Abstract
Natural dyes have emerged as a promising alternative to synthetic dyes for industrial applications due to their advantages, namely, easy availability, low cost, and environmental friendliness. In this sense, natural dyes, due to their potential to react over the pH range, could offer an alternative to conventional pH measuring techniques for industrial products, such as potentiometers, sensors, or indicator drops. Therefore, this project aims to evaluate the potential of several natural organic dyes in response to changes in pH and develop an indicator for determining pH grades. We extracted and analyzed the pigments of forty natural vegetable species using two extraction methods with a mixture of solvents, specifically 70% MeOH/30% H2O. The results find that pigments of cabbage, hibiscus flower, radish, and turmeric in their dry state exhibit the best reaction over a broad pH range, and color can be easily distinguished according to its level. These findings demonstrate the potential of natural dyes as a novel approach for pH verification, providing a sustainable and cost-effective alternative to conventional techniques.
Keywords:
natural dyes; pH; testing strip; pigments; dyes; environment; cabbage; radish; turmeric; sensor 1. Introduction
The measurement of pH plays an important role in understanding chemical reactivity, solubility, and the structural behavior of compounds, as well as in ensuring the proper functioning of biological systems [1,2]. From industrial processes and food biotechnology to environmental and clinical analysis, pH monitoring remains a critical parameter in both research and applied contexts.
Conventional pH meters based on electrochemical glass electrodes offer high precision but can be expensive, require calibration, and are often impractical in low-resource or field settings [3]. Synthetic acid–base indicators are another standard tool, but they raise concerns regarding safety and environmental impact due to their toxicity and lack of biodegradability [4]. Traditional synthetic dyes, such as phenolphthalein, methyl red, and bromothymol blue, although favored for their intense color and sharp responses in volumetric analysis, present significant drawbacks, including potential toxicity, environmental persistence, and restrictions on their use in food or consumer contact applications [5,6]. For example, phenolphthalein has been implicated in intensifying carcinogenic processes and causing alterations in the p53 gene [5]. This necessity has motivated intensive research for safer, more sustainable pH-sensing systems aligned with the principles of green analytical chemistry [7].
In this context, plant-derived natural dyes have emerged as compelling candidates to replace or complement conventional indicators [8,9]. These pigments are typically abundant, inexpensive, and biodegradable, and they are generally regarded as safe, making them attractive for developing disposable sensors in regions with limited resources or strict environmental regulations [10,11].
Natural colorants, especially anthocyanins [12], betalains [13], curcuminoids [14], flavones, and carotenoids, exhibit chromophoric properties that respond sensitively to pH changes and can be recovered from agricultural products or biowaste [5], such as red cabbage [15], berries, beetroot, turmeric [16], and hibiscus flowers [9,17].
The functional mechanism of many of these pigments, especially anthocyanins [12], is based on reversible structural transformations of the flavylium cation into quinoidal bases or chalcone forms as the hydrogen ion concentration changes, resulting in visible color shifts over specific pH ranges [18]. Beyond their pH responsiveness, many natural pigments are bioactive (e.g., antioxidants, antimicrobials), which has spurred their integration into intelligent packaging [10,19,20], optical sensors, and other functional materials, such as solar cells [21] and organic LEDs [22].
A growing body of work has demonstrated that plant extracts can serve as effective acid-base indicators in classical titrations [7,23]. For example, Raghavendra et al. [5] utilized plant biowaste to obtain green acid–base indicators, exploring their phytochemical profiles and quantum-chemical properties. In other work, they evaluated beetroot and pomegranate peel extracts as titration indicators with antioxidant activity [6]. The extracts from fig leaves (Ficus carica), pomegranate leaves (Punica granatum), and Mussaenda flowers (Mussaenda philippica) have been reported to yield titration endpoints that coincide or closely agree with those obtained using phenolphthalein, often with low percentage errors on the order of 1.6–4.5% [5]. Ghatage et al. systematically compared pigment extracts as pH indicators in titrimetric analysis [7], while Korfii et al. [24] demonstrated that red mangrove extracts can serve as practical pH indicators in aqueous systems.
A secondary data analysis from Tuslinah et al. [18] of ethanol extracts from anthocyanin-containing plants such as Adam’s Eve leaves (Rheo discolor), white frangipani flowers, and Telang flowers (Clitoria teratea L.) found no statistically significant differences in endpoint pH detection compared with phenolphthalein (p > 0.05), with relative standard deviations typically ≤1–2%, indicating high precision under the tested conditions. These results support the analytical viability of plant-based dyes as pH indicators, providing robust and low-cost pH indication in liquid assays. However, their implementation as standardized, ready-to-use test strips remains less explored.
Natural pigments have also been explored in more application-oriented formats. Specific applications of natural dyes as indicators are also being reported in food quality control [25]. For example, Sha et al. [23] validated aqueous extracts of Ruellia simplex flowers as indicators for milk quality assessment, demonstrating high accuracy and excellent linearity (R2 ≈ 0.978) comparable to established dye reduction tests, and highlighting the potential of plant-based colorants as field-ready analytical tools in dairy diagnostics.
Wu et al. [15] include red-cabbage puree/poly (vinyl alcohol) films for monitoring fish freshness; Ma et al. [26] developed pH-sensitive chitosan quaternary ammonium films loaded with anthocyanins for pork; Mohseni-Shahri and Moeinpour [27] explored gelatin films co-loaded with curcumin and anthocyanins for shrimp; and Faisal et al. [28] developed composite films that combine anthocyanins with the synthetic dye neutral red to expand the dynamic response range. These systems typically offer improved handling, mechanical robustness, and reduced dye leaching compared with simple liquid indicators.
On the other hand, films and hydrogels containing synthetic pH-sensitive dyes or conducting polymers such as polyaniline (PANI) have been developed for optical readout using simple LED-based devices, illustrating how low-cost optical transduction can complement colorimetric materials in real-time pH monitoring [29]. Overall, these studies show that natural extracts can, at least in certain contexts, match traditional synthetic indicators in terms of accuracy, sensitivity, and precision, while also reducing toxicity and enabling integration into biodegradable materials.
In parallel, paper-based analytical devices (PADs) have emerged as attractive platforms for low-cost sensing, enabling capillary-driven assays and optical readout with minimal equipment [30]. Most PADs reported to date incorporate synthetic dyes or electroactive materials, such as polyaniline (PANI)-based optical pH layers [29], which offer good sensitivity but rely on petrochemical components and may raise environmental or safety concerns. There is, therefore, a growing interest in PADs that use fully bio-derived colorants while retaining quantitative or semi-quantitative performance.
Despite these advances, important challenges remain. Natural pigment sensors frequently exhibit limited stability: anthocyanins and related compounds may degrade or lose color intensity under prolonged exposure to high pH, light, oxygen, or elevated temperature, which can shorten the practical shelf life of test strips and films [12,31]. Ensuring consistent calibration of color changes to precise pH values is also non-trivial, as perceived color is influenced by ambient lighting, dye loading, substrate properties, and the optical characteristics of the imaging system. Moreover, reproducibility can be problematic: differences in plant source, harvest conditions, extraction protocol, or storage can alter the composition of multicomponent extracts, leading to sensor-to-sensor variability in color response [5,11,32]. The secondary analysis of natural dyes [18] versus phenolphthalein further illustrates that, while precision and apparent equivalence can be demonstrated in specific titration settings, key aspects such as long-term storage stability, effects of ionic strength and interfering substances, lighting requirements, and user training remain insufficiently characterized. These gaps underscore the need for systematic studies that not only explore new dye sources but also explicitly address stability, calibration, and reproducibility under practical conditions.
In this context, the present work aims to develop and characterize a simple, paper-based pH test strip utilizing pigments extracted from natural vegetable dyes. We screened forty plant species readily available in the Ecuadorian market, selected based on accessibility and non-endangered status, to identify those with the clearest and broadest pH-responsive color transitions. From this survey, four bio-derived dyes—hibiscus, purple cabbage, radish, and turmeric—were chosen for detailed study based on their distinct color changes over the pH scale. The corresponding extracts were immobilized on mixed cotton–cellulose pads to construct multi-zone paper strips that, in combination, visually cover the full pH range from 0 to 14 using only natural pigments. We then characterized their response to standard buffer solutions under controlled illumination, documented the resulting color matrices, and evaluated short-term stability in both liquid and dry formats over storage periods of 15–30 days.
In addition to visual inspection, we explored a deliberately low-complexity grayscale-based digital image analysis as a semi-quantitative readout, using region-of-interest averaging to mitigate local non-uniformities in dye deposition. This choice was made to approximate a realistic, resource-limited scenario in which only basic camera hardware and straightforward processing are available, while acknowledging that more sophisticated RGB/HSV workflows could further improve precision in future work. Throughout the manuscript, we explicitly frame the system as a bio-derived, semi-quantitative pH test strip and discuss its limitations in stability, calibration, and reproducibility, as well as clear directions for optimization (e.g., long-term aging studies, interference testing, and comparison across multiple harvests and extraction batches). Overall, this study presents a sustainable, low-cost proof-of-concept for biodegradable pH test strips based on natural dyes, with potential applications in educational settings, on-site environmental monitoring, and quality control in food and cosmetic products.
2. Materials and Methods
2.1. Selection of Plant Sources
The experimental process began with the selection of 40 plant species readily available in the Ecuadorian market. The criteria for selection were (1) non-endangered status, ensuring compliance with environmental and conservation regulations, and (2) accessibility in local markets to ensure potential reproducibility and scalability. The selected species included a variety of edible fruits, vegetables, flowers, and spices traditionally used for their natural pigmentation (see Table 1).

Table 1.
Plant species screened as potential natural pH indicators, showing scientific and common names and the plant part (leaf, fruit, or peel) used for pigment extraction.
2.2. Extraction of Natural Pigments
In the present study, all extracts were prepared from plant material acquired in a single region and time period, using a standardized maceration and drying protocol. We did not perform a systematic comparison of different harvests or geographical batches, nor did we conduct chromatographic quantification of individual pigments. Therefore, our data reflect a proof of concept for one set of extract pigments. This research opens the door to the possibility of creating controlled crops if the process is to be industrialized, thereby guaranteeing the colorimetric characteristics of the pigment and eliminating differences that can occur due to various factors, such as geographical origin, climate, and harvesting conditions.
Pigments were extracted using a dynamic-static maceration protocol. Initially, dynamic maceration was performed for 1 hour under continuous agitation to enhance cell wall disruption and pigment release. This step was followed by static maceration for 23 h at room temperature, allowing for the complete diffusion of the pigments into the solvent.
The extraction solvent used was a binary mixture of 70% methanol and 30% distilled water (v/v), chosen for its efficiency in extracting both hydrophilic and moderately lipophilic compounds. A solid-to-solvent ratio of 1:10 (w/v) was maintained to standardize extraction yields across different samples. Although methanol is a toxic solvent and not ideal per green chemistry principles, its use was justified by the high extraction efficiency for a broad range of pigment compounds; moreover, the solvent was completely evaporated after extraction, minimizing its presence in the final product. All extractions were performed with proper safety measures (ventilation, PPE), and the dried pigment extracts contained no residual methanol. In future developments, greener solvent alternatives (e.g., ethanol or water-based systems) will be explored to further improve the eco-friendliness of the process.
After maceration, filtration was performed using vacuum filtration (simply accelerates the process by generating a pressure difference across the filter funnel) and qualitative filter paper (WHATMAN CYTIVA 1440 Grade 40 quantitative cellulose filter paper, 8 µm, Corona, CA, USA) to remove residual plant solids. The resulting filtrate contained the dissolved pigment extract.
To obtain the concentrated colorant, the filtrate was evaporated using a rotary evaporator (model RE-2S-VD, LABFREEZ, Beijing, China) under reduced pressure at 40 °C until a dry pigment concentrate was obtained. This step was crucial for standardizing pigment dosage in subsequent tests and calculating extraction yield per plant material (see Figure 1).
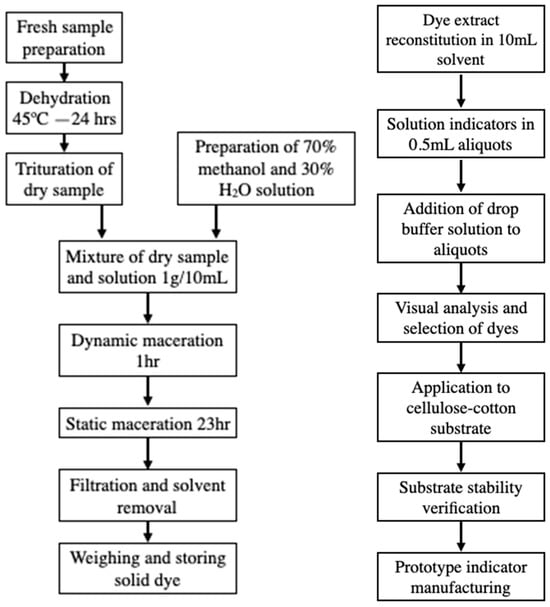
Figure 1.
(Left) Diagram of dye extraction and filtration. (Right) Diagram of experimental testing and prototype manufacturing.
2.3. Preparation of Indicator Solutions
Each pigment extract was reconstituted by dissolving an aliquot of the dried pigment in 10 mL of distilled water, forming a stock solution. From this solution, 14 aliquots of 0.5 mL were dispensed into transparent glass vials, one for each of the 14 buffer pH values.
To each vial, a single drop of buffer solution at a defined pH was added. The buffer solutions spanned a pH range of 1 to 14, prepared using standard analytical-grade buffer reagents. For the most acidic point (pH 0), a diluted hydrochloric acid solution (0.1 M HCl) was used to approximate a pH 0 buffer, extending the range for later tests. The pH of each buffer was verified using a calibrated pH meter (AB 200 ACCUMET, Fisher, Madrid, Spain) to ensure accuracy.
A visual inspection was then carried out under standardized lighting conditions, using a white background and a cold white LED light (BR30, 65W, Sunco, CA, USA) from the same distance and position, to evaluate chromatic transitions. Pigments showing clearly distinguishable color changes across multiple pH levels were considered as preselected candidates (see Figure 2).

Figure 2.
Radish peel dye in 0.5 mL vials exposed to pH levels of 1, 3, 5, 7, 9, 11, and 13, from left to right.
2.4. Application to Paper Substrate
The preselected pigments were further evaluated for stability and responsiveness when applied to a cellulose-cotton matrix. The substrate used was a blend of 50% cellulose and 50% cotton paper, cut into uniform strips and 5 × 5 mm square pads (see Figure 3). Pigment solutions were applied to the paper pads by either immersing the pads in the solution or drop-casting the solution onto the pad until it was fully saturated and then allowing it to dry under ambient conditions. Drying was performed in the dark and at room temperature (avoiding direct sunlight and excessive humidity) to preserve pigment integrity. After drying, each colored paper pad (5 mm × 5 mm) was affixed onto a plastic support strip (transparent acetate, Hygloss, NJ, USA) using a neutral-cure silicone adhesive (SP-0510, Sayer, Mexico). The silicone was applied in a very small amount at the edge of the pad to minimize contact with the pigmented area. Importantly, this silicone adhesive was tested to ensure it had no intrinsic acidity or alkalinity that could influence the indicator (it showed neutral pH and caused no color change in an undyed paper control). The adhesive was allowed to cure for 24 h, yielding assembled test strips with the pigment pads firmly attached.
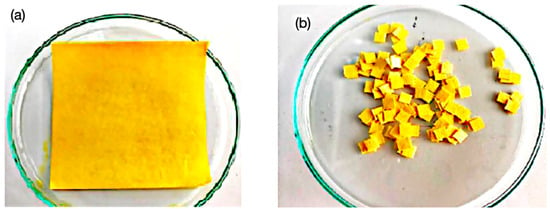
Figure 3.
(a) Turmeric dye used to impregnate the paper substrate, 10 × 10 cm, 50% cotton and 50% cellulose. (b) Impregnated paper substrate in 5 × 5 mm samples ready to be mounted on acetate paper strips.
Pigment solutions were applied to the paper strips by immersion or drop-casting and then dried under ambient conditions, avoiding direct sunlight and excessive humidity. Once dry, the strips were exposed again to standard buffer solutions across the entire pH range to determine if their chromatic response was preserved in a solid-state format (see Figure 4).

Figure 4.
Side-by-side photograph of paper-based pH test pads for five dye systems (rows (a–e)). Rows (a–e) correspond to five distinct natural dye extracts (from top to bottom): turmeric, radish, purple cabbage pigment extracted with water, hibiscus, and purple cabbage. Within each row, pads are ordered from lower to higher pH (left to right), illustrating the changes in hue and intensity across the selected pH set.
The selection criteria at this stage included the following:
- Color contrast between pH values.
- Reversibility of the color response.
Stability of the strips under storage in dry and dark conditions for up to 30 days.
2.5. Digital Image Analysis
To evaluate the analytical potential of the selected indicators, the colored paper strips were subjected to image-based analysis using grayscale intensity profiling. High-resolution photographs (12 MP, f/2.8, Apple iPhone 11, Apple Inc., Cupertino, CA, USA) were taken under controlled lighting and background conditions, and the image data were processed using open-source software, such as ImageJ (ImageJ version 154, NIH, Bethesda, MD, USA) [33].
The color response of each strip to pH was analyzed based on its grayscale intensity profile, enabling a semi-quantitative comparison between samples. This analysis aimed to determine whether the colorimetric variation could be digitally interpreted and correlated to specific pH values, enabling future integration into electronic pH sensing systems. This produced intensity peaks and troughs corresponding to each pad’s position and darkness. However, given that the dye distribution on some pads was not perfectly uniform, the analysis method was refined by sampling multiple points across each pad area. Specifically, we selected three representative regions (center and many opposite side points) on each pad and recorded the grayscale intensity in those regions (see Figure 5), then averaged these values. This multi-point sampling approach provides an average grayscale intensity for the entire pad, thereby mitigating the influence of uneven color distribution or edge effects.
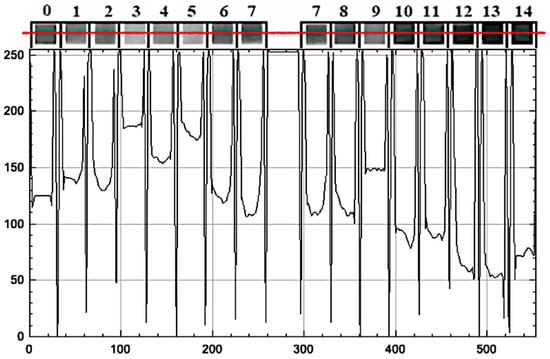
Figure 5.
Grayscale intensity (0–255) along a horizontal scan (red line) of a radish-dye pH strip, with pad boundaries and pH labels (0–14) indicated; peak–trough patterns reflect pH-dependent contrast.
2.6. Final Indicator Selection and Prototype Design
Based on the cumulative results, the four most promising pigments were selected for the development of a prototype. These were chosen for the following qualities:
- Strong and distinguishable chromatic transition across wide pH ranges.
- Adequate stability on paper matrices.
- Clear grayscale profiles suitable for image-based detection.
The four selected pigments were Hibiscus sabdariffa (roselle flower), Brassica oleracea var. capitata f. rubra (purple cabbage), Raphanus sativus (red radish), and Curcuma longa (turmeric). Notably, the first three are anthocyanin-rich extracts, while the last is a curcuminoid-based extract, representing two major classes of natural pH-sensitive pigments.
The final step involved fabricating pH indicator test strips using the selected pigments. These strips were prepared in standardized dimensions and tested for practical usability in laboratory and field conditions.
3. Results
3.1. Visual Response to pH in Preselected Pigments
From the initial screening of 40 plant-based extracts, four pigments were selected based on their strong and distinguishable chromatic response across a broad range of pH values (see Table 2):

Table 2.
Pigments selected and evaluated as pH indicators, and the nominal pH range over which a visible color change was observed.
- Hibiscus sabdariffa (Hibiscus flower)
- Brassica oleracea var. capitata f. rubra (Purple cabbage)
- Raphanus sativus (Radish)
- Curcuma longa (Turmeric)
Each pigment was tested both in aqueous solution and embedded into a solid matrix of paper (50% cellulose, 50% cotton) cut into 5 mm × 5 mm squares. These were mounted onto acetate strips using cold silicone glue (carefully chosen to avoid any alteration with the change in pH of the solutions) to avoid altering the colorant’s stability and chemical behavior (see Figure 6). After drying, the indicator strips were exposed to buffer solutions with pH values ranging from 0 to 14.
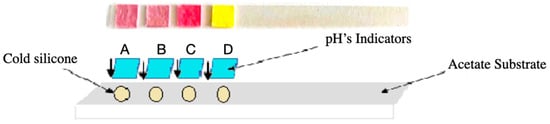
Figure 6.
Assembly of the acetate-based test strip, showing the four dye-impregnated pads (A–D) mounted with cold silicone on the acetate substrate prior to pH exposure.
3.2. Qualitative Visual Evaluation
Upon exposure to the buffer solutions, the samples displayed visually detectable color transitions over different pH ranges. This color shift was immediate for most indicators, particularly in acidic or strongly alkaline conditions (see Figure 7).
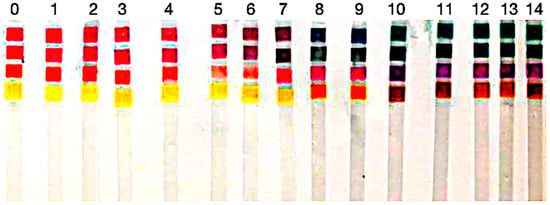
Figure 7.
Photograph of paper strips dyed with the four bio-derived colorants after exposure to buffer solutions from pH 0–14 under controlled illumination. Columns are ordered left-to-right by pH; the labels above indicate the buffer value for each column, and the stacked pads within each column show the dye-dependent color response. The rows (colorant dye) from Table 2 correspond to the rows in this figure.
This visual assessment highlights that the dyes stand out as pigments capable of demonstrating detectable color changes throughout the entire pH spectrum (0–14). In contrast, other pigments displayed limitations in very acidic environments (pH < 3).
Notably, visual inspection remains the most reliable and practical method for determining pH ranges with these natural indicators. While digital processing offers quantitative insights, human vision is still superior in recognizing subtle color gradients across a spectrum, particularly in low-resolution or uneven lighting conditions.
Under uncalibrated conditions, visual perception remains the gold standard for practical pH estimation using natural pigments, particularly when employed outside laboratory settings.
3.3. Digital Image Analysis and Grayscale Profiling
To explore the potential for quantitative digital pH estimation, all samples were photographed under standardized illumination on a white background and using a cold white LED light at a consistent distance. The digital images were then converted to grayscale, and intensity profiles were extracted for each pH condition using image analysis software (ImageJ).
The resulting profiles showed a partial correlation between pH and grayscale intensity, enabling semi-quantitative interpretation in selected pH ranges (see Table 3 and Figure 8):

Table 3.
Dyes selected for the pH test strips and the pH range over which their color response can be reliably distinguished by grayscale image analysis.
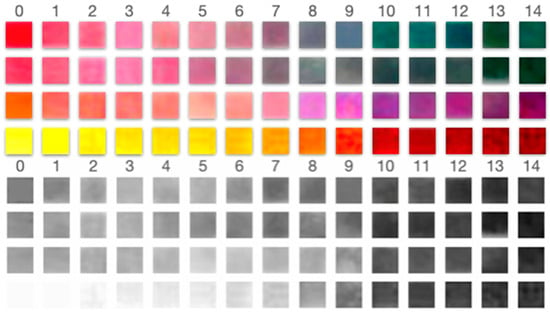
Figure 8.
Color and grayscale matrices for the four dye systems across buffer pH 0–14. Top panels: Original color pads ordered from low to high pH (left to right). Bottom panels: corresponding grayscale renderings used for intensity analysis. These matrices illustrate the pH-dependent hue/brightness shifts that underpin the grayscale detectability reported in Table 3.
The grayscale response was non-linear and pigment-dependent. For example, turmeric exhibited a consistent intensity gradient across all pH values, indicating potential applications in digital or smartphone-assisted sensing platforms. In contrast, hibiscus and radish exhibited plateaus in low-pH regions, limiting their application in highly acidic environments (see Figure 9 and Figure 10).
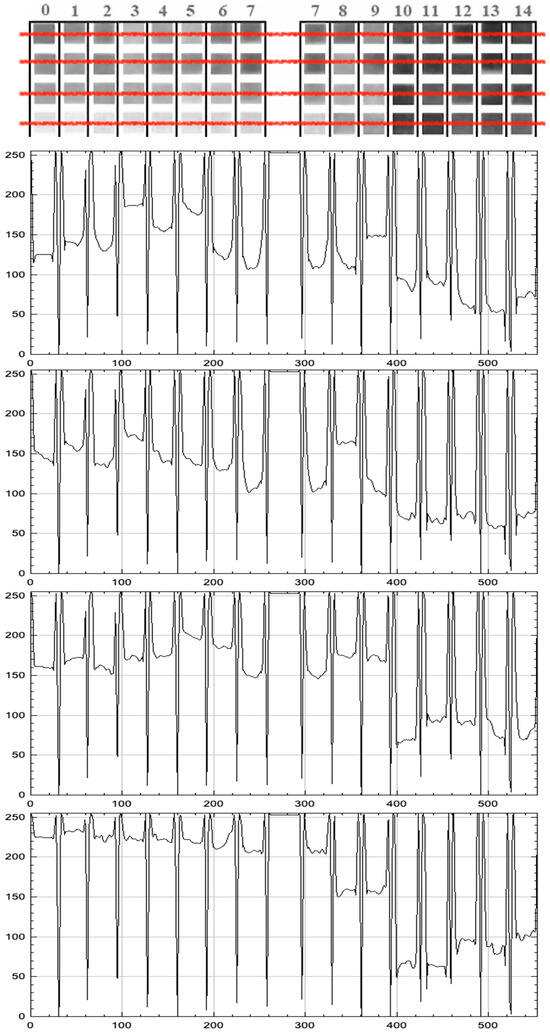
Figure 9.
Grayscale line-scan (horizontal red line) intensity profiles for four dye-based pH strips exposed to buffers from pH 0 to 14. Panels from top to bottom: Hibiscus, Purple cabbage, Radish, Turmeric. Each curve shows pixel intensity (0–255 arbitrary units) along the horizontal position; vertical minima mark inter-pad gaps. The thumbnail mosaics above each plot depict the corresponding pad sequence (0–14).
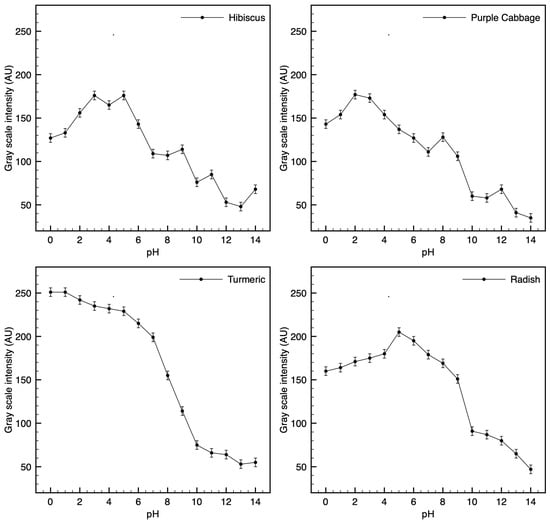
Figure 10.
Grayscale intensity versus pH (0–14) for each dye: Hibiscus, Purple cabbage, Turmeric, and Radish. Curves show the mean pixel intensity (arbitrary units) with error bars indicating the standard deviation from (n = 3) replicate pads per pH. Non-monotonic segments reflect dye-specific hue changes captured in grayscale.
Despite the promise of digital analysis, some limitations remain. Variability in light conditions, sensor sensitivity, and paper texture can affect accuracy. Therefore, visual inspection remains the most effective and accessible method for using these indicators, especially in field settings without image-processing tools.
3.4. Comparison and Application Potential
The four selected pigments were each able to indicate a wide span of pH, but with individual strengths as noted. Summarizing the performance:
- Turmeric was the most versatile and robust pigment, showing a clear color and brightness change across the full pH 0–14 range. It maintained a yellow-to-brown gradation that could potentially allow continuous estimation.
- Purple cabbage and hibiscus offered excellent color contrast in the neutral-to-alkaline pH range (they turn distinctly different colors above pH ~6), making them suitable for applications like food packaging or environmental water testing where neutral-to-basic pH changes are relevant. However, in very acidic conditions (pH < 3), both tended to simply be bright red, limiting their distinguishability in that range.
- Radish performed best in mid-to-high pH levels (it became a strong purple or gray above pH 8) but is less reliable at pH levels below 5 (little visible change in the red-pink region). It could be useful for detecting the onset of alkalinity, but not for differentiating strong acids.
Significantly, all four pigments demonstrated sufficient stability on the paper substrate, maintaining their color and response properties after drying and storage under ambient conditions for at least 30 days. This short-term stability test (up to one month) suggests that the strips do not immediately degrade and can be prepared in advance and used when needed. The prototype strips (kept in closed containers, in the dark, at room temperature) showed no visible color loss or shift over 15 and even 30 days, and the pH response on Day 30 was essentially the same as initially observed. (Figure 11 shows a prototype strip immediately after fabrication and another after 30 days of storage, with no obvious difference in color.) In future work, a comparison will be made between the stability of commercial dyes and that of organic dyes; however, this was not one of the initial objectives of the work. Nevertheless, it is not ruled out for future work to strengthen the research.
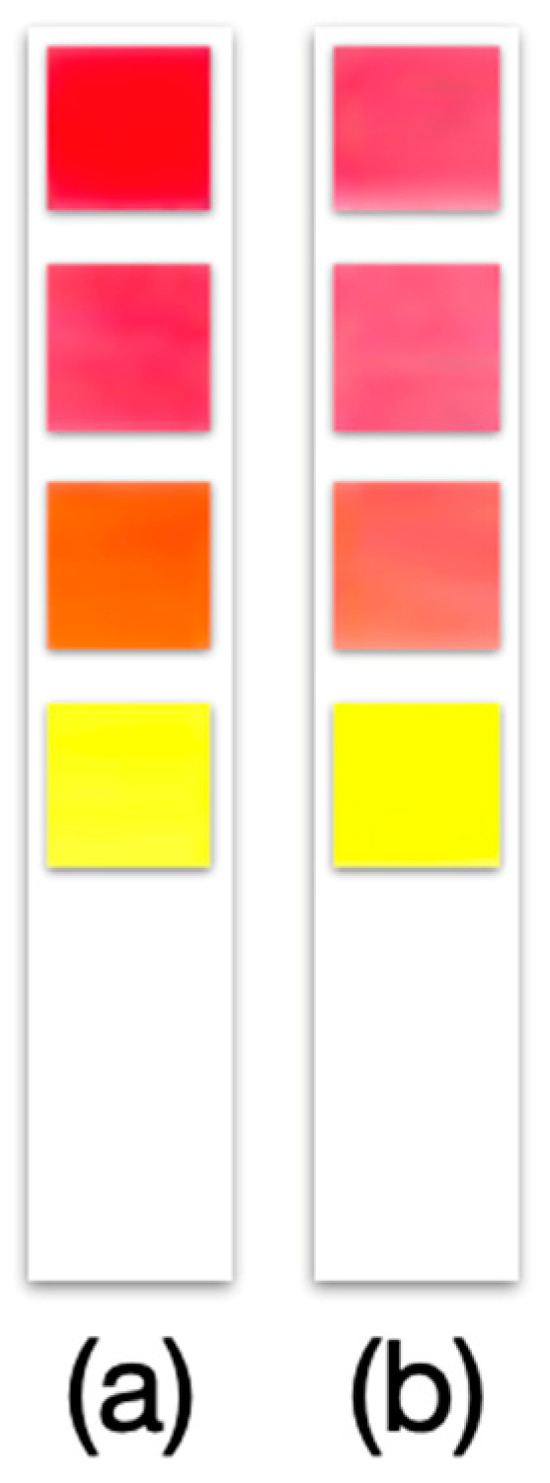
Figure 11.
Prototype of pH test strip assembled with four 50% cotton:50% cellulose papers of 5 × 5 mm impregnated with natural organic dye. (a) Freshly manufactured and (b) after 30 days of storage at 22 ± 5 °C, humidity 30 ± 5%, and light and darkness cycles of 12 h/12 h.
4. Discussion
This study demonstrates the feasibility of paper-based colorimetric pH test strips fabricated with bio-derived dyes (hibiscus, purple cabbage, radish, turmeric). Across pH 0–14, the four systems produced clear hue/intensity changes, with turmeric showing detectable contrast across the full range, while anthocyanin-rich dyes (hibiscus, purple cabbage, radish) exhibited reduced contrast at very low pH. These observations support the intuitive use of plant dyes for affordable pH indication and provide a basis for semi-quantitative readout using image analysis. In particular, turmeric demonstrated full-spectrum responsiveness, confirming its potential as a broad-spectrum natural indicator [16,34,35].
Digital image analysis provided semi-quantitative insights into pigment behavior, revealing that grayscale intensity profiles can partially correlate with pH values in specific ranges. For example, hibiscus and radish allowed grayscale-based detection within the pH range of 5 to 14. At the same time, purple cabbage was detected between pH 3 and 14, and turmeric was detected across the entire pH range of 0 to 14, demonstrating its robustness and reliability [20,25].
The grayscale analysis was chosen rather than an analysis in the color space because (i) it simplifies the processing pipeline, (ii) it is less sensitive to small color-balance shifts than individual RGB channels, and (iii) our device is primarily intended for visual, low-resource applications. We are considering studying it under an experimental design made for that purpose.
However, it is essential to emphasize that, despite the utility of grayscale analysis, visual inspection remains the most effective and accessible method for determining pH using these natural indicators. Human perception excels in detecting complex color gradients, particularly when variability in lighting or image resolution limits digital interpretation [12,30]. This makes these indicators especially suitable for use in educational settings, environmental fieldwork, and low-resource communities, where instrumentation may not be available.
In agreement with prior research on anthocyanins and curcuminoids [12,14,16,27], the findings confirm that plant-based pigments are highly responsive to pH changes, exhibiting sufficient stability under ambient conditions when properly processed and applied. Moreover, the ability to produce test strips using non-toxic, biodegradable materials aligns with the global movement toward greener chemical technologies and eco-friendly analytical tools [36,37].
5. Conclusions and Future Work
This project demonstrates the feasibility and practicality of developing natural pH indicators from plant-derived pigments, offering a sustainable and low-cost alternative to synthetic acid-base indicators. Through a systematic screening of 40 plant species based on availability and extractability, four pigments—Hibiscus sabdariffa, Brassica oleracea var. capitata f. rubra (purple cabbage), Raphanus sativus (radish), and Curcuma longa (turmeric)—were selected for their distinct and reproducible chromatic responses to a wide range of pH values.
These findings support the development of eco-friendly pH test sensors based on plant-derived colorants. Their combination of low cost, biodegradability, and acceptable stability positions them as practical tools for educational, diagnostic, and environmental monitoring applications.
One limitation of field quantitation is that the illumination and camera settings were controlled but not fully standardized across all acquisitions, even with a color reference; small variations in white balance or illuminance (lux) can bias grayscale intensity. Additionally, we did not systematically assess interferences (ionic strength, divalent cations, sugars, proteins, amines) at fixed pH levels. In this sense, the immediate future work should focus on (i) improving long-term stability through encapsulation techniques, (ii) enhancing digital interpretation via calibrated colorimetry and machine learning algorithms applied to RGB/HSV image data, and (iii) exploring combinatorial pigment systems for extended sensitivity and color gradient refinement.
Finally, this research underscores the integration of natural pigments into portable, accessible, and sustainable pH-sensing platforms, thereby bridging traditional knowledge and modern materials science for a diverse range of practical applications.
Author Contributions
Conceptualization, A.A.S., G.J. and G.R.-C.; methodology, A.A.S., G.R.-C. and G.J.; validation, D.C. and V.L.; formal analysis, A.A.S., G.R.-C., D.C. and V.L.; investigation, G.R.-C.; resources, A.A.S. and G.J.; data curation, G.R.-C. and G.J.; writing—original draft preparation, A.A.S.; writing—review and editing, D.C. and V.L.; supervision, A.A.S. and G.R.-C.; project administration, A.A.S.; funding acquisition, A.A.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding. The APC was partially funded by Universidad Técnica Particular de Loja, with funding number POA VIN-56.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding authors.
Acknowledgments
The authors acknowledge the support given by the technicians of every laboratory that helped us reach this research’s objectives, and also R. Palomino-Merino for his academic support in this project. A.A.S. and D.C. acknowledge the partial financial support from the Universidad Técnica Particular de Loja (UTPL). During the preparation of this manuscript, the authors utilized ChatGPT (version 5) and Grammarly for the purposes of improving text, grammar, analysis, and text translation. The authors have reviewed and edited the output and take full responsibility for the content of this publication.
Conflicts of Interest
The authors declare NO conflicts of interest. Aramis A. Sánchez was employed by the company PROSUR Construcción Sustentable del Sur, Puebla 72810, México, but declared NO conflicts of interest. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| pH | Potential hydrogen |
| LED | Light Emitting Diode |
References
- Bates, R.G. Determination of pH: Theory and Practice; Wiley-Interscience: New York, NY, USA, 1973. [Google Scholar]
- Manjakkal, L.; Szwagierczak, D.; Dahiya, R. Metal oxides based electrochemical pH sensors: Current progress and future perspectives. Prog. Mater. Sci. 2020, 109, 100635. [Google Scholar] [CrossRef]
- Kragh, H.S. S.P.L. Sørensen, the pH concept and its early history. Found. Chem. 2025, 27, 237–261. [Google Scholar] [CrossRef]
- Păușescu, I.; Dreavă, D.-M.; Bîtcan, I.; Argetoianu, R.; Dăescu, D.; Medeleanu, M. Bio-Based pH Indicator Films for Intelligent Food Packaging Applications. Polymers 2022, 14, 3622. [Google Scholar] [CrossRef]
- Raghavendra, N.; Chapi, S.; Amberkhed, R.; Hebballi, S.; Shivannagol, V.; Paramshetti, T.; Nidagundi, P.; Raghu, A.V. From plant biowaste to green and sustainable acid-base indicators: Extraction, phytochemical screening, and quantum simulation. Bioresour. Technol. Rep. 2025, 32, 102351. [Google Scholar] [CrossRef]
- Raghavendra, N.; Hublikar, L.V.; Sowmyashree, A.S.; Kale, S.B. Investigation of acid-base indicator and antioxidant properties of beetroot and pomegranate peel extract. Sustain. Chem. One World 2025, 8, 100111. [Google Scholar] [CrossRef]
- Ghatage, T.S.; Killedar, S.G.; Hajare, M.A.; Joshi, M.M. Extraction, characterization, and utilization of different plant pigments as pH indicators in titrimetric analysis. Int. J. Adv. Res. 2017, 3, 462–468. [Google Scholar]
- Liu, D.; Zhang, C.; Pu, Y.; Chen, S.; Liu, L.; Cui, Z.; Zhong, Y. Recent Advances in pH-Responsive Freshness Indicators Using Natural Food Colorants to Monitor Food Freshness. Foods 2022, 11, 1884. [Google Scholar] [CrossRef]
- Saeed, A.A.M.; Al-Salimi, M.S.S.; Manea, Y.K.; Abdulmajeed, W.S.; Mohammed, T.H.A.; Abdallah, S.M.A.; BA Fadhl, O.M.O.; Saleh, A.Y.A.; Abdullah, S.H.H.; Al Asahb, W.A.S. Towards greener analysis: Evaluating plant-derived natural dyes as pH indicators. Mor. J. Chem. 2024, 12, 1511–1530. [Google Scholar] [CrossRef]
- Ndwandwe, B.K.; Malinga, S.P.; Kayitesi, E.; Dlamini, B.C. Recent developments in the application of natural pigments as pH-sensitive food freshness indicators in biopolymer-based smart packaging: Challenges and opportunities. Int. J. Food Sci. Technol. 2024, 59, 2148–2161. [Google Scholar] [CrossRef]
- Panwar, A.; Kumar, S.; Dhiman, A.; Kumar, V.; Gupta, D.; Sharma, A. pH sensitive indicator film based intelligent packaging systems of perishables: Developments and challenges of last decade. Microchem. J. 2024, 207, 111732. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, M.d.L.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. Betalains: Properties, Sources, Applications, and Stability—A Review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Sivakumar, R.; Lim, N.; Park, S.K.; Lee, N.Y. Curcumin–a natural colorant-based pH indicator for molecular diagnostics. Analyst 2025, 150, 1632–1641. [Google Scholar] [CrossRef]
- Wu, H.; Jiao, C.; Li, S.; Li, Q.; Zhang, Z.; Zhou, M.; Yuan, X. A Facile Strategy for Development of pH-Sensing Indicator Films Based on Red Cabbage Puree and Polyvinyl Alcohol for Monitoring Fish Freshness. Foods 2022, 11, 3371. [Google Scholar] [CrossRef]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their derivatives—A review. J. Tradit. Complement. Med. 2017, 7, 205–233. [Google Scholar] [CrossRef] [PubMed]
- Di Salvo, E.; Lo Vecchio, G.; De Pasquale, R.; De Maria, L.; Tardugno, R.; Vadalà, R.; Cicero, N. Natural Pigments Production and Their Application in Food, Health and Other Industries. Nutrients 2023, 15, 1923. [Google Scholar] [CrossRef] [PubMed]
- Tuslinah, L.; Yuliana, A.; Arisnawati, D.; Rizkuloh, L.R. Comparison of color change to pH range and acid–base titration indicator precision test of multiple ethanol extracts. Trends Sci. 2022, 19, 1446. [Google Scholar] [CrossRef]
- Klaric, S.V.; Galvão Maciel, A.; Arend, G.D.; Tres, M.V.; de Lima, M.; Soares, L.S. Application of Plant Extracts Rich in Anthocyanins in the Development of Intelligent Biodegradable Packaging: An Overview. Processes 2025, 13, 191. [Google Scholar] [CrossRef]
- Chiu, I.; Yang, T. Biopolymer-based intelligent packaging integrated with natural colourimetric sensors for food safety and sustainability. Anal. Sci. Adv. 2024, 5, e2300065. [Google Scholar] [CrossRef]
- Castillo, D.; Juárez, A.S.; Tapia, S.E.; Guaman, A.; Calderón, D.O. Twenty natural organic pigments for application in dye sensitized solar cells. In Organic Photovoltaics XVII; SPIE: Bellingham, WA, USA, 2016; Volume 9942, pp. 69–75. [Google Scholar] [CrossRef]
- Sánchez Juárez, A.; Castillo, D.; Guaman, A.; Espinosa, S.; Obregón, D. Study of natural organic dyes as active material for fabrication of organic light emitting diodes. In Organic Light Emitting Materials and Devices XX; SPIE: Bellingham, WA, USA, 2016; Volume 9941, p. 99412E. [Google Scholar] [CrossRef]
- Sha, M.S.; Faisal, F.B.; Ottakath, T.K.; Poovadichalil, N.M.; Sadasivuni, K.K. Natural plant extracts for quality assessment in milk by replacing conventional color reduction methods. J. Food Meas. Charact. 2025, 19, 3768–3777. [Google Scholar] [CrossRef]
- Korfii, U.; Boisa, N.; Tubonimi, I. Application of red mangrove plant (Rhizophora racemosa) extracts as pH indicator. Asian J. Green Chem. 2021, 5, 111–124. [Google Scholar] [CrossRef]
- Terra, A.L.M.; Moreira, J.B.; Costa, J.A.V.; de Morais, M.G. Development of time-pH indicator nanofibers from natural pigments: An emerging processing technology to monitor the quality of foods. LWT 2021, 142, 111020. [Google Scholar] [CrossRef]
- Ma, Y.; Wen, L.; Liu, J.; Du, P.; Liu, Y.; Hu, P.; Cao, J.; Wang, W. Enhanced pH-sensitive anthocyanin film based on chitosan quaternary ammonium salt: A promising colorimetric indicator for visual pork freshness monitoring. Int. J. Biol. Macromol. 2024, 279, 135236. [Google Scholar] [CrossRef]
- Mohseni-Shahri, F.S.; Moeinpour, F. Development of a pH-sensing indicator for shrimp freshness monitoring: Curcumin and anthocyanin-loaded gelatin films. Food Sci. Nutr. 2023, 11, 3898–3910. [Google Scholar] [CrossRef]
- Faisal, M.; Jacobson, T.; Meineret, L.; Vorup, P.; Bordallo, H.N.; Kirkensgaard, J.J.K.; Ulvskov, P.; Blennow, A. Development of pH Indicator Composite Films Based on Anthocyanins and Neutral Red for Monitoring Minced Meat and Fish in Modified Gas Atmosphere (MAP). Coatings 2024, 14, 725. [Google Scholar] [CrossRef]
- Stoukatch, S.; Debliquy, M.; Dupont, F.; Redouté, J.-M. Low-Cost Optical pH Sensor with a Polyaniline (PANI)-Sensitive Layer Based on Commercial Off-the-Shelf (COTS) Components. Micromachines 2023, 14, 2197. [Google Scholar] [CrossRef]
- Meredith, N.A.; Quinn, C.; Cate, D.M.; Reilly, T.H.; Volckens, J.; Henry, C.S. Paper-based analytical devices for environmental analysis. Analyst 2016, 141, 1874–1887. [Google Scholar] [CrossRef]
- Giusti, M.M.; Wrolstad, R.E. Characterization and measurement of anthocyanins by UV–visible spectroscopy. In Current Protocols in Food Analytical Chemistry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001; Unit F1.2. [Google Scholar] [CrossRef]
- Szadkowski, B.; Rogowski, J.; Maniukiewicz, W.; Beyou, E.; Marzec, A. New natural organic–inorganic pH indicators: Synthesis and characterization of pro-ecological hybrid pigments based on anthraquinone dyes and mineral supports. J. Ind. Eng. Chem. 2022, 105, 446–462. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Priyadarsini, K.I. The Chemistry of Curcumin: From Extraction to Therapeutic Agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Castillo, D.; Sánchez, A.; González, J.; Chamba, C.; Lakshminarayanan, V. Natural pigment sensor for solar ultraviolet radiation measurement. In Organic and Hybrid Sensors and Bioelectronics XIV; SPIE: Bellingham, WA, USA, 2021; Volume 11810, p. 118100E. [Google Scholar] [CrossRef]
- Zhao, X.; Zhang, H.; Xiang, H.; Yu, D.; Gao, M.; Yan, R.; Zhang, D. A natural pH indicator with high colorimetric response sensitivity for pork freshness monitoring. Food Biosci. 2024, 57, 103519. [Google Scholar] [CrossRef]
- Zhu, W.; Ma, Z.; Zhang, L.; Yang, C.; Xu, F.; Yu, J. Natural anthocyanin-based wearable colorimetric pH indicator with high color fastness. Dye. Pigment. 2025, 239, 112789. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).