Assessment of the Prescriptions of Systemic Antibiotics in Primary Dental Care in Germany from 2017 to 2021: A Longitudinal Drug Utilization Study
Abstract
1. Introduction
2. Results
2.1. Dental Prescriptions by Year and Substance
2.2. All Antibiotic Prescriptions by Year and Substance
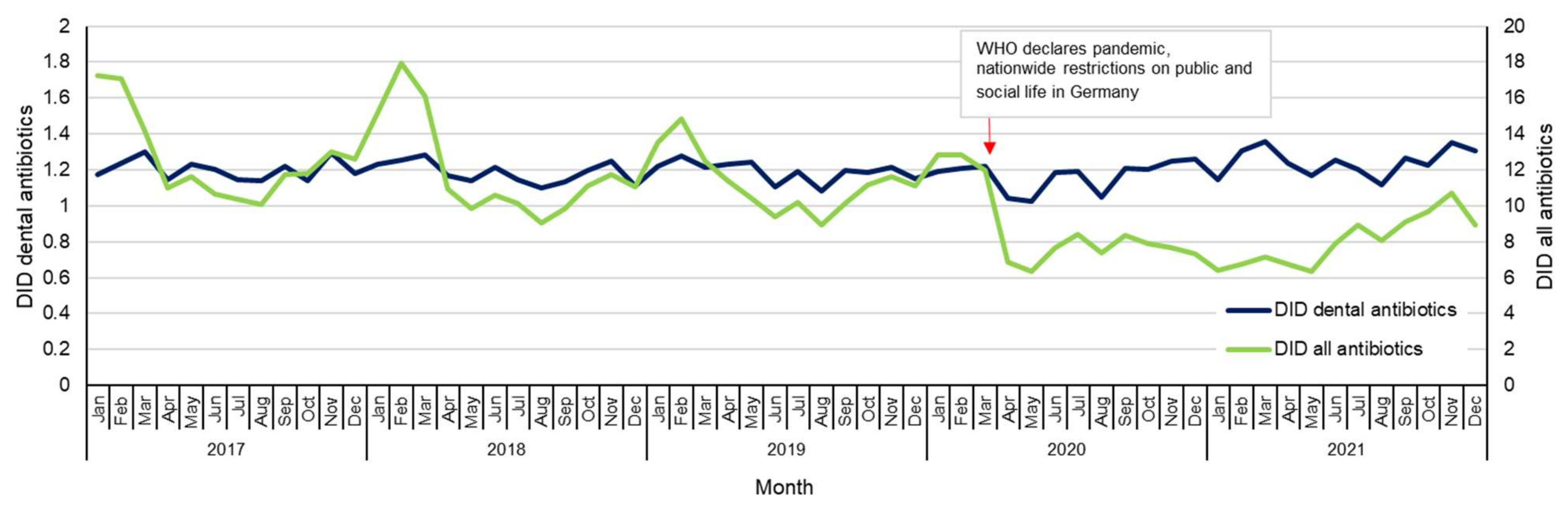
2.3. Antibiotic Prescriptions by Month
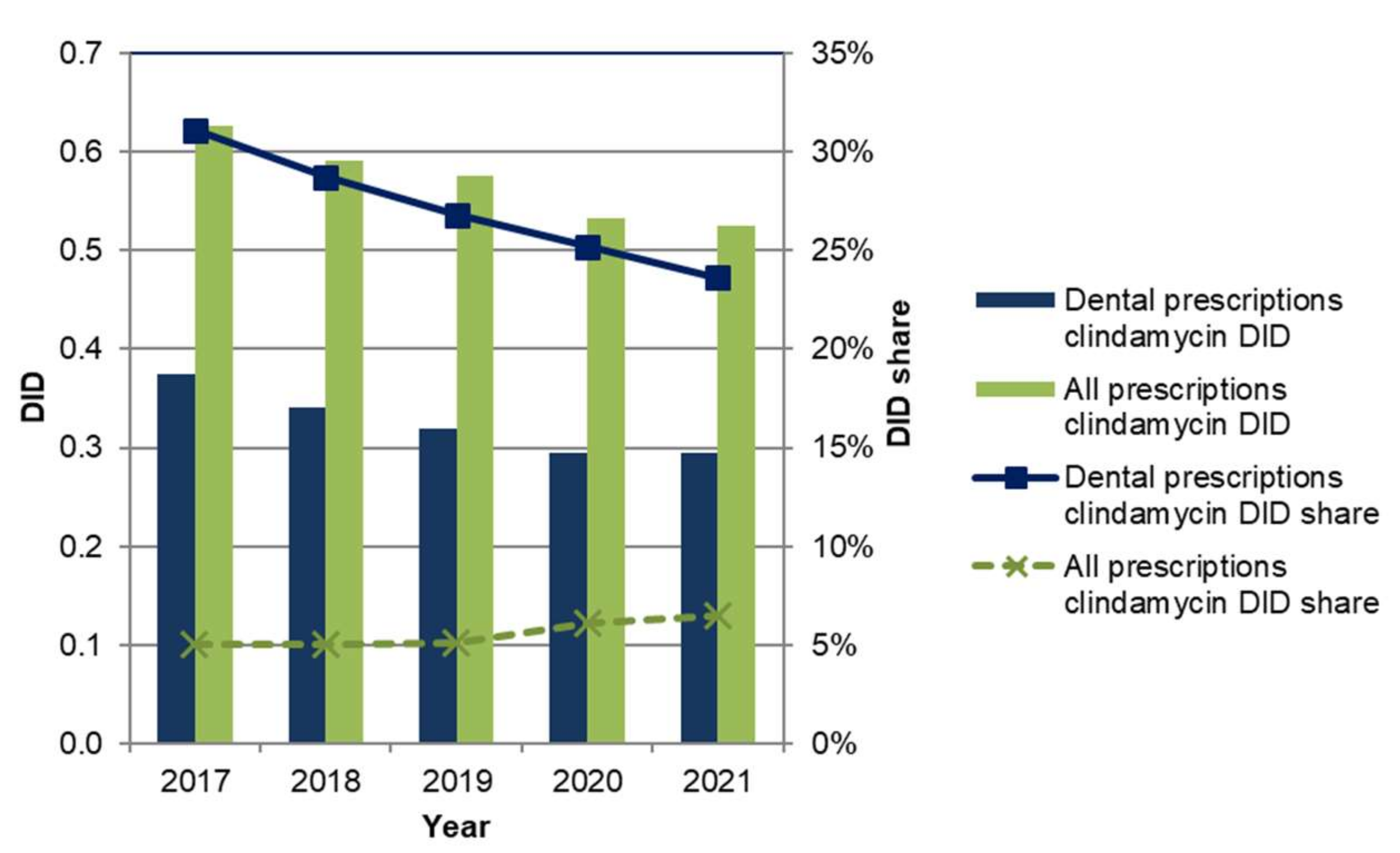
2.4. Analysis of Clindamycin Prescriptions
3. Discussion
3.1. Prescriptions of All Antibiotics in Primary Dental Care
3.2. Prescriptions of Clindamycin in Primary Dental Care
3.3. Prescriptions of Other Antibiotics in Primary Dental Care
3.4. Strengths and Limitations
4. Materials and Methods
4.1. Study Design
4.2. Classification of Data and Measurement of Antibiotic Prescriptions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Llor, C.; Bjerrum, L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Ther. Adv. Drug Saf. 2014, 5, 229–241. [Google Scholar] [CrossRef]
- European Commission. A Pharmaceutical Strategy for Europe. Available online: https://health.ec.europa.eu/medicinal-products/pharmaceutical-strategy-europe_en (accessed on 22 November 2022).
- European Centre for Disease Prevention and Control. Antimicrobial Consumption in the EU/EEA (ESAC-Net)—Annual Epidemiological Report for 2020. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-consumption-europe-2020 (accessed on 27 October 2022).
- Halling, F.; Neff, A.; Heymann, P.; Ziebart, T. Trends in antibiotic prescribing by dental practitioners in Germany. J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2017, 45, 1854–1859. [Google Scholar] [CrossRef]
- Smith, A.; Al-Mahdi, R.; Malcolm, W.; Palmer, N.; Dahlen, G.; Al-Haroni, M. Comparison of antimicrobial prescribing for dental and oral infections in England and Scotland with Norway and Sweden and their relative contribution to national consumption 2010–2016. BMC Oral Health 2020, 20, 172. [Google Scholar] [CrossRef]
- Struyf, T.; Vandael, E.; Leroy, R.; Mertens, K.; Catry, B. Antimicrobial prescribing by Belgian dentists in ambulatory care, from 2010 to 2016. Int. Dent. J. 2019, 69, 480–487. [Google Scholar] [CrossRef]
- Drug Commission of German Dentists (AKZ). Information on Dental Drugs. Available online: https://www.bzaek.de/berufsausuebung/arzneimittel-medizinprodukte/informationen-zahnaerztliche-arzneimittel-iza.html (accessed on 30 September 2022).
- Halling, F. Antibiotics in dentistry. Zahnmed. Up2date 2014, 8, 67–82. [Google Scholar]
- The German Society for Oral and Maxillofacial Surgery, German Society of Dentistry and Oral Medicine. S3 Guideline: Odontogenic Infections; AWMF-Reg.-Nr. 007/006. Available online: https://register.awmf.org/de/leitlinien/detail/007-006 (accessed on 22 November 2022).
- Stein, K.; Farmer, J.; Singhal, S.; Marra, F.; Sutherland, S.; Quiñonez, C. The use and misuse of antibiotics in dentistry: A scoping review. J. Am. Dent. Assoc. 2018, 149, 869–884e5. [Google Scholar] [CrossRef]
- Hussein, R.J.; Krohn, R.; Kaufmann-Kolle, P.; Willms, G. Quality indicators for the use of systemic antibiotics in dentistry. Z. Evidenz Fortbild. Und Qual. Gesundh. 2017, 122, 1–8. [Google Scholar] [CrossRef]
- Hussein, R.J.; Krohn, R.; Wilms, G. The use of systemic antibiotics in endodontic treatments, teeth extractions and emergency treatments within the statutory health insurance system in Germany. DZZ 2018, 73, 22–28. [Google Scholar]
- Halling, F. Dental prescriptions. In Arzneiverordnungs-Report 2017; Schwabe, U., Paffrath, D., Ludwig, W.-D., Klauber, J., Eds.; Springer: Berlin, Germany, 2017; pp. 759–769. [Google Scholar]
- Halling, F. Dental prescriptions. In Arzneiverordnungs-Report 2018; Schwabe, U., Paffrath, D., Ludwig, W.-D., Klauber, J., Eds.; Springer: Berlin, Germany, 2018; pp. 813–824. [Google Scholar]
- Halling, F. Dental prescriptions. In Arzneiverordnungs-Report 2019; Schwabe, U., Paffrath, D., Ludwig, W.-D., Klauber, J., Eds.; Springer: Berlin, Germany, 2019; pp. 1021–1033. [Google Scholar]
- Halling, F. Dental prescriptions. In Arzneiverordnungs-Report 2020; Schwabe, U., Ludwig, W.-D., Eds.; Springer: Berlin, Germany, 2020; pp. 873–885. [Google Scholar]
- Daubländer, M.; Höcherl, K. Dental prescriptions. In Arzneiverordnungs-Report 2021; Ludwig, W.-D., Mühlbauer, B., Seifert, R., Eds.; Springer: Berlin, Germany, 2021; pp. 785–798. [Google Scholar]
- Holstiege, J.; Schulz, M.; Akmatov, M.K.; Steffen, A.; Bätzing, J. Marked reductions in outpatient antibiotic prescriptions for children and adolescents—A population-based study covering 83% of the paediatric population, Germany, 2010 to 2018. Eurosurveillance 2020, 25, 1900599. [Google Scholar] [CrossRef]
- Enners, S.; Gradl, G.; Kieble, M.; Böhm, M.; Laufs, U.; Schulz, M. Utilization of drugs with reports on potential efficacy or harm on COVID-19 before, during, and after the first pandemic wave. Pharmacoepidemiol. Drug Saf. 2021, 30, 1493–1503. [Google Scholar] [CrossRef]
- Shah, S.; Wordley, V.; Thompson, W. How did COVID-19 impact on dental antibiotic prescribing across England? Br. Dent. J. 2020, 229, 601–604. [Google Scholar] [CrossRef]
- Cope, A.L.; Francis, N.A.; Wood, F.; Chestnutt, I.G. Antibiotic prescribing in UK general dental practice: A cross-sectional study. Community Dent. Oral Epidemiol. 2016, 44, 145–153. [Google Scholar] [CrossRef]
- Thompson, W.; Tonkin-Crine, S.; Pavitt, S.H.; McEachan, R.R.C.; Douglas, G.V.A.; Aggarwal, V.R.; Sandoe, J.A.T. Factors associated with antibiotic prescribing for adults with acute conditions: An umbrella review across primary care and a systematic review focusing on primary dental care. J. Antimicrob. Chemother. 2019, 74, 2139–2152. [Google Scholar] [CrossRef]
- Böhmer, F.; Hornung, A.; Burmeister, U.; Köchling, A.; Altiner, A.; Lang, H.; Löffler, C. Factors, perceptions and beliefs associated with inappropriate antibiotic prescribing in german primary dental care: A qualitative study. Antibiotics 2021, 10, 987. [Google Scholar] [CrossRef]
- Köhler, M.; Meyer, J.; Linder, M.; Lambrecht, J.-T.; Filippi, A.; Kulik Kunz, E.M. Prescription of antibiotics in the dental practice: A survey of dentists in Switzerland. Schweiz. Mon. Zahnmed. 2013, 123, 748–759. [Google Scholar]
- Falkenstein, S.; Stein, J.M.; Henne, K.; Conrads, G. Trends in antibiotic use and microbial diagnostics in periodontal treatment: Comparing surveys of German dentists in a ten-year period. Clin. Oral Investig. 2016, 20, 2203–2210. [Google Scholar] [CrossRef]
- Löffler, C.; Böhmer, F.; Hornung, A.; Lang, H.; Burmeister, U.; Podbielski, A.; Wollny, A.; Kundt, G.; Altiner, A. Dental care resistance prevention and antibiotic prescribing modification-the cluster-randomised controlled DREAM trial. Implement. Sci. 2014, 9, 27. [Google Scholar] [CrossRef]
- Meinen, A.; Reuss, A.; Willrich, N.; Feig, M.; Noll, I.; Eckmanns, T.; Al-Nawas, B.; Markwart, R. Antimicrobial resistance and the spectrum of pathogens in dental and oral-maxillofacial infections in hospitals and dental practices in Germany. Front. Microbiol. 2021, 12, 676108. [Google Scholar] [CrossRef]
- Thornhill, M.H.; Dayer, M.J.; Durkin, M.J.; Lockhart, P.B.; Baddour, L.M. Risk of adverse reactions to oral antibiotics prescribed by dentists. J. Dent. Res. 2019, 98, 1081–1087. [Google Scholar] [CrossRef]
- Schindler, C.; Stahlmann, R.; Kirch, W. The Drug Commission of Dentists Informs: These Side Effects Were Reported in 2010. Available online: https://www.bzaek.de/fileadmin/PDFs/za/AKZ/UAW/2010Zmed-AKZ-UAWs.pdf (accessed on 26 October 2022).
- Schindler, C.; Kirch, W. The Drug Commission of Dentists Informs: These Side Effects Were Reported in 2011. Available online: https://www.zm-online.de/archiv/2012/19/zahnmedizin/diese-nebenwirkungen-wurden-2011-gemeldet/ (accessed on 26 October 2022).
- Schindler, C.; Kirch, W. The Drug Commission of Dentists Informs: These Side Effects Were Reported in 2012. Available online: https://www.zm-online.de/archiv/2014/02/zahnmedizin/diese-nebenwirkungen-wurden-2012-gemeldet/ (accessed on 26 October 2022).
- Schindler, C.; Kirch, W.; The Drug Commission of Dentists Informs: Reported Side Effects. 2013/2014. Available online: https://www.zm-online.de/archiv/2015/08/zahnmedizin/gemeldete-nebenwirkungen-20132014/ (accessed on 26 October 2022).
- Schindler, C.; Nagaba, J.; Stahlmann, R. Adverse Event Reports for Clindamycin Decline for the First Time. Available online: https://www.zm-online.de/archiv/2018/09/zahnmedizin/uaw-meldungen-zu-clindamycin-erstmals-ruecklaeufig/ (accessed on 18 October 2022).
- Schindler, C.; Nagaba, J.; Schumacher, C. Adverse Event Reports on Clindamycin on the Rise Again. Available online: https://www.zm-online.de/archiv/2019/07/zahnmedizin/uaw-meldungen-zu-clindamycin-wieder-zunehmend/ (accessed on 26 October 2022).
- Brown, K.A.; Khanafer, N.; Daneman, N.; Fisman, D.N. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob. Agents Chemother. 2013, 57, 2326–2332. [Google Scholar] [CrossRef]
- Ibing, M.; Schäfer, E. Antibiotics in Endodontics. Available online: https://www.zm-online.de/archiv/2022/10/zahnmedizin/antibiotika-in-der-endodontie/ (accessed on 17 October 2022).
- Wurpts, G.; Aberer, W.; Dickel, H.; Brehler, R.; Jakob, T.; Kreft, B.; Mahler, V.; Merk, H.F.; Mülleneisen, N.; Ott, H.; et al. Guideline on diagnostic procedures for suspected hypersensitivity to beta-lactam antibiotics. Allergo J. Int. 2019, 28, 121–151. [Google Scholar] [CrossRef]
- Sakoulas, G.; Geriak, M.; Nizet, V. Is a reported penicillin allergy sufficient grounds to forgo the multidimensional Antimicrobial Benefits of β-Lactam Antibiotics? Clin. Infect. Dis. 2019, 68, 157–164. [Google Scholar] [CrossRef]
- German Dental Association (BZÄK). Annual Report. 2021/2022. Available online: https://www.bzaek.de/fileadmin/PDFs/presse/jahresbericht/jahresbericht_bzaek.pdf (accessed on 28 October 2022).
- Teoh, L.; Stewart, K.; Marino, R.J.; McCullough, M.J. Improvement of dental prescribing practices using education and a prescribing tool: A pilot intervention study. Br. J. Clin. Pharmacol. 2021, 87, 152–162. [Google Scholar] [CrossRef]
- Löffler, C.; Böhmer, F. The effect of interventions aiming to optimise the prescription of antibiotics in dental care-A systematic review. PLoS ONE 2017, 12, e0188061. [Google Scholar] [CrossRef]
- Cosgarea, R.; Jepsen, K. Antibiotics and Resistance Developments. Available online: https://www.zm-online.de/archiv/2022/11/zahnmedizin/antibiotika-und-resistenzentwicklungen/ (accessed on 3 November 2022).
- Cosgarea, R.; Wenzel, S.; Jepsen, K. Systemic Antibiotics in Periodontal Therapy: Benefits and Risks. Available online: https://www.zm-online.de/archiv/2022/11/zahnmedizin/systemische-antibiotika-in-der-parodontaltherapie-nutzen-und-risiken/ (accessed on 3 November 2022).
- Al-Nawas, B. Use of Antibiotics in Dental Practice. Available online: https://www.dgzmk.de/web/suite-dgzmk/antibiotika-in-der-zahnaerztlichen-praxis?p_p_id=56_INSTANCE_7N2A5ZL5qA5X&p_p_lifecycle=0&p_p_state=normal&p_p_mode=view&p_p_col_id=column-1&#p_56_INSTANCE_7N2A5ZL5qA5X (accessed on 21 November 2022).
- Gradl, G.; Teichert, M.; Kieble, M.; Werning, J.; Schulz, M. Comparing outpatient oral antibiotic use in Germany and the Netherlands from 2012 to 2016. Pharmacoepidemiol. Drug Saf. 2018, 27, 1344–1355. [Google Scholar] [CrossRef]
- Gradl, G.; Werning, J.; Enners, S.; Kieble, M.; Schulz, M. Quality appraisal of ambulatory oral cephalosporin and fluoroquinolone use in the 16 german federal states from 2014–2019. Antibiotics 2021, 10, 831. [Google Scholar] [CrossRef]
- World Health Organization. WHO Releases the 2019 AWaRe Classification Antibiotics. Available online: https://www.who.int/publications/i/item/WHOEMPIAU2019.11 (accessed on 21 November 2022).
- Nakai, H.; Hagihara, M.; Kato, H.; Hirai, J.; Nishiyama, N.; Koizumi, Y.; Sakanashi, D.; Suematsu, H.; Yamagishi, Y.; Mikamo, H. Prevalence and risk factors of infections caused by extended-spectrum β-lactamase (ESBL)-producing Enterobacteriaceae. J. Infect. Chemother. 2016, 22, 319–326. [Google Scholar] [CrossRef]
- Eckert, A.W.; Kolk, A. Odontogenic infections and pathogen spectra in maxillofacial surgery. Der MKG-Chir. 2014, 7, 256–260. [Google Scholar] [CrossRef]
- Sobottka, I.; Wegscheider, K.; Balzer, L.; Böger, R.H.; Hallier, O.; Giersdorf, I.; Streichert, T.; Haddad, M.; Platzer, U.; Cachovan, G. Microbiological analysis of a prospective, randomized, double-blind trial comparing moxifloxacin and clindamycin in the treatment of odontogenic infiltrates and abscesses. Antimicrob. Agents Chemother. 2012, 56, 2565–2569. [Google Scholar] [CrossRef]
- Huttner, A.; Bielicki, J.; Clements, M.N.; Frimodt-Møller, N.; Muller, A.E.; Paccaud, J.-P.; Mouton, J.W. Oral amoxicillin and amoxicillin-clavulanic acid: Properties, indications and usage. Clin. Microbiol. Infect. 2020, 26, 871–879. [Google Scholar] [CrossRef]
- Zaidi, S.A. Hepatitis associated with amoxicillin/clavulanic acid and/or ciprofloxacin. Am. J. Med. Sci. 2003, 325, 31–33. [Google Scholar] [CrossRef]
- Gresser, U. Amoxicillin-clavulanic acid therapy may be associated with severe side effects—Review of the literature. Eur. J. Med. Res. 2001, 6, 139–149. [Google Scholar]
- Verband der Privaten Krankenversicherung e.V. Private Insurance Numbers. Available online: https://www.pkv.de/wissen/pkv-zahlenportal/ (accessed on 26 October 2022).
- Elseviers, M.; Wettermark, B.; Almarsdóttir, A.B.; Andersen, M.; Benko, R.; Bennie, M.; Eriksson, I.; Godman, B.; Krska, J.; Poluzzi, E.; et al. Drug Utilization Research: Methods and Applications; John Wiley & Sons, Ltd: Chichester, UK, 2016. [Google Scholar]
- WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD International Language for Drug Utilization Research: The Anatomical Therapeutic Chemical (ATC) Classification System. Available online: https://www.whocc.no/ (accessed on 26 October 2022).
- Federal Institute for Drugs and Medical Devices. ATC-Classification. Available online: https://www.bfarm.de/EN/Code-systems/Classifications/ATC/_node.html (accessed on 13 June 2022).
- Federal Ministry of Health. KM6-Statistics. Available online: https://www.bundesgesundheitsministerium.de/themen/krankenversicherung/zahlen-und-fakten-zur-krankenversicherung/mitglieder-und-versicherte.html (accessed on 26 October 2022).
| Year | 2017 | 2018 | 2019 | 2020 | 2021 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance | DID | % | DID | % | DID | % | DID | % | DID | % | Δ | Δ% |
| All antibiotics | 1.200 | 100.0% | 1.185 | 100.0% | 1.193 | 100.0% | 1.169 | 100.0% | 1.245 | 100.0% | 0.045 | 3.7% |
| Amoxicillin | 0.505 | 42.1% | 0.530 | 44.7% | 0.560 | 47.0% | 0.570 | 48.7% | 0.627 | 50.4% | 0.122 | 24.2% |
| Clindamycin | 0.374 | 31.1% | 0.340 | 28.7% | 0.319 | 26.8% | 0.295 | 25.2% | 0.294 | 23.6% | −0.080 | −21.4% |
| Amoxicillin and beta-lactamase inhibitor | 0.100 | 8.4% | 0.113 | 9.5% | 0.128 | 10.8% | 0.139 | 11.9% | 0.164 | 13.1% | 0.064 | 62.8% |
| Phenoxymethylpenicillin | 0.110 | 9.2% | 0.099 | 8.3% | 0.090 | 7.5% | 0.079 | 6.8% | 0.075 | 6.0% | −0.035 | −32.1% |
| Doxycycline | 0.036 | 3.0% | 0.032 | 2.7% | 0.030 | 2.5% | 0.027 | 2.3% | 0.026 | 2.1% | −0.010 | −26.2% |
| Cefuroxime | 0.028 | 2.3% | 0.026 | 2.2% | 0.024 | 2.0% | 0.022 | 1.9% | 0.022 | 1.8% | −0.006 | −20.7% |
| Metronidazole | 0.015 | 1.3% | 0.015 | 1.3% | 0.016 | 1.3% | 0.014 | 1.2% | 0.014 | 1.1% | −0.001 | −8.9% |
| Other antibiotics | 0.032 | 2.6% | 0.030 | 2.6% | 0.026 | 2.1% | 0.023 | 2.0% | 0.023 | 1.9% | −0.009 | –28.7% |
| Year | 2017 | 2018 | 2019 | 2020 | 2021 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Substance | DID | % | DID | % | DID | % | DID | % | DID | % | Δ | Δ% |
| All antibiotics | 12.581 | 100.0% | 11.925 | 100.0% | 11.242 | 100.0% | 8.786 | 100.0% | 8.056 | 100.0% | −4.525 | −36.0% |
| Amoxicillin | 2.332 | 18.5% | 2.379 | 19.9% | 2.332 | 20.7% | 1.702 | 19.4% | 1.575 | 19.6% | −0.757 | −32.5% |
| Doxycycline | 1.602 | 12.7% | 1.506 | 12.6% | 1.392 | 12.4% | 1.274 | 14.5% | 1.156 | 14.4% | −0.446 | −27.8% |
| Cefuroxime | 2.144 | 17.0% | 1.899 | 15.9% | 1.743 | 15.5% | 1.207 | 13.7% | 0.994 | 12.3% | −1.150 | −53.7% |
| Amoxicillin and beta-lactamase inhibitor | 0.576 | 4.6% | 0.645 | 5.4% | 0.748 | 6.6% | 0.704 | 8.0% | 0.753 | 9.3% | 0.177 | 30.8% |
| Clindamycin | 0.626 | 5.0% | 0.591 | 5.0% | 0.575 | 5.1% | 0.533 | 6.1% | 0.525 | 6.5% | −0.101 | −16.2% |
| Phenoxymethylpenicillin | 0.636 | 5.1% | 0.607 | 5.1% | 0.610 | 5.4% | 0.397 | 4.5% | 0.298 | 3.7% | −0.338 | −53.1% |
| Metronidazole | 0.079 | 0.6% | 0.077 | 0.6% | 0.074 | 0.7% | 0.070 | 0.8% | 0.069 | 0.9% | −0.010 | −13.0% |
| Other antibiotics | 4.586 | 36.5% | 4.221 | 35.5% | 3.768 | 33.6% | 2.899 | 33.0% | 2.686 | 33.3% | −1.900 | −41.4% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gradl, G.; Kieble, M.; Nagaba, J.; Schulz, M. Assessment of the Prescriptions of Systemic Antibiotics in Primary Dental Care in Germany from 2017 to 2021: A Longitudinal Drug Utilization Study. Antibiotics 2022, 11, 1723. https://doi.org/10.3390/antibiotics11121723
Gradl G, Kieble M, Nagaba J, Schulz M. Assessment of the Prescriptions of Systemic Antibiotics in Primary Dental Care in Germany from 2017 to 2021: A Longitudinal Drug Utilization Study. Antibiotics. 2022; 11(12):1723. https://doi.org/10.3390/antibiotics11121723
Chicago/Turabian StyleGradl, Gabriele, Marita Kieble, Jens Nagaba, and Martin Schulz. 2022. "Assessment of the Prescriptions of Systemic Antibiotics in Primary Dental Care in Germany from 2017 to 2021: A Longitudinal Drug Utilization Study" Antibiotics 11, no. 12: 1723. https://doi.org/10.3390/antibiotics11121723
APA StyleGradl, G., Kieble, M., Nagaba, J., & Schulz, M. (2022). Assessment of the Prescriptions of Systemic Antibiotics in Primary Dental Care in Germany from 2017 to 2021: A Longitudinal Drug Utilization Study. Antibiotics, 11(12), 1723. https://doi.org/10.3390/antibiotics11121723






