Multiplexed Optical Nanobiosensing Technologies for Disease Biomarker Detection
Abstract
1. Introduction
2. Nanomaterials for Enhancing the Performance of Biosensors
2.1. Noble Metals
2.2. Silica-Based Nanomaterials
2.3. Carbon-Based Nanomaterials
2.4. Photonic Crystal
3. Fluorescence-Based Multiplex Detection
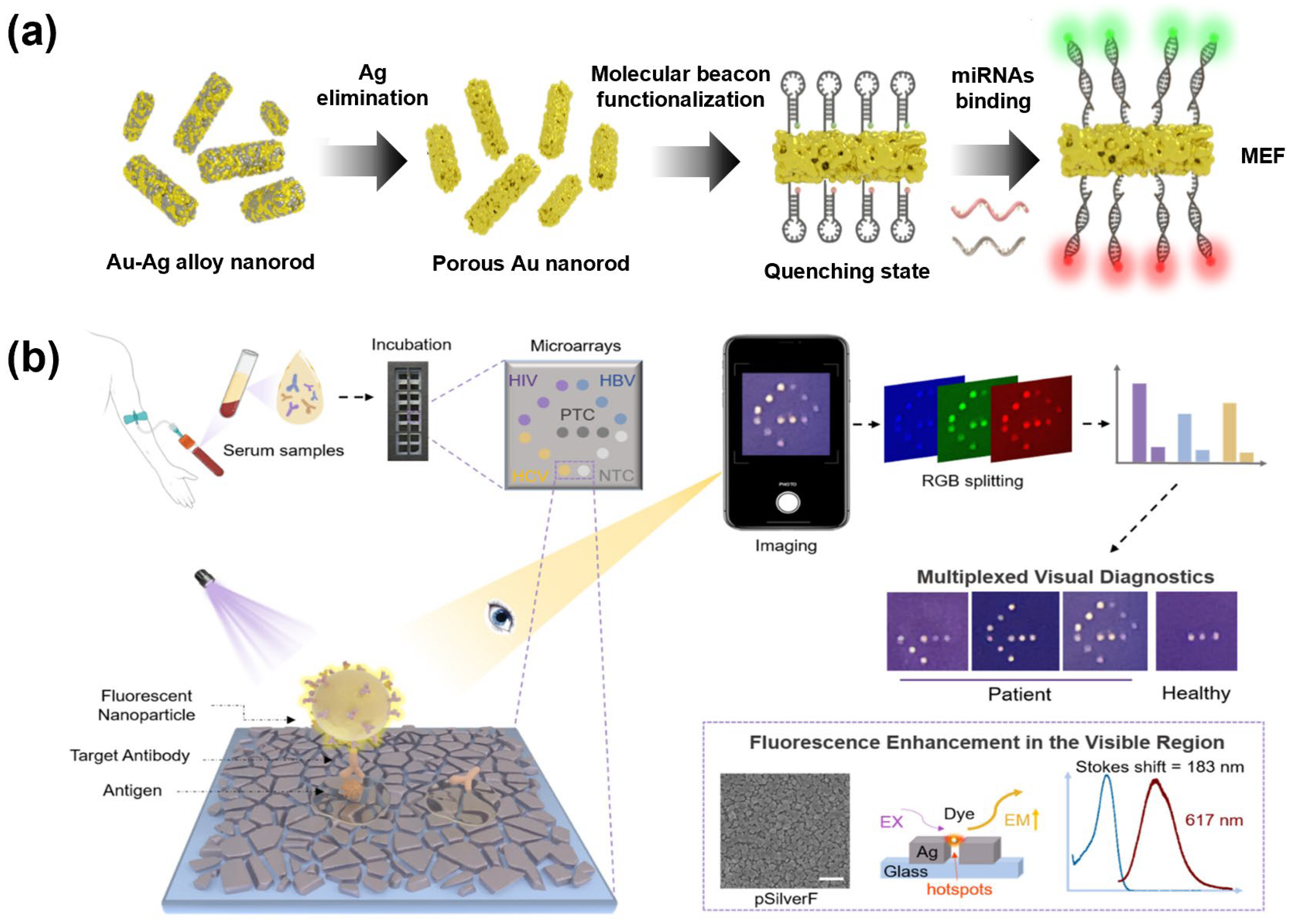
3.1. Fluorescent Particles for an Effective Biosensing System
3.2. Noble Metal-Enhanced Multiplex Fluorescence Nanosensor
3.3. Quantum Dot-Based Multiplex Fluorescence Nanosensor
3.4. Photonic Crystal-Based Multiplex Fluorescence Nanosensor
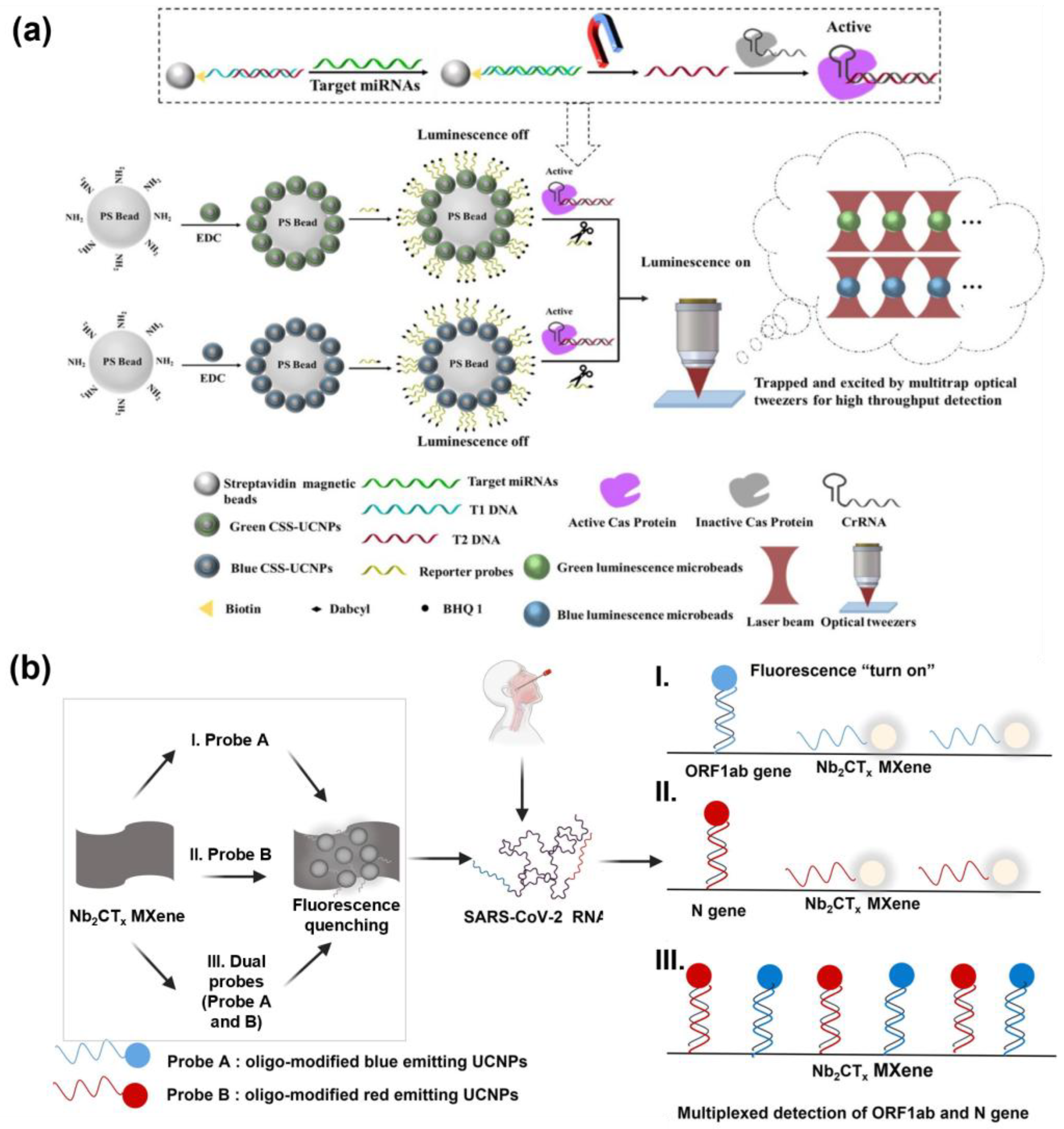
3.5. UCNP-Based Multiplex Fluorescence Nanosensor
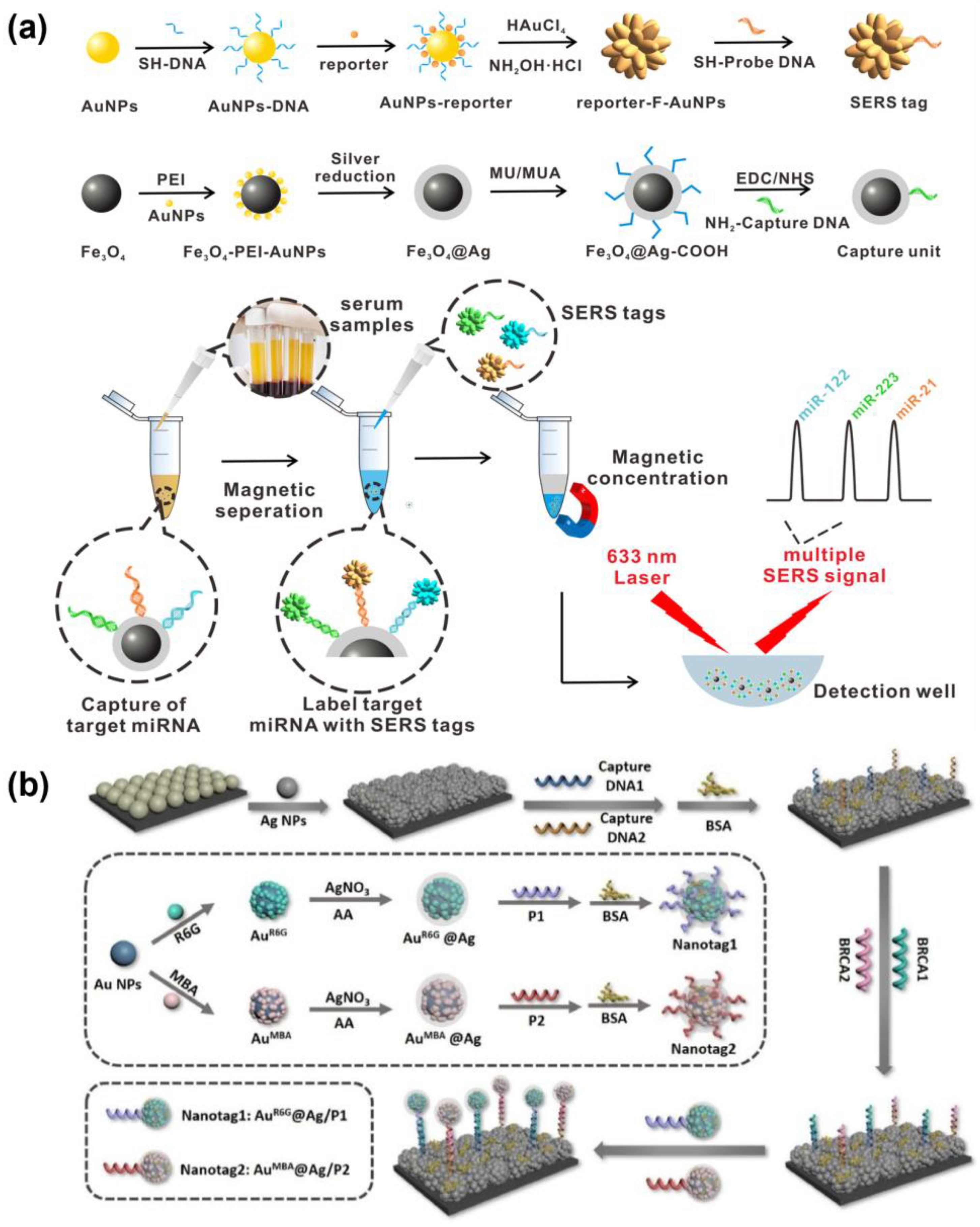
4. SERS-Based Multiplex Detection
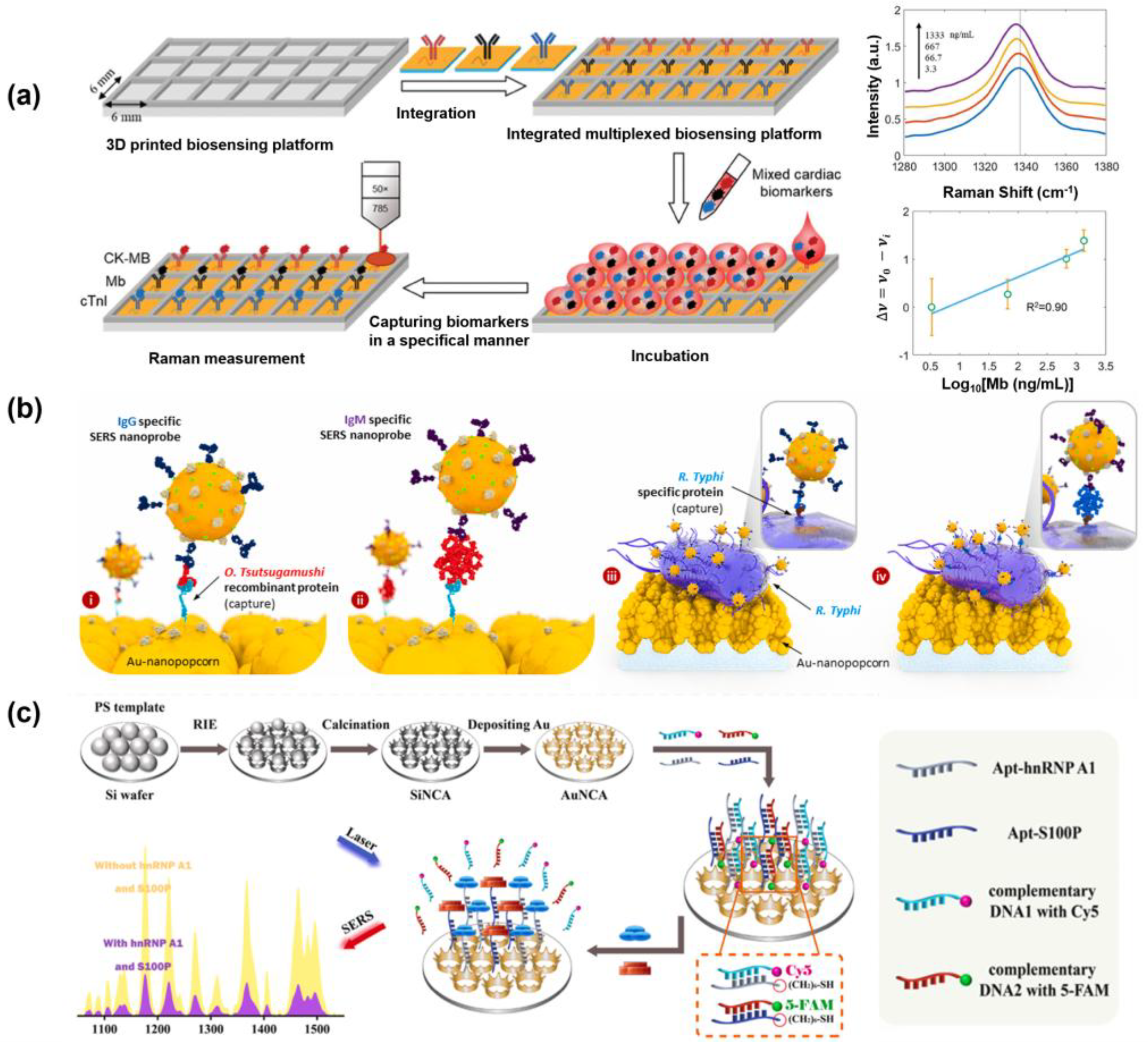
4.1. SERS-Based Detection Platform Using SERS Substrate
4.2. SERS Nanotag-Based Multiplex Biosensor
| Target Biomarkers | Nanoparticles | Method Performance | Sample | Ref. |
|---|---|---|---|---|
| CK-MB, Myoglobin, and cTnI | Gold–silica multilayered pyramidal plasmonic metasurface substrate | LOD: 0.04 ng/mL, 3.6 ng/mL, and 5.2 pg/mL Linear range: 0.1–300 ng/mL, 6–4000 ng/mL, and 8–567 pg/mL | Mixtures of serum | [38] |
| O. tsutsugamushi IgG and IgM, R. typhi IgG and IgM | Microarray chips with gold nanopopcorn nanostructures | LOD: 1:20.4 and 1:7.03, 1:16.8 and 1:12.5 RSD: 5.46%, 4.41%, 4.87%, and 10.15% (Antibody titers) | Human serum (Healthy for negative; Diluted patient sample for positive) | [221] |
| hnRNP A1, S100P | Uniform Au nanocrown array (AuNCA) | LOD: 0.031 pg/mL and 0.057 pg/mL Linear range: 1 pg/mL–1 µg/mL Correlation coefficients: 0.982 and 0.987 | Real clinical serums from healthy and patients | [8] |
| miRNA-21, miRNA-122, miRNA-223 | Star-shaped fractal AuNPs (F-AuNPs) and silver magnetic nanoparticles (AgMNPs) | LOD: 311 aM, 349 aM, and 374 aM Linear range: 1 fM–10 nM | Actual clinical serum samples | [222] |
| Aβ42, Aβ40 | Bumpy core–shell (BCS) SERS nanoprobes | LOD: 87 ag/mL and 1.0 fg/mL RSD: <3% | Human blood plasma and aCSF | [223] |
| miR-214, miR-221 | Gold nanobipyramids (GNBPs) | LOD: 5.14 aM and 5.92 aM Relative error compared with ELISA: <6.85% | Human blood samples from healthy and patients | [219] |
| CEA, NSE | Au–Fe3O4 core–shell nanoparticles | LOD: 0.9 pg/mL and 1.6 pg/mL | - | [218] |
| BRCA1, BRCA2 | Au@Ag core–shell nanoparticles | LOD: 0.61 pM and 0.78 pM Linear range: 1 pM–1 µM RSD: 4.75% | - | [224] |
5. Colorimetric Multiplex Biomarker Detection
5.1. Paper-Based Multiplex Colorimetric Biosensor
5.2. Dual-Mode, Including Colorimetric Methods for Multiplex Detection
6. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Klebes, A.; Ates, H.C.; Verboket, R.D.; Urban, G.A.; von Stetten, F.; Dincer, C.; Früh, S.M. Emerging Multianalyte Biosensors for the Simultaneous Detection of Protein and Nucleic Acid Biomarkers. Biosens. Bioelectron. 2024, 244, 115800. [Google Scholar] [CrossRef]
- Xi, H.; Jiang, H.; Juhas, M.; Zhang, Y. Multiplex Biosensing for Simultaneous Detection of Mutations in SARS-CoV-2. ACS Omega 2021, 6, 25846–25859. [Google Scholar] [CrossRef]
- van Dongen, J.E.; Berendsen, J.T.W.; Steenbergen, R.D.M.; Wolthuis, R.M.F.; Eijkel, J.C.T.; Segerink, L.I. Point-of-Care CRISPR/Cas Nucleic Acid Detection: Recent Advances, Challenges and Opportunities. Biosens. Bioelectron. 2020, 166, 112445. [Google Scholar] [CrossRef]
- Semeniak, D.; Cruz, D.F.; Chilkoti, A.; Mikkelsen, M.H. Plasmonic Fluorescence Enhancement in Diagnostics for Clinical Tests at Point-of-Care: A Review of Recent Technologies. Adv. Mater. 2023, 35, 2107986. [Google Scholar] [CrossRef]
- Kitchawengkul, N.; Prakobkij, A.; Saenmuangchin, R.; Citterio, D.; Nacapricha, D.; Jarujamrus, P. Ratiometric Fluorometry on Microfluidic Paper-Based Analytical Device for Simultaneous Glucose and Cholesterol Detection Using MnFe-Layered Double Hydroxides as Peroxidase Mimic. Sens. Actuators B Chem. 2025, 435, 137671. [Google Scholar] [CrossRef]
- Chi, Y.J.; Ryu, B.; Ahn, S.; Koh, W.-G. A Colorimetric Biosensor Based on a Biodegradable Fluidic Device Capable of Efficient Saliva Sampling and Salivary Biomarker Detection. Sens. Actuators B Chem. 2023, 396, 134601. [Google Scholar] [CrossRef]
- Tian, T.; Qiu, Z.; Jiang, Y.; Zhu, D.; Zhou, X. Exploiting the Orthogonal CRISPR-Cas12a/Cas13a Trans-Cleavage for Dual-Gene Virus Detection Using a Handheld Device. Biosens. Bioelectron. 2022, 196, 113701. [Google Scholar] [CrossRef]
- Cao, X.; Liu, Z.; Qin, X.; Gu, Y.; Huang, Y.; Qian, Y.; Wang, Z.; Li, H.; Zhu, Q.; Wei, W. LoC-SERS Platform for Rapid and Sensitive Detection of Colorectal Cancer Protein Biomarkers. Talanta 2024, 270, 125563. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, J.; Zhao, X.; Chen, H.; Xu, H.; Bai, L.; Wang, W.; Yang, H.; Wei, D.; Yuan, B. Highly Sensitive Electrochemical Immunosensor for the Simultaneous Detection of Multiple Tumor Markers for Signal Amplification. Talanta 2021, 226, 122133. [Google Scholar] [CrossRef]
- Yang, Y.; Su, Z.; Wu, D.; Liu, J.; Zhang, X.; Wu, Y.; Li, G. Low Background Interference SERS Aptasensor for Highly Sensitive Multiplex Mycotoxin Detection Based on Polystyrene Microspheres-Mediated Controlled Release of Raman Reporters. Anal. Chim. Acta 2022, 1218, 340000. [Google Scholar] [CrossRef]
- Choi, M.Y.; Haizan, I.; Choi, J.H. Simultaneous Detection for Breast Cancer-Associated Cell-Free DNA and MicroRNA Using Multiplex CRISPR Cas12a/Cas13a System. Sens. Actuators B Chem. 2025, 439, 137830. [Google Scholar] [CrossRef]
- Hasan, M.R.; Sharma, P.; Pilloton, R.; Khanuja, M.; Narang, J. Colorimetric Biosensor for the Naked-Eye Detection of Ovarian Cancer Biomarker PDGF Using Citrate Modified Gold Nanoparticles. Biosens. Bioelectron. X 2022, 11, 100142. [Google Scholar] [CrossRef]
- Yin, L.; You, T.; Arslan, M.; El-Seedi, H.R.; Guo, Z.; Zou, X.; Cai, J. Dual-Layers Raman Reporter-Tagged Au@Ag Combined with Core-Satellite Assemblies for SERS Detection of Zearalenone. Food Chem. 2023, 429, 136834. [Google Scholar] [CrossRef]
- Lee, J.-C.; Kim, S.Y.; Song, J.; Jang, H.; Kim, M.; Kim, H.; Choi, S.Q.; Kim, S.; Jolly, P.; Kang, T.; et al. Micrometer-Thick and Porous Nanocomposite Coating for Electrochemical Sensors with Exceptional Antifouling and Electroconducting Properties. Nat. Commun. 2024, 15, 711. [Google Scholar] [CrossRef]
- Biswas, D.S.; Gaki, P.; Da Silva, E.C.; Combes, A.; Reisch, A.; Didier, P.; Klymchenko, A.S. Long-range Energy Transfer Between Dye-loaded Nanoparticles: Observation and Amplified Detection of Nucleic Acids. Adv. Mater. 2023, 35, e2301402. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, L.; Liu, X.; Chang, Y.; Xia, R.; Zhang, J.; Kong, Y.; Gong, Y.; Li, T.; Wang, G.; et al. Colored Cellulose Nanoparticles with High Stability and Easily Modified Surface for Accurate and Sensitive Multiplex Lateral Flow Assay. ACS Nano 2025, 19, 4704–4717. [Google Scholar] [CrossRef]
- Shin, M.; Kim, W.; Yoo, K.; Cho, H.-S.; Jang, S.; Bae, H.-J.; An, J.; Lee, J.; Chang, H.; Kim, D.-E.; et al. Highly Sensitive Multiplexed Colorimetric Lateral Flow Immunoassay by Plasmon-Controlled Metal–Silica Isoform Nanocomposites: PINs. Nano Converg. 2024, 11, 42. [Google Scholar] [CrossRef]
- Sun, J.; Shi, Z.; Wang, L.; Zhang, X.; Luo, C.; Hua, J.; Feng, M.; Chen, Z.; Wang, M.; Xu, C. Construction of a Microcavity-Based Microfluidic Chip with Simultaneous SERS Quantification of Dual Biomarkers for Early Diagnosis of Alzheimer’s Disease. Talanta 2023, 261, 124677. [Google Scholar] [CrossRef]
- Nicholson, R.I.; Gee, J.M.W.; Harper, M.E. EGFR and Cancer Prognosis. Eur. J. Cancer 2001, 37, 9–15. [Google Scholar] [CrossRef]
- Hunt, A.; Torati, S.R.; Slaughter, G. Paper-Based DNA Biosensor for Rapid and Selective Detection of MiR-21. Biosensors 2024, 14, 485. [Google Scholar] [CrossRef] [PubMed]
- Syed, R.U.; Banu, H.; Alshammrani, A.; Alshammari, M.D.; Kumar, S.G.; Kadimpati, K.K.; Khalifa, A.A.S.; Aboshouk, N.A.M.; Almarir, A.M.; Hussain, A.; et al. MicroRNA-21 (MiR-21) in Breast Cancer: From Apoptosis Dysregulation to Therapeutic Opportunities. Pathol. Res. Pract. 2024, 262, 155572. [Google Scholar] [CrossRef]
- Chen, C.; Demirkhanyan, L.; Gondi, C.S. The Multifaceted Role of MiR-21 in Pancreatic Cancers. Cells 2024, 13, 948. [Google Scholar] [CrossRef]
- Hariri, M.; Alivirdiloo, V.; Ardabili, N.S.; Gholami, S.; Masoumi, S.; Mehraban, M.R.; Alem, M.; Hosseini, R.S.; Mobed, A.; Ghazi, F.; et al. Biosensor-Based Nanodiagnosis of Carcinoembryonic Antigen (CEA): An Approach to Classification and Precise Detection of Cancer Biomarker. Bionanoscience 2024, 14, 429–446. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, S.; Zhang, F. Optical Multiplexed Bioassays for Improved Biomedical Diagnostics. Angew. Chem. 2019, 131, 13342–13353. [Google Scholar] [CrossRef]
- Wei, C.; Lei, X.; Yu, S. Multiplexed Detection Strategies for Biosensors Based on the CRISPR-Cas System. ACS Synth. Biol. 2024, 13, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, Y.; Liu, J.; Wei, S.; Li, N.; Yao, X.; Wang, M.; Su, X.; Jing, G.; Xu, J.; et al. Concurrent Detection of Protein and MiRNA at the Single Extracellular Vesicle Level Using a Digital Dual CRISPR-Cas Assay. ACS Nano 2025, 19, 1271–1285. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Huang, Q.; Fu, R.; Xun, Z.; Ou, Q.; Xianyu, Y.; Liu, C. A Triple-Modal Biosensing Strategy for Hepatitis B Virus Based on Mg2+-Mediated Modulation of CRISPR/Cas12a and Au@Pt Nanoparticles. Small 2025, 21, e05341. [Google Scholar] [CrossRef]
- Zhu, Y.; Xing, C.; Yang, L.; Li, Q.; Wang, X.; Zhou, J.; Zhang, C.; Ren, C.; Liu, F.; He, J.; et al. Dual-Gene Detection in a Single-Tube System Based on CRISPR-Cas12a/Cas13a for Severe Fever Thrombocytopenia Syndrome Virus. Front. Microbiol. 2022, 13, 977382. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, R.; Zhang, Z.; Jiang, Y.; Miao, Y.; Zhou, S.; Ji, M.; Hsu, C.-W.; Xu, H.; Li, Z.; et al. An Ultrasensitive One-Pot Cas13a-Based Microfluidic Assay for Rapid Multiplexed Detection of MicroRNAs. Biosens. Bioelectron. 2025, 274, 117212. [Google Scholar] [CrossRef]
- Duy Mac, K.; Su, J. Optical Biosensors for Diagnosing Neurodegenerative Diseases. npj Biosensing 2025, 2, 20. [Google Scholar] [CrossRef]
- Kaur, B.; Kumar, S.; Kaushik, B.K. Recent Advancements in Optical Biosensors for Cancer Detection. Biosens. Bioelectron. 2022, 197, 113805. [Google Scholar] [CrossRef]
- Aslan, M.; Seymour, E.; Brickner, H.; Clark, A.E.; Celebi, I.; Townsend, M.B.; Satheshkumar, P.S.; Riley, M.; Carlin, A.F.; Ünlü, M.S.; et al. A Label-Free Optical Biosensor-Based Point-of-Care Test for the Rapid Detection of Monkeypox Virus. Biosens. Bioelectron. 2025, 269, 116932. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Qiu, J.; Ma, F.; Zhang, C. Advances in Single-molecule Fluorescent Nanosensors. WIREs Nanomed. Nanobiotechnol. 2021, 13, e1716. [Google Scholar] [CrossRef] [PubMed]
- Plou, J.; Valera, P.S.; García, I.; de Albuquerque, C.D.L.; Carracedo, A.; Liz-Marzán, L.M. Prospects of Surface-Enhanced Raman Spectroscopy for Biomarker Monitoring toward Precision Medicine. ACS Photonics 2022, 9, 333–350. [Google Scholar] [CrossRef]
- Testa-Anta, M.; Ramos-Docampo, M.A.; Comesaña-Hermo, M.; Rivas-Murias, B.; Salgueiriño, V. Raman Spectroscopy to Unravel the Magnetic Properties of Iron Oxide Nanocrystals for Bio-Related Applications. Nanoscale Adv. 2019, 1, 2086–2103. [Google Scholar] [CrossRef]
- Wang, H.; Wu, T.; Li, M.; Tao, Y. Recent Advances in Nanomaterials for Colorimetric Cancer Detection. J. Mater. Chem. B 2021, 9, 921–938. [Google Scholar] [CrossRef]
- Choi, M.Y.; Park, D.H.; Choi, J.-H. Multiplex Metal Enhanced Fluorescence (MEF) Effect on Porous Au Nanorod for Highly Sensitive Multi-MicroRNA (MiRNA) Detection. Sens. Actuators B Chem. 2023, 393, 134280. [Google Scholar] [CrossRef]
- Zheng, P.; Wu, L.; Raj, P.; Kim, J.H.; Paidi, S.K.; Semancik, S.; Barman, I. Multiplexed SERS Detection of Serum Cardiac Markers Using Plasmonic Metasurfaces. Adv. Sci. 2024, 11, 2405910. [Google Scholar] [CrossRef]
- Viswambari Devi, R.; Doble, M.; Verma, R.S. Nanomaterials for Early Detection of Cancer Biomarker with Special Emphasis on Gold Nanoparticles in Immunoassays/Sensors. Biosens. Bioelectron. 2015, 68, 688–698. [Google Scholar] [CrossRef]
- Lee, J.; Takemura, K.; Park, E. Plasmonic Nanomaterial-Based Optical Biosensing Platforms for Virus Detection. Sensors 2017, 17, 2332. [Google Scholar] [CrossRef]
- Kim, Y.J.; Rho, W.-Y.; Park, S.; Jun, B.-H. Optical Nanomaterial-Based Detection of Biomarkers in Liquid Biopsy. J. Hematol. Oncol. 2024, 17, 10. [Google Scholar] [CrossRef]
- Samadi Pakchin, P.; Fathi, F.; Samadi, H.; Adibkia, K. Recent Advances in Receptor-Based Optical Biosensors for the Detection of Multiplex Biomarkers. Talanta 2025, 281, 126852. [Google Scholar] [CrossRef]
- Martinez-Banderas, A.I.; Malki, A.; Froehlich, T.; Petrich, W. High-Performance Nanobiosensing Technologies for Future Diagnostic Needs. Trends Biotechnol. 2025, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, T.; Yuan, X.; Wang, Y.; Yue, X.; Wang, L.; Zhang, J.; Wang, J. Plasmonic Nanostructure Biosensors: A Review. Sensors 2023, 23, 8156. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, C.; Sharma, S.; Yougbaré, S.; Chen, C.-Y.; Kuo, T.-R. Nanoplasmonic Biosensors: A Comprehensive Overview and Future Prospects. Int. J. Nanomed. 2025, 20, 5817–5836. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, M.; Fan, Z. Current Trends in Colorimetric Biosensors Using Nanozymes for Detecting Biotoxins (Bacterial Food Toxins, Mycotoxins, and Marine Toxins). Anal. Methods 2024, 16, 6771–6792. [Google Scholar] [CrossRef]
- Xiao, Y.; Zhang, Z.; Yin, S.; Ma, X. Nanoplasmonic Biosensors for Precision Medicine. Front. Chem. 2023, 11, 1209744. [Google Scholar] [CrossRef]
- Li, M.; Cushing, S.K.; Wu, N. Plasmon-Enhanced Optical Sensors: A Review. Analyst 2015, 140, 386–406. [Google Scholar] [CrossRef]
- Yu, H.; Peng, Y.; Yang, Y.; Li, Z.-Y. Plasmon-Enhanced Light–Matter Interactions and Applications. npj Comput. Mater. 2019, 5, 45. [Google Scholar] [CrossRef]
- Singh, M.P.; Strouse, G.F. Involvement of the LSPR Spectral Overlap for Energy Transfer between a Dye and Au Nanoparticle. J. Am. Chem. Soc. 2010, 132, 9383–9391. [Google Scholar] [CrossRef]
- Pellas, V.; Hu, D.; Mazouzi, Y.; Mimoun, Y.; Blanchard, J.; Guibert, C.; Salmain, M.; Boujday, S. Gold Nanorods for LSPR Biosensing: Synthesis, Coating by Silica, and Bioanalytical Applications. Biosensors 2020, 10, 146. [Google Scholar] [CrossRef] [PubMed]
- Mayer, K.M.; Hafner, J.H. Localized Surface Plasmon Resonance Sensors. Chem. Rev. 2011, 111, 3828–3857. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.; Bhattacharya, A.; Nag, A. Metal-Enhanced Fluorescence Study in Aqueous Medium by Coupling Gold Nanoparticles and Fluorophores Using a Bilayer Vesicle Platform. ACS Omega 2019, 4, 5983–5990. [Google Scholar] [CrossRef]
- Anger, P.; Bharadwaj, P.; Novotny, L. Enhancement and Quenching of Single-Molecule Fluorescence. Phys. Rev. Lett. 2006, 96, 113002. [Google Scholar] [CrossRef]
- Choi, J.-H.; Lim, J.; Shin, M.; Paek, S.-H.; Choi, J.-W. CRISPR-Cas12a-Based Nucleic Acid Amplification-Free DNA Biosensor via Au Nanoparticle-Assisted Metal-Enhanced Fluorescence and Colorimetric Analysis. Nano Lett. 2021, 21, 693–699. [Google Scholar] [CrossRef]
- Camposeo, A.; Persano, L.; Manco, R.; Wang, Y.; Del Carro, P.; Zhang, C.; Li, Z.Y.; Pisignano, D.; Xia, Y. Metal-Enhanced Near-Infrared Fluorescence by Micropatterned Gold Nanocages. ACS Nano 2015, 9, 10047–10054. [Google Scholar] [CrossRef]
- Verma, S.; Pathak, A.K.; Rahman, B.M.A. Review of Biosensors Based on Plasmonic-Enhanced Processes in the Metallic and Meta-Material-Supported Nanostructures. Micromachines 2024, 15, 502. [Google Scholar] [CrossRef]
- Choi, J.-H.; Choi, J.-W. Metal-Enhanced Fluorescence by Bifunctional Au Nanoparticles for Highly Sensitive and Simple Detection of Proteolytic Enzyme. Nano Lett. 2020, 20, 7100–7107. [Google Scholar] [CrossRef]
- Choi, J.-H.; Ha, T.; Shin, M.; Lee, S.-N.; Choi, J.-W. Nanomaterial-Based Fluorescence Resonance Energy Transfer (FRET) and Metal-Enhanced Fluorescence (MEF) to Detect Nucleic Acid in Cancer Diagnosis. Biomedicines 2021, 9, 928. [Google Scholar] [CrossRef]
- Badshah, M.A.; Koh, N.Y.; Zia, A.W.; Abbas, N.; Zahra, Z.; Saleem, M.W. Recent Developments in Plasmonic Nanostructures for Metal Enhanced Fluorescence-Based Biosensing. Nanomaterials 2020, 10, 1749. [Google Scholar] [CrossRef]
- Romero, M.R.; Veglia, A.V.; Amé, M.V.; Bracamonte, A.G. Multimodal Spectroscopy Assays for Advanced Nano-Optics Approaches by Tuning Nano-Tool Surface Chemistry and Metal-Enhanced Fluorescence. Crystals 2024, 14, 338. [Google Scholar] [CrossRef]
- Liu, Y.-T.; Luo, X.-F.; Lee, Y.-Y.; Chen, I.-C. Investigating the Metal-Enhanced Fluorescence on Fluorescein by Silica Core-Shell Gold Nanoparticles Using Time-Resolved Fluorescence Spectroscopy. Dye. Pigment. 2021, 190, 109263. [Google Scholar] [CrossRef]
- Ribeiro, T.; Baleizão, C.; Farinha, J.P.S. Artefact-Free Evaluation of Metal Enhanced Fluorescence in Silica Coated Gold Nanoparticles. Sci. Rep. 2017, 7, 2440. [Google Scholar] [CrossRef]
- Bastús, N.G.; Comenge, J.; Puntes, V. Kinetically Controlled Seeded Growth Synthesis of Citrate-Stabilized Gold Nanoparticles of up to 200 Nm: Size Focusing versus Ostwald Ripening. Langmuir 2011, 27, 11098–11105. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and Growth Mechanism of Gold Nanorods (NRs) Using Seed-Mediated Growth Method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Mei, Z.; Tang, L. Surface-Plasmon-Coupled Fluorescence Enhancement Based on Ordered Gold Nanorod Array Biochip for Ultrasensitive DNA Analysis. Anal. Chem. 2017, 89, 633–639. [Google Scholar] [CrossRef]
- Tóth, E.; Ungor, D.; Novák, T.; Ferenc, G.; Bánhelyi, B.; Csapó, E.; Erdélyi, M.; Csete, M. Mapping Fluorescence Enhancement of Plasmonic Nanorod Coupled Dye Molecules. Nanomaterials 2020, 10, 1048. [Google Scholar] [CrossRef]
- Chakraborty, D.; Mukherjee, A.; Ethiraj, K.R. Gold Nanorod-Based Multiplex Bioanalytical Assay for the Detection of CYFRA 21-1 and CA-125: Towards Oral Cancer Diagnostics. Anal. Methods 2022, 14, 3614–3622. [Google Scholar] [CrossRef]
- Zhao, T.; Pang, X.; Wang, C.; Wang, L.; Yang, Y.; Wang, J.; Jia, J.; Liu, X.; Xu, S.; Luo, X. Plasmonic Gold Nanostar-Based Probes with Distance-Dependent Plasmon-Enhanced Fluorescence for Ultrasensitive DNA Methyltransferase Assay. Anal. Chem. 2024, 96, 4402–4409. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Wang, W.; Zhao, T.; Cui, Y.; Liu, P.; Xu, S.; Luo, X. More Symmetrical “Hot Spots” Ensure Stronger Plasmon-Enhanced Fluorescence: From Au Nanorods to Nanostars. Anal. Chem. 2021, 93, 2480–2489. [Google Scholar] [CrossRef]
- Shan, F.; Zhang, X.-Y.; Fu, X.-C.; Zhang, L.-J.; Su, D.; Wang, S.-J.; Wu, J.-Y.; Zhang, T. Investigation of Simultaneously Existed Raman Scattering Enhancement and Inhibiting Fluorescence Using Surface Modified Gold Nanostars as SERS Probes. Sci. Rep. 2017, 7, 6813. [Google Scholar] [CrossRef]
- Theodorou, I.G.; Jawad, Z.A.R.; Jiang, Q.; Aboagye, E.O.; Porter, A.E.; Ryan, M.P.; Xie, F. Gold Nanostar Substrates for Metal-Enhanced Fluorescence through the First and Second Near-Infrared Windows. Chem. Mater. 2017, 29, 6916–6926. [Google Scholar] [CrossRef]
- Pazos-Perez, N.; Guerrini, L.; Alvarez-Puebla, R.A. Plasmon Tunability of Gold Nanostars at the Tip Apexes. ACS Omega 2018, 3, 17173–17179. [Google Scholar] [CrossRef]
- Breausche, F.E.; Somerlot, A.; Walder, J.; Osei, K.; Okyem, S.; Driskell, J.D. Immobilization of Thiol-Modified Horseradish Peroxidase on Gold Nanoparticles Enhances Enzyme Stability and Prevents Proteolytic Digestion. Langmuir 2024, 40, 13957–13967. [Google Scholar] [CrossRef]
- Qin, W.; Wang, J.; Tang, Z.; Tian, H.; Wu, Z. Tris(2-Carboxyethyl)Phosphine-Mediated Immobilization of Thiolated DNA on Gold Nanoclusters and Its Application in Multiplex MicroRNA Detection. Sens. Actuators B Chem. 2024, 401, 135028. [Google Scholar] [CrossRef]
- San Juan, A.M.; Jaitpal, S.; Ng, K.W.; Martinez, C.; Tripathy, S.; Phillips, C.; Coté, G.L.; Mabbott, S. Freeze-Driven Synthesis of DNA Hairpin-Conjugated Gold Nanoparticle Biosensors for Dual-Mode Detection. ACS Appl. Bio Mater. 2024, 7, 3005–3013. [Google Scholar] [CrossRef]
- Sebben, D.; Li, H. Intracellular Metal Enhanced Fluorescence Utilizing Gold Nanoparticles Embedded in Hydrogel Droplets for Sensitive Protein Detection in Cells. Talanta Open 2023, 8, 100265. [Google Scholar] [CrossRef]
- Cobley, C.M.; Skrabalak, S.E.; Campbell, D.J.; Xia, Y. Shape-Controlled Synthesis of Silver Nanoparticles for Plasmonic and Sensing Applications. Plasmonics 2009, 4, 171–179. [Google Scholar] [CrossRef]
- Gahlaut, S.K.; Pathak, A.; Gupta, B.D. Recent Advances in Silver Nanostructured Substrates for Plasmonic Sensors. Biosensors 2022, 12, 713. [Google Scholar] [CrossRef]
- Abel, B.; Coskun, S.; Mohammed, M.; Williams, R.; Unalan, H.E.; Aslan, K. Metal-Enhanced Fluorescence from Silver Nanowires with High Aspect Ratio on Glass Slides for Biosensing Applications. J. Phys. Chem. C 2015, 119, 675–684. [Google Scholar] [CrossRef]
- Lu, Z.; Ji, J.; Ye, H.; Zhang, H.; Zhang, S.; Xu, H. Quantifying the Ultimate Limit of Plasmonic Near-Field Enhancement. Nat. Commun. 2024, 15, 8803. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, Y.; Chowdhury, M.H.; Lakowicz, J.R. Metal-Enhanced Single-Molecule Fluorescence on Silver Particle Monomer and Dimer: Coupling Effect between Metal Particles. Nano Lett. 2007, 7, 2101–2107. [Google Scholar] [CrossRef]
- Jin, F.; Li, H.; Xu, D. Enzyme-Free Fluorescence Microarray for Determination of Hepatitis B Virus DNA Based on Silver Nanoparticle Aggregates-Assisted Signal Amplification. Anal. Chim. Acta 2019, 1077, 297–304. [Google Scholar] [CrossRef]
- Saad, Y.; Gazzah, M.H.; Mougin, K.; Selmi, M.; Belmabrouk, H. Sensitive Detection of SARS-CoV-2 Using a Novel Plasmonic Fiber Optic Biosensor Design. Plasmonics 2022, 17, 1489–1500. [Google Scholar] [CrossRef]
- Yun, B.J.; Kwon, J.E.; Lee, K.; Koh, W.-G. Highly Sensitive Metal-Enhanced Fluorescence Biosensor Prepared on Electrospun Fibers Decorated with Silica-Coated Silver Nanoparticles. Sens. Actuators B Chem. 2019, 284, 140–147. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, B. Recent Advances in Porous Pt-Based Nanostructures: Synthesis and Electrochemical Applications. Chem. Soc. Rev. 2014, 43, 2439. [Google Scholar] [CrossRef]
- Wang, J.; Gao, H.; Sun, F.; Xu, C. Nanoporous PtAu Alloy as an Electrochemical Sensor for Glucose and Hydrogen Peroxide. Sens. Actuators B Chem. 2014, 191, 612–618. [Google Scholar] [CrossRef]
- Das, N.; Kumar, A.; Kumar Roy, S.; Kumar Satija, N.; Raja Gopal, R. Bare Plasmonic Metal Nanoparticles: Synthesis, Characterisation and in Vitro Toxicity Assessment on a Liver Carcinoma Cell Line. IET Nanobiotechnol. 2020, 14, 851–857. [Google Scholar] [CrossRef]
- Wang, L.; Zeng, Y.; Shen, A.; Fu, Y.; Zeng, L.; Hu, J. Facile and Controllable Synthesis of Triplex Au@Ag–Pt@infinite Coordination Polymer Core–Shell Nanoparticles for Highly Efficient Immobilization of Enzymes and Enhanced Electrochemical Biosensing Activity. RSC Adv. 2016, 6, 86025–86033. [Google Scholar] [CrossRef]
- Wang, C.; Daimon, H.; Lee, Y.; Kim, J.; Sun, S. Synthesis of Monodisperse Pt Nanocubes and Their Enhanced Catalysis for Oxygen Reduction. J. Am. Chem. Soc. 2007, 129, 6974–6975. [Google Scholar] [CrossRef]
- Charoenkitamorn, K.; Tue, P.; Kawai, K.; Chailapakul, O.; Takamura, Y. Electrochemical Immunoassay Using Open Circuit Potential Detection Labeled by Platinum Nanoparticles. Sensors 2018, 18, 444. [Google Scholar] [CrossRef]
- Chakari-Khiavi, F.; Mirzaie, A.; Khalilzadeh, B.; Yousefi, H.; Abolhasan, R.; Kamrani, A.; Pourakbari, R.; Shahpasand, K.; Yousefi, M.; Rashidi, M.-R. Application of Pt@ZIF-8 Nanocomposite-Based Electrochemical Biosensor for Sensitive Diagnosis of Tau Protein in Alzheimer’s Disease Patients. Sci. Rep. 2023, 13, 16163. [Google Scholar] [CrossRef]
- Leonardi, A.A.; Lo Faro, M.J.; Fazio, B.; Spinella, C.; Conoci, S.; Livreri, P.; Irrera, A. Fluorescent Biosensors Based on Silicon Nanowires. Nanomaterials 2021, 11, 2970. [Google Scholar] [CrossRef]
- Muhammad, W.; Song, J.; Kim, S.; Ahmed, F.; Cho, E.; Lee, H.; Kim, J. Silicon-Based Biosensors: A Critical Review of Silicon’s Role in Enhancing Biosensing Performance. Biosensors 2025, 15, 119. [Google Scholar] [CrossRef]
- Unksov, I.N.; Anttu, N.; Verardo, D.; Höök, F.; Prinz, C.N.; Linke, H. Fluorescence Excitation Enhancement by Waveguiding Nanowires. Nanoscale Adv. 2023, 5, 1760–1766. [Google Scholar] [CrossRef]
- Alagarasan, J.K.; Shasikala, S.; Ganesan, S.; Arunachalam, M.; Manojkumar, U.; Palaninaicker, S.; Nguyen, D.D.; Chang, S.W.; Lee, M.; Lo, H.-M. Silicon Nanoparticles as a Fluorometric Probe for Sensitive Detection of Cyanide Ion and Its Application in C. Elegans Bio-Imaging. Environ. Res. 2023, 224, 115402. [Google Scholar] [CrossRef]
- Henriksson, A.; Neubauer, P.; Birkholz, M. Functionalization of Oxide-Free Silicon Surfaces for Biosensing Applications. Adv. Mater. Interfaces 2021, 8, 2100927. [Google Scholar] [CrossRef]
- Mariani, S.; Robbiano, V.; Strambini, L.M.; Debrassi, A.; Egri, G.; Dähne, L.; Barillaro, G. Layer-by-Layer Biofunctionalization of Nanostructured Porous Silicon for High-Sensitivity and High-Selectivity Label-Free Affinity Biosensing. Nat. Commun. 2018, 9, 5256. [Google Scholar] [CrossRef]
- Anttu, N. Fluorophore Signal Detection and Imaging Enhancement in High Refractive Index Nanowire Biosensors. Nano Express 2025, 6, 015005. [Google Scholar] [CrossRef]
- Zhang, Y.; Cai, N.; Chan, V. Recent Advances in Silicon Quantum Dot-Based Fluorescent Biosensors. Biosensors 2023, 13, 311. [Google Scholar] [CrossRef]
- Thomas, S.A.; Sefannaser, M.; Petersen, R.J.; Anderson, K.J.; Kilin, D.S.; Pringle, T.A.; Hobbie, E.K. Size-Dependent Radiative Relaxation in Silicon Quantum Dots: Impact of Targeted Size. Phys. Rev. Mater. 2025, 9, 076004. [Google Scholar] [CrossRef]
- Moretta, R.; De Stefano, L.; Terracciano, M.; Rea, I. Porous Silicon Optical Devices: Recent Advances in Biosensing Applications. Sensors 2021, 21, 1336. [Google Scholar] [CrossRef]
- Hong, D.; Jo, E.-J.; Bang, D.; Jung, C.; Lee, Y.E.; Noh, Y.-S.; Shin, M.G.; Kim, M.-G. Plasmonic Approach to Fluorescence Enhancement of Mesoporous Silica-Coated Gold Nanorods for Highly Sensitive Influenza A Virus Detection Using Lateral Flow Immunosensor. ACS Nano 2023, 17, 16607–16619. [Google Scholar] [CrossRef]
- Arshavsky-Graham, S.; Ward, S.J.; Massad-Ivanir, N.; Scheper, T.; Weiss, S.M.; Segal, E. Porous Silicon-Based Aptasensors: Toward Cancer Protein Biomarker Detection. ACS Meas. Sci. Au 2021, 1, 82–94. [Google Scholar] [CrossRef]
- Lu, M.; Pan, C.; Qin, X.; Wu, M. Silicon Nanoparticle-Based Ratiometric Fluorescence Probes for Highly Sensitive and Visual Detection of VB2. ACS Omega 2023, 8, 14499–14508. [Google Scholar] [CrossRef]
- Awawdeh, K.; Buttkewitz, M.A.; Bahnemann, J.; Segal, E. Enhancing the Performance of Porous Silicon Biosensors: The Interplay of Nanostructure Design and Microfluidic Integration. Microsyst. Nanoeng. 2024, 10, 100. [Google Scholar] [CrossRef]
- Myres, G.J.; Harris, J.M. Stable Immobilization of DNA to Silica Surfaces by Sequential Michael Addition Reactions Developed with Insights from Confocal Raman Microscopy. Anal. Chem. 2023, 95, 3499–3506. [Google Scholar] [CrossRef]
- Lo Faro, M.; Leonardi, A.; Priolo, F.; Fazio, B.; Irrera, A. Future Prospects of Luminescent Silicon Nanowires Biosensors. Biosensors 2022, 12, 1052. [Google Scholar] [CrossRef]
- Yu, W.; Sisi, L.; Haiyan, Y.; Jie, L. Progress in the Functional Modification of Graphene/Graphene Oxide: A Review. RSC Adv. 2020, 10, 15328–15345. [Google Scholar] [CrossRef]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and Applications of Graphene Oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Gul, W.; Alrobei, H. Effect of Graphene Oxide Nanoparticles on the Physical and Mechanical Properties of Medium Density Fiberboard. Polymers 2021, 13, 1818. [Google Scholar] [CrossRef]
- Du, D.; Wang, L.; Shao, Y.; Wang, J.; Engelhard, M.H.; Lin, Y. Functionalized Graphene Oxide as a Nanocarrier in a Multienzyme Labeling Amplification Strategy for Ultrasensitive Electrochemical Immunoassay of Phosphorylated P53 (S392). Anal. Chem. 2011, 83, 746–752. [Google Scholar] [CrossRef]
- Lewandowska-Andralojc, A.; Gacka, E.; Pedzinski, T.; Burdzinski, G.; Lindner, A.; O’Brien, J.M.; Senge, M.O.; Siklitskaya, A.; Kubas, A.; Marciniak, B.; et al. Understanding Structure–Properties Relationships of Porphyrin Linked to Graphene Oxide through π–π-Stacking or Covalent Amide Bonds. Sci. Rep. 2022, 12, 13420. [Google Scholar] [CrossRef]
- Liu, X.; Aizen, R.; Freeman, R.; Yehezkeli, O.; Willner, I. Multiplexed Aptasensors and Amplified DNA Sensors Using Functionalized Graphene Oxide: Application for Logic Gate Operations. ACS Nano 2012, 6, 3553–3563. [Google Scholar] [CrossRef] [PubMed]
- Baruah, A.; Newar, R.; Das, S.; Kalita, N.; Nath, M.; Ghosh, P.; Chinnam, S.; Sarma, H.; Narayan, M. Biomedical Applications of Graphene-Based Nanomaterials: Recent Progress, Challenges, and Prospects in Highly Sensitive Biosensors. Discov. Nano 2024, 19, 103. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Yuan, S.; Wang, L.; Guan, X. Joint Entropy-Assisted Graphene Oxide-Based Multiplexing Biosensing Platform for Simultaneous Detection of Multiple Proteases. Anal. Chem. 2020, 92, 15042–15049. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Huang, P.J.; Kelly, E.Y.; Liu, J. Graphene Oxide Surface Blocking Agents Can Increase the DNA Biosensor Sensitivity. Biotechnol. J. 2016, 11, 780–787. [Google Scholar] [CrossRef]
- Battisti, A.; Samal, S.K.; Puppi, D. Biosensing Systems Based on Graphene Oxide Fluorescence Quenching Effect. Micromachines 2023, 14, 1522. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, C.; Wang, C.; Wang, P.; Chang, X.; Han, L.; Zhang, Y. Multiple Biomarker Simultaneous Detection in Serum via a Nanomaterial-Functionalized Biosensor for Ovarian Tumor/Cancer Diagnosis. Micromachines 2022, 13, 2046. [Google Scholar] [CrossRef]
- Dai, B.; Zhou, R.; Ping, J.; Ying, Y.; Xie, L. Recent Advances in Carbon Nanotube-Based Biosensors for Biomolecular Detection. TrAC Trends Anal. Chem. 2022, 154, 116658. [Google Scholar] [CrossRef]
- Ferrier, D.C.; Honeychurch, K.C. Carbon Nanotube (CNT)-Based Biosensors. Biosensors 2021, 11, 486. [Google Scholar] [CrossRef] [PubMed]
- Murjani, B.O.; Kadu, P.S.; Bansod, M.; Vaidya, S.S.; Yadav, M.D. Carbon Nanotubes in Biomedical Applications: Current Status, Promises, and Challenges. Carbon. Lett. 2022, 32, 1207–1226. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Yang, L.; Deng, S.; Hao, Y.; Zhang, K.; Wang, X.; Liu, Y.; Liu, H.; Chen, Y.; Xie, M. Development of Nanosensor by Bioorthogonal Reaction for Multi-Detection of the Biomarkers of Hepatocellular Carcinoma. Sens. Actuators B Chem. 2021, 334, 129653. [Google Scholar] [CrossRef]
- Hendler-Neumark, A.; Wulf, V.; Bisker, G. Single-Walled Carbon Nanotube Sensor Selection for the Detection of MicroRNA Biomarkers for Acute Myocardial Infarction as a Case Study. ACS Sens. 2023, 8, 3713–3722. [Google Scholar] [CrossRef]
- Chen, J.; Liu, X.; Tang, J.; Jiang, J.; Gu, C.; Jiang, T.; Wu, K. Quantitative Detection and Intelligent Distinguishing of Urinary Tract Infection Pathogens Based on SERS-Active PDMS@BP-CNT Ternary Substrate. Sens. Actuators B Chem. 2025, 426, 137047. [Google Scholar] [CrossRef]
- Fang, W.; Zhang, Z.; Miao, N.; Yang, R.; Xiao, Z.; Shen, X.; Cao, Y.; Xin, W. Highly Uniform Fabrication of Gold Nanoparticles on Carbon Nanotube Sheets for Sensors Based on Surface-Enhanced Raman Spectroscopy with Improved Reproducibility. ACS Appl. Nano Mater. 2023, 6, 9949–9957. [Google Scholar] [CrossRef]
- Ali, S.G.; Alwan, A.M.; Jabbar, A.A. Novel Approach of SERS Sensors AgNPs/PSi by Incorporated MWCNT. Results Chem. 2024, 10, 101727. [Google Scholar] [CrossRef]
- Ackermann, J.; Metternich, J.T.; Herbertz, S.; Kruss, S. Biosensing with Fluorescent Carbon Nanotubes. Angew. Chem. Int. Ed. 2022, 61, e202112372. [Google Scholar] [CrossRef]
- Nißler, R.; Ackermann, J.; Ma, C.; Kruss, S. Prospects of Fluorescent Single-Chirality Carbon Nanotube-Based Biosensors. Anal. Chem. 2022, 94, 9941–9951. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, X.; Lu, X.; Cao, X.; Ao, L.; Ma, L.; Shen, C.; Fu, Y.; Yang, Y. BODIPY-Labeled Aptasensor Based on Multi-Walled Carbon Nanotubes as the Quencher for “off–on” Detection of Catechin. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 306, 123597. [Google Scholar] [CrossRef]
- Langenbacher, R.; Budhathoki-Uprety, J.; Jena, P.V.; Roxbury, D.; Streit, J.; Zheng, M.; Heller, D.A. Single-Chirality Near-Infrared Carbon Nanotube Sub-Cellular Imaging and FRET Probes. Nano Lett. 2021, 21, 6441–6448. [Google Scholar] [CrossRef]
- Xiong, Y.; Shepherd, S.; Tibbs, J.; Bacon, A.; Liu, W.; Akin, L.D.; Ayupova, T.; Bhaskar, S.; Cunningham, B.T. Photonic Crystal Enhanced Fluorescence: A Review on Design Strategies and Applications. Micromachines 2023, 14, 668. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, X.; Gu, Z. Photonic Crystals in Bioassays. Adv. Funct. Mater. 2010, 20, 2970–2988. [Google Scholar] [CrossRef]
- Liu, J.; Nero, M.; Jansson, K.; Willhammar, T.; Sipponen, M.H. Photonic Crystals with Rainbow Colors by Centrifugation-Assisted Assembly of Colloidal Lignin Nanoparticles. Nat. Commun. 2023, 14, 3099. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xie, Z.; Gu, H.; Jin, L.; Zhao, X.; Wang, B.; Gu, Z. Multifunctional Photonic Crystal Barcodes from Microfluidics. NPG Asia Mater. 2012, 4, e25. [Google Scholar] [CrossRef]
- Wang, J.; Pinkse, P.W.H.; Segerink, L.I.; Eijkel, J.C.T. Bottom-Up Assembled Photonic Crystals for Structure-Enabled Label-Free Sensing. ACS Nano 2021, 15, 9299–9327. [Google Scholar] [CrossRef]
- Sizova, S.; Shakurov, R.; Mitko, T.; Shirshikov, F.; Solovyeva, D.; Konopsky, V.; Alieva, E.; Klinov, D.; Bespyatykh, J.; Basmanov, D. The Elaboration of Effective Coatings for Photonic Crystal Chips in Optical Biosensors. Polymers 2021, 14, 152. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Zhao, X.-W.; Zhao, Y.-J.; Li, J.; Xu, W.-Y.; Wen, Z.-Y.; Xu, M.; Gu, Z.-Z. Photonic Crystal Hydrogel Beads Used for Multiplex Biomolecular Detection. J. Mater. Chem. 2009, 19, 5730. [Google Scholar] [CrossRef]
- Rizk, S.; Abd-Elsamee, S.; Marzouk, E.S.A.; Areed, N.F.F. Photonic Crystal Biosensor Featuring an Eye-Shaped Cavity for Precise Identification of Cancerous Cells. Sci. Rep. 2025, 15, 23926. [Google Scholar] [CrossRef]
- Wei, X.; Bian, F.; Cai, X.; Wang, Y.; Cai, L.; Yang, J.; Zhu, Y.; Zhao, Y. Multiplexed Detection Strategy for Bladder Cancer MicroRNAs Based on Photonic Crystal Barcodes. Anal. Chem. 2020, 92, 6121–6127. [Google Scholar] [CrossRef]
- Drummen, G. Fluorescent Probes and Fluorescence (Microscopy) Techniques—Illuminating Biological and Biomedical Research. Molecules 2012, 17, 14067–14090. [Google Scholar] [CrossRef]
- Liu, H.; Jiang, G.; Ke, G.; Ren, T.; Yuan, L. Organic Fluorophores with Large Stokes Shift for Bioimaging and Biosensing. ChemPhotoChem 2024, 8, e202300277. [Google Scholar] [CrossRef]
- Yao, J.; Yang, M.; Duan, Y. Chemistry, Biology, and Medicine of Fluorescent Nanomaterials and Related Systems: New Insights into Biosensing, Bioimaging, Genomics, Diagnostics, and Therapy. Chem. Rev. 2014, 114, 6130–6178. [Google Scholar] [CrossRef] [PubMed]
- Bauch, M.; Toma, K.; Toma, M.; Zhang, Q.; Dostalek, J. Plasmon-Enhanced Fluorescence Biosensors: A Review. Plasmonics 2014, 9, 781–799. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Han, L.; Chen, M.; Pan, L.; Tu, K. Recent Progress in Nanomaterial-Based Fluorescence Assays for the Detection of Food-Borne Pathogens. Sensors 2024, 24, 7715. [Google Scholar] [CrossRef]
- Kshirsagar, A.; Politza, A.J.; Guan, W. Deep Learning Enabled Universal Multiplexed Fluorescence Detection for Point-of-Care Applications. ACS Sens. 2024, 9, 4017–4027. [Google Scholar] [CrossRef]
- Singh, H.; Thakur, B.; Bhardwaj, S.K.; Khatri, M.; Kim, K.-H.; Bhardwaj, N. Nanomaterial-Based Fluorescent Biosensors for the Detection of Antibiotics in Foodstuffs: A Review. Food Chem. 2023, 426, 136657. [Google Scholar] [CrossRef]
- Sharma, A.; Majdinasab, M.; Khan, R.; Li, Z.; Hayat, A.; Marty, J.L. Nanomaterials in Fluorescence-Based Biosensors: Defining Key Roles. Nano-Struct. Nano Objects 2021, 27, 100774. [Google Scholar] [CrossRef]
- Huang, F.; Xie, Z.; Zhang, Q.; Zada, S.; Lin, R.; Deng, Y.; Liu, Q.; Chen, H.; Zhou, H.; Miao, H.; et al. Recent Advances in Fluorescence Resonance Energy Transfer (FRET) Biosensors for Exosomes. Curr. Issues Mol. Biol. 2025, 47, 235. [Google Scholar] [CrossRef]
- Mal, D.K.; Pal, H.; Chakraborty, G. A Comprehensive Review on Recent Advances in Fluorescence-Based Bio-Analytes Sensing. TrAC Trends Anal. Chem. 2024, 171, 117493. [Google Scholar] [CrossRef]
- Zhong, W. Nanomaterials in Fluorescence-Based Biosensing. Anal. Bioanal. Chem. 2009, 394, 47–59. [Google Scholar] [CrossRef]
- Kochuveedu, S.T.; Kim, D.H. Surface Plasmon Resonance Mediated Photoluminescence Properties of Nanostructured Multicomponent Fluorophore Systems. Nanoscale 2014, 6, 4966–4984. [Google Scholar] [CrossRef]
- Jana, J.; Ganguly, M.; Pal, T. Enlightening Surface Plasmon Resonance Effect of Metal Nanoparticles for Practical Spectroscopic Application. RSC Adv. 2016, 6, 86174–86211. [Google Scholar] [CrossRef]
- Quesada-González, D.; Merkoçi, A. Quantum Dots for Biosensing: Classification and Applications. Biosens. Bioelectron. 2025, 273, 117180. [Google Scholar] [CrossRef] [PubMed]
- Arai, M.S.; de Camargo, A.S.S. Exploring the Use of Upconversion Nanoparticles in Chemical and Biological Sensors: From Surface Modifications to Point-of-Care Devices. Nanoscale Adv. 2021, 3, 5135–5165. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, Z.A.; Dabash, H.; Ponnamma, D.; Abbas, M.K.G. Carbon Dots as Versatile Nanomaterials in Sensing and Imaging: Efficiency and Beyond. Heliyon 2024, 10, e31634. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, K.; Rai, H.; Mondal, S. Quantum Dots: An Overview of Synthesis, Properties, and Applications. Mater. Res. Express 2023, 10, 062001. [Google Scholar] [CrossRef]
- Martins, C.S.M.; LaGrow, A.P.; Prior, J.A.V. Quantum Dots for Cancer-Related MiRNA Monitoring. ACS Sens. 2022, 7, 1269–1299. [Google Scholar] [CrossRef]
- Wegner, K.D.; Hildebrandt, N. Near Infrared Quantum Dots for Biosensing and Bioimaging. TrAC Trends Anal. Chem. 2024, 180, 117922. [Google Scholar] [CrossRef]
- Shen, W.; Wang, Y.; Liao, L. Near-Infrared Quantum Dots for Electroluminescence: Balancing Performance and Sustainability. Laser Photon. Rev. 2025, 19, 2401947. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Hashemi, S.A.; Yari Kalashgrani, M.; Omidifar, N.; Lai, C.W.; Vijayakameswara Rao, N.; Gholami, A.; Chiang, W.-H. The Pivotal Role of Quantum Dots-Based Biomarkers Integrated with Ultra-Sensitive Probes for Multiplex Detection of Human Viral Infections. Pharmaceuticals 2022, 15, 880. [Google Scholar] [CrossRef]
- Yang, Y.; Dev, A.; Sychugov, I.; Hägglund, C.; Zhang, S.-L. Plasmon-Enhanced Fluorescence of Single Quantum Dots Immobilized in Optically Coupled Aluminum Nanoholes. J. Phys. Chem. Lett. 2023, 14, 2339–2346. [Google Scholar] [CrossRef] [PubMed]
- Jessy Mercy, D.; Girigoswami, K.; Girigoswami, A. A Mini Review on Biosensor Advancements-Emphasis on Quantum Dots. Results Chem. 2024, 7, 101271. [Google Scholar] [CrossRef]
- Hildebrandt, N.; Lim, M.; Kim, N.; Choi, D.Y.; Nam, J.-M. Plasmonic Quenching and Enhancement: Metal–Quantum Dot Nanohybrids for Fluorescence Biosensing. Chem. Commun. 2023, 59, 2352–2380. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Pons, T.; Medintz, I.L.; Mattoussi, H. Biosensing with Luminescent Semiconductor Quantum Dots. Sensors 2006, 6, 925–953. [Google Scholar] [CrossRef]
- Davodabadi, F.; Mirinejad, S.; Fathi-Karkan, S.; Majidpour, M.; Ajalli, N.; Sheervalilou, R.; Sargazi, S.; Rozmus, D.; Rahdar, A.; Diez-Pascual, A.M. Aptamer-functionalized Quantum Dots as Theranostic Nanotools against Cancer and Bacterial Infections: A Comprehensive Overview of Recent Trends. Biotechnol. Prog. 2023, 39, e3366. [Google Scholar] [CrossRef]
- Bhatt, S.; Pathak, R.; Punetha, V.D.; Punetha, M. Graphene Quantum Dots-Based Materials as an Emerging Nanoplatform in Disease Diagnosis and Therapy. Part. Part. Syst. Charact. 2025, 42, 2400221. [Google Scholar] [CrossRef]
- Rasheed, P.A.; Ankitha, M.; Pillai, V.K.; Alwarappan, S. Graphene Quantum Dots for Biosensing and Bioimaging. RSC Adv. 2024, 14, 16001–16023. [Google Scholar] [CrossRef]
- Li, M.; Chen, T.; Gooding, J.J.; Liu, J. Review of Carbon and Graphene Quantum Dots for Sensing. ACS Sens. 2019, 4, 1732–1748. [Google Scholar] [CrossRef]
- Khan, Z.G.; Patil, P.O. A Comprehensive Review on Carbon Dots and Graphene Quantum Dots Based Fluorescent Sensor for Biothiols. Microchem. J. 2020, 157, 105011. [Google Scholar] [CrossRef]
- Tajik, S.; Dourandish, Z.; Zhang, K.; Beitollahi, H.; Van Le, Q.; Jang, H.W.; Shokouhimehr, M. Carbon and Graphene Quantum Dots: A Review on Syntheses, Characterization, Biological and Sensing Applications for Neurotransmitter Determination. RSC Adv. 2020, 10, 15406–15429. [Google Scholar] [CrossRef] [PubMed]
- Mansuriya, B.; Altintas, Z. Applications of Graphene Quantum Dots in Biomedical Sensors. Sensors 2020, 20, 1072. [Google Scholar] [CrossRef] [PubMed]
- Al Ja’fArawy, M.S.; Thirumalai, D.; Lee, J.; Jung, H.S.; Chang, S.-C.; Yoon, J.-H.; Kim, D.-H. Graphene Quantum Dot Nanocomposites: Electroanalytical and Optical Sensor Technology Perspective. J. Anal. Sci. Technol. 2023, 14, 29. [Google Scholar] [CrossRef]
- Li, K.; Tu, J.; Zhang, Y.; Jin, D.; Li, T.; Li, J.; Ni, W.; Xiao, M.-M.; Zhang, Z.-Y.; Zhang, G.-J. Ultrasensitive Detection of Exosomal MiRNA with PMO-Graphene Quantum Dots-Functionalized Field-Effect Transistor Biosensor. iScience 2022, 25, 104522. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Dong, H.; Yang, Z.; Zhong, X.; Chen, Y.; Dai, W.; Zhang, X. Aptamer-Conjugated Graphene Quantum Dots/Porphyrin Derivative Theranostic Agent for Intracellular Cancer-Related MicroRNA Detection and Fluorescence-Guided Photothermal/Photodynamic Synergetic Therapy. ACS Appl. Mater. Interfaces 2017, 9, 159–166. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, J.X. Upconversion Nanomaterials: Synthesis, Mechanism, and Applications in Sensing. Sensors 2012, 12, 2414–2435. [Google Scholar] [CrossRef]
- Dong, H.; Sun, L.-D.; Yan, C.-H. Energy Transfer in Lanthanide Upconversion Studies for Extended Optical Applications. Chem. Soc. Rev. 2015, 44, 1608–1634. [Google Scholar] [CrossRef]
- Máčala, J.; Makhneva, E.; Hlaváček, A.; Kopecký, M.; Gorris, H.H.; Skládal, P.; Farka, Z. Upconversion Nanoparticle-Based Dot-Blot Immunoassay for Quantitative Biomarker Detection. Anal. Chem. 2024, 96, 10237–10245. [Google Scholar] [CrossRef]
- Cheng, S.; Huang, B.; Lu, K.-Q.; Li, J.; Wen, H.-R.; Zhang, J. Recent Advances of Upconversion Nanoparticles for Biomolecules Detection-A Review. Anal. Methods 2025, 17, 5563–5578. [Google Scholar] [CrossRef]
- Yang, L.; Che, C.; Guo, M.; Fan, C.; Sun, L.; Chen, S. An Upconversion Fluorescent Resonant Energy Transfer Biosensor for the Detection of MicroRNA through DNA Hybridization. ACS Omega 2024, 9, 47156–47166. [Google Scholar] [CrossRef]
- Jiang, W.; Yi, J.; Li, X.; He, F.; Niu, N.; Chen, L. A Comprehensive Review on Upconversion Nanomaterials-Based Fluorescent Sensor for Environment, Biology, Food and Medicine Applications. Biosensors 2022, 12, 1036. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Li, F.; Ågren, H.; Chen, G. Inhibiting Concentration Quenching in Yb3+-Tm3+ Upconversion Nanoparticles by Suppressing Back Energy Transfer. Nat. Commun. 2025, 16, 4218. [Google Scholar] [CrossRef] [PubMed]
- Würth, C.; Grauel, B.; Pons, M.; Frenzel, F.; Rissiek, P.; Rücker, K.; Haase, M.; Resch-Genger, U. Yb- and Er Concentration Dependence of the Upconversion Luminescence of Highly Doped NaYF4:Yb,Er/NaYF4:Lu Core/Shell Nanocrystals Prepared by a Water-Free Synthesis. Nano Res. 2022, 15, 9639–9646. [Google Scholar] [CrossRef]
- Esmaeili, S.; Rajil, N.; Hazrathosseini, A.; Neuman, B.W.; Alkahtani, M.H.; Sen, D.; Hu, Q.; Wu, H.-J.; Yi, Z.; Brick, R.W.; et al. Quantum-Enhanced Detection of Viral CDNA via Luminescence Resonance Energy Transfer Using Upconversion and Gold Nanoparticles. Nanophotonics 2025. [Google Scholar] [CrossRef]
- Zhao, L.; Song, Y.; Xu, H. Catalytic Hairpin Self-Assembly for Biosensing: Classification, Influencing Factors, and Applications. TrAC Trends Anal. Chem. 2024, 171, 117508. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Liu, R.; Wang, S.; Li, L.; Zhao, P.; Wang, Y.; Ge, S.; Yu, J. Entropy-Driven Catalysis Cycle Assisted CRISPR/Cas12a Amplification Photoelectrochemical Biosensor for MiRNA-21 Detection. Sens. Actuators B Chem. 2023, 394, 134334. [Google Scholar] [CrossRef]
- Yang, S.; Zhan, X.; Yuan, L.; Lamy de la Chapelle, M.; Fu, W.; Yang, X. Entropy Driven-Based Catalytic Biosensors for Bioanalysis: From Construction to Application-A Review. Anal. Chim. Acta 2025, 1338, 343549. [Google Scholar] [CrossRef]
- Zhou, W.; Gao, X.; Liu, D.; Chen, X. Gold Nanoparticles for In Vitro Diagnostics. Chem. Rev. 2015, 115, 10575–10636. [Google Scholar] [CrossRef]
- Esporrín-Ubieto, D.; Fraire, J.C.; Sánchez-deAlcázar, D.; Sánchez, S. Engineered Plasmonic and Fluorescent Nanomaterials for Biosensing, Motion, Imaging, and Therapeutic Applications. Adv. Mater. 2025, 2502171. [Google Scholar] [CrossRef]
- Joyce, C.; Fothergill, S.M.; Xie, F. Recent Advances in Gold-Based Metal Enhanced Fluorescence Platforms for Diagnosis and Imaging in the near-Infrared. Mater. Today Adv. 2020, 7, 100073. [Google Scholar] [CrossRef]
- Tan, X.; Yang, J.; Liu, Y.; Tang, Z.; Xiao, H.; Lv, J.; He, Y.; Hu, R.; Jin, Z.; Chen, S.; et al. Field Detection of Multiple Infectious Diseases with Naked Eye Using Plasmonic-Enhanced Fluorescent Nanoparticles. Anal. Chem. 2025, 97, 7359–7368. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, D.; Chen, J.; Shi, M.; Yuan, K.; Sun, H.; Meng, H.-M.; Li, Z. DNA Logical Device Combining an Entropy-Driven Catalytic Amplification Strategy for the Simultaneous Detection of Exosomal Multiplex MiRNAs In Situ. Anal. Chem. 2024, 96, 1733–1741. [Google Scholar] [CrossRef]
- Chin, L.K.; Yang, J.-Y.; Chousterman, B.; Jung, S.; Kim, D.-G.; Kim, D.-H.; Lee, S.; Castro, C.M.; Weissleder, R.; Park, S.-G.; et al. Dual-Enhanced Plasmonic Biosensing for Point-of-Care Sepsis Detection. ACS Nano 2023, 17, 3610–3619. [Google Scholar] [CrossRef]
- Ratre, P.; Nazeer, N.; Kumari, R.; Thareja, S.; Jain, B.; Tiwari, R.; Kamthan, A.; Srivastava, R.K.; Mishra, P.K. Carbon-Based Fluorescent Nano-Biosensors for the Detection of Cell-Free Circulating MicroRNAs. Biosensors 2023, 13, 226. [Google Scholar] [CrossRef]
- Zheng, P.; Wu, N. Fluorescence and Sensing Applications of Graphene Oxide and Graphene Quantum Dots: A Review. Chem. Asian J. 2017, 12, 2343–2353. [Google Scholar] [CrossRef]
- Gaviria, M.I.; Barrientos, K.; Arango, J.P.; Cano, J.B.; Peñuela, G.A. Highly Sensitive Fluorescent Biosensor Based on Acetylcholinesterase and Carbon Dots–Graphene Oxide Quenching Test for Analytical and Commercial Organophosphate Pesticide Detection. Front. Environ. Sci. 2022, 10, 825112. [Google Scholar] [CrossRef]
- Gao, Y.; Yu, H.; Tian, J.; Xiao, B. Nonenzymatic DNA-Based Fluorescence Biosensor Combining Carbon Dots and Graphene Oxide with Target-Induced DNA Strand Displacement for MicroRNA Detection. Nanomaterials 2021, 11, 2608. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Zhang, J.; Ju, H.; Lu, H.; Wang, S.; Jin, S.; Hao, K.; Du, H.; Zhang, X. Highly Sensitive Multiple MicroRNA Detection Based on Fluorescence Quenching of Graphene Oxide and Isothermal Strand-Displacement Polymerase Reaction. Anal. Chem. 2012, 84, 4587–4593. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Ma, L.; Huang, Z.; Hu, H.; Wu, P.; Liu, J. Janus DNA Orthogonal Adsorption of Graphene Oxide and Metal Oxide Nanoparticles Enabling Stable Sensing in Serum. Mater. Horiz. 2018, 5, 65–69. [Google Scholar] [CrossRef]
- He, S.; Song, B.; Li, D.; Zhu, C.; Qi, W.; Wen, Y.; Wang, L.; Song, S.; Fang, H.; Fan, C. A Graphene Nanoprobe for Rapid, Sensitive, and Multicolor Fluorescent DNA Analysis. Adv. Funct. Mater. 2010, 20, 453–459. [Google Scholar] [CrossRef]
- Lu, C.; Yang, H.; Zhu, C.; Chen, X.; Chen, G. A Graphene Platform for Sensing Biomolecules. Angew. Chem. Int. Ed. 2009, 48, 4785–4787. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; He, J.H.; Wu, X.Q.; Liu, J.J.; Lv, W.Y.; Huang, C.Z.; Liu, H.; Li, C.M. Simultaneous Detection of Multiple MicroRNAs Based on Fluorescence Resonance Energy Transfer under a Single Excitation Wavelength. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2024, 322, 124788. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Du, H.; Yan, X.; Jie, G. Carbon Quantum Dot-Based Fluorescence Quenching Coupled with Enzyme-Assisted Multiple Cycle Amplification for Biosensing of MiRNA. Microchem. J. 2022, 183, 108116. [Google Scholar] [CrossRef]
- Li, L.; Tan, K.; Bai, Y.; Chen, J.; Dong, R.; Li, Z.; Wang, J. Real-Time Detection of Multiple Intracellular MicroRNAs Using an Ultrasound-Propelled Nanomotor-Based Dynamic Fluorescent Probe. Anal. Chem. 2024, 96, 10274–10282. [Google Scholar] [CrossRef]
- Wang, P.; Wei, X.; Shen, L.; Xu, K.; Wen, Z.; Gao, N.; Fan, T.; Xun, S.; Zhu, Q.; Qu, X.; et al. Amplification-Free Analysis of Bladder Cancer MicroRNAs on Wrinkled Silica Nanoparticles with DNA-Functionalized Quantum Dots. Anal. Chem. 2024, 96, 4860–4867. [Google Scholar] [CrossRef]
- Hu, O.; Li, Z.; Tong, Y.; Wang, Q.; Chen, Z. DNA Functionalized Double Quantum Dots-Based Fluorescence Biosensor for One-Step Simultaneous Detection of Multiple MicroRNAs. Talanta 2021, 235, 122763. [Google Scholar] [CrossRef]
- Cheng, N.; Fu, J. An Approach to the Simultaneous Detection of Multiple Biomarkers for the Early Diagnosis of Liver Cancer Using Quantum Dot Nanoprobes. Infect. Microbes Dis. 2022, 4, 34–40. [Google Scholar] [CrossRef]
- Ji, D.-D.; Wu, M.-X.; Ding, S.-N. Photonic Crystal Barcodes Assembled from Dendritic Silica Nanoparticles for the Multiplex Immunoassays of Ovarian Cancer Biomarkers. Anal. Methods 2022, 14, 298–305. [Google Scholar] [CrossRef]
- Zhang, D.; Cai, L.; Wei, X.; Wang, Y.; Shang, L.; Sun, L.; Zhao, Y. Multiplexed CRISPR/Cas9 Quantifications Based on Bioinspired Photonic Barcodes. Nano Today 2021, 40, 101268. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, Y.; Li, X.; Luan, Q. CRISPR/Cas13a-Enhanced Porous Hydrogel Encapsulated Photonic Barcodes for Multiplexed Detection of Virus. Small 2025, 21, 2408725. [Google Scholar] [CrossRef]
- Yu, H.; Xu, P.-F.; Liu, Y.; Jia, Z.-S.; Li, Y.-Y.; Tang, H.-W. LRET-Based Simultaneous Detection of Dual MiRNAs via Multitrap Optical Tweezers Assisted Suspension Array Tagged by Two Different Luminescent Quenchable UCNPs Combining CRISPR/Cas12a Amplification. Anal. Chem. 2025, 97, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Ma, Y.; Li, L.; Wong, M.C.; Wang, P.; Chen, J.; Chen, H.; Wang, F.; Hao, J. Multiplexed Detection of SARS-CoV-2 Based on Upconversion Luminescence Nanoprobe/MXene Biosensing Platform for COVID-19 Point-of-Care Diagnostics. Mater. Des. 2022, 223, 111249. [Google Scholar] [CrossRef] [PubMed]
- Kneipp, J.; Kneipp, K. Surface Enhanced Nonlinear Raman Processes for Advanced Vibrational Probing. ACS Nano 2024, 18, 20851–20860. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Huo, H.; Wu, Y.; Chen, L.; Su, L.; Zhang, X.; Song, J.; Yang, H. Design and Synthesis of SERS Materials for In Vivo Molecular Imaging and Biosensing. Adv. Sci. 2023, 10, 2202051. [Google Scholar] [CrossRef]
- Awiaz, G.; Lin, J.; Wu, A. Recent Advances of Au@Ag Core–Shell SERS-based Biosensors. Exploration 2023, 3, 20220072. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Wang, P.; Fan, L.; Shi, Y. Highly Sensitive Multiplex Detection of Foodborne Pathogens Using a SERS Immunosensor Combined with Novel Covalent Organic Frameworks Based Biologic Interference-Free Raman Tags. Talanta 2022, 243, 123369. [Google Scholar] [CrossRef]
- Wang, C.; Wang, C.; Li, J.; Tu, Z.; Gu, B.; Wang, S. Ultrasensitive and Multiplex Detection of Four Pathogenic Bacteria on a Bi-Channel Lateral Flow Immunoassay Strip with Three-Dimensional Membrane-like SERS Nanostickers. Biosens. Bioelectron. 2022, 214, 114525. [Google Scholar] [CrossRef]
- Mousavi, S.D.; Li, J.; Gordon, J.; Cheng, H.-W.; Skeete, Z.; Filippone, N.; Walter, L.; Hakimi, S.; Hader, M.; Feldman, H.; et al. Printed Paper Substrates with Plasmonic and Magnetic Nanoprobes for SERS Detection of Cancer Biomarkers. ACS Appl. Nano Mater. 2025, 8, 15395–15404. [Google Scholar] [CrossRef]
- Ge, S.; Chen, G.; Deng, J.; Gu, Y.; Mao, Y.; Zhou, X.; Li, G. Multiplex Signal Amplification Strategy-Based Early-Stage Diagnosis of Parkinson’s Disease on a SERS-Enabled LoC System. Anal. Chim. Acta 2023, 1247, 340890. [Google Scholar] [CrossRef]
- Choi, J.-H.; Shin, M.; Yang, L.; Conley, B.; Yoon, J.; Lee, S.-N.; Lee, K.-B.; Choi, J.-W. Clustered Regularly Interspaced Short Palindromic Repeats-Mediated Amplification-Free Detection of Viral DNAs Using Surface-Enhanced Raman Spectroscopy-Active Nanoarray. ACS Nano 2021, 15, 13475–13485. [Google Scholar] [CrossRef]
- Das, A.; Kim, K.; Park, S.-G.; Choi, N.; Choo, J. SERS-Based Serodiagnosis of Acute Febrile Diseases Using Plasmonic Nanopopcorn Microarray Platforms. Biosens. Bioelectron. 2021, 192, 113525. [Google Scholar] [CrossRef]
- Wu, J.; Zhou, X.; Li, P.; Lin, X.; Wang, J.; Hu, Z.; Zhang, P.; Chen, D.; Cai, H.; Niessner, R.; et al. Ultrasensitive and Simultaneous SERS Detection of Multiplex MicroRNA Using Fractal Gold Nanotags for Early Diagnosis and Prognosis of Hepatocellular Carcinoma. Anal. Chem. 2021, 93, 8799–8809. [Google Scholar] [CrossRef]
- Shim, J.-E.; Kim, Y.J.; Hahm, E.; Choe, J.-H.; Baek, A.; Kim, R.M.; You, E.-A. Ultrasensitive SERS Nanoprobe-Based Multiplexed Digital Sensing Platform for the Simultaneous Quantification of Alzheimer’s Disease Biomarkers. Biosens. Bioelectron. 2025, 274, 117216. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zhao, J.; Zuo, Z.; Song, L.; Han, X.X.; Yang, J.; Xu, B.; Zhao, B. SERS Nanotags for Ultrasensitive and Dual Biomarker Detection of Breast Cancer. Sens. Actuators Rep. 2025, 9, 100344. [Google Scholar] [CrossRef]
- Sloan-Dennison, S.; Wallace, G.Q.; Hassanain, W.A.; Laing, S.; Faulds, K.; Graham, D. Advancing SERS as a Quantitative Technique: Challenges, Considerations, and Correlative Approaches to Aid Validation. Nano Converg. 2024, 11, 33. [Google Scholar] [CrossRef]
- Dey, P. Aiming for Maximized and Reproducible Enhancements in the Obstacle Race of SERS. ACS Meas. Sci. Au 2023, 3, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Fang, L.; Wang, Y.; Xiong, Z. Current Trends of Raman Spectroscopy in Clinic Settings: Opportunities and Challenges. Adv. Sci. 2024, 11, 2300668. [Google Scholar] [CrossRef]
- Lee, Y.; Haizan, I.; Sim, S.B.; Choi, J.-H. Colorimetric Biosensors: Advancements in Nanomaterials and Cutting-Edge Detection Strategies. Biosensors 2025, 15, 362. [Google Scholar] [CrossRef]
- Sivakumar, R.; Dinh, V.P.; Lee, N.Y. Copper Sulfate-Induced Diphenylamine for Rapid Colorimetric Point-of-Care Detection of Contagious Pathogens Combined with Loop-Mediated Isothermal Amplification. ACS Sustain. Chem. Eng. 2023, 11, 2079–2088. [Google Scholar] [CrossRef]
- Alafeef, M.; Moitra, P.; Dighe, K.; Pan, D. RNA-Extraction-Free Nano-Amplified Colorimetric Test for Point-of-Care Clinical Diagnosis of COVID-19. Nat. Protoc. 2021, 16, 3141–3162. [Google Scholar] [CrossRef]
- Pinheiro, T.; Marques, A.C.; Carvalho, P.; Martins, R.; Fortunato, E. Paper Microfluidics and Tailored Gold Nanoparticles for Nonenzymatic, Colorimetric Multiplex Biomarker Detection. ACS Appl. Mater. Interfaces 2021, 13, 3576–3590. [Google Scholar] [CrossRef]
- Zhu, W.; Li, L.; Zhou, Z.; Yang, X.; Hao, N.; Guo, Y.; Wang, K. A Colorimetric Biosensor for Simultaneous Ochratoxin A and Aflatoxins B1 Detection in Agricultural Products. Food Chem. 2020, 319, 126544. [Google Scholar] [CrossRef]
- Bordbar, M.M.; Samadinia, H.; Sheini, A.; Aboonajmi, J.; Javid, M.; Sharghi, H.; Ghanei, M.; Bagheri, H. Non-Invasive Detection of COVID-19 Using a Microfluidic-Based Colorimetric Sensor Array Sensitive to Urinary Metabolites. Microchim. Acta 2022, 189, 316. [Google Scholar] [CrossRef]
- Ivrigh, Z.J.-N.; Bigdeli, A.; Jafarinejad, S.; Hormozi-Nezhad, M.R. Multiplex Detection of Antidepressants with a Single Component Condition-Based Colorimetric Sensor Array. Sens. Actuators B Chem. 2022, 363, 131855. [Google Scholar] [CrossRef]
- Song, Y.; Wei, W.; Qu, X.; Song, Y.; Wei, W.; Qu, X. Colorimetric Biosensing Using Smart Materials. Adv. Mater. 2011, 23, 4215–4236. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, B.; Bruylants, G.; Jabin, I. Tailored Ultrastable Core–Shell Au@Ag Nanoparticles for Enhanced Colorimetric Detection in Lateral Flow Assays. ACS Appl. Nano Mater. 2024, 7, 6169–6177. [Google Scholar] [CrossRef]
- Xu, C.; Zheng, S.; Xia, X.; Li, J.; Yu, Q.; Wang, Y.; Jin, Q.; Wang, C.; Gu, B. Core–Satellite-Structured Magnetic Nanozyme Enables the Ultrasensitive Colorimetric Detection of Multiple Drug Residues on Lateral FLow Immunoassay. Anal. Chim. Acta 2024, 1325, 343115. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.; Cho, H.H.; Moon, H.; Song, H.; Ro, J.C.; Lee, J.H.; Lee, J. Simultaneous Triplex Detection in a Single-Test-Line Lateral Flow Immunoassay Utilizing Distinct Nanoparticle Colorimetry. Biochip J. 2024, 18, 247–256. [Google Scholar] [CrossRef]
- Cao, X.E.; Kim, J.; Mehta, S.; Erickson, D. Two-Color Duplex Platform for Point-of-Care Differential Detection of Malaria and Typhoid Fever. Anal. Chem. 2021, 93, 12175–12180. [Google Scholar] [CrossRef]
- Su, J.; Ge, W.; Su, Y.; Chen, X.; Zhao, X.; Ding, R.; Shi, K.; Liu, Z. Dual-Colorimetric Amplification Biosensor Based on GNPs and G-Quadruplex/Hemin DNAzyme for Multiplexed MicroRNAs. Talanta 2026, 297, 128682. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Jiang, J.; Charconnet, M.; Peng, Y.; Zhang, L.; Lawrie, C.H. Shape-Specific Gold Nanoparticles for Multiplex Biosensing Applications. ACS Omega 2024, 9, 37163–37169. [Google Scholar] [CrossRef]
- Parakh, A.; Awate, A.; Barman, S.M.; Kadu, R.K.; Tulaskar, D.P.; Kulkarni, M.B.; Bhaiyya, M. Artificial Intelligence and Machine Learning for Colorimetric Detections: Techniques, Applications, and Future Prospects. Trends Environ. Anal. Chem. 2025, 48, e00280. [Google Scholar] [CrossRef]
- Poosinuntakul, N.; Chanmee, T.; Porntadavity, S.; Chailapakul, O.; Apilux, A. Silver-Enhanced Colloidal Gold Dip Strip Immunoassay Integrated with Smartphone-Based Colorimetry for Sensitive Detection of Cardiac Marker Troponin I. Sci. Rep. 2022, 12, 19866. [Google Scholar] [CrossRef]
- Yan, X.-H.; Ji, B.; Fang, F.; Guo, X.-L.; Zhao, S.; Wu, Z.-Y. Fast and Sensitive Smartphone Colorimetric Detection of Whole Blood Samples on a Paper-Based Analytical Device. Talanta 2024, 270, 125515. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, L.; Ma, G.; Qiu, S.; Shan, G.; Zhao, L.; Sun, Y.; Cui, A.; Zhang, R.; Liu, X. Portable Paper-Based Microfluidic Devices with Cu1-XAgxS NPs Modification for Multiplex Intelligent Visualized Detection of Adrenaline and Glucose Simultaneously. Anal. Chim. Acta 2025, 1336, 343489. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Mu, K.; Wei, H.; Chen, H.; Wang, Y.; Zhang, W.; Rong, Z. Paper-Based Multiplex Colorimetric Vertical Flow Assay with Smartphone Readout for Point-of-Care Detection of Acute Kidney Injury Biomarkers. Sens. Actuators B Chem. 2023, 390, 134029. [Google Scholar] [CrossRef]
- Mansouri, S.; Boulares, S.; Chabchoub, S.; Alharbi, Y.; Alqahtani, A. Recent Progress of Smartphone-Assisted Paper-Based Analytical Devices (PADs) for Multiplex Sensing: Focusing on Colorimetric and Optical Sensors for Environmental Monitoring, Food Safety, and Biomedical Application. Microchem. J. 2025, 209, 112670. [Google Scholar] [CrossRef]
- Xiong, J.; Qin, L.; Zhang, H.; Zhang, S.; He, S.; Xu, Y.; Zhang, L.; Wang, Z.; Jiang, H. Sensitive and Simultaneous Detection of Ractopamine and Salbutamol Using Multiplex Lateral Flow Immunoassay Based on Polyethyleneimine-Mediated SiO2@QDs Nanocomposites: Comparison and Application. Microchem. J. 2022, 181, 107730. [Google Scholar] [CrossRef]
- Yang, Y.-C.; Liu, M.-H.; Yang, S.-M.; Chan, Y.-H. Bimodal Multiplexed Detection of Tumor Markers in Non-Small Cell Lung Cancer with Polymer Dot-Based Immunoassay. ACS Sens. 2021, 6, 4255–4264. [Google Scholar] [CrossRef]
- Wen, C.-Y.; Yang, X.; Zhao, T.-Y.; Qu, J.; Tashpulatov, K.; Zeng, J. Dual-Mode and Multiplex Lateral Flow Immunoassay: A Powerful Technique for Simultaneous Screening of Respiratory Viruses. Biosens. Bioelectron. 2025, 271, 117030. [Google Scholar] [CrossRef]
- Lin, D.; Li, B.; Fu, L.; Qi, J.; Xia, C.; Zhang, Y.; Chen, J.; Choo, J.; Chen, L. A Novel Polymer-Based Nitrocellulose Platform for Implementing a Multiplexed Microfluidic Paper-Based Enzyme-Linked Immunosorbent Assay. Microsyst. Nanoeng. 2022, 8, 53. [Google Scholar] [CrossRef]

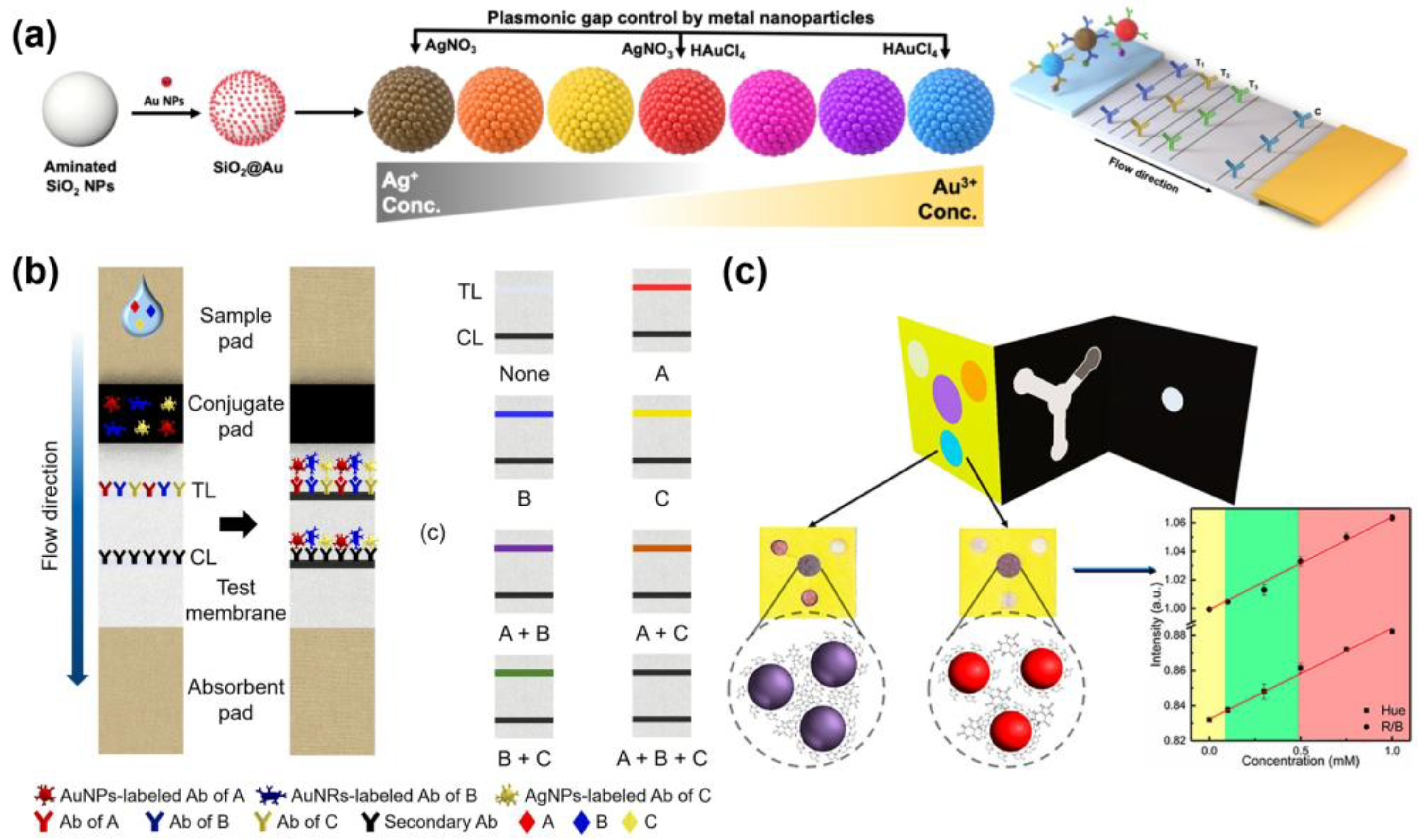
| Target Biomarkers | Nanoparticles | Method Performance | Sample | Ref. |
|---|---|---|---|---|
| miR-21, miR-141 | pAuNRs | LOD: 0.1 pM | Human sample (5%) | [37] |
| SARS-CoV-2 spike protein, Influenza A (H1N1) hemagglutinin, RSV fusion protein, Adenovirus hexon protein | PDDA-conjugated polystyrene NPs on Ag island substrate (pSilverF) | LOD: 0.168 NCU/mL, 0.023 NCU/mL and 0.168 NCU/mL | Human serum (42 patient samples, 26 healthy controls) | [191] |
| miR-21, miR-122 | AuNPs with hairpin DNA probes + EDC DNA logic circuit | LOD: 2.4 × 105 to 6.8 × 106 particles/μL Sensitivity: 93.3%, Accuracy: 93.3%, AUC: 0.92 | Exosomes derived from HCC patient serum | [192] |
| MCP-1, IL-6, IL-10, IL-3, IL-1β, TNF-α | Au nanodimple substrate decorated with AuNPs and TSA amplification | LOD: <1 pg/mL 100% accuracy | Clinical plasma samples 100% accuracy | [193] |
| miR-21, miR-141 | CDs or QDs (fluorescent reporters) and GO quencher | LOD: 60 pM, 50 pM | Human serum | [202] |
| miR-21, miR-141 | CQD-labeled probes and GO quencher | LOD: 4.7 fM Recoveries: 93.3–109.2% | Human serum samples | [203] |
| miR-21, miR-10b | Au nanowire/GO nanomotors with multicolor QD-labeled ssDNA probes | LOD: 0.5–10 pM OFF–ON switching within 15 min | Intracellular detection in living cells | [204] |
| AFP, DKK1, GPC3 | CdSe/ZnS core–shell QDs (525, 585, 625 nm) conjugated with specific antibodies | LOD: 0.625 ng/mL, 1.25 ng/mL and 2.5 ng/mL | Mixed reference antigen sample | [207] |
| miR-135b, miR-21, miR-96 | WSNs with QD–DNA | LOD: 5 fM, 19 fM and 8 fM Linear range: 10 fM–100 nM RSD: <5% | Clinical serum samples | [205] |
| miR-33, miR-125b | Dual QDs (donors) conjugated with ssDNA and BHQ-functionalized multifunctional nanomaterials (quencher) | LOD: 0.06 nM, 0.02 nM RSD: <2.1% | Human serum samples | [206] |
| CA125, CEA, AFP | Porous silica-based PhC beads (dSiO2) functionalized with antibodies and CdTe QDs | LOD: 0.52 ng/mL, 0.64 ng/mL and 0.79 U/mL RSD: <5.5% | Human serum samples | [208] |
| HPV16, HPV18, HPV33 | Bioinspired PhC barcodes: Structural colors at 418 nm, 520 nm, 652 nm | LOD: 0.025 pM | Synthetic nucleic acids | [211] |
| SARS-CoV-2 N gene, IAV, IBV | PhC barcodes encapsulated in porous hydrogel | LOD: 200 aM Recovery rates range: 88.9–112.6% | Clinical RNA samples | [210] |
| miR-155, miR-let-7a | Luminescence-quenched UCNP–microbead assemblies (green 541 nm, blue 475 nm) with quencher-labeled reporters | LOD: 17 aM, 22 aM | Single-cell analysis | [211] |
| SARS-CoV-2 ORF1ab gene, N gene | UCNPs and Ti3C2Tx MXene quencher | LOD: 15 fM, 194 fM | Spiked samples | [212] |
| Principle | Target Biomarkers | Nanoparticles | Method Performance | Sample | Ref. |
|---|---|---|---|---|---|
| Colorimetric | miR-10b, miR-21 | MBs, AuNPs, G-quadruplex/hemin DNAzyme | LOD: 1.5 nM, 2.2 nM Spike recoveries: 93.3% to 109.2% RSDs: 2.1% and 7.2% | Spiked into the diluted healthy human serum sample | [240] |
| M gene in the influenza A virus, E gene in SARS-CoV-2 | GNSp, GNSh | LOD: 33 nM, 10 nM | - | [241] | |
| ICAM1, CA19-9, PSA | Plasmon-controlled metal–silica isoform nanocomposites (PINs) | LOD: 6.65 ng/mL, 0.04 U/mL, 0.12 ng/mL | - | [17] | |
| AFP, NSE, CEA | AuNPs, AgNPs, AuNRs | LOD: 50 ng/mL (each) | - | [238] | |
| Glucose, uric acid, cholesterol | AuNPs | LOD: 1.25 mM, 71μM, 81μM | - | [231] | |
| NGAL, CysC, RBP4 | AuNPs | LOD: 1.04 ng/mL, 0.96 ng/mL, 1.17 ng/mL Sensitivity: 92% Specificity: 88% | Clinical serum samples | [245] | |
| Adrenaline, Glucose | Cu1−xAgx S NP | LOD: 10.2 nM, 11.5 μM Linear range: 0.05–30 μM, 0.02–12 mM | Real sample | [246] | |
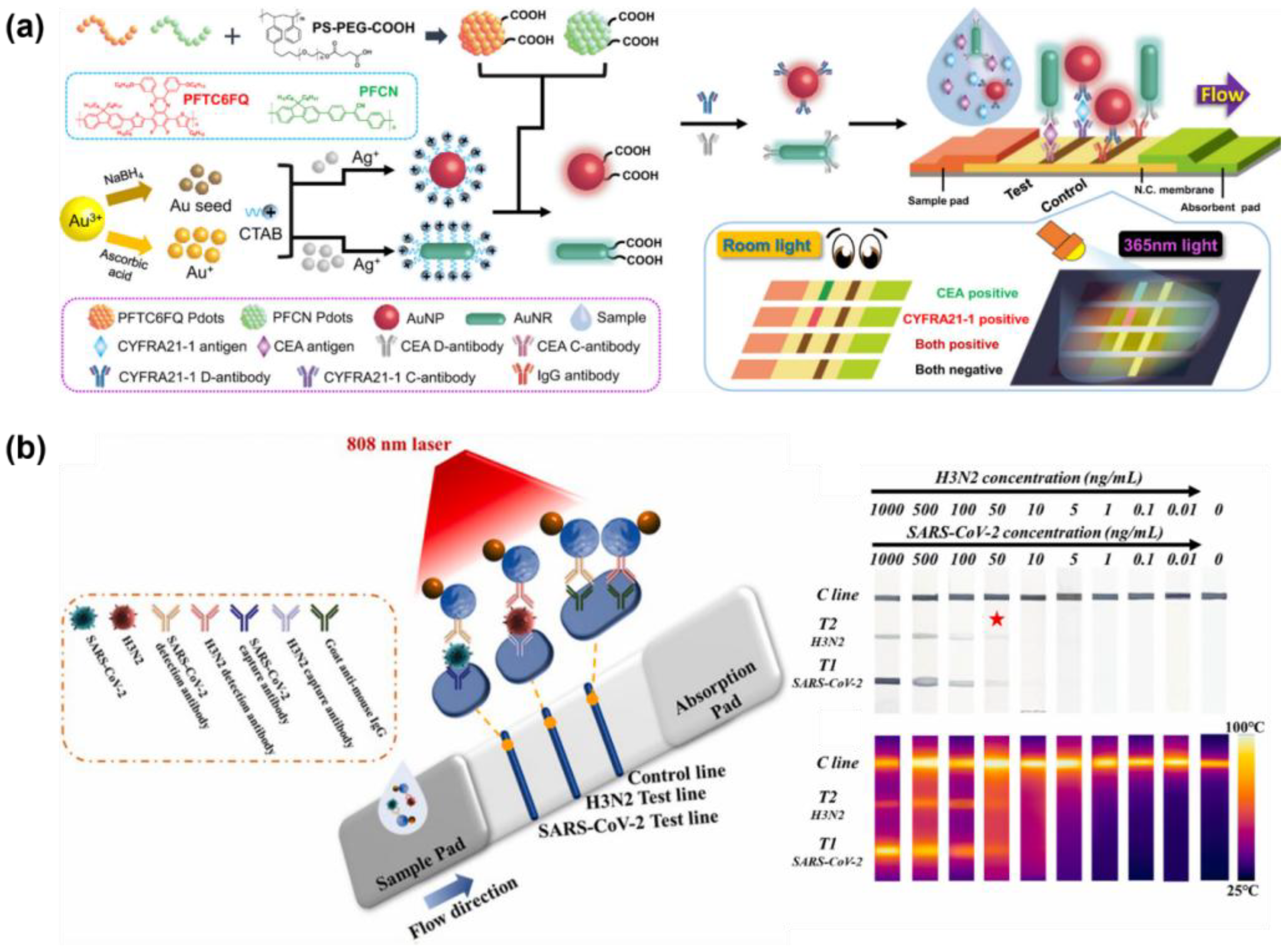
| Dual (Colorimetric and fluorescence) | CYFRA21-1, CEA | Au@Pdot, AuNRs, AuNPs | Cutoff value: 3.3 ng/mL, 5 ng/mL (Colorimetric) 0.07 ng/mL, 0.12 ng/mL (Fluorescence) Specificity: 95% | Clinical serum sample | [249] |
| Dual (Colorimetric and photothermal effects) | H3N2 influenza, SARS-CoV-2 | Janus Aushell–Fe3O4 | LOD: 50 ng/mL (Each) (Naked eye), 2 pg/mL, 7 pg/mL (Photothermal effects) | - | [250] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, P.; Choi, M.Y.; Lee, Y.; Lee, K.-B.; Choi, J.-H. Multiplexed Optical Nanobiosensing Technologies for Disease Biomarker Detection. Biosensors 2025, 15, 682. https://doi.org/10.3390/bios15100682
Kim P, Choi MY, Lee Y, Lee K-B, Choi J-H. Multiplexed Optical Nanobiosensing Technologies for Disease Biomarker Detection. Biosensors. 2025; 15(10):682. https://doi.org/10.3390/bios15100682
Chicago/Turabian StyleKim, Pureum, Min Yu Choi, Yubeen Lee, Ki-Bum Lee, and Jin-Ha Choi. 2025. "Multiplexed Optical Nanobiosensing Technologies for Disease Biomarker Detection" Biosensors 15, no. 10: 682. https://doi.org/10.3390/bios15100682
APA StyleKim, P., Choi, M. Y., Lee, Y., Lee, K.-B., & Choi, J.-H. (2025). Multiplexed Optical Nanobiosensing Technologies for Disease Biomarker Detection. Biosensors, 15(10), 682. https://doi.org/10.3390/bios15100682





