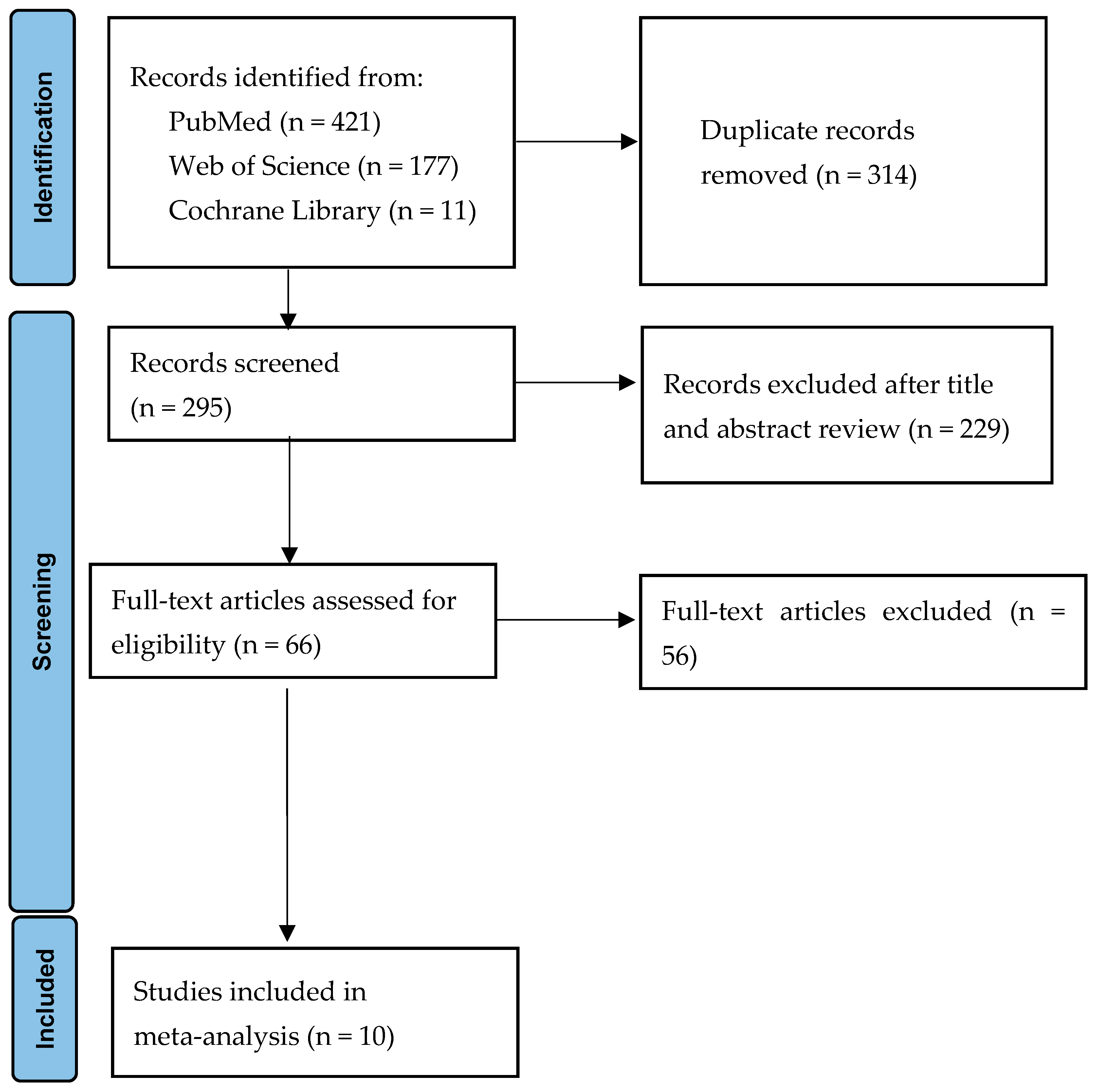
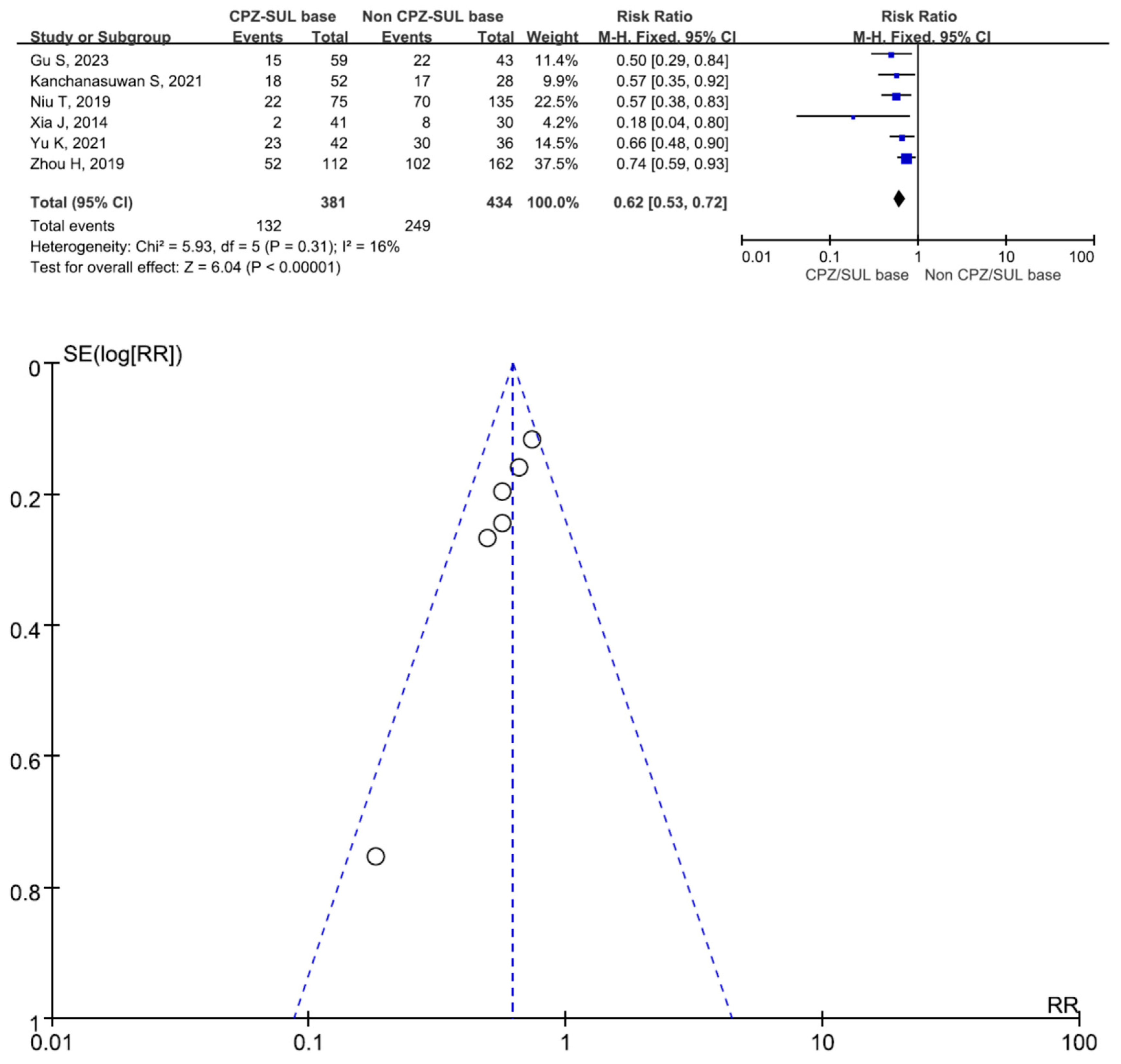
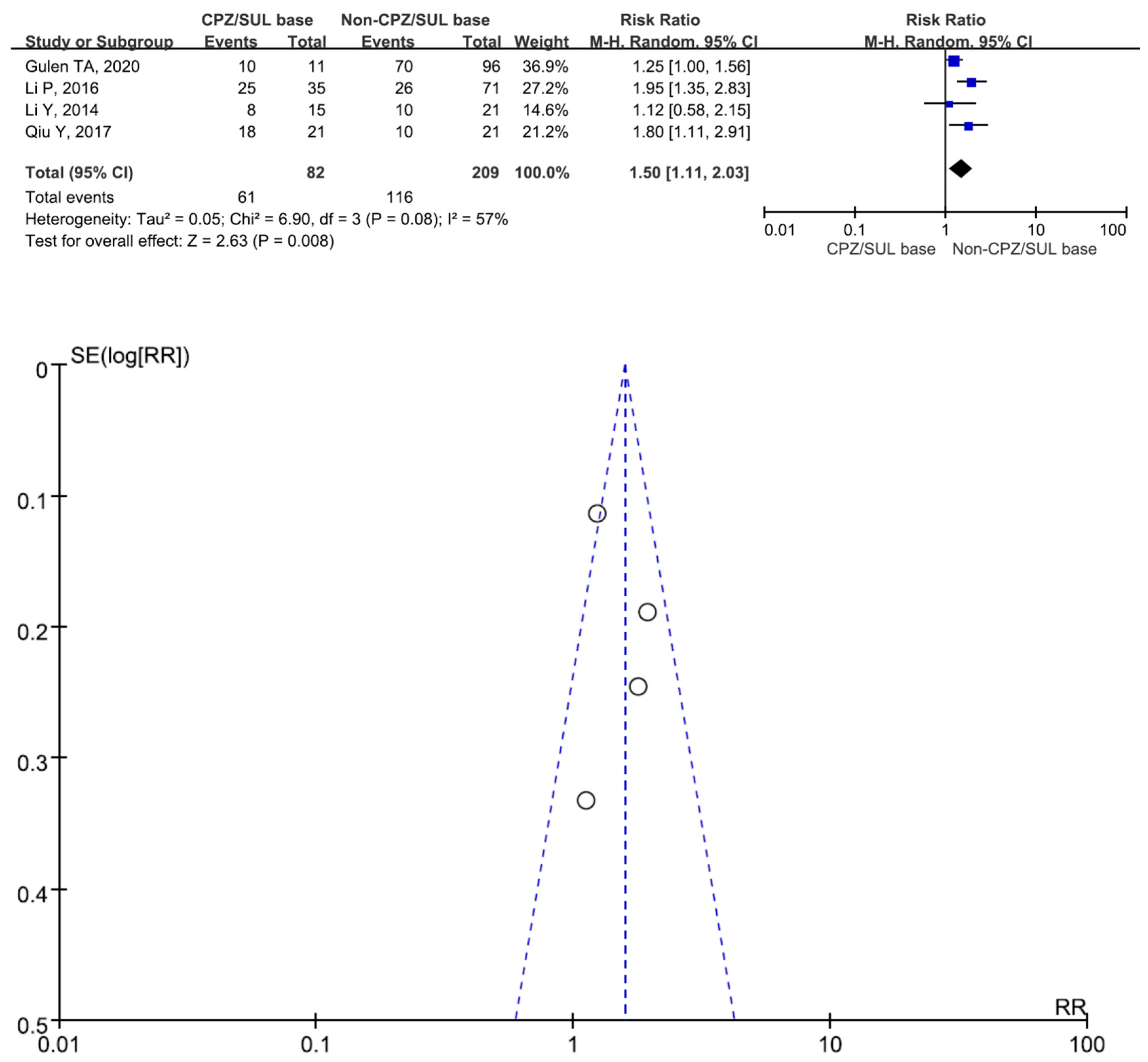
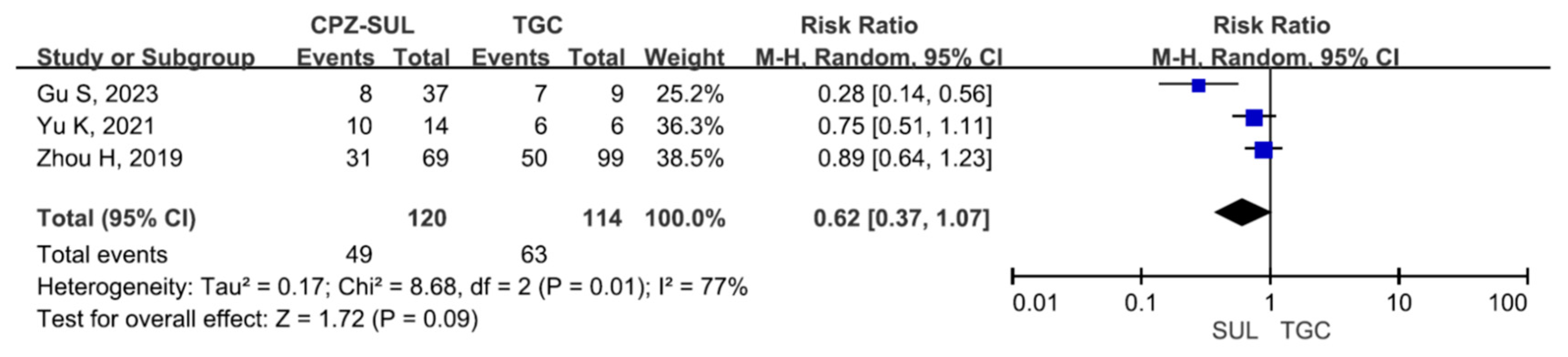
3. Results
4. Discussion
Limitations
5. Conclusions
6. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Eliopoulos, G.M.; Maragakis, L.L.; Perl, T.M. Acinetobacter baumannii: Epidemiology, antimicrobial resistance, and treatment options. Clin. Infect. Dis. 2008, 46, 1254–1263. [Google Scholar] [CrossRef]
- Gales, A.C.; Castanheira, M.; Jones, R.N.; Sader, H.S. Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: Results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008–2010). Diagn. Microbiol. Infect. Dis. 2012, 73, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Nutman, A.; Glick, R.; Temkin, E.; Hoshen, M.; Edgar, R.; Braun, T.; Carmeli, Y. A case-control study to identify predictors of 14-day mortality following carbapenem-resistant Acinetobacter baumannii bacteraemia. Clin. Microbiol. Infect. 2014, 20, O1028–O1034. [Google Scholar] [CrossRef]
- Hu, F.P.; Guo, Y.; Zhu, D.M.; Wang, F.; Jiang, X.F.; Xu, Y.C.; Zhang, X.J.; Zhang, C.X.; Ji, P.; Xie, Y.; et al. Resistance trends among clinical isolates in China reported from CHINET surveillance of bacterial resistance, 2005–2014. Clin. Microbiol. Infect. 2016, 22 (Suppl. S1), S9–S14. [Google Scholar] [CrossRef]
- Chiang, T.T.; Tang, T.H.; Chiu, C.H.; Chen, T.L.; Ho, M.W.; Lee, C.H.; Yang, Y.S. Antimicrobial activities of cefoperazone-sulbactam in comparison to cefoperazone against clinical organisms from medical centers in Taiwan. J. Med. Sci. 2016, 36, 229–233. [Google Scholar] [CrossRef]
- Chang, P.C.; Chen, C.C.; Lu, Y.C.; Lai, C.C.; Huang, H.L.; Chuang, Y.C.; Tang, H.J. The impact of inoculum size on the activity of cefoperazone-sulbactam against multidrug resistant organisms. J. Microbiol. Immunol. Infect. 2018, 51, 207–213. [Google Scholar] [CrossRef]
- Choi, J.Y.; Kim, C.O.; Park, Y.S.; Yoon, H.J.; Shin, S.Y.; Kim, Y.K.; Kim, M.S.; Kim, Y.A.; Song, Y.G.; Yong, D.; et al. Comparison of efficacy of cefoperazone/sulbactam and imipenem/cilastatin for treatment of Acinetobacter bacteremia. Yonsei Med. J. 2006, 47, 63–69. [Google Scholar] [CrossRef]
- Xia, J.; Zhang, D.; Xu, Y.; Gong, M.; Zhou, Y.; Fang, X. A retrospective analysis of carbapenem-resistant Acinetobacter baumannii-mediated nosocomial pneumonia and the in vitro therapeutic benefit of cefoperazone/sulbactam. Int. J. Infect. Dis. 2014, 23, 90–93. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Atkins, D.; Eccles, M.; Flottorp, S.; Guyatt, G.H.; Henry, D.; Hill, S.; Liberati, A.; O’Connell, D.; Oxman, A.D.; Phillips, B.; et al. GRADE Working Group. Systems for grading the quality of evidence and the strength of recommendations I: Critical appraisal of existing approaches The GRADE Working Group. BMC Health Serv. Res. 2004, 4, 38. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J. GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Q.; Wang, P.; Zhu, D.; Ye, X.; Wu, S.; Wang, M. Clonal dissemination of extensively drug-resistant Acinetobacter baumannii producing an OXA-23 β-lactamase at a teaching hospital in Shanghai, China. J. Microbiol. Immunol. Infect. 2015, 48, 101–108. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, P.; Wang, X.; Wang, W.; Zhao, X. Comparison of the efficacies of three empirically-selected antibiotics for treating Acinetobacter baumannii pulmonary infection: Experience from a teaching hospital in China. Int. J. Clin. Pharmacol. Ther. 2017, 55, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Zhang, J.; Wu, L.; Zhang, D.; Fu, L.; Xue, X. Comparison of the treatment efficacy between tigecycline plus high-dose cefoperazone-sulbactam and tigecycline monotherapy against ventilator-associated pneumonia caused by extensively drug-resistant Acinetobacter baumannii. Int. J. Clin. Pharmacol. Ther. 2018, 56, 120–129. [Google Scholar] [CrossRef]
- Zhou, H.; Yao, Y.; Zhu, B.; Ren, D.; Yang, Q.; Fu, Y.; Yu, Y.; Zhou, J. Risk factors for acquisition and mortality of multidrug-resistant Acinetobacter baumannii bacteremia: A retrospective study from a Chinese hospital. Medicine 2019, 98, e14937. [Google Scholar] [CrossRef]
- Niu, T.; Luo, Q.; Li, Y.; Zhou, Y.; Yu, W.; Xiao, Y. Comparison of Tigecycline or Cefoperazone/Sulbactam therapy for bloodstream infection due to Carbapenem-resistant Acinetobacter baumannii. Antimicrob. Resist. Infect. Control 2019, 8, 52. [Google Scholar] [CrossRef]
- Gulen, T.A.; Ayfer, I.; Odenmis, I.; Kayabas, U. Acinetobacter baumannii infections and antibiotic resistance in hospitalized patients in an education and research hospital: A six-year analysis. FLORA 2020, 25, 563–571. [Google Scholar] [CrossRef]
- Yu, K.; Zeng, W.; Xu, Y.; Liao, W.; Xu, W.; Zhou, T.; Cao, J.; Chen, L. Bloodstream infections caused by ST2 Acinetobacter baumannii: Risk factors, antibiotic regimens, and virulence over 6 years period in China. Antimicrob. Resist. Infect. Control 2021, 10, 16. [Google Scholar] [CrossRef]
- Kanchanasuwan, S.; Kositpantawong, N.; Singkhamanan, K.; Hortiwakul, T.; Charoenmak, B.; Ozioma, F.N.; Doi, Y.; Chusri, S. Outcomes of Adjunctive Therapy with Intravenous Cefoperazone-Sulbactam for Ventilator-Associated Pneumonia Due to Carbapenem-Resistant Acinetobacter baumannii. Infect. Drug Resist. 2021, 14, 1255–1264. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Xiong, J.; Peng, S.; Hu, L.; Zhu, H.; Xiao, Y.; Luo, H.; Hang, Y.; Chen, Y.; Fang, X.; et al. Assessment of Effective Antimicrobial Regimens and Mortality-Related Risk Factors for Bloodstream Infections Caused by Carbapenem-Resistant Acinetobacter baumannii. Infect. Drug Resist. 2023, 16, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Landman, D.; Georgescu, C.; Martin, D.A.; Quale, J. Polymyxins revisited. Clin. Microbiol. Rev. 2008, 21, 449–465. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.S.; Murray, C.K.; Jorgensen, J.H. Colistin heteroresistance in Acinetobacter and its association with previous colistin therapy. Antimicrob. Agents Chemother. 2008, 52, 351–352. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.S.; Suh, J.Y.; Kwon, K.T.; Jung, S.I.; Park, K.H.; Kang, C.I.; Chung, D.R.; Peck, K.R.; Song, J.H. High rates of resistance to colistin and polymyxin B in subgroups of Acinetobacter baumannii isolates from Korea. J. Antimicrob. Chemother. 2007, 60, 1163–1167. [Google Scholar] [CrossRef]
- Lo-Ten-Foe, J.R.; de Smet, A.M.; Diederen, B.M.; Kluytmans, J.A.; van Keulen, P.H. Comparative evaluation of the VITEK 2, disk diffusion, Etest, broth microdilution, and agar dilution susceptibility testing methods for colistin in clinical isolates, including heteroresistant Enterobacter cloacae and Acinetobacter baumannii strains. Antimicrob. Agents Chemother. 2007, 51, 3726–3730. [Google Scholar] [CrossRef]
- Markou, N.; Markantonis, S.L.; Dimitrakis, E.; Panidis, D.; Boutzouka, E.; Karatzas, S.; Rafailidis, P.; Apostolakos, H.; Baltopoulos, G. Colistin serum concentrations after intravenous administration in critically ill patients with serious multidrug-resistant, Gram-negative bacilli infections: A prospective, open-label, uncontrolled study. Clin. Ther. 2008, 30, 143–151. [Google Scholar] [CrossRef]
- Taccone, F.S.; Rodriguez-Villalobos, H.; De Backer, D.; De Moor, V.; Deviere, J.; Vincent, J.L.; Jacobs, F. Successful treatment of septic shock due to pan-resistant Acinetobacter baumannii using combined antimicrobial therapy including tigecycline. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 257–260. [Google Scholar] [CrossRef]
- Gordon, N.C.; Wareham, D.W. A review of clinical and microbiological outcomes following treatment of infections involving multidrug-resistant Acinetobacter baumannii with tigecycline. J. Antimicrob. Chemother. 2009, 63, 775–780. [Google Scholar] [CrossRef]
- Schafer, J.; Goff, D.; Stevenson, K.; Mangino, J.E. Early experience with tigecycline for ventilator-associated pneumonia and bacteraemia caused by multidrug-resistant Acinetobacter baumannii. Pharmacotherapy 2007, 27, 980–987. [Google Scholar] [CrossRef]
- Peleg, A.Y.; Potoski, B.A.; Rea, R.; Adams, J.; Sethi, J.; Capitano, B.; Husain, S.; Kwak, E.J.; Bhat, S.V.; Paterson, D.L. Acinetobacter baumannii bloodstream infection while receiving tigecycline: A cautionary report. J. Antimicrob. Chemother. 2007, 59, 128–131. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Lin, Y.C.; Lu, P.L.; Chen, H.C.; Chang, H.L.; Sheu, C.C. Antibiotic strategies and clinical outcomes in critically ill patients with pneumonia caused by carbapenem-resistant Acinetobacter baumannii. Clin. Microbiol. Infect. 2018, 24, 908.e1–908.e7. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.T.; Wang, Y.C.; Kuo, S.C.; Chen, C.T.; Liu, C.P.; Liu, Y.M.; Chen, T.L.; Yang, Y.S. Multicenter Study of Clinical Features of Breakthrough Acinetobacter Bacteremia during Carbapenem Therapy. Antimicrob. Agents Chemother. 2017, 61, e00931-17. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.; Han, Y.; Zhao, J.; Wei, C.; Cui, J.; Wang, R.; Liu, Y. Tigecycline treatment experience against multidrug-resistant Acinetobacter baumannii infections: A systematic review and meta-analysis. Int. J. Antimicrob. Agents. 2016, 47, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Isler, B.; Doi, Y.; Bonomo, R.A.; Paterson, D.L. New Treatment Options against Carbapenem-Resistant Acinetobacter baumannii Infections. Antimicrob. Agents Chemother. 2018, 63, e01110-18. [Google Scholar] [CrossRef]
- Gu, W.J.; Wang, F.; Tang, L.; Bakker, J.; Liu, J.C. Colistin for the treatment of ventilator associated pneumonia caused by multidrug-resistant gram-negative bacteria: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2014, 44, 477–485. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Fang, Y.; Wang, X.; Chen, Y.; Qi, Q.; Huang, F.; Xiao, X. Meta-analysis of colistin for the treatment of Acinetobacter baumannii infection. Sci. Rep. 2015, 5, 17091. [Google Scholar] [CrossRef]
- Tasina, E.; Haidich, A.B.; Kokkali, S.; Arvanitidou, M. Efficacy and safety of tigecycline for the treatment of infectious diseases: A meta-analysis. Lancet Infect. Dis. 2011, 834, 834–844. [Google Scholar] [CrossRef]
- Penwell, W.F.; Shapiro, A.B.; Giacobbe, R.A.; Gu, R.F.; Gao, N.; Thresher, J.; McLaughlin, R.E.; Huband, M.D.; DeJonge, B.L.; Ehmann, D.E.; et al. Molecular mechanisms of sulbactam antibacterial activity and resistance determinants in Acinetobacter baumannii. Antimicrob. Agents Chemother. 2015, 59, 1680–1689. [Google Scholar] [CrossRef]
- Beganovic, M.; Daffinee, K.E.; Luther, M.K.; LaPlante, K.L. Minocycline Alone and in Combination with Polymyxin B, Meropenem, and Sulbactam against Carbapenem-Susceptible and -Resistant Acinetobacter baumannii in an In Vitro Pharmacodynamic Model. Antimicrob. Agents Chemother. 2021, 65, e01680-20. [Google Scholar] [CrossRef]
- Findlay, J.; Poirel, L.; Bouvier, M.; Nordmann, P. In vitro activity of sulbactam-durlobactam against carbapenem-resistant Acinetobacter baumannii and mechanisms of resistance. J. Glob. Antimicrob. Resist. 2022, 30, 445–450. [Google Scholar] [CrossRef] [PubMed]
- McLeod, S.M.; Moussa, S.H.; Hackel, M.A.; Miller, A.A. In Vitro Activity of Sulbactam-Durlobactam against Acinetobacter baumannii-calcoaceticus Complex Isolates Collected Globally in 2016 and 2017. Antimicrob Agents Chemother. 2020, 64, e02534-19. [Google Scholar] [CrossRef] [PubMed]
- Jaruratanasirikul, S.; Wongpoowarak, W.; Aeinlang, N.; Jullangkoon, M. Pharmacodynamics modeling to optimize dosage regimens of sulbactam. Antimicrob. Agents Chemother. 2013, 57, 3441–3444. [Google Scholar] [CrossRef]
- Jaruratanasirikul, S.; Wongpoowarak, W.; Wattanavijitkul, T.; Sukarnjanaset, W.; Samaeng, M.; Nawakitrangsan, M.; Ingviya, N. Population pharmacokinetics and pharmacodynamics modeling to optimize dosage regimens of sulbactam in critically ill patients with severe sepsis caused by Acinetobacter baumannii. Antimicrob. Agents Chemother. 2016, 60, 7236–7244. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shu, Y.; Zhu, F.; Feng, B.; Zhang, Z.; Liu, L.; Wang, G. Comparative efficacy and safety of combination therapy with high-dose sulbactam or colistin with additional antibacterial agents for multiple drug-resistant and extensively drug-resistant Acinetobacter baumannii infections: A systematic review and network meta-analysis. J. Glob. Antimicrob. Resist. 2020, 24, 136–147. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, L.; Yue, M.; Huang, X.; Yang, Y.; Yu, H. Sulbactam combined with tigecycline improves outcomes in patients with severe multidrug-resistant Acinetobacter baumannii pneumonia. BMC Infect. Dis. 2022, 22, 795. [Google Scholar] [CrossRef]
- Jellison, T.K.; Mckinnon, P.S.; Rybak, M.J. Epidemiology, resistance, and outcomes of Acinetobacter baumannii bacteremia treated with imipenem-cilastatin or ampicillin-sulbactam. Pharmacotherapy 2001, 21, 142–148. [Google Scholar] [CrossRef]
- Makris, D.; Petinaki, E.; Tsolak, V.; Manoulakas, E.; Mantzarlis, K.; Apostolopoulou, O.; Sfyras, D.; Zakynthinos, E. Colistin versus Colistin Combined with Ampicillin-Sulbactam for Multiresistant Acinetobacter baumannii Ventilator-associated Pneumonia Treatment: An Open-label Prospective Study. Indian. J. Crit. Care Med. 2018, 22, 67–77. [Google Scholar] [CrossRef]
- Betrosian, A.P.; Frantzeskaki, F.; Xanthaki, A.; Georgiadis, G. High-dose ampicillin-sulbactam as an alternative treatment of late-onset VAP from multidrug-resistant Acinetobacter baumannii. Scand. J. Infect. Dis. 2007, 39, 38–43. [Google Scholar] [CrossRef]
- Assimakopoulos, S.F.; Karamouzos, V.; Lefkaditi, A.; Sklavou, C.; Kolonitsiou, F.; Christofidou, M.; Fligou, F.; Gogos, C.; Marangos, M. Triple combination therapy with high-dose ampicillin/sulbactam.; high-dose tigecycline and colistin in the treatment of ventilator-associated pneumonia caused by pan-drug resistant Acinetobacter baumannii: A case series study. Infez. Med. 2019, 27, 11–16. [Google Scholar]
- Jung, S.Y.; Lee, S.H.; Lee, S.Y.; Yang, S.; Noh, H.; Chung, E.K.; Lee, J.I. Antimicrobials for the treatment of drug-resistant Acinetobacter baumannii pneumonia in critically ill patients: A systemic review and Bayesian network meta-analysis. Crit. Care 2017, 21, 319. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Aitken, S.L.; Bonomo, R.A.; Mathers, A.J.; van Duin, D.; Clancy, C.J. Infectious Diseases Society of America 2023 Guidance on the Treatment of Antimicrobial Resistant Gram-Negative Infections. Clin Infect Dis. 2023, ciad428. [Google Scholar] [CrossRef]
- Jorgensen, S.C.J.; Rybak, M.J. Meropenem and vaborbactam: Stepping up the battle against carbapenem-resistant Enterobacteriaceae. Pharmacotherapy 2018, 38, 444–461. [Google Scholar] [CrossRef] [PubMed]
- Zhanel, G.G.; Lawrence, C.K.; Adam, H.; Schweizer, F.; Zelenitsky, S.; Zhanel, M.; Lagacé-Wiens, P.R.S.; Walkty, A.; Denisuik, A.; Golden, A.; et al. Imipenem-Relebactam and Meropenem-Vaborbactam: Two Novel Carbapenem-β-Lactamase Inhibitor Combinations. Drugs 2018, 78, 65–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, Y.; Han, R.; Huang, Z.; Zhang, X.; Hu, F.; Yang, F. Sulbactam Enhances in vitro Activity of β-Lactam Antibiotics Against Acinetobacter baumannii. Infect. Drug Resist. 2021, 14, 3971–3977. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.H.; Yu, W.L. Cefoperazone/sulbactam: New composites against multiresistant gram negative bacteria? Infect. Genet. Evol. 2021, 88, 104707. [Google Scholar] [CrossRef]
- Lai, C.C.; Chen, C.C.; Lu, Y.C.; Chuang, Y.C.; Tang, H.J. In vitro activity of cefoperazone and cefoperazone-sulbactam against carbapenem-resistant Acinetobacter baumannii and Pseudomonas aeruginosa. Infect. Drug Resist. 2018, 12, 25–29. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, M.; Zhou, S. Observation of clinical efficacy of the cefoperazone/sulbactam anti-infective regimen in the treatment of multidrug-resistant Acinetobacter baumannii lung infection. J. Clin. Pharm. Ther. 2022, 47, 1020–1027. [Google Scholar] [CrossRef]
- Liu, B.; Bai, Y.; Liu, Y.; Di, X.; Zhang, X.; Wang, R.; Wang, J. In vitro activity of tigecycline in combination with cefoperazone-sulbactam against multidrug-resistant Acinetobacter baumannii. J. Chemother. 2015, 27, 271–276. [Google Scholar] [CrossRef]
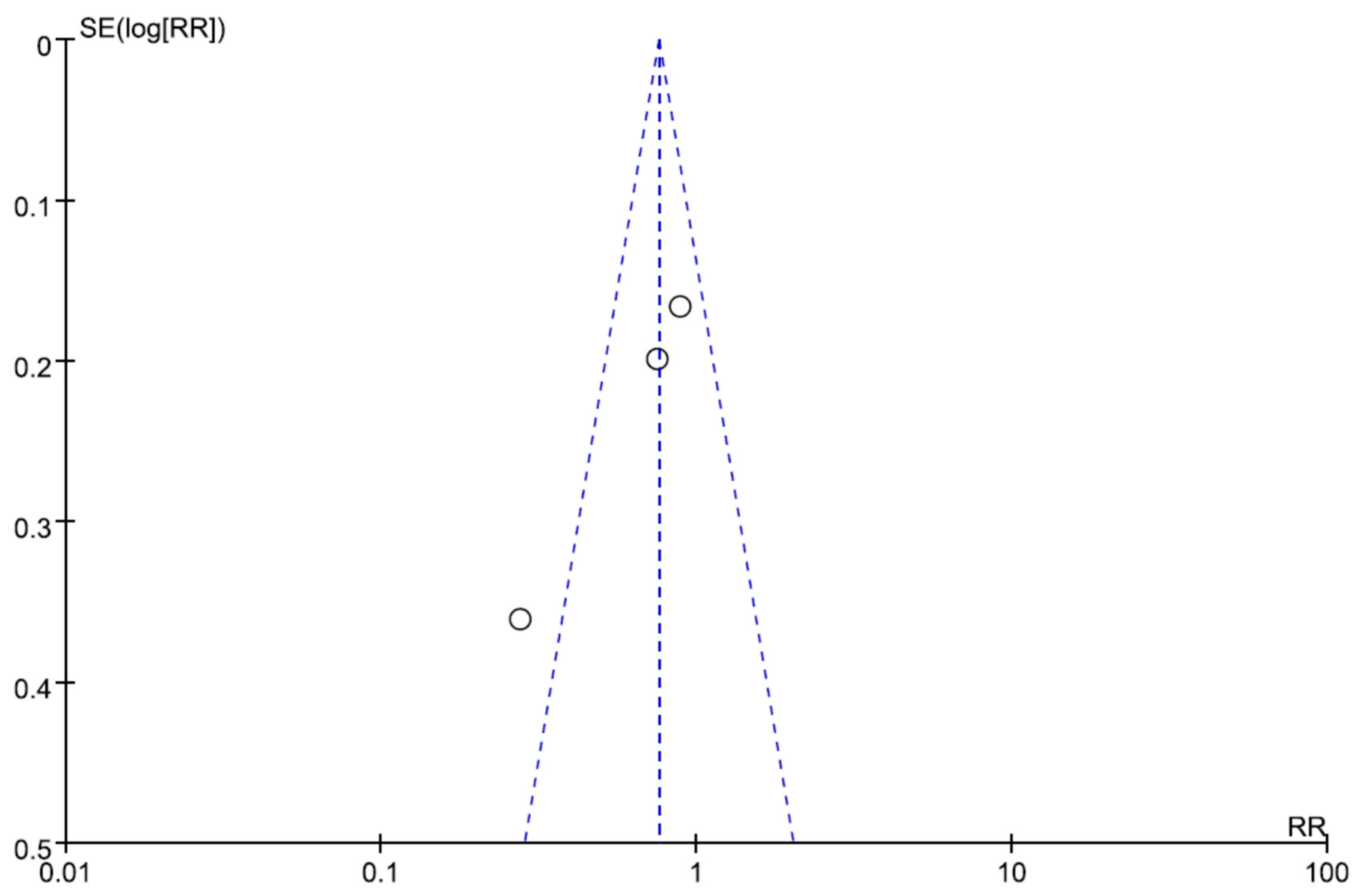
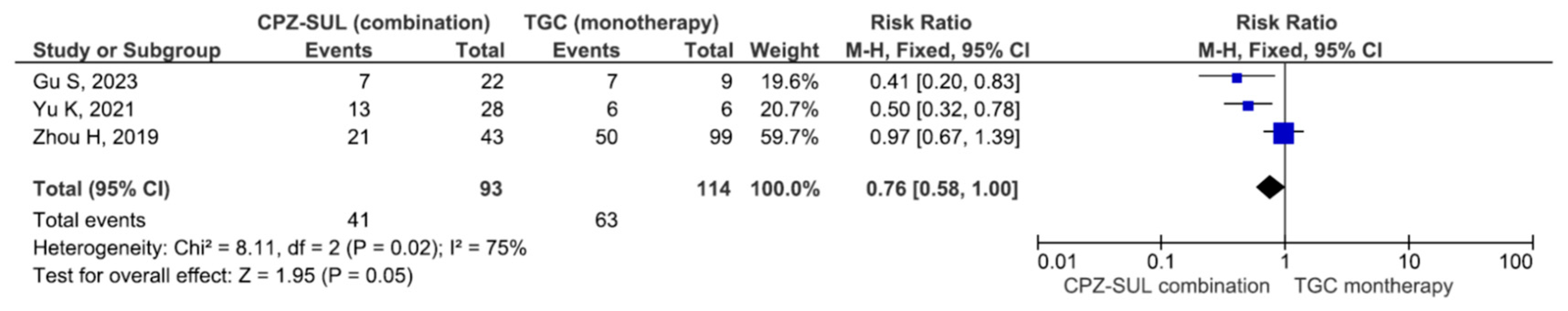
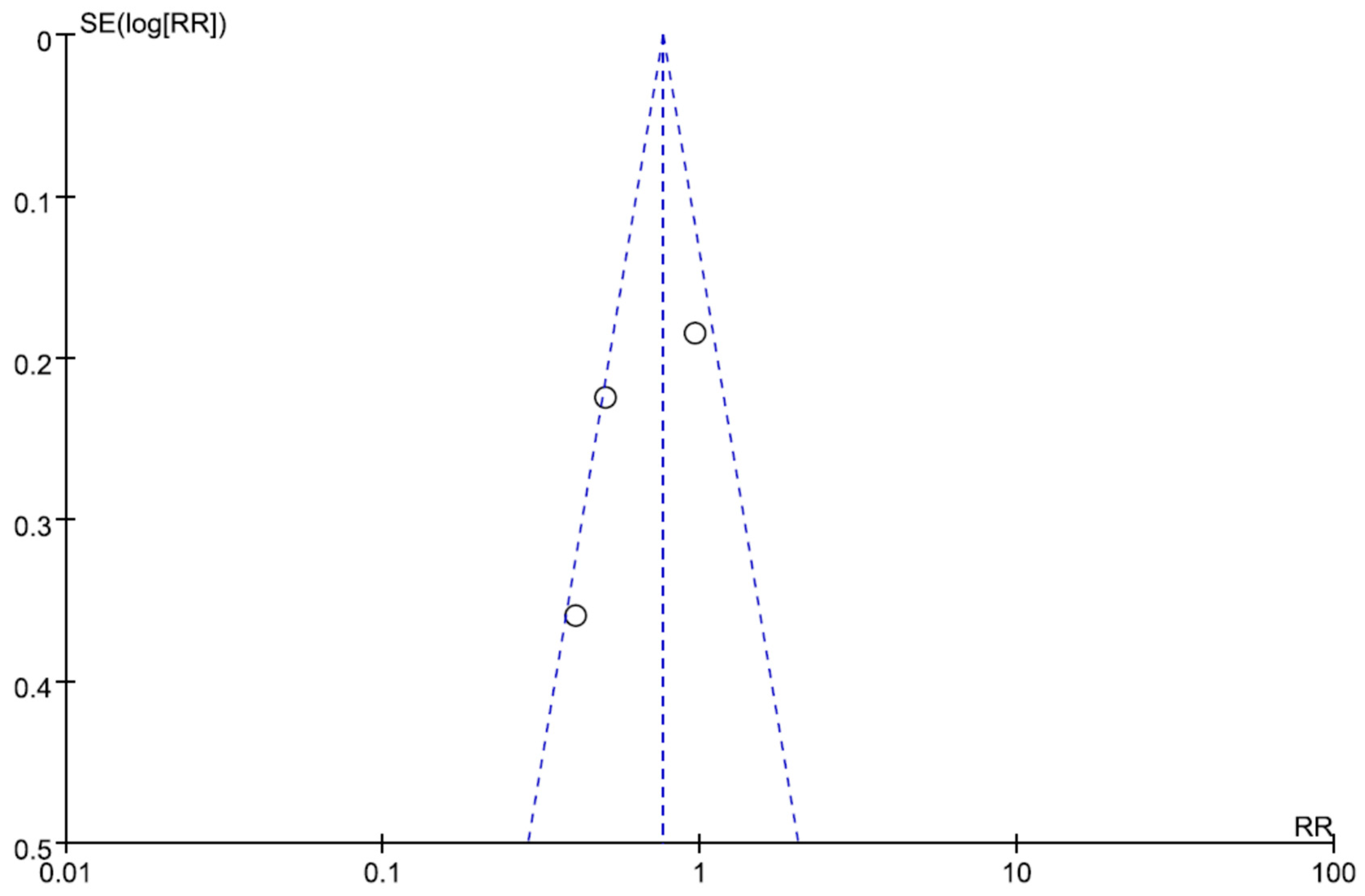
| Author/Year | Region/ Study Type | Bacteria | Infection Type | No of Patients (CPZ/SUL) | No of Patients (Non-CPZ/SUL) | Drug Dosage of CPZ/SUL | Drugs of Non-CPZ/SUL Groups | Combination Drugs of CPZ/SUL Groups |
|---|---|---|---|---|---|---|---|---|
| Xia J/2014 [9] | China/ RET | CRAB | HAP | 41 | 30 | No data | No data | Minocycline Meropenem, Levofloxacin |
| Li Y/2014 [14] | China RET | XDRAB | Mixed | 15 | 21 | CPZ/SUL (2:1) 3.0 gm q12h | Carbapemen | Carbapenem, Minocycline |
| Li P/2016 [15] | China/ RET | MDRAB XDRAB | HAP | 35 | 71 | No data | Carbapenem, Tigecycline | No antibiotic |
| Qin Y/2017 [16] | China/ RET | XDRAB | VAP | 21 | 21 | No data | Tigecycline | Tigecycline |
| Zhou H/2019 [17] | China/ RET | MDRAB | BSI | 112 | 162 | No data | Tigecycline | Tigecycline |
| Niu T/2019 [18] | China/ RET | CRAB | BSI | 75 | 135 | CPZ/SUL (2:1) 3.0 gm q8h–6.0 gm q6h | Tigecycline | Carbapenem |
| Gulin TA/2020 [19] | Turkey/ RET | MDRAB XDRAB | Mixed | 11 | 96 | No data | Colistin, Carbapenem, Tigecycline | Colistin |
| Yu K/2021 [20] | China/ RET | MDRAB | BSI | 42 | 36 | No data | Polymyxin B, Carbapenem, Tigecycline | Tigecycline |
| Kanchanasuwan S/2021 [21] | Thailand/ RET | CRAB | VAP | 52 | 28 | CPZ/SUL (1:1) 2.0 gm q8h–q4h | Imipenem, Tigecycline, Other antibiotics | Imipenem, Tigecycline, Other antibiotics |
| GU S/2023 [22] | China/ RET | CRAB | BSI | 59 | 43 | CPZ/SUL (2:1) 3.00 gm q8h–q6h | Carbapenem, Tigecycline, Other antibiotics | Tigecycline |
| Author/Year | Confounding | Selection | Interventions Classification | Interventions Deviations | Missing Data | Measurement of Outcomes | Selective Results |
|---|---|---|---|---|---|---|---|
| Xia J/2014 [9] | moderate risk | high risk | moderate risk | moderate risk | serious risk | serious risk | high risk |
| Li Y/2014 [14] | low risk | moderate risk | low risk | low risk | low risk | moderate risk | moderate risk |
| Li P/2016 [15] | moderate risk | moderate risk | high risk | high risk | serious risk | serious risk | serious risk |
| Qin Y/2017 [16] | high risk | high risk | high risk | high risk | serious risk | serious risk | serious risk |
| Zhou H/2019 [17] | low risk | low risk | low risk | moderate risk | serious risk | serious risk | high risk |
| Niu T/2019 [18] | low risk | low risk | low risk | moderate risk | low risk | low risk | low risk |
| Gulin TA/2020 [19] | high risk | high risk | moderate risk | high risk | serious risk | serious risk | serious risk |
| Yu K/2021 [20] | low risk | low risk | moderate risk | moderate risk | serious risk | serious risk | high risk |
| Kanchanasuwan S/2021 [21] | low risk | low risk | moderate risk | moderate risk | low risk | low risk | moderate risk |
| GU S/2023 [22] | low risk | low risk | moderate risk | moderate risk | low risk | low risk | low risk |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Lin, L.; Kuo, S. Comparing the Outcomes of Cefoperazone/Sulbactam-Based and Non-Cefoperazone/Sulbactam-Based Therapeutic Regimens in Patients with Multiresistant Acinetobacter baumannii Infections—A Meta-Analysis. Antibiotics 2024, 13, 907. https://doi.org/10.3390/antibiotics13090907
Huang C, Lin L, Kuo S. Comparing the Outcomes of Cefoperazone/Sulbactam-Based and Non-Cefoperazone/Sulbactam-Based Therapeutic Regimens in Patients with Multiresistant Acinetobacter baumannii Infections—A Meta-Analysis. Antibiotics. 2024; 13(9):907. https://doi.org/10.3390/antibiotics13090907
Chicago/Turabian StyleHuang, Chienhsiu, Lichen Lin, and Sufang Kuo. 2024. "Comparing the Outcomes of Cefoperazone/Sulbactam-Based and Non-Cefoperazone/Sulbactam-Based Therapeutic Regimens in Patients with Multiresistant Acinetobacter baumannii Infections—A Meta-Analysis" Antibiotics 13, no. 9: 907. https://doi.org/10.3390/antibiotics13090907
APA StyleHuang, C., Lin, L., & Kuo, S. (2024). Comparing the Outcomes of Cefoperazone/Sulbactam-Based and Non-Cefoperazone/Sulbactam-Based Therapeutic Regimens in Patients with Multiresistant Acinetobacter baumannii Infections—A Meta-Analysis. Antibiotics, 13(9), 907. https://doi.org/10.3390/antibiotics13090907







