A Seven-Year Microbiological and Molecular Study of Bacteremias Due to Carbapenemase-Producing Klebsiella Pneumoniae: An Interrupted Time-Series Analysis of Changes in the Carbapenemase Gene’s Distribution after Introduction of Ceftazidime/Avibactam
Abstract
1. Introduction
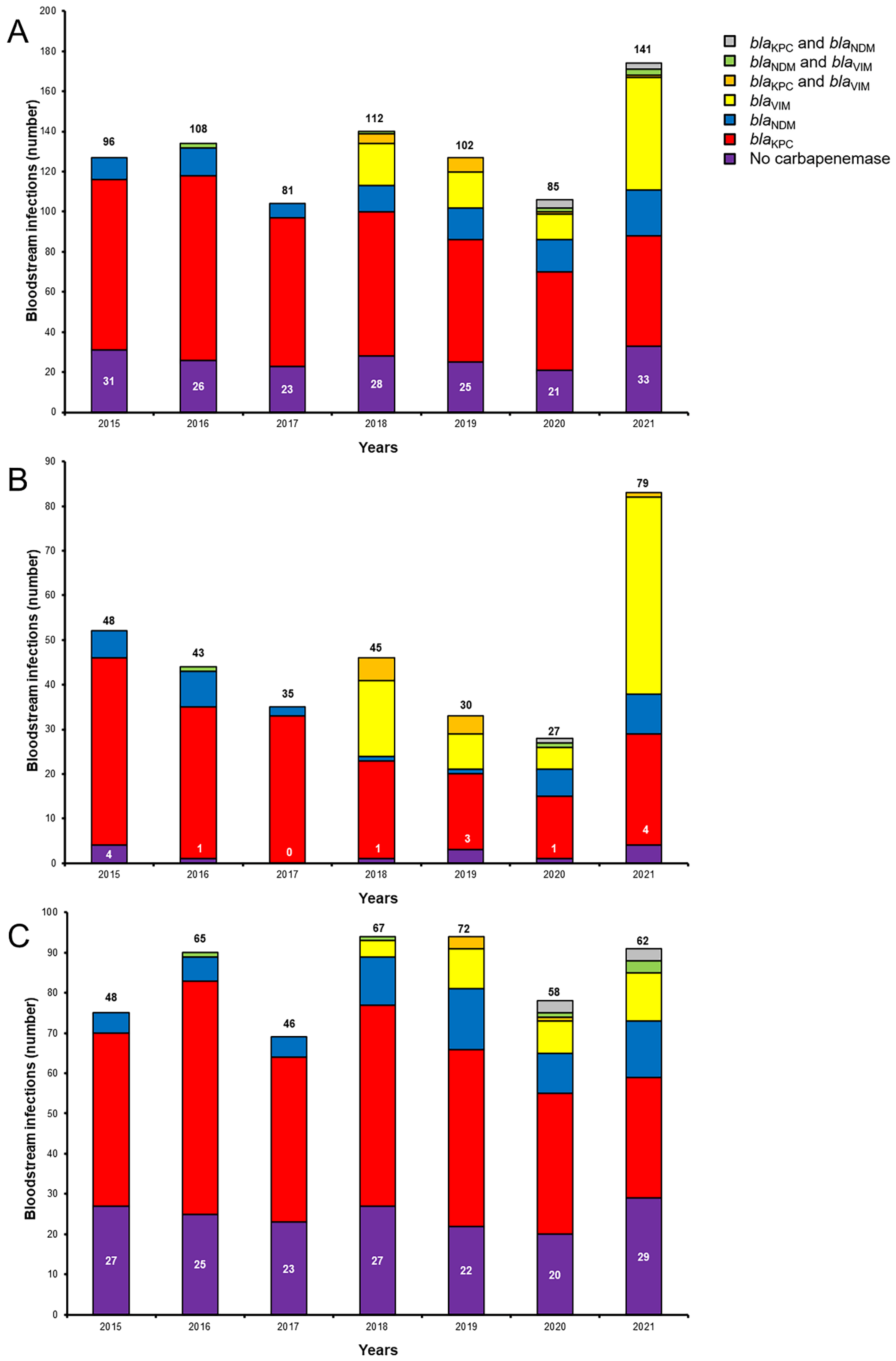
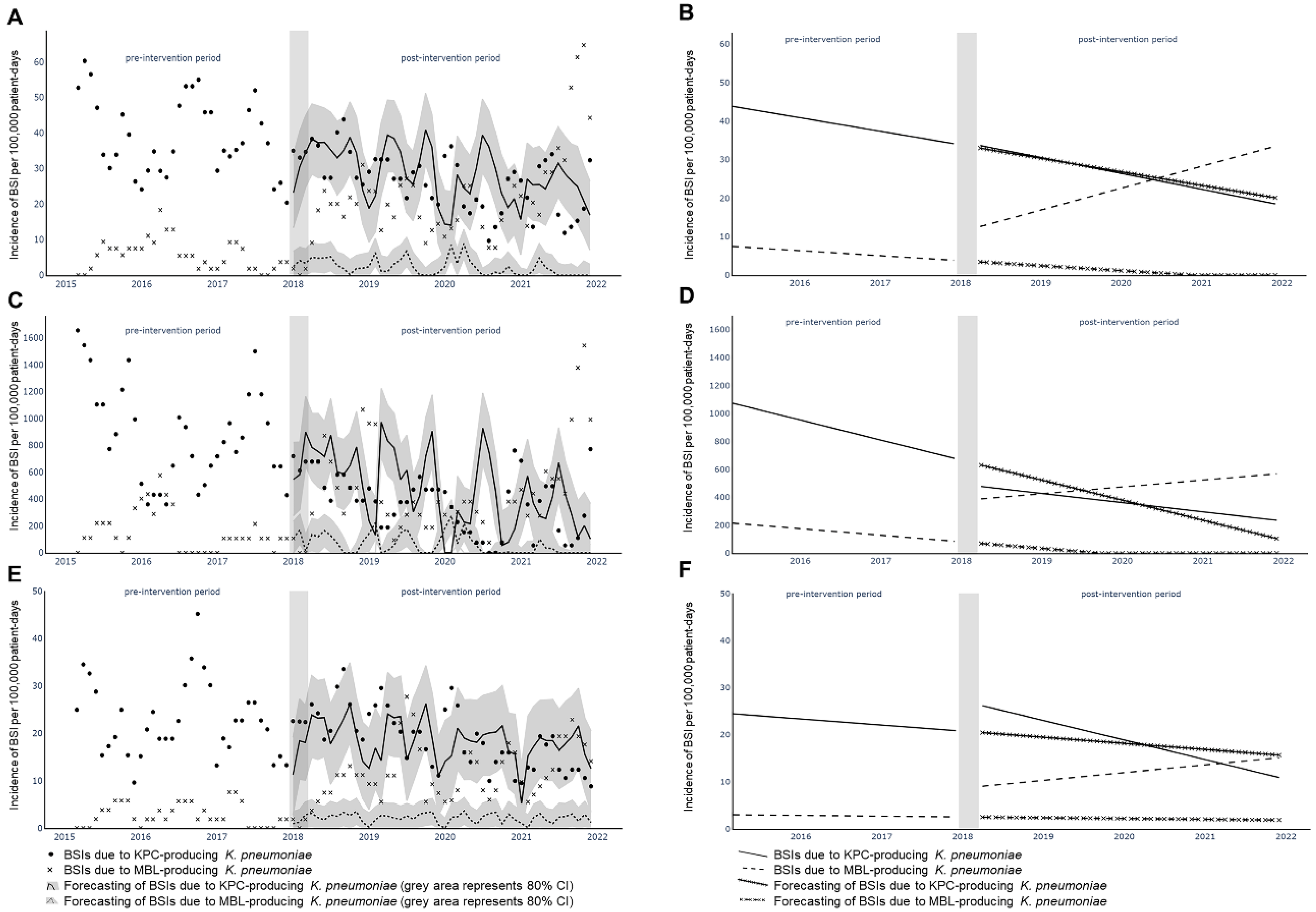
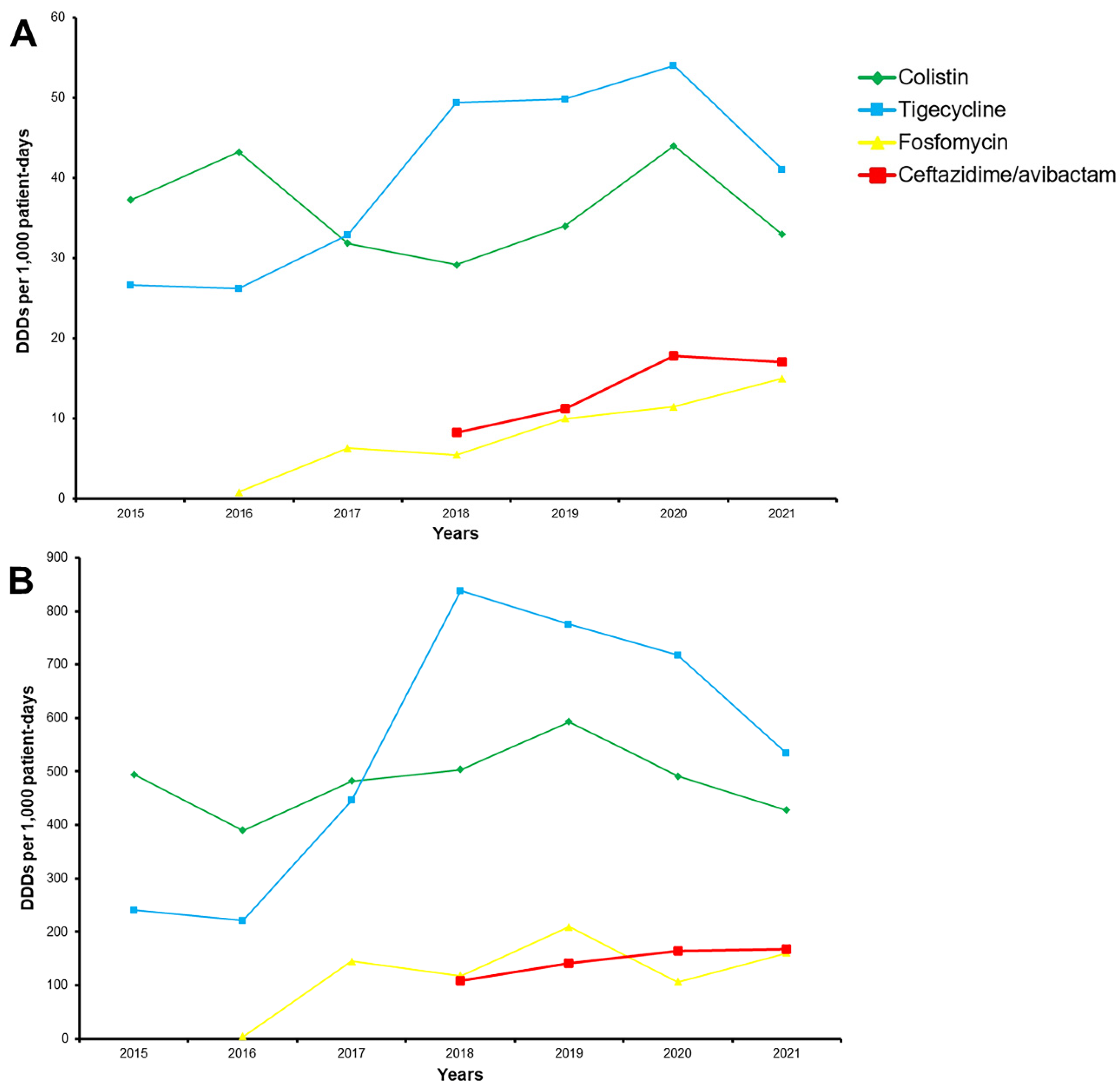
2. Results
2.1. Antimicrobial Susceptibility and Genotypes
2.2. Interrupted Time-Series Analysis
2.3. Colistin, Tigecycline, Fosfomycin, Aminoglycoside and Ceftazidime/Avibactam Resistance
2.4. Antibiotic Consumption
2.5. Other Pathogens
3. Discussion
4. Materials and Methods
4.1. Identification and Antimicrobial Susceptibility Testing
4.2. Genotypes of Isolates
4.3. Antibiotic Consumption
4.4. Other Pathogens
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Albiger, B.; Glasner, C.; Struelens, M.J.; Grundmann, H.; Monnet, D.L. European Survey of Carbapenemase-Producing Enterobacteriaceae working, g. Carbapenemase-producing Enterobacteriaceae in Europe: Assessment by national experts from 38 countries, May 2015. Euro Surveill. Bull. 2015, 20, 30062. [Google Scholar] [CrossRef]
- Spyropoulou, A.; Papadimitriou-Olivgeris, M.; Bartzavali, C.; Vamvakopoulou, S.; Marangos, M.; Spiliopoulou, I.; Anastassiou, E.D.; Christofidou, M. A ten-year surveillance study of carbapenemase-producing Klebsiella pneumoniae in a tertiary care Greek university hospital: Predominance of KPC- over VIM- or NDM-producing isolates. J. Med. Microbiol. 2016, 65, 240–246. [Google Scholar] [CrossRef]
- Cristina, M.L.; Alicino, C.; Sartini, M.; Faccio, V.; Spagnolo, A.M.; Bono, V.D.; Cassola, G.; De Mite, A.M.; Crisalli, M.P.; Ottria, G.; et al. Epidemiology, management, and outcome of carbapenem-resistant Klebsiella pneumoniae bloodstream infections in hospitals within the same endemic metropolitan area. J. Infect. Public Health 2018, 11, 171–177. [Google Scholar] [CrossRef]
- Queenan, A.M.; Bush, K. Carbapenemases: The versatile beta-lactamases. Clin. Microbiol. Rev. 2007, 20, 440–458. [Google Scholar] [CrossRef]
- Papadimitriou-Olivgeris, M.; Fligou, F.; Bartzavali, C.; Zotou, A.; Spyropoulou, A.; Koutsileou, K.; Vamvakopoulou, S.; Sioulas, N.; Karamouzos, V.; Anastassiou, E.D.; et al. Carbapenemase-producing Klebsiella pneumoniae bloodstream infection in critically ill patients: Risk factors and predictors of mortality. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2017, 36, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Tansarli, G.S.; Papaparaskevas, J.; Balaska, M.; Samarkos, M.; Pantazatou, A.; Markogiannakis, A.; Mantzourani, M.; Polonyfi, K.; Daikos, G.L. Colistin resistance in carbapenemase-producing Klebsiella pneumoniae bloodstream isolates: Evolution over 15 years and temporal association with colistin use by time series analysis. Int. J. Antimicrob. Agents 2018, 52, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Alicino, C.; Giacobbe, D.R.; Orsi, A.; Tassinari, F.; Trucchi, C.; Sarteschi, G.; Copello, F.; Del Bono, V.; Viscoli, C.; Icardi, G. Trends in the annual incidence of carbapenem-resistant Klebsiella pneumoniae bloodstream infections: A 8-year retrospective study in a large teaching hospital in northern Italy. BMC Infect. Dis. 2015, 15, 415. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Skalidis, T.; Vardakas, K.Z.; Legakis, N.J.; Hellenic Cefiderocol Study, G. Activity of cefiderocol (S-649266) against carbapenem-resistant Gram-negative bacteria collected from inpatients in Greek hospitals. J. Antimicrob. Chemother. 2017, 72, 1704–1708. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou-Olivgeris, M.; Bartzavali, C.; Lambropoulou, A.; Solomou, A.; Tsiata, E.; Anastassiou, E.D.; Fligou, F.; Marangos, M.; Spiliopoulou, I.; Christofidou, M. Reversal of carbapenemase-producing Klebsiella pneumoniae epidemiology from blaKPC- to blaVIM-harbouring isolates in a Greek ICU after introduction of ceftazidime/avibactam. J. Antimicrob. Chemother. 2019, 74, 2051–2054. [Google Scholar] [CrossRef] [PubMed]
- van Duin, D.; Lok, J.J.; Earley, M.; Cober, E.; Richter, S.S.; Perez, F.; Salata, R.A.; Kalayjian, R.C.; Watkins, R.R.; Doi, Y.; et al. Colistin Versus Ceftazidime-Avibactam in the Treatment of Infections Due to Carbapenem-Resistant Enterobacteriaceae. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 66, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Iacchini, S.; Sabbatucci, M.; Gagliotti, C.; Rossolini, G.M.; Moro, M.L.; Iannazzo, S.; D’Ancona, F.; Pezzotti, P.; Pantosti, A. Bloodstream infections due to carbapenemase-producing Enterobacteriaceae in Italy: Results from nationwide surveillance, 2014 to 2017. Euro Surveill. 2019, 24, 1800159. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Regional Outbreak of New Delhi Metallo-Beta-Lactamase Producing Carbapenem-Resistant Enterobacteriaceae, Italy, 2018–2019; European Centre for Disease Prevention and Control: Stockholm, Sweden, 2019. [Google Scholar]
- Lenhard, J.R.; Rana, A.P.; Wenzler, E.; Huang, Y.; Kreiswirth, B.N.; Chen, L.; Bulman, Z.P. A coup d’etat by NDM-producing Klebsiella pneumoniae overthrows the major bacterial population during KPC-directed therapy. Diagn. Microbiol. Infect. Dis. 2020, 98, 115080. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Mao, J.; Chen, Y.; Wang, J.; Zhang, P.; Jiang, Y.; Yang, Q.; Yu, Y.; Qu, T. Clinical and Microbiological Characteristics of Community-Onset Carbapenem-Resistant Enterobacteriaceae Isolates. Infect. Drug Resist. 2020, 13, 3131–3143. [Google Scholar] [CrossRef] [PubMed]
- Goodman, K.E.; Simner, P.J.; Klein, E.Y.; Kazmi, A.Q.; Gadala, A.; Toerper, M.F.; Levin, S.; Tamma, P.D.; Rock, C.; Cosgrove, S.E.; et al. Predicting probability of perirectal colonization with carbapenem-resistant Enterobacteriaceae (CRE) and other carbapenem-resistant organisms (CROs) at hospital unit admission. Infect. Control. Hosp. Epidemiol. 2019, 40, 541–550. [Google Scholar] [CrossRef] [PubMed]
- Pano-Pardo, J.R.; Lopez Quintana, B.; Lazaro Perona, F.; Ruiz Carrascoso, G.; Romero-Gomez, M.P.; Loeches Yague, B.; Diaz-Pollan, B.; Martinez-Virto, A.; Mingorance, J.; Garcia Rodriguez, J.; et al. Community-Onset Bloodstream and Other Infections, Caused by Carbapenemase-Producing Enterobacteriaceae: Epidemiological, Microbiological, and Clinical Features. Open Forum Infect. Dis. 2016, 3, ofw136. [Google Scholar] [CrossRef] [PubMed]
- Skjot-Arkil, H.; Mogensen, C.B.; Lassen, A.T.; Johansen, I.S.; Chen, M.; Petersen, P.; Andersen, K.V.; Ellermann-Eriksen, S.; Moller, J.M.; Ludwig, M.; et al. Carrier prevalence and risk factors for colonisation of multiresistant bacteria in Danish emergency departments: A cross-sectional survey. BMJ Open 2019, 9, e029000. [Google Scholar] [CrossRef]
- Salomao, M.C.; Guimaraes, T.; Duailibi, D.F.; Perondi, M.B.M.; Letaif, L.S.H.; Montal, A.C.; Rossi, F.; Cury, A.P.; Duarte, A.J.S.; Levin, A.S.; et al. Carbapenem-resistant Enterobacteriaceae in patients admitted to the emergency department: Prevalence, risk factors, and acquisition rate. J. Hosp. Infect. 2017, 97, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Assimakopoulos, S.F.; Lazaris, V.; Papadimitriou-Olivgeris, M.; Lagadinou, M.; Verigou, E.; Tzouvara, E.; Kolonitsiou, F.; Christofidou, M.; Symeonidis, A.; Marangos, M. Predictors of mortality for KPC-producing Klebsiella pneumoniae bloodstream infections in adult neutropenic patients with haematological malignancies. Infect. Dis. 2020, 52, 446–449. [Google Scholar] [CrossRef]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters; Version 10.0; EUCAST: Stockholm, Sweden, 2020. [Google Scholar]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters; Version 7.1; EUCAST: Stockholm, Sweden, 2017. [Google Scholar]
- Papalini, C.; Sabbatini, S.; Monari, C.; Mencacci, A.; Francisci, D.; Perito, S.; Pasticci, M.B. In vitro antibacterial activity of ceftazidime/avibactam in combination against planktonic and biofilm carbapenemase-producing Klebsiella pneumoniae isolated from blood. J. Glob. Antimicrob. Resist. 2020, 23, 4–8. [Google Scholar] [CrossRef]
- Cui, X.; Shan, B.; Zhang, X.; Qu, F.; Jia, W.; Huang, B.; Yu, H.; Tang, Y.W.; Chen, L.; Du, H. Reduced Ceftazidime-Avibactam Susceptibility in KPC-Producing Klebsiella pneumoniae From Patients Without Ceftazidime-Avibactam Use History—A Multicenter Study in China. Front. Microbiol. 2020, 11, 1365. [Google Scholar] [CrossRef]
- The European Committee on Antimicrobial Susceptibility Testing. Guidance Document on Tigecycline Dosing in Association with Revision of Breakpoints for Enterobacterales and other Species with an “Intermediate” Category. 2018. Available online: http://www.eucast.org (accessed on 18 August 2022).
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 12.0. 2022. Available online: http://www.eucast.org (accessed on 18 August 2022).
- Nordmann, P.; Poirel, L.; Carrer, A.; Toleman, M.A.; Walsh, T.R. How to detect NDM-1 producers. J. Clin. Microbiol. 2011, 49, 718–721. [Google Scholar] [CrossRef] [PubMed]
- WHO Collaborating Centre for Drug Statistics Methodology; Oslo, N. Guidelines for ATC Classification and DDD Assignment 2020; WHO: Geneva, Switzerland, 2019. [Google Scholar]
- Hyndman, R.J.; Athanasopoulos, G. Forecasting: Principles and Practice, 2nd ed.; OTexts: Melbourne, VIC, Australia, 2018; Available online:OTexts.com/fpp2 (accessed on 18 August 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadimitriou-Olivgeris, M.; Bartzavali, C.; Karachalias, E.; Spiliopoulou, A.; Tsiata, E.; Siakallis, G.; Assimakopoulos, S.F.; Kolonitsiou, F.; Marangos, M. A Seven-Year Microbiological and Molecular Study of Bacteremias Due to Carbapenemase-Producing Klebsiella Pneumoniae: An Interrupted Time-Series Analysis of Changes in the Carbapenemase Gene’s Distribution after Introduction of Ceftazidime/Avibactam. Antibiotics 2022, 11, 1414. https://doi.org/10.3390/antibiotics11101414
Papadimitriou-Olivgeris M, Bartzavali C, Karachalias E, Spiliopoulou A, Tsiata E, Siakallis G, Assimakopoulos SF, Kolonitsiou F, Marangos M. A Seven-Year Microbiological and Molecular Study of Bacteremias Due to Carbapenemase-Producing Klebsiella Pneumoniae: An Interrupted Time-Series Analysis of Changes in the Carbapenemase Gene’s Distribution after Introduction of Ceftazidime/Avibactam. Antibiotics. 2022; 11(10):1414. https://doi.org/10.3390/antibiotics11101414
Chicago/Turabian StylePapadimitriou-Olivgeris, Matthaios, Christina Bartzavali, Eleftherios Karachalias, Anastasia Spiliopoulou, Ekaterini Tsiata, Georgios Siakallis, Stelios F. Assimakopoulos, Fevronia Kolonitsiou, and Markos Marangos. 2022. "A Seven-Year Microbiological and Molecular Study of Bacteremias Due to Carbapenemase-Producing Klebsiella Pneumoniae: An Interrupted Time-Series Analysis of Changes in the Carbapenemase Gene’s Distribution after Introduction of Ceftazidime/Avibactam" Antibiotics 11, no. 10: 1414. https://doi.org/10.3390/antibiotics11101414
APA StylePapadimitriou-Olivgeris, M., Bartzavali, C., Karachalias, E., Spiliopoulou, A., Tsiata, E., Siakallis, G., Assimakopoulos, S. F., Kolonitsiou, F., & Marangos, M. (2022). A Seven-Year Microbiological and Molecular Study of Bacteremias Due to Carbapenemase-Producing Klebsiella Pneumoniae: An Interrupted Time-Series Analysis of Changes in the Carbapenemase Gene’s Distribution after Introduction of Ceftazidime/Avibactam. Antibiotics, 11(10), 1414. https://doi.org/10.3390/antibiotics11101414








