Abstract
Background: Candida albicans and non-Candida albicans Candida species (NCACs) are known to colonize and invade various tissues, including the oral mucosa. In this work, we aimed to characterize mature biofilms of several Candida spp. clinical isolates (n = 33) obtained from the oral mucosa of children, adults, and elders of Eastern Europe and South America. Methods: Each strain was evaluated for its capacity to form biofilms in terms of total biomass using the crystal violet assay and for matrix components production (proteins and carbohydrates) using the BCA and phenol-sulfuric tests, respectively. The effect of different antifungals on biofilm formation was studied. Results: in the children’s group, a predominance of C. krusei (81%) was observed, while, among adults, the main species was C. albicans (59%). Most strains showed a reduced response to antimicrobial drugs when in biofilm form (p < 0.01). Moreover, it was observed that strains isolated from children produced more matrix, with higher levels of protein and polysaccharides. Conclusions: children were more likely to be infected by NCACs than adults. More importantly, these NCACs were able to form biofilms richer in matrix components. This finding is of clinical importance, particularly in pediatric care, since stronger biofilms are highly associated with antimicrobial resistance, recurrent infections, and higher therapeutic failure.
1. Introduction
Candidiasis has been increasing in recent decades, mainly due to the growing number of individuals with immunocompromised conditions and the higher antifungal resistance of Candida spp. [1]. Candida spp. are common colonizers in the oral cavity, vagina, and skin, but it is possible that they might be located in the endocardium and meninges. Systemic infections are mainly observed in adults, however, the clinical features of disseminated disease in adults have only been recently described [2].
In the oral cavity, in which Candida spp. is the most common fungus, oral candidiasis (OC) is particularly critical due to its high prevalence in all ages and genders [3]. OC is characterized by oral discomfort, pain, burning sensation, parageusia, and aversion to food [1,4]. It is usually associated with age (childhood or older), smoking, diabetes mellitus, nutritional disorders, endocrinopathies, immunosuppressive conditions, and malignancies [4]. Nevertheless, and importantly, OC can also be observed in 60% of the healthy population and non-immunocompromised individuals [5].
It is known that pathogenic mechanisms of Candida spp. depend upon both host conditions and Candida virulence factors [6]. Thus, healthy members of the population presenting candidiasis might host Candida spp. with virulence factors that are able to switch to the pathogenic form even in healthy immune systems [6]. One of the most important virulence factors of the Candida spp. is its ability to form biofilms, which are difficult to control, resulting in chronic or recurrent infections [6]. Biofilms are a sessile community of microorganisms adhered to biotic or abiotic surfaces and embedded in an extracellular polymeric substance (EPS) that forms a matrix [7,8]. This extracellular matrix plays a key role in antimicrobial resistance by preventing antibiotic penetration, avoiding phagocytosis through immune system cells, acting as physical barrier to environmental changes, and promoting microbial adhesion to biotic and abiotic surfaces. The matrix is also responsible for the structural organization of biofilms, forming water channels and allowing intercellular interactions [7,9]. Its composition consists of exopolysaccharides, nucleic acids (eDNA and eRNA), proteins, lipids (e.g., ergosterol), and other biomolecules [8,9]. Biofilm matrix composition is directly associated to the pathogenicity of species/strains, as well as to antifungal drug resistance.
This study might improve our understanding on how to better treat each species/strain according to a species’ biofilm profile. In addition, the colonization of Candida spp. biofilms in different age groups is still poorly explored. Hence, in order to better understand Candida species’ oral biofilm formation and their effects in different age groups from different continents, we isolated strains of C. albicans and NCACs from Eastern Europe and South America and analyzed the biofilms in terms of chemical composition and antifungal drug susceptibility.
2. Results
2.1. Demographic Data of Study Population and Ethical Aspects
Participants (n = 31) were divided into three groups: children (1–12 years), adults (13–59 years), and elders (+60 years). The average age of participants from the children, adults, and elders’ groups was 3.9±1.97, 34±11.61, and 70±2.83 years old, respectively. Most of the participants in the children’s group were female, while adults and elders were predominantly male. In the children’s group, a predominance of C. krusei (81%) was observed, while among adults and elders, the main species was C. albicans (63% and 67%, respectively) (See Appendix A).
It is relevant to note that, during the isolation process, only one species was isolated from each person. This could probably be a limitation of the isolation technique itself. CHROMagar Candida screening was routinely performed in order to confirm that the isolate was not contaminated (common protocol).
2.2. pBiofilm Formation of Candida spp.
We measured the total biomass of Candida spp. biofilms formed by strains isolated from the oral mucosa of the participants of this study through crystal violet (CV) staining (Figure 1). Results showed that all strains are moderate or strong biofilm formers, and the most-isolated Candida spp. in children had a higher biofilm mass than the one from the adult group. C. krusei BC06 presented the highest biofilm biomass (2.14 Abs/cm2 ± 0.08), followed by C. krusei BC18 (1.95 Abs/cm2 ± 0.17) and C. krusei BC34 (1.92 Abs/cm2 ± 0.41). It is relevant to note that all of these strains were isolated from children.
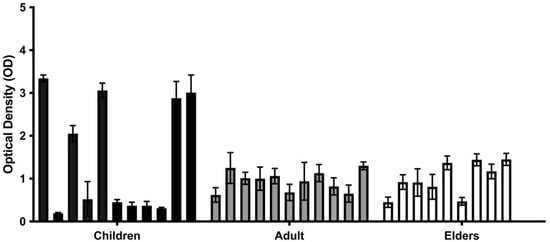
Figure 1.
In vitro biofilm production of clinical isolates from children, adults, and elders. The black and grey bars are each from different age groups. Biofilm was quantified through staining with crystal violet (OD570) after 48 h of incubation. Each value is the average of three independent experiments conducted in triplicate.
Surprisingly, if we group the results based on the species of Candida, the highest biofilm former was also found to be C. krusei (Figure 2). On the other hand, the in vitro average of matrix production by the other Candida spp. (i.e., the average of all strain species) was similar (Figure 2). Hence, when comparing the biofilm formation among species of Candida from all groups (Figure 2), C. krusei (
= 0.94 Abs/cm2 ± 0.80) produced the highest quantity of biofilm biomass, followed by C. glabrata ( = 0.80 Abs/cm2), C. intermedia ( = 0.71 Abs/cm2), and C. valida ( = 0.71 Abs/cm2 ± 0.13). Curiously, in this study, C. albicans ( = 0.42 Abs/cm2 ± 0.19) was the species presenting the lowest ability to form biofilms. On the other hand, these averages show no statistically significant differences.
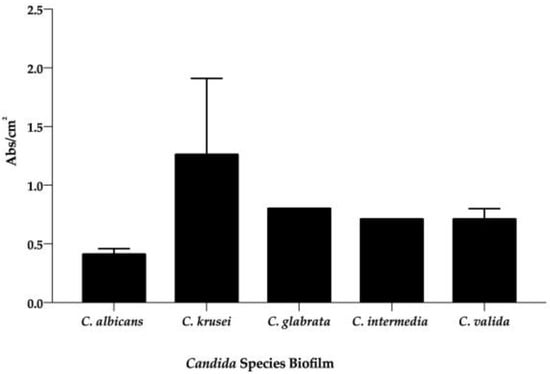
Figure 2.
Average of in vitro biofilm (Abs/cm2) production from each species of Candida. The black bars are the mean of biofilm (Abs/cm2), which was quantified through staining with crystal violet (OD570) after 48 h of incubation. Each value is the average of three independent experiments conducted in triplicate.
The biofilms of all clinical isolates were compared to the reference strain, C. albicans SC5314, since it presents a biofilm with high hyphal quantity and entanglement [8,10]. In the children’s group, we observed that BC06, BC12, BC18, BC31, BC34 (p > 0.01), and BC10 (p > 0.05) had a higher ability to produce biofilms (Figure 3A). For strains isolated from adults, all biofilms presented higher absorbance values than the reference (meaning bigger biofilm production) except for C. albicans CA1, C. albicans CA11, and C. krusei CK13 (Figure 3B). Similarly, in the elders’ group, most strains were stronger producers of biofilm when compared to C. albicans SC5314, excepting C. albicans CA3 and C. albicans CAMYK 2738 (Figure 3C). Among all C. albicans strains, C. albicans MYK 2760 was the strongest biofilm producer (0.76 Abs/cm2 ± 0.09) and the weakest were C. albicans BC29 (0.15 Abs/cm2 ± 0.02) and C. albicans CA3 (0.15 Abs/cm2 ± 0.03). On the other hand, the strain control, C. albicans SC5314, presented intermediary values (0.29 Abs/cm2 ± 0.21) (Figure 3).
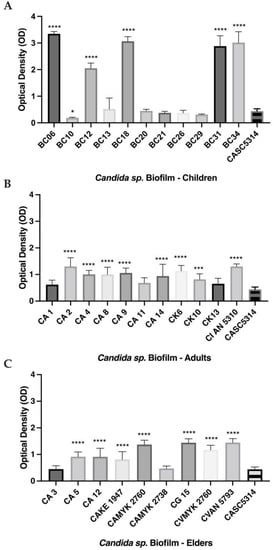
Figure 3.
In vitro biofilm production of clinical isolates from children (A), adults (B), and elders (C). Biofilm was quantified through staining with crystal violet (OD570) after 48 h of incubation. The grey bars are the biofilm biomass of each strain. Each value is the average of three independent experiments conducted in triplicate. Error bars represent the mean and standard deviations. **** p < 0.0001, *** p < 0.001 and * p < 0.05 as compared to biofilm formed by the reference strain (C. albicans SC5314). Note: CA—C. albicans; CK—C. krusei; CG—C. glabrata; CI—C. intermedia; CV—C. valida.
2.3. Biofilm Matrix Composition
For the study of biofilm composition, the polysaccharide content was determined using the phenol/sulfuric acid method and the protein content was determined with the BCA Kit (Figure 4 and Figure 5) [10]. The highest level of polysaccharides was observed in C. glabrata BC21 from the children’s group and the lowest in biofilm of C. albicans MYK 2760 biofilm from the adults’ group (Figure 4).
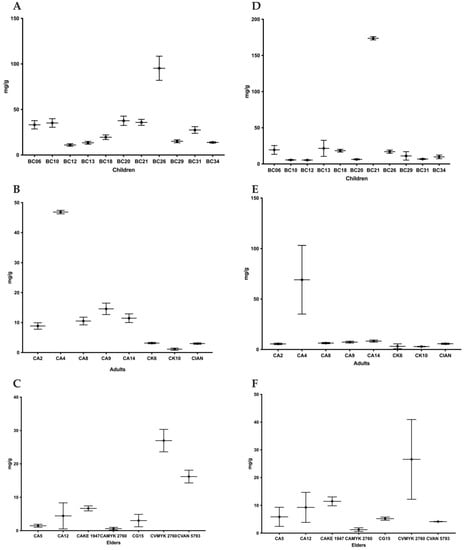
Figure 4.
Average quantity of protein (A–C) and polysaccharides (D–F) isolated from a matrix of in vitro biofilm produced by clinical isolates from children, adults, and elders. Each value is the average (protein mg/g of biofilm) ± standard deviation (SD). Note: CA—C. albicans; CK—C. krusei; CG—C. glabrata; CI—C. intermedia; CV—C. valida.
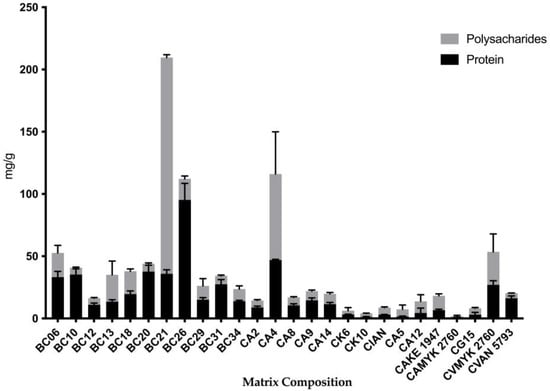
Figure 5.
Proportion of protein (OD562) and polysaccharide (OD490) quantity from each strain from different ages. The gray bars are the polysaccharide level, and the black bars are the protein level of each strain. Each value is the average (mg/g of biofilm) of three independent experiments conducted in triplicate ± standard deviation (SD). Note: CA—C. albicans; CK—C. krusei; CG—C. glabrata; CI—C. intermedia; CV—C. valida.
On the other hand, the quantities of proteins were the highest in the biofilms of C. krusei BC26 from the children’s group and the lowest in the matrix of C. albicans MYK 2760 biofilm from the adults’ group (Figure 4). In addition, the strains that had the highest polysaccharide levels (BC13 and BC21) also had more polysaccharide than protein in their extracellular matrices (Figure 5).
2.4. Effect of Antifungals against Biofilm Formation of Different Candida Species
In order to determine the susceptibility of biofilms to antifungals, fluconazole (1250 mg/L), voriconazole (800 mg/L), anidulafungin (2 mg/L), and amphotericin B (2 mg/L) were added at the start of the experiment. The goal was to evaluate the effect of these drugs on the development of the biofilms. These concentrations were carefully chosen, taking into account several previous studies conducted by our group with the same conditions (antifungal drugs used to treat matured biofilms of Candida spp.) [10,11,12,13,14,15].
Figure 6 presents the percentage of biomass reduction in the presence of the different antifungals. Generally, all strains showed significant biomass reduction in the presence of antifungals. The exceptions were the biofilms of C. glabrata BC10, which presented a significant increase in total biomass values (p < 0.01) with amphotericin B (2 mg/L) when compared with the controls (biofilms without antifungals).
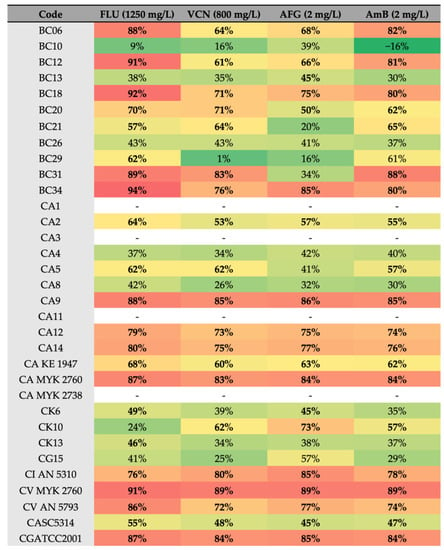
Figure 6.
Heatmap of the reduction (%) of biofilm formation in the presence of antifungals. Red > 80%, orange > 65%, yellow > 50%, and green < 50% reduction. Note: FLU—Fluconazole, VCN—voriconazole, AFG—anidulafungin, AmB—amphotericin B. Bold: above 50%.
The lowest values of biofilm reduction were obtained for C. albicans BC29 treated with Vcn at 800 mg/L (Figure 6). Furthermore, strains isolated from children and elders showed greater susceptibility to Flu than strains isolated from adults (Figure 7).
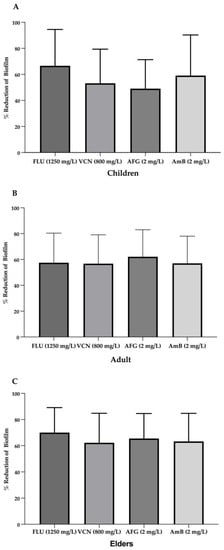
Figure 7.
Average of the reduction (%) of biofilm formation in the presence of antifungals in children group (A), adult group (B) and elders’ group (C). Note: FLU—Fluconazole, VCN—voriconazole, AFG—anidulafungin, AmB—amphotericin B.
2.5. Confocal Laser Scanning Microscopy
Confocal scanning laser microscopy (CLSM) was performed in order to evaluate the organization and architecture of the biofilms. The images of the biofilms were presented individually or reconstructed in 3D projections (Figure 8, Panel A–I). In addition, vertical (x-z) sections or side views of the 3D reconstructed images were used to determine biofilm thickness and architecture.
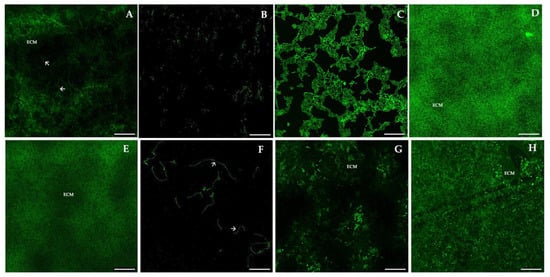
Figure 8.
Representative images of confocal laser scanning microscopy (CLSM) of mature biofilms of Candida spp. from children, adults, and elders, analyzed by PNA-FISH using the C. albicans PNA probe. (A): Mature biofilm of C. albicans SC5314; (B): C. glabrata BC21; (C): C. albicans BC29; (D): C. valida AN5793; (E): C. krusei 6; (F): C. albicans CAMYK 2760; (G): C. intermedia AN5310; (H): C. krusei BC06. Magnification: 600×. Laser: 488 nm (ALEXA®-488). Arrows indicate hyphae form, and ECM means extracellular matrix. Scale bar: 100 μm.
C. albicans SC5314 (A) showed an expected heterogeneous architecture of a mature biofilm, with cells and hyphae embedded within the extracellular matrix (ECM). Differences between the biofilms formed by all species and strains are clearly visible in Figure 8, which is reflected in the architecture, the amount of extracellular polymeric substances (EPS), and the thickness. Panels H (C. krusei BC06) and G (C. intermedia AN5310) show biofilms covering the entire surface of the slide, similarly to A, but no hyphae cells are observed, as these species are non-dimorphic fungi. In panels B (C. glabrata BC21) and F (C. albicans CAMYK 2760), we can see microcolonies of predominant yeast forms visible (panel B) and yeast and hyphae forms (panel F), while panel D (C. valida AN5793) shows a mature biofilm with a lower quantity of EPS. In contrast, in panel E (C. krusei 6), there are cells in mature biofilms, enclosed in ECM, appearing as diffuse green fluorescence. Finally, C. albicans BC29 (C) biofilm shows yeast cells and hyphae as the control but not the formation of a uniformly flat surface.
3. Discussion
The genus Candida includes approximately 300 species, and it is a member of the healthy microbiota, asymptomatically colonizing several human tissues [16,17,18,19]. Candida represents one of the most common causes of systemic infection in hospitalized patients [20]. For hospitalized children, Candida spp. are the second most common pathogen identified in the setting of sepsis [21] and are the leading infectious cause of death in children with cancer or following an organ or hematopoietic stem cell transplant [21].
In oral microbiota, Candida spp. have high probability to infect other tissues, developing systemic infection [22,23]. C. albicans is still the most prevalent species in the oral cavity [18,21,24], but NCACs (e.g., C. glabrata, C. tropicalis, C. parapsilosis, and C. krusei) have increased [25]. The identification of Candida spp. present in oral mucosa is a critical issue for the successful treatment of infected patients. This is particularly important for species such as NCACs which have shown high antimicrobial resistance rates [21,22,24].
In this study, C. krusei was the predominant species in the oral cavities of children, which, curiously, in not in accordance with the current literature, which indicated that C. albicans is the predominant species [24]. However, it is relevant to note that there are limited studies including Candida spp. involving pediatric patients, because, typically, they involve healthy and/or adult patients [21,24]. Additionally, past studies that focused candidemia observed that NCACs were predominant in pediatric patients, and C. tropicalis was the most common species (50% of the infections) [26]. Previously, a multi-national study of pediatric candidiasis observed a higher proportion of C. guilliermondii and C. krusei in non-US sites [25]. In the same study, of the 449 Candida isolates recovered, 40% were C. albicans, and NCACs predominated in 60% of the cases [25]. Similarly, a prospective epidemiologic study of invasive candidiasis in children and neonates enrolled 196 pediatric and 25 neonatal patients with invasive candidiasis in different countries. It was observed that NCACs prevailed in the pediatric (56%) and neonatal (52%) age groups [27]. In this same study, a difference was also observed among antimicrobial responses. The antimicrobial treatment was successful in most of the children, but when C. glabrata was involved, there was a lower successful outcome rate (55%). The most-used antifungal therapies for pediatric and neonatal invasive candidiasis were fluconazole (21%), liposomal amphotericin B (20%), and micafungin (18%), and no significant differences were observed among the classes of antifungals [27]. Children are thought to be more susceptible to antimicrobials due to their reduced exposure and also to their shorter lifetimes and limited number of illnesses. However, we cannot put aside environmental exposure to drugs, which has been shown to have a critical role in selection pressure and the presence of multiresistant strains in volunteers’ flora [20,21].
Not surprisingly, as other studies have shown [1,2,16], our results indicated that, in adults and elders, C. albicans was the most common species, followed by C. krusei. Epidemiological studies have shown that, among NCACs, the predominant species in candidemia is C. glabrata in the USA, Northern Europe, and Australia, while C. parapsilosis is the most prevalent in Latin America, Southern Europe, and Asia [28].
The association between antimicrobial resistance and biofilm production ability is known [7,19,29,30,31,32,33,34]. Thus, biofilm characterization is essential in order to find therapeutic protocols capable of preventing and treating chronic infections. Patients with oral candidiasis are mostly treated with antifungal agents such as polyenes (e.g., AmB and nystatin) and azole derivatives (e.g., fluconazole, ketoconazole, clotrimazole, and miconazole) [35]. It has been reported that 20% of patients with oral candidiasis experience recurrence of infection and that around 30% of these recurrences are caused by different Candida strains than from the first episode of infection [3,35]. Usually, recurrent infections are correlated to biofilm formation [1,3,7,12,25,28,30,36].
In this study, the biofilms’ comparisons were made while taking into account C. albicans SC5314 biofilm, which has high hyphae quantity and entanglement [8,11]. The results showed that all of the strains were biofilm formers, regardless of the age group. Still, the characteristics of biofilms were different between age groups. The biofilm formed by isolates from children presented a higher biomass and a matrix composition richer in polysaccharides than the ones from adults. This it probably is due to the fact that NCACs were predominant in the children’s group. According to Kumari et al. [36], the total carbohydrate and protein content in the biofilm matrices was significantly higher in NCACs when compared with C. albicans. The matrix carbohydrate content was the highest in C. tropicalis, followed by C. krusei, and the lowest content was observed in C. albicans, which was predominant in the adult group. Rodrigues et al. also reported similar results in NCACs [8,36].
C. albicans are dimorphic fungi and hyphae are an important structural component of its biofilms [19,36]. The hyphae in biofilms contribute to the overall architectural stability of the biofilm and to adherence to normal biofilm development and maintenance [19]. Indeed, the genes associated to hyphal Efg1, Tec1, Ndt80, and Rob1 are also necessary for biofilm formation [19,20,29]. There is a direct relationship between C. albicans morphogenesis and exposure to antifungal agents [37,38]. It has been previously observed that fluconazole-susceptible yeasts show reduced hyphae production while resistant isolates were little affected by this antifungal agent [38,39,40]. Azoles are known to act by inhibiting the production of ergosterol, which is the main constituent of a fungal cell membrane [37,38,39]. These antifungal agents probably reduce the production of hyphae due to the increased surface area of hyphal cells compared to those of spherical forms. Previous work shows that alterations in the ERG11 gene, which is responsible for ergosterol production, limit hyphae formation, presumably due to the lack of ergosterol [19,37,38,39]. In addition, treatment with azole leads to an accumulation of the sterol biosynthetic intermediate farnesyl pyrophosphate, which indirectly stimulates the overproduction of farnesol and is capable of inhibiting filamentous growth in C. albicans [7,39]. The development of efflux pumps by Candida spp. is the most frequent mechanism of azole resistance. Therefore, the two gene families which encode the efflux pump deserve to be highlighted. The CDR1 and CDR2 genes belong to the ATP-binding cassette superfamily, as do the MDR1 genes of the key facilitator superfamily. Thus, increased expression levels of CDR1, CDR2, and MDR1 in C. albicans cause resistance to fluconazole [41].
The presence polysaccharides in the extracellular matrix, such as β-1,3 glucan, β-1,6 glucan, and α-1,2-branched α-1,6 mannan, contribute to the antimicrobial resistance of Candida biofilms [34,42,43]. The mechanism of the drug–polysaccharide interaction was first described for C. albicans biofilm matrix and the azole drug, Flu [40]. The glucan and mannan components for the extracellular matrix form a complex that sequesters drugs, likely through non-covalent interactions [33]. Interestingly, there is a correlation between cell wall glucan and biofilm growth [40]. Moreover, the mechanisms of drug sequestration for other Candida spp., including C. tropicalis, C. parapsilosis, C. glabrata, and C. auris, were associated with presence of glucan [13,19,33].
In addition, the matrix polysaccharides of Candida biofilms also sequester antimicrobial drugs, for instance, AmB, anidulafungin, and flucytosine [13,42]. Furthermore, a higher level of biomass and polysaccharides can be associated with FKS1, FKS2, BGL2, and XOG1 genes’ expression [13,43,44]. The importance of these genes is related to the delivery and production of the β-1,3 glucans matrix and to the matrix structure and adherence of biofilm cells to a surface, or in other words, the antimicrobial resistance phenotype [15,42].
All of the strains that presented a low ability to produce biofilm (O.D. inferior to 0,5) were not tested. We would like to emphasize the lack of a direct correlation between a high concentration of polysaccharide in the matrix and biofilm antifungal resistance. However, and surprisingly, most of the strains presenting high levels of polysaccharide in their biofilm matrix were less sensible to antifungal drugs (drug susceptibility lower than 70%) (e.g., strains BC21, CA4, and BC13; Figure 5 and Figure 6). Of this group, BC21 displayed the highest drug susceptibility. This probably occurred because it was isolated from a 1-year-old child, and exposure to antifungals—which promotes the selection of resistant microorganisms—is low at this age. A higher susceptibility of C. albicans strains to antifungal drugs, as compared to NCACs, was also observed in the adult group. No direct correlation could be made with other virulence factors not considered in this work.
Importantly, it was observed that the proportion of protein/polysaccharides was directly associated with the susceptibility of the Candida spp. to the antimicrobial drugs in the children’s group, except in strains with a higher level of polysaccharides, such as BC13 (C. krusei) and BC21 (C. glabrata). Still, in the adult group, a relationship between the matrix composition and antimicrobial sensibility was not observed, probably because adult microbiota present other virulence factors than matrix production, such as ergosterol biosynthesis [20,45]. This is, for example, the case for the biofilm resistance to Flu and AmB, which is associated with a significant decrease in total ergosterol content as well as changes in the levels of other sterols by the expression of ERG genes, which act as binding sites for antifungal molecules, inhibiting their binding to ergosterol [3,40,45,46].
Previously, our study on the adult strains (planktonic form) showed that half of the isolates presented resistance to 5′-fluorouracil and that almost 29% had resistance to Flu [16]. All isolates were sensible to AmB, and two samples had an intermediate profile to Vcn [16]. Hence, almost 82% of the collected samples showed resistance to at least one antifungal class, which is clinically remarkable [16]. In this study, in biofilm form, a reduction of biomass was observed in all strains treated with antifungal agents except BC10 (C. glabrata) cultivated with AmB. This difference might be associated to the tested concentrations of the antifungal drugs. In the present study, the concentrations of antifungals used were previously known to be effective against Candida biofilms.
Beforehand, our group showed that the minimum fungicidal concentration (MFC) for Flu and Vcn for C. glabrata ATCC 2001 was almost 200-fold and 4-fold higher than the MIC, respectively [12,13]. Also, Fonseca et al. [47] demonstrated that Flu at 1250 mg/L can be effective for biomass reduction (p < 0.01), even in the resistant strains, according to the MIC breakpoint of EUCAST [48]. When comparing Flu and Vcn, another study observed that C. glabrata biofilms were more susceptible to Vcn and, at 1000 mg/mL, the latter was effective in inhibiting the biofilms [12]. Curiously, there was an overexpression of the three ERG genes in the presence of both drugs and an increase of β-1,3 glucans in matrices [12]. Importantly, the use of Vcn is recommended for esophageal and oropharyngeal candidiasis in cases of fluconazole-refractory disease [12,49].
Similarly, Ramage et al. [50] described that AmB was up to 32 times less active on biofilm cells than on planktonic cells, with variable resistance being observed among the strains. At 2 mg/L, AmB reduced the biofilm biomass by 64.2% (p < 0.0001) and promoted a reduction of viable cells on C. glabrata ATCC2001 biofilms [15]. Other reports presented similar results for other Candida spp., demonstrating that AmB is still among the most effective drugs for the treatment of Candida spp. infections [15,37,51,52].
Anidulafungin was tested against the biofilm of reference strains and isolates of C. albicans and NCACs, and the inhibition of sessile cells was around 50% of the metabolic activity [53]. The effective concentration against biofilms of C. albicans ATCC10231, ATCC90028, C. tropicalis ATCC750, C. albicans, and C. tropicalis isolates was between 0.5 and 1 μg/ mL, more than five or six dilutions higher than their planktonic MICs [53]. In this study, it was used two times, and no resistance was observed. A previous study using caspofungin and micafungin against biofilm of Candida spp. showed that the concentration needed to eradicate the biofilm (MBEC) was five to six times higher than the MFC (planktonic cells). The MBEC was between 0.5–3 mg/L and 3.5–17 mg/L for caspofungin and micafungin, respectively [8].
In conclusion, children were more likely to be infected by NCACs when comparing with adults and elders. As a key factor, these NCACs produced biofilms richer in matrix components. This is a very relevant clinical finding, since stronger biofilms are directly linked with higher antifungal resistance (and, thus, therapeutic failure).
As a limitation of our study, we have no further information on the source of the samples (previous antibiotic therapy and co-morbidities), and the studied populations were heterogenous.
4. Materials and Methods
4.1. Collection and Identification of the Clinical Isolates
Some clinical isolates of Candida spp. (n = 31) were collected by a lab technician from the oral mucosa (swab from tongue or oral mucosa) of patients of the primary health care unit of Acarape city and were maintained at the laboratory of the Department of Microbiology of the University of International Integration of Afro-Brazilian Lusophony (UNILAB) in Brazil. Others were collected (following the same procedure) and maintained by the Department of Clinical Microbiology, from several wards of the Nitra Faculty Hospital in Slovakia. Samples were immediately stored and kept at −80 °C, until accurate identification by biomolecular methods. Then, all of the isolates were sent to the LEPABE laboratory at the University of Porto for the development of this work.
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board (or Ethics Committee) of the Nitra Faculty Hospital in Slovakia (Code: 020322; Date: 2 March 2022). In Brazil, the study was preceded by the approval of the committee of ethics in research (CEP) under the number 4.432.501, following the ethical aspects of the resolution 466/12 and 510/16 of the Conselho Nacional de Saúde. All of the names and private information of patients were kept confidential.
The clinical isolates were coded as:
- -
- Children: BC06, BC10, BC12, BC13, BC18, BC20, BC21, BC26, BC29, BC31, and BC34.
- -
- Adults: CA1, CA2, CA4, CA8, CA9, CA11, CA14, CK6, CK10, and CIAN 5310.
- -
- Elders: CA3, CA5, CA12, CAKE 1947, CAMYK 2760, CA MYK 2738, CG15, CVMYK 2760, and CVAN 5793.
Reference strains Candida albicans SC5314 and Candida glabrata ATCC2001 were acquired from the American Type Culture Collection.
In all cases, for routine identification, Candida isolates were grown in Sabouraud Dextrose Agar (SDA) (Merck, Darmstadt, Germany) under aerobic conditions for 24 h at 37 °C. The procedures for identification were performed by standard mycological methods at 30 °C for 48 h using chromogenic medium CHROMagar Candida (CHROMagar Microbiology, Paris, France) [14,15].
All samples from Slovakia have the patient data published in the article Černáková et al. [16]. Regarding the samples from the children, they were all clinically healthy.
4.2. Inoculum Preparation
Candida species were grown on SDA and incubated for 24 h at 37 °C. In order to prepare the inoculum, cells were then inoculated in SDB Sabouraud dextrose broth (SDB) (Merck, Darmstadt, Germany) and incubated for 18 h at 37 °C under agitation at 120 rpm. After incubation, the inoculum density was adjusted to 1 × 105 cells/mL using a Neubauer chamber with RPMI-1640 (Sigma-Aldrich, St. Louis, MO, USA) [46].
4.3. Antifungal Drugs
Fluconazole, voriconazole, and anidulafungin were provided by Pfizer® (New York, NY, USA)—Pfizer) in their pure form. Amphotericin came from Sigma® (Sigma-Aldrich, Buffalo, NY, USA). Aliquots of 5000 mg/L of fluconazole (Flu) and voriconazole (Vcn) and 40 mg/L of amphotericin B (AmB) and anidulafungin (Afg) were prepared using dimethyl sulfoxide (DMSO) for all drugs. The final concentrations used were prepared in RPMI-1640 (Sigma-Aldrich, St. Louis, MO, USA). For DMSO, 1% was also used as a control.
4.4. Biofilm Formation and Crystal Violet Assay
The characterization of biofilm formation by Candida spp. was performed according to Rodrigues et al. [13]. Briefly, after the inoculum, a total of 100 μL of each strain inoculum was transferred to each well of the 96-well micro-plate, and then 100 μL of RPMI-1640 was added, supplemented or not with antifungals for 48 h at 37 °C. The antifungals tested were fluconazole (1250 mg/L), voriconazole (800 mg/L), anidulafungin (caspofungin/micafungin) (2 mg/L), and amphotericin B (2 mg/L). It is relevant to indicate that these concentrations were chosen, according to previous studies of our group, as the minimum concentrations to eradicate the biofilm (MBEC) in several Candida spp. [8,10,11,12,13,14,15]. Wells containing only culture medium without inoculum were used as negative control.
After incubation, the biofilm biomasses were analyzed by the crystal violet (CV) assay. For this, the supernatant was carefully aspirated, and the wells were washed twice with 200 μL PBS (phosphate-buffered saline, 0.1 M, pH = 7.2). Subsequently, biofilms were fixed by 100% (v/v) methanol, 200 μL/well, for 20 min. After drying, the supernatants were aspirated, and 200 μL of 1% (v/v aqueous CV was added to each well (Sigma–Aldrich, St. Louis, MO, USA). After 5 min, the dye solution was aspirated, and the wells were washed twice with sterile distilled water. Subsequently, 200 μL of a 33% acetic acid solution was added to each well and immediately transferred to a new 96-well plate. Finally, the plates were read at 570 nm (FLUOStar Omega Plate Reader, BMG LABTECH, Ortenberg, Germany) [8]. The cut-off optical density (ODc) was defined as three standard deviations above the mean OD of the negative control, and the strains were classified as follows: OD ≤ Odc = no biofilm producer; Odc < OD ≤ 2 × Odc = weak biofilm producer; 2 × Odc < OD ≤ 4 × Odc = moderate biofilm producer; and 4 × Odc < OD = strong biofilm producer [10].
4.5. Quantification of Matrix Polysaccharides
The quantification of polysaccharides was performed by the phenol-sulfuric acid method, according to Rodrigues et al. [8]. Briefly, the biofilm matrix was collected after the 48 h incubation period, then the biofilms were scraped (two times, using a 100 uL tip) from the 24-well plates, resuspended in ultra-pure water, sonicated for 60 s (Sonopuls HD 2200, Bandelin, Berlin, Germany), vortexed for 2 min, and centrifuged at 2898× g (4000 rpm) for 8 min. The supernatant was sterilized with a 0.22 μm filter membrane [10]. In a small test tube with 0.5 mL of supernatant (matrix), 0.5 mL of phenol (50 g/L) and 2.5 mL of sulfuric acid (95–97%) were added. The mixture was vortexed and allowed to stand for 15 min at room temperature. Then, the absorbance was measured at 490 nm by a multilabel plate reader, and the results were expressed as absorbance [10]. A blank without glucose was set for each assay. The quantity of polysaccharides was extrapolated from a standard curve made with standard glucose concentrations. The quantity of polysaccharides was then normalized by dry weight of biofilm (mg polysaccharides/g biofilm) [10].
4.6. Quantification of Matrix Protein
The extracted biofilm matrix (25 μL) was transferred to 96-well plates (triplicate) and 200 μL of reagent mixture of Novagen® BCA Protein Assay Kit (Merck KGaA, Darmstadt, Germany) were added to each well. The solution was homogenized by vortex and incubated for 30 min at 37 °C. Then, the absorbance at 562 nm was determined using PBS as control. The amount of protein was extrapolated from a standard curve performed with standard BSA concentrations. The amount of protein was then normalized by dry weight of biofilm (mg protein/g biofilm) [8].
4.7. Assessment of the Spatial Arrangement of Candida spp. Cells in Biofilms by CLSM
A specific 23S rRNA PNA probe developed and optimized by our group was used for Candida spp. detection: 5′-Alexa488-OO-CACCCACAAAATCAA-3′ (melting temperature: 75.69 °C; specificity: 96.04%; sensibility: 84.79%). The probe was synthesized (Panagene, Daejoen, Republic of Korea), attached to the Alexa®-488 fluorochrome, and tested with C. albicans SC5314.
For the biofilm spatial organization, the PNA-FISH were performed directly in the coverslip Thermanox™, plastic slices coated by poly-L-lysine (to enhance cells adhesion) on which biofilms were grown, over the course of 48 h. Briefly, Candida spp. biofilms were formed on commercially available, presterilized, polystyrene, 12-well microtiter plates. In each well, there was a Thermanox™ coverslip (5 mm) for the development and growth of the biofilms. After the period of biofilm formation, all of the medium was aspired, and the biofilms were washed once with PBS to remove non-adherent cells (and to avoid biofilm loss). Then, biofilms were fixed in 4% (w/v) paraformaldehyde (Sigma-Aldrich, St Louis, MO, USA) followed by 50% (v/v) ethanol for 30 min at −20 °C and were incubated with PNA Probe at 54 °C. After a 30 min incubation period in the dark, the sample was observed by CLSM (LSM 710, Carl Zeiss, Germany). Image acquisition was performed using a 60× oil-immersion objective (60×/1.2 W) and the 488 nm laser line. Z-stacks with 1 µm Z-steps were collected. All microscope settings were identical among the analyzed groups. Zeiss Zen software was used for confocal image acquisition and processing, and ImageJ software was used for analysis.
4.8. Statistical Analysis
The experimental data were evaluated by GraphPad Prism v.9.1.1 software (San Diego, CA, USA). The data were analyzed using one-way ANOVA followed by Dunnett’s test. In all cases, statistical significance was set as p < 0.05. Data are presented as the mean ± standard deviation (SD). All experiments were performed three times independently, in triplicate.
5. Conclusions
The resistance of Candida spp. biofilms to different antifungal agents is multifactorial [10,30,36]. Features of virulence, such as membrane and cell wall barriers, dimorphism, the signal transduction pathway, proteins related to stress tolerance, hydrolytic enzymes (e.g., proteases, lipases, and hemolysins), and toxin production should be studied in the future in order to better understand the isolated strains as well as the pathogenesis of the genus Candida [6].
Here we show that the oral biofilm displays different species of Candida in terms of quantity, matrix composition, and, consequently, susceptibility to antifungal drugs, depending, for instance, on the age group. These might be related to the maturity of the immune system and metabolism, but also to habits such as diet, physical activity, smoking, antibiotic use, and associated diseases. The results of this study might help us to better control these infections and to choose adequate therapy depending on the species, immunity state, and age. The present work opens the door to new studies that can deepen our understanding of virulence factors and pathogenicity in Candida spp. among different ages, allowing for the reduction of resistant strains and promoting more efficient treatments.
Author Contributions
A.M.C.V.A.: conception of the protocol, biofilm analysis, data analysis, and writing—original draft preparation; B.O.L. and L.Č.: collection, isolation, and maintenance of Candida spp. strains; G.S.C., A.C.R.d.M.L., É.H.S.d.B., and L.Č.: collection, isolation, and maintenance of Candida spp. strains and writing—review and editing; C.F.R. and N.F.A.: conception of the protocol, writing—review and editing, funding acquisition, and supervision; L.F.d.L.: methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Pfizer® the Global Medical Grants—Reference 60395063 and by (i) LA/P/0045/2020 (ALiCE), UIDB/00511/2020, and UIDP/00511/2020 (LEPABE), funded by national funds through FCT/MCTES (PIDDAC).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board (or Ethics Committee) of the Nitra Faculty Hospital in Slovakia (Code: 020322; Date: 2 March 2022). In Brazil, the study was preceded by the approval of the committee of ethics in research (CEP) under the number 4.432.501, following the ethical aspects of the resolution 466/12 and 510/16 of the Conselho Nacional de Saúde. All of the names and private information of patients were kept confidential.
Acknowledgments
L.Č. thanks the grant of VEGA 1/0240/23 from the Ministry of Education, Science, Research, and the Sport of the Slovak Republic and to the Operation Program of Integrated Infrastructure for the project, UpScale of Comenius University Capacities and Competence in Research, Development and Innovation, ITMS2014+: 313021BUZ3, co-financed by the European Regional Development Fund. The authors would like to thank Ana Paula Ribeiro Rodrigues, the LAMOFOPA/UECE, and Fundação Cearense de Apoio ao Dessenvolvimento Cientifico e Tecnológico—FUNCAP for technical support in obtaining confocal microscope (CLSM) images.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Demographic data associated with biofilm production for each strain.
Table A1.
Demographic data associated with biofilm production for each strain.
| Strain Code | Species | O.D.570 | Age | Gender |
|---|---|---|---|---|
| BC06 | C. krusei | 3.34 ± 0.08 | 7 | Female |
| BC10 | C. glabrata | 0.19 ± 0.02 | 4 | Female |
| BC12 | C. krusei | 2.05 ± 0.19 | 3 | Male |
| BC13 | C. krusei | 0.52 ± 0.41 | 6 | Female |
| BC18 | C. krusei | 3.06 ± 0.17 | 2 | Female |
| BC20 | C. krusei | 0.45 ± 0.06 | 3 | Male |
| BC21 | C. glabrata | 0.37 ± 0.08 | 1 | Female |
| BC26 | C. krusei | 0.37 ± 0.10 | 1 | Female |
| BC29 | C. albicans | 0.31 ± 0.02 | 5 | Female |
| BC31 | C. krusei | 2.88 ± 0.39 | 5 | Female |
| BC34 | C. krusei | 3.01 ± 0.41 | 6 | Female |
| CA1 | C. albicans | 0.62 ± 0.17 | 14 | Male |
| CA2 | C. albicans | 1.25 ± 0.36 | 46 | Female |
| CA3 | C. albicans | 0.45 ± 0.12 | 66 | Male |
| CA4 | C. albicans | 1.01± 0.14 | 55 | Male |
| CA5 | C. albicans | 0.92 ± 0.17 | 70 | Male |
| CA8 | C. albicans | 1.00 ± 0.27 | 34 | Male |
| CA9 | C. albicans | 1.06 ± 0.18 | 20 | Female |
| CA11 | C. albicans | 0.68 ± 0.19 | 29 | Female |
| CA12 | C. albicans | 0.91 ± 0.32 | 72 | Female |
| CA14 | C. albicans | 0.94 ± 0.44 | 28 | Male |
| CA KE 1947 | C. albicans | 0.81 ± 0.29 | 66 | Female |
| CA MYK 2760 | C. albicans | 1.37 ± 0.16 | 74 | Male |
| CA MYK 2738 | C. albicans | 0.47± 0.09 | 71 | Male |
| CK6 | C. krusei | 1.13 ± 0.20 | 26 | Male |
| CK10 | C. krusei | 0.82 ± 0.20 | 56 | Male |
| CK13 | C. krusei | 0.65 ± 0.20 | 49 | Male |
| CI AN 5310 | C. intermedia | 1.30 ± 0.09 | 37 | Female |
| CG15 | C. glabrata | 1.44 ± 0.14 | 68 | Male |
| CV MYK 2760 | C. valida | 1.17 ± 0.17 | 78 | Female |
| CV AN 5793 | C. valida | 1.45 ± 0.14 | 70 | Male |
Note: CA—C. albicans; CK—C. krusei; CG—C. glabrata; CI—C. intermedia; CV—C. valida.
Appendix B

Table A2.
Matrix composition of Candida spp. biofilms isolated from children, including mean values of protein quantity (mg/g of biofilm) and mean values of polysaccharide quantity (mg/g of biofilm) ± standard deviation (SD).
Table A2.
Matrix composition of Candida spp. biofilms isolated from children, including mean values of protein quantity (mg/g of biofilm) and mean values of polysaccharide quantity (mg/g of biofilm) ± standard deviation (SD).
| BC06 | BC10 | BC12 | BC13 | BC18 | BC20 | BC21 | BC26 | BC29 | BC31 | BC34 | CASC 5314 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein (mg/g) | 33.19 ± 4.60 | 35.22 ± 4.70 | 11.01 ± 1.35 | 13.45 ± 1.59 | 19.55 ± 2.55 | 37.63 ± 5.10 | 35.84 ± 3.30 | 95.26 ± 13.27 | 15.05 ± 1.63 | 27.48 ± 3.70 | 13.82 ± 0.70 | 1.24 ± 0.05 |
| Polysaccharides (mg/g) | 19.43 ± 6.07 | 5.55 ± 0.49 | 5.36 ± 0.44 | 21.57 ± 11.11 | 18.34 ± 1.87 | 6.34 ± 0.64 | 173.78 ± 2.26 | 16.96 ± 2.24 | 11.13 ± 5.86 | 6.80 ± 0.61 | 9.79 ± 2.54 | 0.03 ± 0.05 |
Note: CA—C. albicans.

Table A3.
Matrix composition of Candida spp. biofilms isolated from the adult group, including mean values of protein quantity (mg/g of biofilm) and mean values of polysaccharide quantity (mg/g of biofilm) ± standard deviation (SD).
Table A3.
Matrix composition of Candida spp. biofilms isolated from the adult group, including mean values of protein quantity (mg/g of biofilm) and mean values of polysaccharide quantity (mg/g of biofilm) ± standard deviation (SD).
| CA2 | CA4 | CA8 | CA9 | CA14 | CK6 | CK10 | CIAN 5310 | CASC 5314 | |
|---|---|---|---|---|---|---|---|---|---|
| Protein (mg/g) | 8.87 ± 1.08 | 46.86 ± 0.58 | 10.52 ± 1.29 | 14.59 ± 1.91 | 11.46 ± 1.44 | 3.16 ± 0.23 | 1.13 ± 0.36 | 2.99 ± 0.24 | 1.24 ± 0.05 |
| Polysaccharides (mg/g) | 5.54 ± 0.74 | 69.13 ± 33.98 | 6.36 ± 0.66 | 7.31 ± 0.90 | 8.34 ± 1.04 | 3.15 ± 2.45 | 2.91 ± 0.26 | 5.70 ± 0.62 | 0.03 ± 0.05 |
Note: CA—C. albicans, CK—C. krusei; CI—C. intermedia.

Table A4.
Matrix composition of Candida spp. biofilms isolated from elders, including mean values of protein quantity (mg/g of biofilm) and mean values of polysaccharide quantity (mg/g of biofilm) ± standard deviation (SD).
Table A4.
Matrix composition of Candida spp. biofilms isolated from elders, including mean values of protein quantity (mg/g of biofilm) and mean values of polysaccharide quantity (mg/g of biofilm) ± standard deviation (SD).
| CA5 | CA12 | CAKE 1947 | CAMYK 2760 | CG15 | CVMYK 2760 | CVAN 5793 | CASC 5314 | |
|---|---|---|---|---|---|---|---|---|
| Protein (mg/g) | 1.48 ± 0.44 | 4.43 ± 3.90 | 6.68 ± 0.75 | 0.60 ± 0.39 | 3.02 ± 1.85 | 26.97 ± 3.36 | 16.19 ± 1.91 | 1.24 ± 0.05 |
| Polysaccharides (mg/g) | 5.88 ± 3.45 | 9.28 ± 5.42 | 11.47 ± 1.59 | 1.27 ± 0.66 | 5.22 ± 0.56 | 26.58 ± 14.36 | 4.16 ± 0.07 | 0.03 ± 0.05 |
Note: CA—C. albicans, CK—C. krusei; CI—C. intermedia; CG—C. glabrata; CV—C. valida.
References
- Rajendra Santosh, A.B.; Muddana, K.; Bakki, S.R. Fungal Infections of Oral Cavity: Diagnosis, Management, and Association with COVID-19. SN Compr. Clin. Med. 2021, 3, 1373–1384. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa-García, E.; Olalde-Hernández, M.J.; Irigoyen-Camacho, M.E.; Mondragón-Padilla, A.; Mendoza-Juache, A.; Sánchez-Vargas, L.O. Antifungal Susceptibility of Oral Isolates of Candida Species from Chronic Kidney Disease Patients on Chronic Dialysis. J. Mycol. Med. 2020, 30, 101009. [Google Scholar] [CrossRef] [PubMed]
- Gallè, F.; Sanguinetti, M.; Colella, G.; Di Onofrio, V.; Torelli, R.; Rossano, F.; Liguori, G. Oral Candidosis: Characterization of a Sample of Recurrent Infections and Study of Resistance Determinants. New Microbiol. 2011, 34, 379–389. [Google Scholar] [PubMed]
- Taylor, M.; Raja, A. Oral Candidiasis (Thrush); StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Fotos, P.G.; Vincent, S.D.; Hellstein, J.W. Oral Candidosis. Oral Surg. Oral Med. Oral Pathol. 1992, 74, 41–49. [Google Scholar] [CrossRef]
- Staniszewska, M. Virulence Factors in Candida Species. Curr. Protein Pept. Sci. 2020, 21, 313–323. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial Biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Rodrigues, M.E.; Henriques, M. Susceptibility of Candida glabrata Biofilms to Echinocandins: Alterations in the Matrix Composition. Biofouling 2018, 34, 569–578. [Google Scholar] [CrossRef]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm Matrixome: Extracellular Components in Structured Microbial Communities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Rodrigues, M.; Silva, S.; Henriques, M. Candida glabrata Biofilms: How Far Have We Come? J. Fungi 2017, 3, 11. [Google Scholar] [CrossRef]
- Pokhrel, S.; Boonmee, N.; Tulyaprawat, O.; Pharkjaksu, S.; Thaipisutikul, I.; Chairatana, P.; Ngamskulrungroj, P.; Mitrpant, C. Assessment of Biofilm Formation by Candida albicans Strains Isolated from Hemocultures and Their Role in Pathogenesis in the Zebrafish Model. J. Fungi 2022, 8, 1014. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Gonçalves, B.; Rodrigues, M.E.; Silva, S.; Azeredo, J.; Henriques, M. The Effectiveness of Voriconazole in Therapy of Candida glabrata’s Biofilms Oral Infections and Its Influence on the Matrix Composition and Gene Expression. Mycopathologia 2017, 182, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Henriques, M. Portrait of Matrix Gene Expression in Candida glabrata Biofilms with Stress Induced by Different Drugs. Genes 2018, 9, 205. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Correia, A.; Vilanova, M.; Henriques, M. Inflammatory Cell Recruitment in Candida glabrata Biofilm Cell-Infected Mice Receiving Antifungal Chemotherapy. J. Clin. Med. 2019, 8, 142. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.F.; Alves, D.F.; Henriques, M. Combination of Posaconazole and Amphotericin b in the Treatment of Candida glabrata Biofilms. Microorganisms 2018, 6, 123. [Google Scholar] [CrossRef]
- Černáková, L.; Líšková, A.; Lengyelová, L.; Rodrigues, C.F. Prevalence and Antifungal Susceptibility Profile of Oral Candida spp. Isolates from a Hospital in Slovakia. Medicina 2022, 58, 576. [Google Scholar] [CrossRef]
- Fornari, G.; Vicente, V.A.; Gomes, R.R.; Muro, M.D.; Pinheiro, R.L.; Ferrari, C.; Herkert, P.F.; Takimura, M.; de Carvalho, N.S.; Queiroz-Telles, F. Susceptibility and Molecular Characterization of Candida Species from Patients with Vulvovaginitis. Braz. J. Microbiol. 2016, 47, 373–380. [Google Scholar] [CrossRef]
- Sucupira, P.H.F.; Moura, T.R.; Gurgel, I.L.S.; Pereira, T.T.P.; Padovan, A.C.B.; Teixeira, M.M.; Bahia, D.; Soriani, F.M. In Vitro and in Vivo Characterization of Host–Pathogen Interactions of the L3881 Candida albicans Clinical Isolate. Front. Microbiol. 2022, 13, 901442. [Google Scholar] [CrossRef]
- Nobile, C.J.; Johnson, A.D. Candida albicans Biofilms and Human Disease. Annu. Rev. Microbiol. 2015, 69, 71–92. [Google Scholar] [CrossRef]
- Watamoto, T.; Samaranayake, L.P.; Egusa, H.; Yatani, H.; Seneviratne, C.J. Transcriptional Regulation of Drug-Resistance Genes in Candida albicans Biofilms in Response to Antifungals. J. Med. Microbiol. 2011, 60, 1241–1247. [Google Scholar] [CrossRef]
- Steinbach, W.J. Pediatric Invasive Candidiasis: Epidemiology and Diagnosis in Children. J. Fungi 2016, 2, 5. [Google Scholar] [CrossRef]
- Childers, N.K.; Stinnett, E.A.; Wheeler, P.; Wright, J.T.; Castleberry, R.P.; Dasanayake, A.P. Oral Complications in Children with Cancer. Oral Surg. Oral Med. Oral Pathol. 1993, 75, 41–47. [Google Scholar] [CrossRef] [PubMed]
- González Gravina, H.; González De Morán, E.; Zambrano, O.; Chourio, M.L.; Rodríguez De Valero, S.; Robertis, S.; Mesa, L.; Mesa, D.L. Oral Candidiasis in Children and Adolescents with Cancer: Identification of Candida spp. Med. Oral Patol. Oral Cir. Bucal (Int.) 2007, 12, E419–E423. [Google Scholar]
- Shirazi, J.H.; Ali, M.I.; Akhtar, Z.; Jamal, A.; Rashid, A. Pediatric Oropharyngeal Candidiasis: A Comprehensive Study on Risk Factors and Most Prevalent Species of Candida. Pak. J. Pharm. Sci. 2019, 32, 2873–2878. [Google Scholar] [CrossRef] [PubMed]
- Palazzi, D.L.; Arrieta, A.; Castagnola, E.; Halasa, N.; Hubbard, S.; Brozovich, A.A.; Fisher, B.T.; Steinbach, W.J. Candida Speciation, Antifungal Treatment and Adverse Events in Pediatric Invasive Candidiasis: Results from 441 Infections in a Prospective, Multi-National Study. Pediatr. Infect. Dis. J. 2014, 33, 1294–1296. [Google Scholar] [CrossRef] [PubMed]
- Rajeshwari, R.; Vyasam, S.; Chandran, J.; Porwal, S.; Ebenezer, K.; Thokchom, M.; James, E.J.; Karuppusami, R. Risk Factors for Candida Infection among Children Admitted to a Pediatric Intensive Care Unit in a Tertiary Care Centre in Southern India. Indian J. Crit. Care Med. 2022, 26, 717–722. [Google Scholar] [CrossRef]
- Steinbach, W.J.; Roilides, E.; Berman, D.; Hoffman, J.A.; Groll, A.H.; Bin-Hussain, I.; Palazzi, D.L.; Castagnola, E.; Halasa, N.; Velegraki, A.; et al. Results From a Prospective, International, Epidemiologic Study of Invasive Candidiasis in Children and Neonates. Pediatr. Infect. Dis. J. 2012, 31, 1252–1257. [Google Scholar] [CrossRef]
- Kim, E.J.; Lee, E.; Kwak, Y.G.; Yoo, H.M.; Choi, J.Y.; Kim, S.R.; Shin, M.J.; Yoo, S.Y.; Cho, N.H.; Choi, Y.H. Trends in the Epidemiology of Candidemia in Intensive Care Units From 2006 to 2017: Results From the Korean National Healthcare-Associated Infections Surveillance System. Front. Med. (Lausanne) 2020, 7, 606976. [Google Scholar] [CrossRef]
- Kumari, A.; Mankotia, S.; Chaubey, B.; Luthra, M.; Singh, R. Role of Biofilm Morphology, Matrix Content and Surface Hydrophobicity in the Biofilm-Forming Capacity of Various Candida Species. J. Med. Microbiol. 2018, 67, 889–892. [Google Scholar] [CrossRef]
- Singh, R.; Kumari, A.; Kaur, K.; Sethi, P.; Chakrabarti, A. Relevance of Antifungal Penetration in Biofilm-Associated Resistance of Candida albicans and Non-albicans Candida Species. J. Med. Microbiol. 2018, 67, 922–926. [Google Scholar] [CrossRef]
- Ferreira, J.A.G.; Carr, J.H.; Starling, C.E.F.; de Resende, M.A.; Donlan, R.M. Biofilm Formation and Effect of Caspofungin on Biofilm Structure of Candida Species Bloodstream Isolates. Antimicrob. Agents Chemother. 2009, 53, 4377–4384. [Google Scholar] [CrossRef]
- Gebremedhin, S.; Dorocka-Bobkowska, B.; Prylinski, M.; Konopka, K.; Duzgunes, N. Miconazole Activity Against Candida Biofilms Developed On Acrylic Discs. J. Physiol. Pharmacol. 2014, 4, 593–600. [Google Scholar]
- Mitchell, K.F.; Zarnowski, R.; Sanchez, H.; Edward, J.A.; Reinicke, E.L.; Nett, J.E.; Mitchell, A.P.; Andes, D.R. Community Participation in Biofilm Matrix Assembly and Function. Proc. Natl. Acad. Sci. USA 2015, 112, 4092–4097. [Google Scholar] [CrossRef] [PubMed]
- Nett, J.E.; Andes, D.R. Contributions of the Biofilm Matrix to Candida Pathogenesis. J. Fungi 2020, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Thiyahuddin, N.M.; Lamping, E.; Rich, A.M.; Cannon, R.D. Yeast Species in the Oral Cavities of Older People: A Comparison between People Living in Their Own Homes and Those in Rest Homes. J. Fungi 2019, 5, 30. [Google Scholar] [CrossRef]
- Rodrigues, C.F. Candida Glabrata Biofilms: Mechanisms of Antifungal Resistance and Matrix Role. Doctoral Thesis, Universidade do Minho, Braga, Portugal, 2018. [Google Scholar]
- Pierce, C.G.; Srinivasan, A.; Uppuluri, P.; Ramasubramanian, A.K.; López-Ribot, J.L. Antifungal Therapy with an Emphasis on Biofilms. Curr. Opin. Pharmacol. 2013, 13, 726–730. [Google Scholar] [CrossRef]
- Sharma, J.; Rosiana, S.; Razzaq, I.; Shapiro, R.S. Linking Cellular Morphogenesis with Antifungal Treatment and Susceptibility in Candida Pathogens. J. Fungi 2019, 5, 17. [Google Scholar] [CrossRef]
- Ha, K.C.; White, T.C. Effects of Azole Antifungal Drugs on the Transition from Yeast Cells to Hyphae in Susceptible and Resistant Isolates of the Pathogenic Yeast Candida albicans. Antimicrob. Agents Chemother. 1999, 43, 763–768. [Google Scholar] [CrossRef]
- Nett, J.; Lincoln, L.; Marchillo, K.; Massey, R.; Holoyda, K.; Hoff, B.; VanHandel, M.; Andes, D. Putative Role of β-1,3 Glucans in Candida albicans Biofilm Resistance. Antimicrob. Agents Chemother. 2007, 51, 510–520. [Google Scholar] [CrossRef]
- Rad, K.K.; Falahati, M.; Roudbary, M.; Farahyar, S.; Nami, S. Overexpression of MDR-1 and CDR-2 genes in fluconazole resistance of Candida albicans isolated from patients with vulvovaginal candidiasis. Curr. Med. Mycol. 2016, 2, 24–29. [Google Scholar] [CrossRef]
- Mitchell, K.F.; Zarnowski, R.; Andes, D.R. Fungal Super Glue: The Biofilm Matrix and Its Composition, Assembly, and Functions. PLoS Pathog. 2016, 12, e1005828. [Google Scholar] [CrossRef]
- Al-Fattani, M.A.; Douglas, L.J. Biofilm Matrix of Candida albicans and Candida tropicalis: Chemical Composition and Role in Drug Resistance. J. Med. Microbiol. 2006, 55, 999–1008. [Google Scholar] [CrossRef] [PubMed]
- Douglas, C.M. Fungal ß(1,3)-D-Glucan Synthesis. Med. Mycol. 2001, 39, 55–66. [Google Scholar] [CrossRef]
- Rodrigues, C.F.; Boas, D.V.; Haynes, K.; Henriques, M. The MNN2 Gene Knockout Modulates the Antifungal Resistance of Biofilms of Candida glabrata. Biomolecules 2018, 8, 130. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Chandra, J.; Kuhn, D.M.; Ghannoum, M.A. Mechanism of Fluconazole Resistance in Candida albicans Biofilms: Phase-Specific Role of Efflux Pumps and Membrane Sterols. Infect. Immun. 2003, 71, 4333–4340. [Google Scholar] [CrossRef]
- Fonseca, E.; Silva, S.; Rodrigues, C.F.; Alves, C.T.; Azeredo, J.; Henriques, M. Effects of Fluconazole on Candida Glabrata Biofilms and Its Relationship with ABC Transporter Gene Expression. Biofouling 2014, 30, 447–457. [Google Scholar] [CrossRef] [PubMed]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs for Antifungal Agents Version 10.0, Valid from 2020-02-04. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Clinical_breakpoints/AFST_BP_v10.0_200204_updatd_links_200924.pdf (accessed on 23 February 2023).
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Ramage, G.; Vande, W.K.; Wickes, B.L.; López-Ribot, J.L. Standardized method for in vitro antifungal susceptibility testing of Candida albicans biofilms. Antimicrob Agents Chemother 2001, 45, 2475–2479. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Delattin, N.; Cammue, B.P.; Thevissen, K. Reactive Oxygen Species-Inducing Antifungal Agents and Their Activity against Fungal Biofilms. Future Med. Chem. 2014, 6, 77–90. [Google Scholar] [CrossRef]
- Radwan, M.A.; AlQuadeib, B.T.; Šiller, L.; Wright, M.C.; Horrocks, B. Oral Administration of Amphotericin B Nanoparticles: Antifungal Activity, Bioavailability and Toxicity in Rats. Drug Deliv. 2017, 24, 40–50. [Google Scholar] [CrossRef]
- Rosato, A.; Piarulli, M.; Schiavone, B.P.I.; Catalano, A.; Carocci, A.; Carrieri, A.; Carone, A.; Caggiano, G.; Franchini, C.; Corbo, F.; et al. In Vitro Effectiveness of Anidulafungin against Candida spp. Biofilms. J. Antibiot. (Tokyo) 2013, 66, 701–704. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).