Efficacy of Antimicrobial Agents in Dentifrices: A Systematic Review
Abstract
1. Introduction
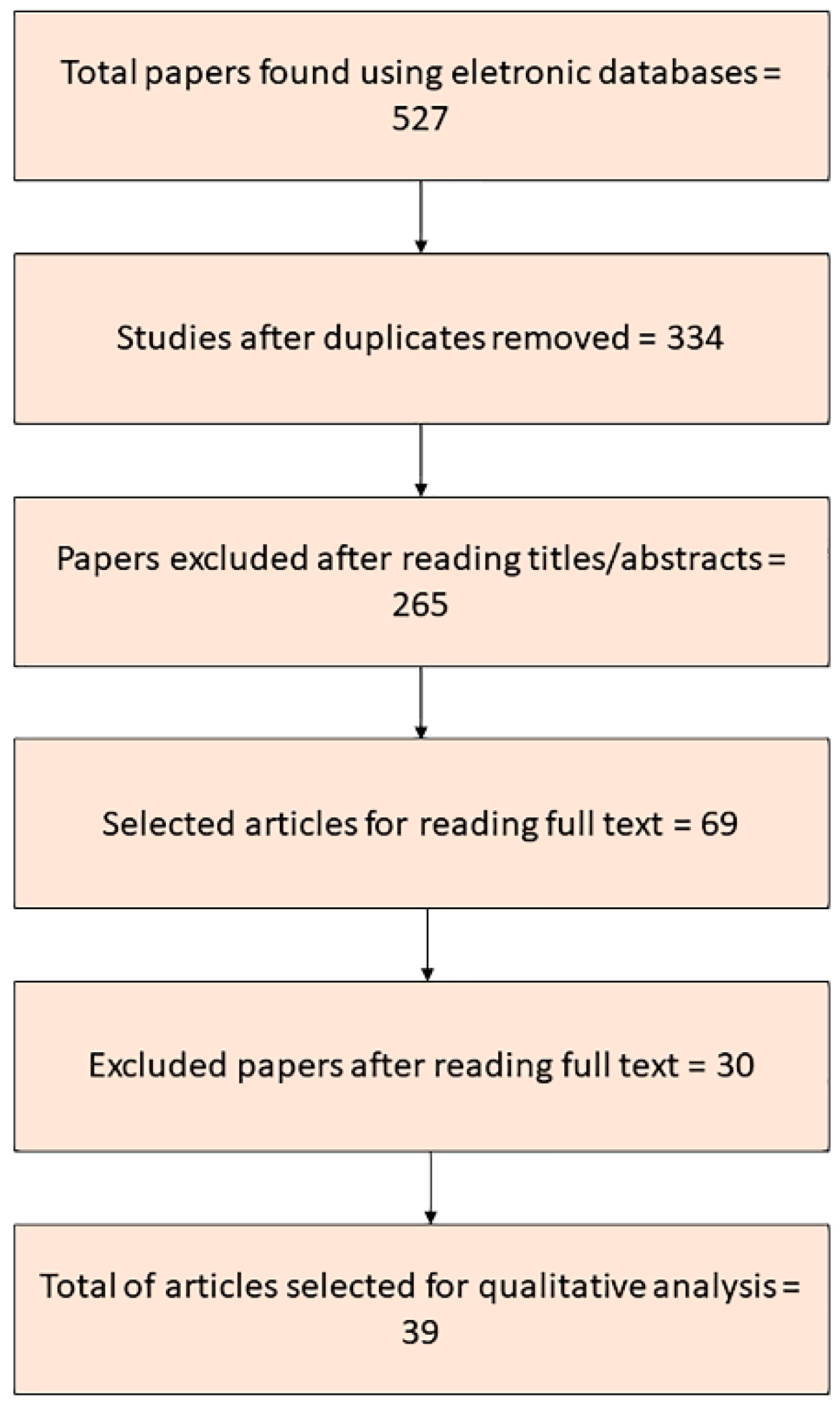
2. Materials and Methods
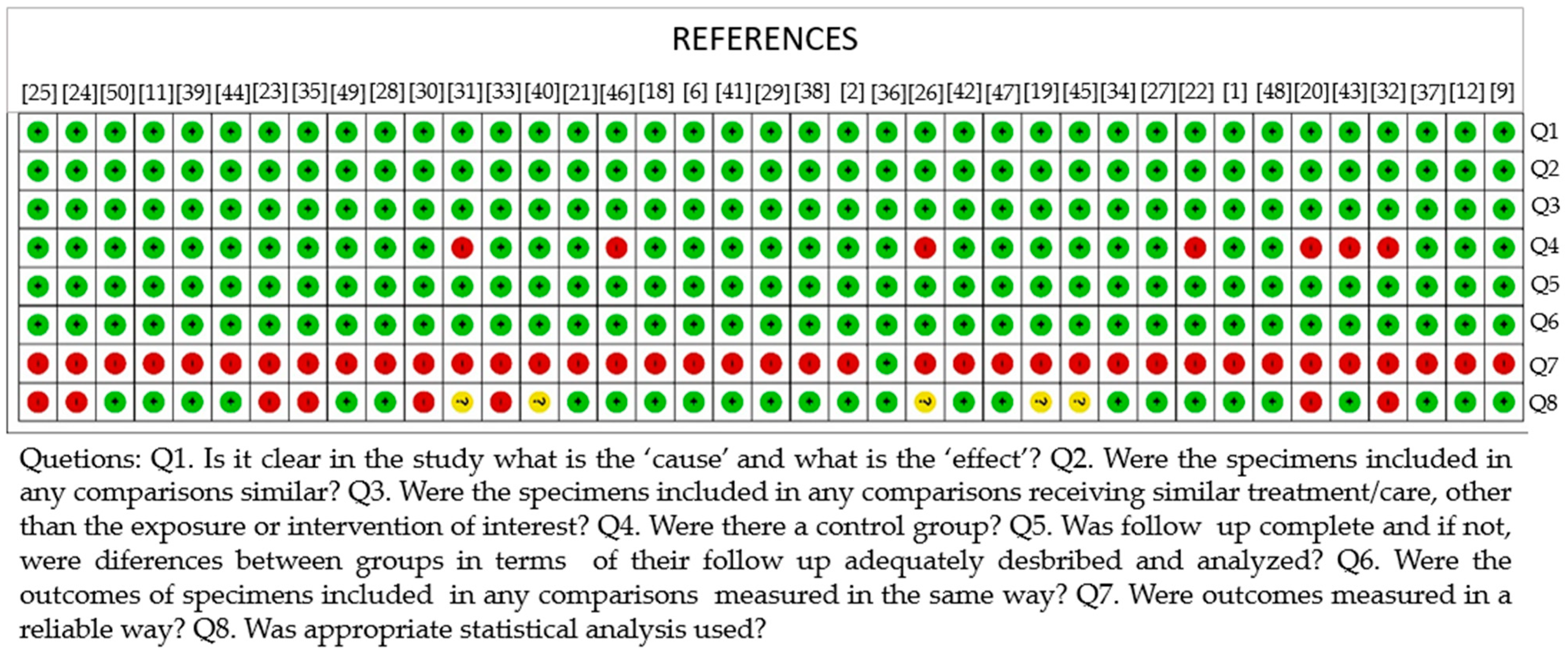
3. Results
4. Discussion
5. Conclusions
- The presence of antimicrobial agents in dentifrice formulations can promote the reduction of the number of microorganisms involved in oral diseases, but with variations in their effectiveness, depending on the agent used and the microorganism evaluated.
- Some dentifrice formulations with herbal ingredients, such as miswak and neem extracts, can be as effective as dentifrice formulations with chemical antimicrobial agents, such as sodium monofluorophosphate and sodium fluoride.
- The antimicrobial activity of a dentifrice with antimicrobial agents can be reduced when it is diluted.
- The interaction between the different components of a dentifrice can influence the effectiveness of its antimicrobial activity, and thus the synergism between the ingredients is of great importance.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Oliveira Carvalho, I.; Purgato, G.A.; Píccolo, M.S.; Pizziolo, V.R.; Coelho, R.R.; Diaz-Muñoz, G.; Diaz, M.A.N. In vitro anticariogenic and antibiofilm activities of toothpastes formulated with essential oils. Arch. Oral. Biol. 2020, 117, 104834. [Google Scholar] [CrossRef]
- Lee, S.S.; Zhang, W.; Li, Y. The antimicrobial potential of 14 natural herbal dentifrices: Results of an in vitro diffusion method study. J. Am. Dent. Assoc. 2004, 135, 1133–1141. [Google Scholar] [CrossRef]
- van Houte, J. Role of micro-organisms in caries etiology. J. Dent. Res. 1994, 73, 672–681. [Google Scholar] [CrossRef] [PubMed]
- Corby, P.M.; Lyons-Weiler, J.; Bretz, W.A.; Hart, T.C.; Aas, J.A.; Boumenna, T.; Goss, J.; Corby, A.L.; Junior, H.M.; Weyant, R.J.; et al. Microbial risk indicators of early childhood caries. J. Clin. Microbiol. 2005, 43, 5753–5759. [Google Scholar] [CrossRef] [PubMed]
- Tanzer, J.M.; Livingston, J.; Thompson, A.M. The microbiology of primary dental caries in humans. J. Dent. Educ. 2001, 65, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Prasanth, M. Antimicrobial efficacy of different toothpastes and mouthrinses: An in vitro study. Dent. Res. J. 2011, 8, 85–94. [Google Scholar]
- Desai, J.V. Candida albicans Hyphae: From Growth Initiation to Invasion. J. Fungi 2018, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Tada, A.; Senpuku, H.; Motozawa, Y.; Yoshihara, A.; Hanada, N.; Tanzawa, H. Association between commensal bacteria and opportunistic pathogens in the dental plaque of elderly individuals. Clin. Microbiol. Infect. 2006, 12, 776–781. [Google Scholar] [CrossRef]
- Ahmed, F.; Prashanth, S.T.; Sindhu, K.; Nayak, A.; Chaturvedi, S. Antimicrobial efficacy of nanosilver and chitosan against Streptococcus mutans, as an ingredient of toothpaste formulation: An in vitro study. J. Indian Soc. Pedod. Prev. Dent 2019, 37, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Malic, S.; Emanuel, C.; Lewis, M.A.; Williams, D.W. Antimicrobial activity of novel mouthrinses against planktonic cells and biofilms of pathogenic microorganisms. Microbiol. Discov. 2013, 1, 11. [Google Scholar] [CrossRef]
- Valones, M.A.A.; Higino, J.S.; Souza, P.; Crovella, S.; Júnior, A.D.F.C.; Carvalho, A.D.A.T. Dentifrice Containing Extract of Rosmarinus officinalis Linn.: An Antimicrobial Evaluation. Braz. Dent. J. 2016, 27, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Lim, X.Y.; Wahida, P.F. The fundamental study of antimicrobial activity of Piper betle extract in commercial toothpastes. J. Herb. Med. 2018, 14, 29–34. [Google Scholar] [CrossRef]
- Pannuti, C.M.; De Mattos, J.P.; Ranoya, P.N.; De Jesus, A.M.; Lotufo, R.F.M.; Romito, G.A. Clinical effect of a herbal dentifrice on the control of plaque and gingivitis: A double-blind study. Pesqui. Odontol. Bras. 2003, 17, 314–318. [Google Scholar] [CrossRef]
- Bou-Chacra, N.A.; Gobi, S.S.; Ohara, M.T.; Andreoli, T.D.J.P. Antimicrobial activity of four different dental gel formulas on cariogenic bacteria evaluated using the linear regression method. Rev. Bras. Cienc. Farm. J. Pharm. Sci. 2005, 41, 323–331. [Google Scholar] [CrossRef]
- Wiegand, A.; Buchalla, W.; Attin, T. Review on fluoride-releasing restorative materials--fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent. Mater. 2007, 23, 343–362. [Google Scholar] [CrossRef] [PubMed]
- McMurry, L.M.; Oethinger, M.; Levy, S.B. Triclosan targets lipid synthesis. Nature 1998, 394, 531–532. [Google Scholar] [CrossRef]
- Shubhra, V.; Dakshi, A.; Vidya, D.; Hari, P. Comparative Evaluation of 0.2% Chlorhexidine Versus Herbal Oral Rinse on Plaque Induced Gingivitis. J. Indian Assoc. Public Health Dent. 2012, 10, 55–62. [Google Scholar]
- Randall, J.P.; Seow, W.K.; Walsh, L.J. Antibacterial activity of fluoride compounds and herbal toothpastes on Streptococcus mutans: An in vitro study. Aust. Dent. J. 2015, 60, 368–374. [Google Scholar] [CrossRef] [PubMed]
- Junevičius, J.; Žilinskas, J.; Česaitis, K.; Česaitienė, G.; Gleiznys, D. Antimicrobial activity of silver and gold in toothpastes: A comparative analysis. Stomatologija 2015, 17, 9–12. [Google Scholar] [PubMed]
- Camargo, S.E.A.; Milhan, N.V.M.; Saraiva, F.D.O.; De Oliveira, J.R.; De Oliveira, L.D.; Camargo, C.H.R. Are Desensitizing Toothpastes Equally Biocompatible and Effective Against Microorganisms? Braz. Dent. J. 2017, 28, 604–611. [Google Scholar] [CrossRef]
- Sadeghi, M.; Assar, S. An in vitro antimicrobial activity of ten Iranian-made toothpastes. Dent. Res. J. 2009, 6, 87–92. [Google Scholar]
- Evans, A.; Leishman, S.J.; Walsh, L.J.; Seow, W.K. Inhibitory effects of children’s toothpastes on Streptococcus mutans, Streptococcus sanguinis and Lactobacillus acidophilus. Eur. Arch. Paediatr. Dent. 2015, 16, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Shewale, J.G.; Gelhaus, H.C.; Ratcliff, J.L.; Hernandez-Kapila, Y.L. In vitro antiviral activity of stabilized chlorine dioxide containing oral care products. Oral Dis. 2021, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wade, W.G.; Addy, M. Antibacterial activity of some triclosan-containing toothpastes and their ingredients. J. Periodontol. 1992, 63, 280–282. [Google Scholar] [CrossRef]
- Wade, W.; Addy, M.; Hughes, J.; Milsom, S.; Doherty, F. Studies on stannous fluoride toothpaste and gel (1). Antimicrobial properties and staining potential in vitro. J. Clin. Periodontol. 1997, 24, 81–85. [Google Scholar] [CrossRef]
- Kiesow, A.; Sarembe, S.; Pizzey, R.L.; Axe, A.S.; Bradshaw, D.J. Material compatibility and antimicrobial activity of consumer products commonly used to clean dentures. J. Prosthet. Dent. 2016, 115, 189–198.e8. [Google Scholar] [CrossRef]
- Fernández, E.; Sánchez, M.D.C.; Llama-Palacios, A. Antibacterial Effects of Toothpastes Evaluated in an In Vitro Biofilm Model. Oral Health Prev. Dent. 2017, 15, 251–257. [Google Scholar] [CrossRef]
- Shaheena, S.; Chintagunta, A.D.; Dirisala, V.R.; Kumar, N.S.S. Extraction of bioactive compounds from Psidium guajava and their application in dentistry. AMB Express 2019, 9, 208. [Google Scholar] [CrossRef]
- Sacsaquispe-Contreras, S. Development of New Experimental Dentifrice of Peruvian Solanum tuberosum (Tocosh) Fermented by Water Stress: Antibacterial and Cytotoxic Activity. J. Contemp. Dent. Pract. 2019, 20, 1206–1211. [Google Scholar] [CrossRef]
- Sekar, M.; Abdullah, M.Z. Formulation, evaluation and antimicrobial properties of polyherbal toothpaste. Int. J. Curr. Pharm. Rev. Res. 2016, 8, 105–107. [Google Scholar]
- Sato, M.; Fujiwara, S.; Nagayama, M.; Yamaguchi, R.; Tokuda, C.; Takeuchi, H.; Yamada, H.; Sugimoto, H.; Okihara, K. Effect of propolis and propolis-containing toothpaste on the formation of dental plaque in vitro. Oral Ther. Pharmacol. 2001, 20, 5–10. [Google Scholar]
- Babu, N.K.; Arumugham, I.M.; Kumar, R.P.; Sakthi, D.S.; Shanmugham, S.S. Anti-microbial Efficacy of Commercially Available Herbal Dentifrices on Streptococcus mutans and Candida albicans. J. Pharm. Res. Int. 2020, 32, 87–94. [Google Scholar] [CrossRef]
- Sartini, F.; Hamudeng, A.M. Antibacterial activity of ethanolic extract of green tea (Camellia sinensis L.) and its toothpaste products against Streptococcus mutans and Lactobacillus acidophilus. Asian J. Microbiol. Biotechnol. Environ. Sci. 2015, 17, 879–882. [Google Scholar]
- Gbedema, S.Y.; Adu, F.; Bayor, M.T.; Arhin-Sam, V.E.; Annan, K. In vitro antimicrobial study of the efficacy of a toothpaste formulated from Garcinia kola stem wood extract. Int. J. Pharm. Pharm. Sci. 2010, 2, 98–101. [Google Scholar]
- Sharma, S.; Agarwal, S.S.; Prakash, J.; Pandey, M.; Singh, A. Formulation development and quality evaluation of polyherbal toothpaste “oral s”. J. Pharm. Res. Allied Sci. 2014, 3, 30–39. [Google Scholar]
- Kooshki, F.; Tabatabaei, F.S.; Tajik, S.; Aayan, A. The comparison of antimicrobial effects of herbal and chemical agents on toothpaste: An experimental study. Dent. Res. J. 2018, 15, 289–294. [Google Scholar]
- Anushree, B.; Fawaz, M.A.; Narahari, R. Comparison of Antimicrobial Efficacy of Triclosan- Containing, Herbal and Homeopathy Toothpastes- An Invitro Study. J. Clin. Diagn. Res. 2015, 9, DC05–DC08. [Google Scholar] [CrossRef]
- Leite, V.M.F.; Pinheiro, J.B.; Pisani, M.X.; Watanabe, E.; De Souza, R.F.; Paranhos, H.D.F.O.; Lovato-Silva, C.H. In vitro antimicrobial activity of an experimental dentifrice based on Ricinus communis. Braz. Dent. J. 2014, 25, 191–196. [Google Scholar] [CrossRef]
- Pal, S.; Thounaojam, N.; Sanjenbam, N.; Singh, D.K.; Kumar, K.; Shah, M. The Efficacy of Commercially Available Herbal Dentifrices in Comparison with Conventional Dentifrices against Two Common Oral Microbes: An In vitro Study. J. Pharm. Bioallied Sci. 2021, 13 (Suppl. S1), 176–179. [Google Scholar] [CrossRef]
- Sari, Y.W.; Nuzulia, N.A.; Wahyuni, W.T.; Bahtiar, A.; Saputra, A.; Subroto, M.H.A.; Ariesanti, Y.; Syafitri, U.; Bachtiar, I. Remineralization and antibacterial/antibiofilm effects of toothpaste containing nanohydroxyapatite and Curcuma aeruginosa extract. Nat. Prod. Res. 2021, 27, 1–5. [Google Scholar] [CrossRef]
- Oluwasina, O.O.; Ezenwosu, I.V.; Ogidi, C.O.; Oyetayo, V.O. Antimicrobial potential of toothpaste formulated from extracts of Syzygium aromaticum, Dennettia tripetala and Jatropha curcas latex against some oral pathogenic microorganisms. AMB Express 2019, 9, 20. [Google Scholar] [CrossRef] [PubMed]
- Karadağlıoğlu, I.; Ulusoy, N.; Başer, K.H.C.; Hanoğlu, A.; Şık, I. Antibacterial Activities of Herbal Toothpastes Combined with Essential Oils against Streptococcus mutans. Pathogens 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Benlatef, L.; Malinee, M.; Norrapong, B.; Cowawintaweewat, S.; Pootong, A. Inhibitory activities of herbal based toothpaste on germ tube and adhesion of Candida albicans. J. Pure Appl. Microbiol. 2016, 10, 2551–2556. [Google Scholar] [CrossRef]
- Sunitha, J.; Ananthalakshmi, R.; Jeeva, J.S.; Jeddy, N.; Dhakshininamoorthy, S.; Meenakshi, R.M. Antimicrobial effect of herbal dentifrices: An in vitro study. J. Pharm. Bioallied Sci. 2015, 7, S628–S631. [Google Scholar] [CrossRef]
- Gibraiel, F.; Rajput, M.; Rajput, M.S.; Singh, M.; Saxena, N.; Vishal, A.; Kumar, A. In vitro study to investigate the antimicrobial efficacy of different toothpastes and mouth rinses. Res. J. Pharm. Biol. Chem. Sci. 2014, 5, 245–257. [Google Scholar]
- Roopavathi, K.M.; Gopal, S.V.; Pushpalatha, G.; Bennadi, D.; Renushri, B.V.; Madhura, D.B. Antimicrobial efficacy of commercially available toothpastes-An in vitro study. J. Young Pharm. 2015, 7, 187–193. [Google Scholar] [CrossRef]
- Kamarazaman, K.; Mokhtar, K.; Norhashim, M.Z.; Mohamed, Z.; Alam, M.K. A study on antibacterial activity of commercial dentifrices against Streptococcus mutans. Int. Med. J. 2014, 21, 204–207. [Google Scholar]
- Carvalho, F.G.; Negrini, T.D.C.; Sacramento, L.V.S.; Hebling, J.; Spolidorio, D.M.P.; Duque, C. The in vitro antimicrobial activity of natural infant fluoride-free toothpastes on oral micro-organisms. J. Dent. Child. 2011, 78, 3–8. [Google Scholar]
- Sharma, V.K.; Mazumder, B.; Sharma, P.P. Antimicrobial & powder characterization of herbal Dentifrices. Indian Drugs 2013, 50, 39–47. [Google Scholar] [CrossRef]
- Vanni, R.; Waldner-Tomic, N.M.; Belibasakis, G.N.; Attin, T.; Schmidlin, P.R.; Thurnheer, T. Antibacterial Efficacy of a Propolis Toothpaste and Mouthrinse Against a Supragingival Multispecies Biofilm. Oral Health Prev. Dent. 2015, 13, 531–535. [Google Scholar] [CrossRef]
- Diaz, M.A.N.; Carvalho, I.O.; Diaz, G. Herbal Dentifrices for Children. In Emerging Trends in Oral Health Sciences and Dentistry; Virdi, M.S., Ed.; IntechOpen: London, UK, 2015. [Google Scholar]
- Haraszthy, V.I.; Zambon, J.J.; Sreenivasan, P.K. Evaluation of the antimicrobial activity of dentifrices on human oral bacteria. J. Clin. Dent. 2010, 21, 96–100. [Google Scholar]
- Binney, A.; Addy, M.; McKeowr, S.; Everatt, L. The choice of controls in toothpaste studies. The effect of a number of commercially available toothpastes compared to water on 4-day plaque regrowth. J. Clin. Periodontol. 1996, 23, 456–459. [Google Scholar] [CrossRef]
- Gupta, P.; Agarwal, N.; Anup, N.; Manujunath, B.C.; Bhalla, A. Evaluating the anti-plaque efficacy of meswak (Salvadora persica) containing dentifrice: A triple blind controlled trial. J. Pharm. Bioallied Sci. 2012, 4, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Davies, R.; Scully, C.; Preston, A.J. Dentifrices—An update. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e976–e982. [Google Scholar] [CrossRef] [PubMed]
- Suller, M.T.; Russell, A.D. Triclosan and antibiotic resistance in Staphylococcus aureus. J. Antimicrob. Chemother. 2000, 46, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Blinkhorn, A.; Bartold, P.M.; Cullinan, M.P.; Madden, T.E.; Marshall, R.I.; Raphael, S.L.; Seymour, G.J. Is there a role for triclosan/copolymer toothpaste in the management of periodontal disease? Br. Dent. J. 2009, 207, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Rice, K.C.; Liu, X.-M.; Reinhardt, R.A.; Bayles, K.W.; Wang, D. Triclosan-loaded tooth-binding micelles for prevention and treatment of dental biofilm. Pharm. Res. 2010, 27, 2356–2364. [Google Scholar] [CrossRef] [PubMed]
- Forbes, S.; Latimer, J.; Sreenivasan, P.K.; McBain, A.J. Simultaneous Assessment of Acidogenesis-Mitigation and Specific Bacterial Growth-Inhibition by Dentifrices. PLoS ONE 2016, 11, e0149390. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, S.G. Comparative study of in Vitro antibacterial activity of miswak extracts and different toothpastes. Am. J. Agric. Biol. Sci. 2013, 8, 82–88. [Google Scholar] [CrossRef]
- Sivakumar, P.; Geetha, R.V. In vitro study of anti-bacterial efficacy of herbal Toothpastes vs Triclosan and Fluoride containing control. J. Pharm. Sci. Res. 2016, 8, 680–683. [Google Scholar]
- Adwan, G.; Salameh, Y.; Adwan, K.; Barakat, A. Assessment of antifungal activity of herbal and conventional toothpastes against clinical isolates of Candida albicans. Asian. Pac. J. Trop. Biomed. 2012, 2, 375–379. [Google Scholar] [CrossRef]
- Pai, M.R.; Acharya, L.D.; Udupa, N. Evaluation of antiplaque activity of Azadirachta indica leaf extract gel—A 6-week clinical study. J. Ethnopharmacol. 2004, 90, 99–103. [Google Scholar] [CrossRef]
- Wolinsky, L.E.; Mania, S.; Nachnani, S.; Ling, S. The inhibiting effect of aqueous Azadirachta indica (Neem) extract upon bacterial properties influencing in vitro plaque formation. J. Dent. Res. 1996, 75, 816–822. [Google Scholar] [CrossRef] [PubMed]
- Kulshrestha, R.; Kranthi, J.; Rao, P.K.; Jenner, F.; Jaleel, V.A.; Maheswar, G. Evaluating the antimicrobial activity of commercially available herbal toothpastes on microorganisms associated with diabetes mellitus. J. Contemp. Dent. Pract. 2013, 14, 924–929. [Google Scholar] [CrossRef]
- Shafiq, H.B.; Nawaz, S.; Amin, U.; Rasool, M.H. Role of chemical and herbal dentifrices against indigenous oral Pathogens. Pak. J. Pharm. Sci. 2018, 31, 1323–1331. [Google Scholar]
- De Rossi, A.; Ferreira, D.C.A.; Da Silva, R.A.B.; De Queiroz, A.M.; Da Silva, L.A.B.; Nelson-Filho, P. Antimicrobial activity of toothpastes containing natural extracts, chlorhexidine or triclosan. Braz. Dent. J. 2014, 25, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.; Addy, M.; Newcombe, R.G.; Marlow, I. A study to assess the plaque inhibitory action of a newly formulated triclosan toothpaste. J. Clin. Periodontol. 2001, 28, 86–89. [Google Scholar] [CrossRef] [PubMed]

| Material/Antimicrobial Agent | Dentifrices Used | Organisms | Method of Antimicrobial Activity Evaluation | Main Conclusions | Ref. |
|---|---|---|---|---|---|
| Cinnamon, rosemary, nutmeg, orange, mint, ginger, oregano, thyme, clove, eucalyptus, tangerine, lime and tea tree oil | 18 dentifrices formulated with essential oils of cinnamon, clove, oregano and thyme | Staphylococcus aureus, Streptococcus mutans, Lactobacillus lactis and Enterococcus faecalis | Hole-plate diffusion method Broth microdilution method (MIC) | In general, the best results were obtained with cinnamon essential oil and dentifrice against S. mutans | [1] |
| Triclosan, bee propolis and plant extracts, such as Peelu, neem, licorice, pomegranate rind, clove, Persian walnut, cinnamon, peppermint, echinacea, grapefruit seed, among others | 14 dentifrices: Acu-Herb, Auromere Herbal Toothpaste, Dental Gel, Dental Herb (powder), Dentifrico de Echinacea, Healthy Mouth, Herbal Brite, Nature’s Gate Natural Toothpaste, Nutrismile C, Peelu, Pink Toothpaste with Myrrh, Pure Herb, Sea Fresh and Tom’s of Maine Natural Toothpaste | Streptococcus mutans, Streptococcus sanguis, Actinomyces viscosus and Candida albicans | Standard diffusion method | 6 herbal dentifrices were effective in inhibiting the growth of microorganisms Only one herbal dentifrice showed consistent antimicrobial activity against all 4 microorganisms (Dental Gel) | [2] |
| Triclosan, sodium monofluorophosphate, sodium fluoride and herbal products, such as Salvadora persica, Azadirachta indica, Babhul, among others | 5 dentifrices (A, B, C, D and E) and 5 mouthwashes | Streptococcus mutans, Escherichia coli and Candida albicans | Modified agar well diffusion method | Triclosan containing dentifrice formulations were more effective compared to non-triclosan containing synthetic dentifrices Herbal dentifrices exhibited lower efficacy compared to the others | [6] |
| Nanosilver, chitosan and fluoride | 3 dentifrices: containing nanosilver (TruCare Nanosilver); containing chitosan (Conybio Plus Chitosan); and containing fluoride (Oral B Pro-Health) | Streptococcus mutans | Modified agar well diffusion method | The dentifrice containing nanosilver has the highest antibacterial efficacy against S. mutans, followed by the dentifrice containing fluoride and chitosan | [9] |
| Rosemary extract, propolis, mauve, cinnamon, peppermint, triclosan and sodium fluoride | Dentifrices based on rosemary (TR) and propolis (TH—Sorriso Herbal com Própolis) and Colgate Total 12 (TPC) | Streptococcus mutans, Streptococcus oralis and Lactobacillus rhamnosus | Macrodilution method Standardized single disk method | The antimicrobial activity of rosemary-based dentifrice (TR) was similar to the commercial dentifrice (TH) For the inhibition of L. rhamnosus, the propolis-based commercial dentifrice was more effective than TR dentifrice | [11] |
| P. betle extract, triclosan, sodium fluoride, sodium bicarbonate, corn mint, peppermint, sage oil and coneflower | 3 dentifrices: Colgate Total, Paradontax and Darlie | Escherichia coli, Staphylococcus aureus, Streptococcus mutans, Streptococcus salivarius and Candida albicans | Standard procedure for determining MIC Agar disk diffusion assay | A statistically significant increase in the zone of inhibition after addition of P. betle extract was observed with all 3 dentifrices on all pathogens tested | [12] |
| Triclosan, sodium monofluorophosphate, sodium fluoride, sodium lauryl sulfate and plant extracts, such as peppermint, eucalyptus, cinnamon, clove, Carum petroselinum (parsley), among others | 15 dentifrices and a stannous fluoride gel: Sensodyne Repair, Sensodyne Total Care, Pronamel, Colgate Pro Relief, Colgate Sensitive Multi, Colgate Total, Colgate Neutrafluor 5000 plus, Colgate Sparkling Gel, Alfree, Macleans Advanced Enamel Lock, Gel Kam, Herbal Brite, Herbal Fresh, Grants and Woolworthse Select | Streptococcus mutans | Agar diffusion method | Colgate Total has the greatest effect and Colgate Gel Kam has the least. Only two herbal toothpastes showed antimicrobial activity (Herbal Fresh and Macro) Most of the antimicrobial activity against S. mutans depends on the presence of non-fluoride agents, such as triclosan and sodium lauryl sulfate | [18] |
| Silver and gold nanoparticles | Royal Denta dentifrice containing silver and gold nanoparticles | Staphylococcus aureus, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Bacillus cereus and Candida albicans | Agar diffusion method | Silver in dentifrice has a greater antimicrobial effect than gold, but its effect is still lower than that of a chemical antimicrobial agent | [19] |
| Sodium fluoride, sodium monofluorophosphate and triclosan | 3 desensitizing dentifrices (Colgate Sensitive Pró-Alívio, Sensodyne Rápido Alívio and Oral B Pro Sensitive) and a common dentifrice (Colgate Total 12 Professional Clean) | Candida albicans, Streptococcus mutans and Staphylococcus aureus | Microdilution method (MIC) | Colgate dentifrice was able to eliminate all microorganisms evaluated at lower concentrations compared to Colgate Sensitive and Oral B Sensitive C. albicans was the least resistant to the dentifrices. S. aureus was the most resistant. | [20] |
| Sodium monofluorophosphate, sodium lauryl sulfate, triclosan, bromochlorophene and sodium fluoride | 10 Iranian-made dentifrices: Paveh, Saviz, Latifeh II, Bath, Darugar II, Darugar I, Close up, Tage, Pooneh III and Nasim | Streptococcus mutans, Streptococcus sanguis, Actinomyces viscosus and Candida albicans | Agar diffusion method | All dentifrices showed antimicrobial activity. Those containing more than one antimicrobial showed higher activity The antimicrobial property of Pooneh III was similar and Bath higher than the positive control | [21] |
| Sodium monofluorophosphate, stannous fluoride and sodium fluoride in various concentrations | Dentifrices with low fluoride concentration: Colgate My First, Oral B Stages and Macleans Milk Teeth Dentifrices with standard fluoride concentration for adults: Colgate Total, Oral B Tooth and Gums, Macleans Extreme Clean, Colgate Sensitive Pro Relief and Oral B Pro Health Dentifrice with high fluoride concentration: Colgate Neutrafluor 5000 | Streptococcus mutans, Streptococcus sanguinis and Lactobacillus acidophilus | Agar diffusion assay | Dentifrices containing 1450 ppm fluoride produced greater growth inhibition of S. mutans and S. sanguinis than those with <500 ppm Colgate Total produced the highest average growth inhibition on S. mutans and S. sanguinis. Colgate Pro Relief produced the lowest | [22] |
| Stabilized chlorine dioxide | ClōSYS oral care products: two mouth rinses, one oral spray and one fluoride dentifrice | SARS-CoV-2, SARS-CoV, human coronavirus (HCoV) 229E, influenza A (H3N2), rhinovirus type 14, adenovirus type 5 and herpes simplex virus (HSV) type 1 and 2 | In vitro suspension virucidal assays | Stabilized chlorine dioxide contained in oral care products reduced the viral load of several viruses | [23] |
| Sodium monofluorophosphate, sodium fluoride, triclosan and zinc citrate | 5 dentifrices: Colgate Regular, Macleans Anti-plaque Formula, Mentadent P Gum Health Formula, Crest Gum Health and Colgate Gum Protection | 17 bacterias: A. actinomy-cetemcomitans (2), A. odontolyticus, A. viscosus, C. rectus, C. orchracea, Capnocytophaga species, E. timidum, S. oralis, P. anaerobius, P. micros, P. gingivalis, P. intermedia (2), S. constellatus, V. parvula and Veillonella species | Agar dilution method Maximum inhibitory dilution (MID) | Mentadent Gum Health Formula, which contains triclosan and zinc citrate, had the highest antimicrobial activity of the dentifrices containing triclosan Crest Gum Health was generally less active than all other dentifrices, including Colgate Regular, except against A. actinomycetemcomitans | [24] |
| Stannous fluoride, sodium fluoride and stannous pyrophosphate | 2 stannous fluoride dentifrices (SF1, SF2), 2 experimental dentifrices with SF and stannous pyrophosphate (SFSP1, SFSP2), a gel with SF (G) and a dentifrice with NaF (C) | 20 species: Actinobacillus actinomycetemcomitans (2), Campylobacter rectus, Capynocytophaga sputigena, Capynocytophaga species, Fusobacterium nucleatum, Porphyromonas gingivalis (2), Prevotella nigrescens, Prevotella intermedia, Veillonella parvala, Veillonella species, Actinomyces viscosus, Actinomyces odontolyricus, Eubacterium timidum, Peptostreptococcus anaerobius, Peptostreptococcus micros, Streptococcus constellatus, Streptococcus mutans and Streptococcus oralis | Maximum inhibitory dilution (MID) | All formulations showed antimicrobial activity with the average order of highest activity descending being C, SF2, SF1, SFSP1, SFSP2 and G SF1 containing stannous fluoride was less active than C, a conventional dentifrice containing sodium fluoride (NaF) | [25] |
| Triclosan and fluoride | Colgate Total dentifrice | Klebsiella pneumoniae, Streptococcus mutans, Candida albicans, Porphyromonas gingivalis and Fusobacterium nucleatum | Microbial kill time assay European suspension test method | In antimicrobial assays, the dentifrice was effective against bacteria, but not against C. albicans | [26] |
| Sodium fluoride, triclosan and stannous fluoride | 4 commercially available dentifrices, 2 containing sodium fluoride (NaF) at different concentrations (1450 and 2500 ppm) and 2 NaF with triclosan or stannous fluoride | Streptococcus oralis, Actinomyces naeslundii, Veillonella parvula, Fusobacterium nucleatum, Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans | Subgingival biofilm model | Dentifrices containing NaF and stannous fluoride demonstrated higher antimicrobial activity for A. actinomycetencomitans, P. gingivalis, and F. nucleatum when compared to those containing NaF and triclosan, 1450 ppm NaF, or 2500 ppm NaF | [27] |
| Guava leaf extract | Three prepared toothpaste formulations varying in ingredient concentrations (F1, F2 and F3) | Bacillus subtilis, Proteus vulgaris, Staphylococcus aureus, Streptococcus mutantes and Streptococcus oralis | Well diffusion method | F3 was very effective against the bacteria tested, followed by F2 and F1 The highest zone of inhibition was observed against P. vulgaris and B. subtilis and the lowest was observed against S. aureus | [28] |
| Solanum tuberosum powder | Experimental dentifrice containing Solanum tuberosum (Tocosh) | Staphylococcus aureus, Streptococcus mutans, Streptococcus mitis and Candida albicans | Agar diffusion method | Dentifrice with Tocosh has an antibacterial effect against S. aureus, S. mutans and S. mitis. No antimicrobial effect was found against C. albicans | [29] |
| Pomegranate peel, lemon peel and mango peel extract, mint oil and sodium lauryl sulfate | A polyherbal dentifrice containing pomegranate, lemon and mango peel methanolic extract formulated in different concentrations (100, 250 and 500 mg/mL) | Staphylococcus aureus, Bacillus cereus, Escherichia coli and Pseudomonas aeruginosa | Disk-diffusion method | The polyherbal dentifrice has promising antimicrobial effects against Gram-positive and Gram-negative organisms, and may be safer compared to a synthetic dentifrice S. aureus was the most sensitive, followed by E. coli, B. cereus and P. aeruginosa | [30] |
| Propolis (10% ethanol solution) | Yamada Yohojo Propolis Hamigaki dentifrice, which contains an ethanolic solution of 10% propolis sample at a concentration of 2% (w/w) | 20 strains representing 12 species: S. mutans (3), S. sobrinus (3), S. cricetus, S. rattus, S. sanguis (2), S. oralis, S. gordonii, S. mitis (2), S. salivarius, A. naeslundii, A. viscosus (3) and L. casei | Minimum inhibitory concentration (MIC) | The propolis-containing dentifrice inhibited the growth of 20 bacterial strains in a concentration range of 3–7 mg/mL | [31] |
| Ingredients not specified | 6 dentifrices: Ayush, Dant kanti, Colgate vedshakti, Meswak, Dantajeevan and Dabur red | Streptococcus mutans and Candida albicans | Antibiotic susceptibility testing Agar disk-diffusion method | The herbal dentifrice formulations were effective in controlling the oral microflora For S. mutans, the zone of inhibition is greatest in Ayush. For C. albicans, the zone of inhibition is greatest in Ayush and Dant kanti | [32] |
| Green tea ethanolic extract and sodium lauryl sulfate | Two experimental dentifrice formulas containing 5% green tea extract in a gel base. Formula A was enriched with 30% CaCO3 as an abrasive. Formula B was made without CaCO3 | Streptococcus mutans and Lactobacillus acidophilus | Agar diffusion method | In comparison with herbal dentifrice patent containing a combination of green tea extract and P. bettle leaves extract, this had higher antibacterial activity against S. mutans than the green tea dentifrice, but not against L. acidophilus | [33] |
| G. kola extract, sodium lauryl sulfate and sodium bicarbonate Close up, Aquafresh and Colgate do not have their ingredients specified | Experimental dentifrice containing G. kola extract, Close up, Aquafresh and Colgate | Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, Klebsiella pneumoniae, Bacillus subtilis, Staphylococcus aureus, α-haemolytic, Streptococcus pneumoniae, Streptococcus pyogenes and Candida albicans | Standard agar diffusion and broth dilution methods | All microorganisms were significantly susceptible to the extract and the dentifrice The herbal dentifrice inhibited all tested microorganisms, while none of the commercial dentifrices used showed any antimicrobial activity | [34] |
| Herbal extracts, such as Stevia rebaudiana leaves (SR), Azadirachta indica bark (AZ), Piper longum fruit (PL), Curcuma longa rhizome (CL), Salvadora persica bark (SP), among others | Polyherbal dentifrice (formulations 1, 2 and 3), Herbodent and Apollo | Streptococcus mutans, Streptococcus oralis, staphylococcus aureus, Candida albicans and Lactobacillus acidophilus | Agar well diffusion method Serial micro dilution method (MIC) | The methanol extract of polyherbal formulation 1 and 3 showed lower activity when compared to polyherbal formulation 2. However, the activity was lower than gentamicin | [35] |
| Calendula extracts, sage clay, sodium fluoride and sodium lauryl sulfate | 2 dentifrices: Iranian herbal dentifrice and Iranian chemical dentifrice | Streptococcus mutans, Lactobacillus and Candida albicans | Agar disk-diffusion method | At the total concentration, herbal and chemical dentifrices have the same antimicrobial effect, but when reducing the concentration, the effect of the herbal dentifrice is reduced compared to the chemical one | [36] |
| Triclosan, sodium monofluorophosphate and plant extracts, such as miswak, neem, Babool, Pudina, Long, Vajradanti, turmeric, calendula, eucalyptus, and others | 9 dentifrices in three groups | Escherichia coli, Staphylococcus aureus, Streptococcus mutans and Candida albicans | Agar well-diffusion method | Of the herbal groups, the only dentifrice containing several phytochemicals was found to be significantly effective and comparable to the triclosan and fluoride formulation. Homeopathic products showed lower antimicrobial activity | [37] |
| Ricinus communis, sodium, monofluorophosphate, chloramine T and sodium bicarbonate | Experimental dentifrice based on Ricinus communis Colgate, Corega Brite and Trihydral commercial toothpastes | Staphylococcus aureus, Escherichia coli, Streptococcus mutans, Enterococcus faecalis, Candida albicans and Candida glabrata | Microdilution technique in 96-well plates (MIC) Well agar diffusion method | Comparing the experimental dentifrices, the product with 10% R. communis produced the largest inhibition halos and showed antimicrobial activity similar to commercial dentifrices, except against S. aureus None of the dentifrices were effective against E. coli | [38] |
| Fluoride and herbal extracts | Herbal dentifrices: Himalaya herbals (A1) and Dabur red (A2) Conventional dentifrices: Colgate super shakti (B1) and Pepsodent complete germicheck (B2) | Streptococcus mutans and Candida albicans | Disk-diffusion method | Herbal dentifrices are equally and sometimes better than conventional ones At 50% concentration, B2 showed the maximum zone of inhibition for S. mutans, while at 100% concentration A1 showed better effects. For C. albicans, A2 was the most effective | [39] |
| Nanohydroxyapatite (nanoHAP) and Curcuma aeruginosa extract | 20 dentifrices with different concentrations of nanoHAP and C. aeruginosa, plus OF1 and OF2 | Streptococcus mutans | Agar diffusion and microdilution methods | Most of the dentifrice formulations showed antibacterial activity. OF1 and OF2 were shown to have antibacterial activity comparable to that achieved with the control | [40] |
| Ethanolic extracts of Dennettia tripetala seeds, Syzygium aromaticum buds, Jatropha curcas latex, sodium fluoride and Aloe vera | 3 commercial dentifrices labeled Com A, Com B and Com C The formulated dentifrices were labeled DenSyzJat, DenSyz and SyzJat | Escherichia coli, Bacillus sp., Staphylococcus aureus, Staphylococcus aureus resistente à meticilina, Staphylococcus epidermidis, Micrococcus luteus, Streptococcus mutans, Streptococcus pyogenes, Lactobacillus acidophilus, Candida albicans and Pseudomonas aeruginosa | Agar well diffusion method Minimum inhibitory concentration (MIC) | The formulated dentifrices exhibited antimicrobial property against all tested microorganisms and showed better and significant antimicrobial effect when compared to commercial dentifrices The dentifrice formulated with S. aromaticum extract alone seems to be much more active among the 3 bioactive materials used for formulation | [41] |
| Origanum dubium and Cinnamomum cassia oils, triclosan, sodium fluoride, sodium bicarbonate and plant extracts, such as Calendula officinalis, Aloe barbadensis and Melaleuca alternifolia oil | 4 dentifrices: Splat Organic, Splat Biocalcium, Jack N ‘Jill and Colgate Total | Streptococcus mutans | Agar diffusion method in disk (pure oil) and well (dentifrice) | The antibacterial activity of the dentifrices was higher than positive control Herbal dentifrices showed higher antibacterial activity than their initial forms after the addition of essential oils. C. cassia showed higher antibacterial activity than O. dubium | [42] |
| Sodium monofluorophosphate, sodium fluoride, triclosan, sodium lauryl sulfate, herbal extracts and oils, such as Clinacanthus nutans, Streblus asper and Murraya paniculata leaves, peppermint oil, eucalyptus oil, among others | 6 dentifrices: Darly, Close up, Systema, Herbal Twin Lotus, Salz and Colgate | Candida albicans | Agar diffusion assay Broth microdilution technique (MIC and MFC) | All dentifrices inhibited the growth of C. albicans with a mean range of the zone of inhibition between 8 and 16.92 mm Herbal Twin Lotus showed the largest mean zone of inhibition. Saltz showed the lowest mean zone of inhibition | [43] |
| Triclosan, sodium fluoride and herbal extracts such as neem, chamomile, Babul and Miswalk | 6 dentifrices: neem, Vicco Vajradanti, Himalaya Herbal, Colgate Herbal, Dabur Red and Dabur Babool | Streptococcus mutans and Lactobacillus acidophilus | well method of microbial culture | Herbal dentifrices are more effective against lactobacilli organisms at the same level as conventional dentifrice and less effective for S. mutans when compared to non-herbal dentifrices | [44] |
| Triclosan, sodium monofluorophosphate, sodium bicarbonate, sodium lauryl sulfate, sodium fluoride and plant extracts, such as Anacyclus pyrethrum, Azadirachta indica (neem), Acacia arabica (Babool), Salvadora persica (miswak), Pudina, among others | 7 dentifrices (A, B, C, D, E, F, and G) and 2 mouthwashes (H and I) | Escherichia coli and Candida albicans | Modified agar well diffusion method | The formulations containing natural antimicrobials were more effective compared to dentifrices containing synthetic antimicrobials The antibacterial activity of A is lower compared to formulation B at higher dilutions | [45] |
| Sodium monofluorophosphate, triclosan, zinc sulfate, sodium lauryl sulfate, amine fluoride, sodium fluoride and plant extracts, such as neem and miswak | 7 commercial dentifrices | Streptococcus mutans, Escherichia coli and Candida albicans | Well agar diffusion assay | Dentifrice formulations containing triclosan are more effective The zone of inhibition decreases with increasing dilution | [46] |
| Sodium fluoride, triclosan, sodium monofluorophosphate, sodium lauryl sulfate, eugenol, clove oil and plant extracts, such as miswak and neem, | 6 dentifrices: 3 fluoride (Colgate Total, Fresh and White and Safi–clove) and 3 3 non-fluoride dentifrices (Mukmin, Halagel and Pureen) | Streptococcus mutans | Standard disk diffusion method | All dentifrices showed antibacterial activity at both concentrations tested, but better results at full strength compared to the diluted. The antibacterial activity of the non-fluoridated toothpastes is as good as the fluoridated ones | [47] |
| Cashew extract, mango extract, calendula extract (Callendula officinalis), Aloe vera, natural banana, natural apple, sodium fluoride and sodium lauryl sulfate | 6 infant dentifrices: experimental cashew-based dentifrice; experimental mango-based dentifrice; experimental dentifrice without plant extract and fluoride; First Teeth dentifrice; Weleda dentifrice; and Tandy dentifrice | Streptococcus mutans, Streptococcus sobrinus, Lactobacillus acidophilus and Candida albicans | Agar plate diffusion test | First Teeth, Weleda, mango-based dentifrice and dentifrice without plant extract showed no antimicrobial effect against any of the microorganisms tested The cashew dentifrice showed significant antimicrobial activity against S. mutans, S. sobrinus and L. acidophilus. Tandy had antimicrobial activity against all microorganisms | [48] |
| Sodium monofluorophosphate, sodium lauryl sulfate, and herbal extracts, such as maricha, pippali, sunthi, kapor, akarkara, khadir, lawang, among others | Marketed allopathic powdered toothpastes (brand I and II) and herbal powdered toothpastes (brand III and IV) | Staphylococcus sorbinus, Staphylococcus salivarius and Lactobacillus acidophilus | Agar well diffusion method | The diameter of the zone of inhibition observed in brands I and II for test microorganisms was not significantly different from the diameter observed in brands III and IV | [49] |
| Propolis and chlorhexidine | 3 dentifrices (with propolis, without propolis and with chlorhexidine) and 2 mouthwashes (with propolis and chlorhexidine) | Actinomyces naeslundii, Veillonella dispar, Fusobacterium nucleatum, Streptococcus mutans, Streptococcus oralis and Candida albicans | In vitro multispecies biofilm model | Propolis seems to have no effect in respect to reducing CFU in the supragingival biofilm model used, neither in the dentifrice nor in the mouthwash | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinho, V.T.; dos Reis, A.C.; da Costa Valente, M.L. Efficacy of Antimicrobial Agents in Dentifrices: A Systematic Review. Antibiotics 2022, 11, 1413. https://doi.org/10.3390/antibiotics11101413
Marinho VT, dos Reis AC, da Costa Valente ML. Efficacy of Antimicrobial Agents in Dentifrices: A Systematic Review. Antibiotics. 2022; 11(10):1413. https://doi.org/10.3390/antibiotics11101413
Chicago/Turabian StyleMarinho, Vanessa Teixeira, Andréa Cândido dos Reis, and Mariana Lima da Costa Valente. 2022. "Efficacy of Antimicrobial Agents in Dentifrices: A Systematic Review" Antibiotics 11, no. 10: 1413. https://doi.org/10.3390/antibiotics11101413
APA StyleMarinho, V. T., dos Reis, A. C., & da Costa Valente, M. L. (2022). Efficacy of Antimicrobial Agents in Dentifrices: A Systematic Review. Antibiotics, 11(10), 1413. https://doi.org/10.3390/antibiotics11101413






