Optical Devices for the Diagnosis and Management of Spinal Cord Injuries: A Review
Abstract
1. Introduction
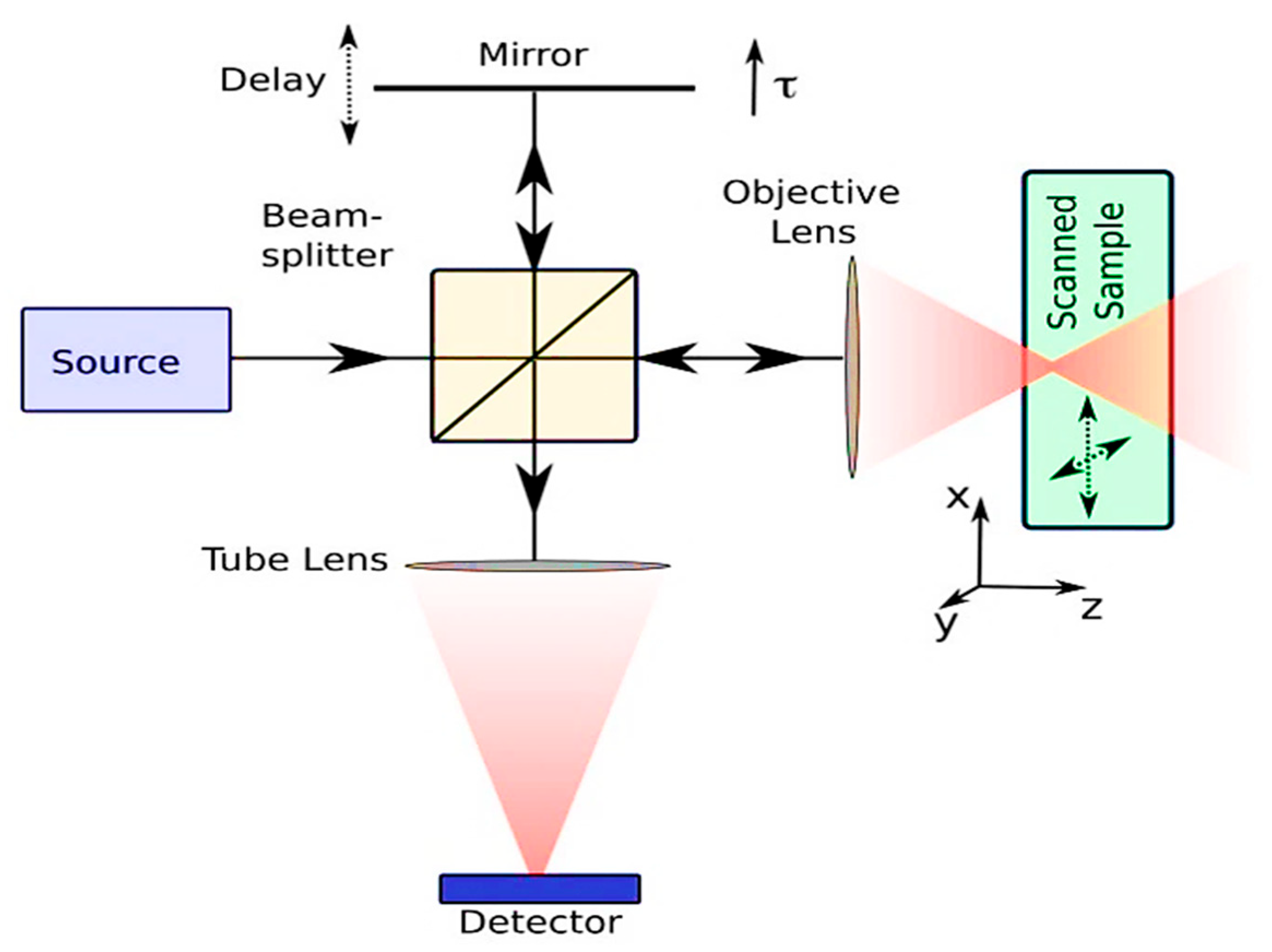
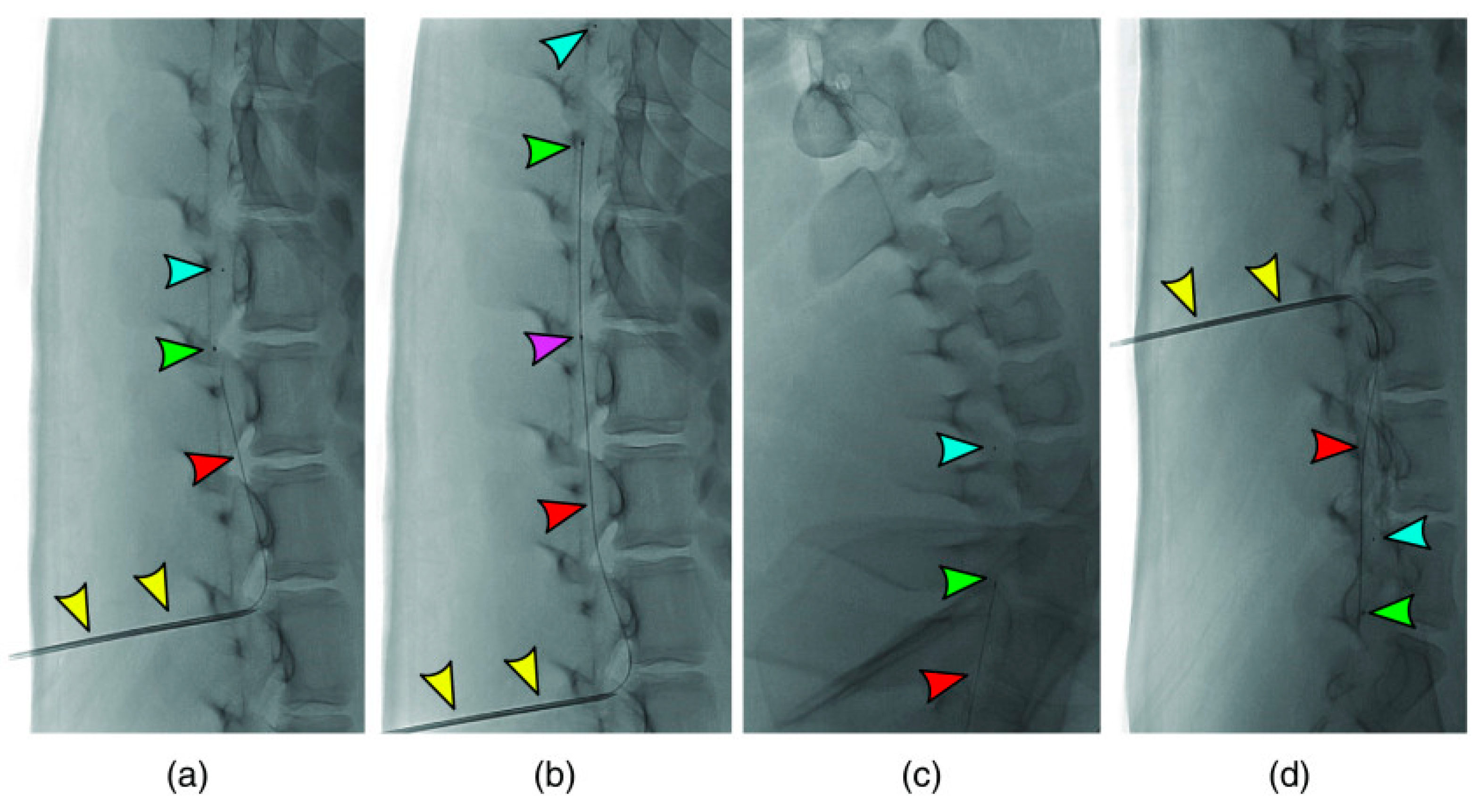
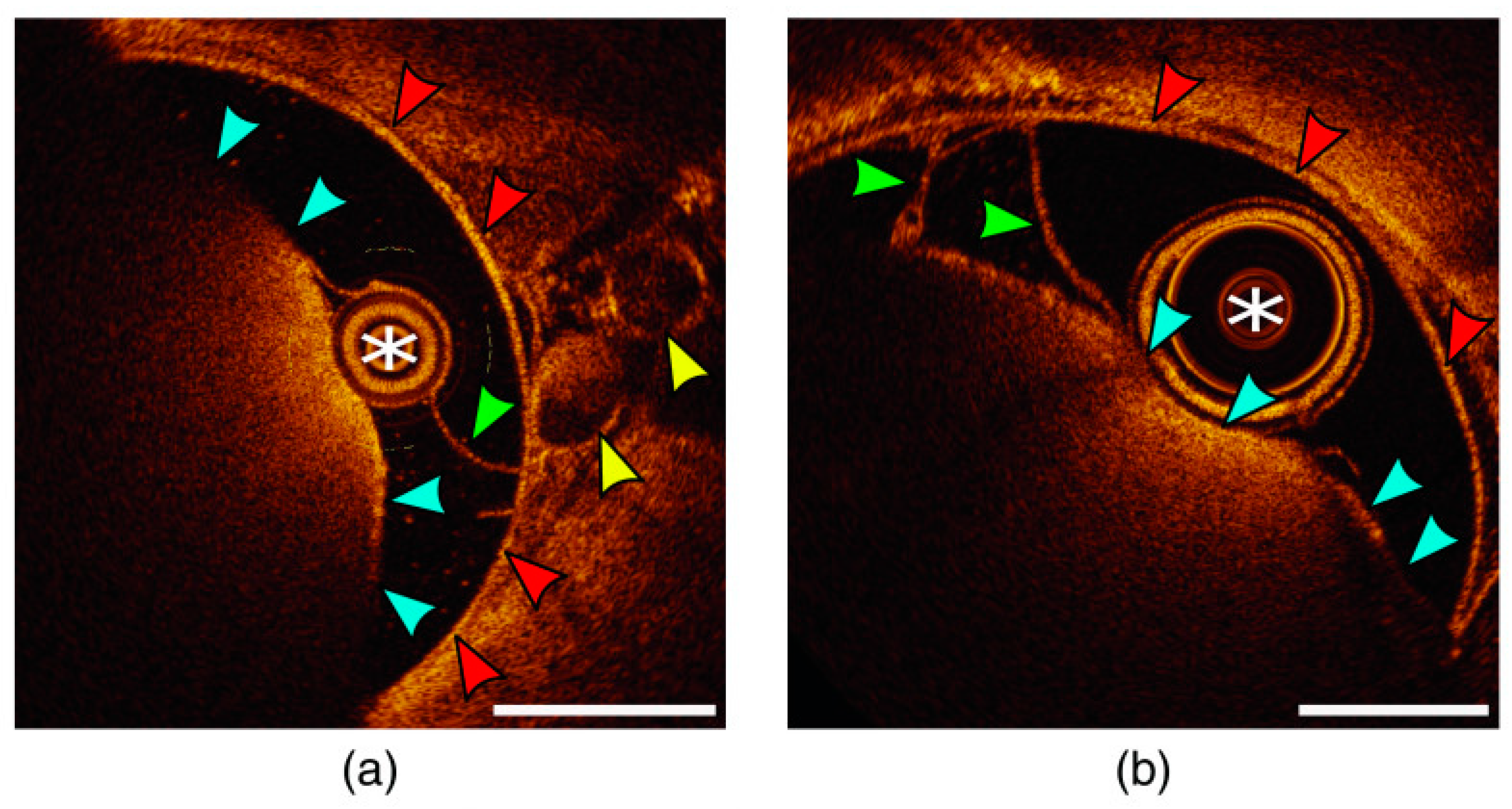
2. Optical Coherence Tomography (OCT)
3. Fluorescence Imaging
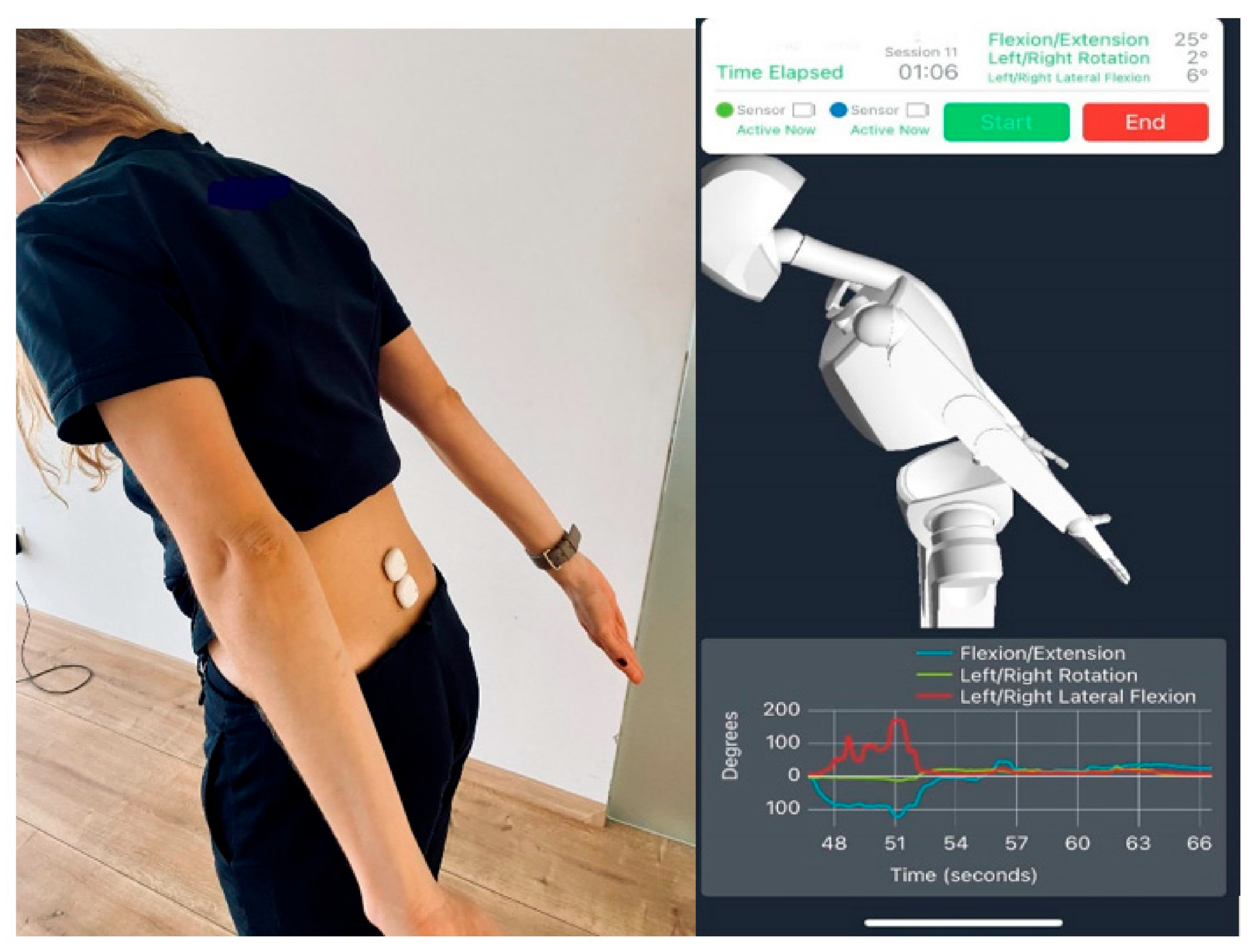
4. Wearable Optical Technology
5. Neuroimaging with Optical Techniques
- (a)
- The 2PE imaging method is a nonlinear laser-scanning fluorescence microscopy technique with sub-micrometer spatial resolution and 500 μm~1 mm depth of view, and has become more popular in neuroscience. It has been demonstrated that near-infrared two-photon excitation wavelengths can be used as a stimulus for structural fluorescence imaging as well as for CaSDI and VSDI for the imaging of neural activity in a wide field of view. Its wavelength is about twice as long as conventional wavelengths used for confocal or epifluorescence excitation [77,78,79]. Due to recent advances in MPE, especially 3PE, it is now possible to obtain even deeper functional imaging below a depth of 1 mm.
- (b)
- Using OISI with spatial and temporal resolutions of up to 100 mm and 2 s, we can visualize local microcirculation and cortical functional architecture label-free, with resolutions of up to 100 mm and 2 s, respectively. Earlier studies used this technique to examine the functional connections in the large-area cortex of mice, as well as to examine the functional connections on the exposed mouse skulls [80,81,82]. It has been shown that the use of multicolored light illumination can be used to assess the concentrations of deoxy- and oxyhemoglobin in different areas of the brain [83,84,85]. It has been used for a long time to infer the activity of the brain, based on changes in cortical reflectance caused by hemodynamic responses, which can be measured by OISI.
- (c)
- A laser speckle is a random interference pattern that appears when coherent light is scattered within tissue, often referred to as a laser beam. A dynamic movement within the live tissue generates changes in the speckle pattern as a result of the dynamic movement of scatterers, such as red blood cells, within the tissue. Using this technique called laser speckle color imaging (LSCI), we can produce a two-dimensional representation of tissue perfusion or blood flow in a two-dimensional space. An LSCI system is capable of achieving a time resolution of 10 msec to 10 sec as well as a spatial resolution of 10 μm, depending on the application. As a result of its shallow light-penetration depth, this technology can provide only a superficial blood flow map [86,87].
- (d)
- CaSDI and VSDI can be used simultaneously to measure the activity of several categories of neurons at the same time, since these methods provide temporal and spatial resolutions in the msec and μm ranges, respectively. In these optical neuronal imaging methods, certain dyes that are sensitive to neural activity are used to detect neural activity. Depending on the type of measurement, these methods can be used to monitor cellular or sub-cellular neural activity using dyes that glow when an action potential is generated [88,89].
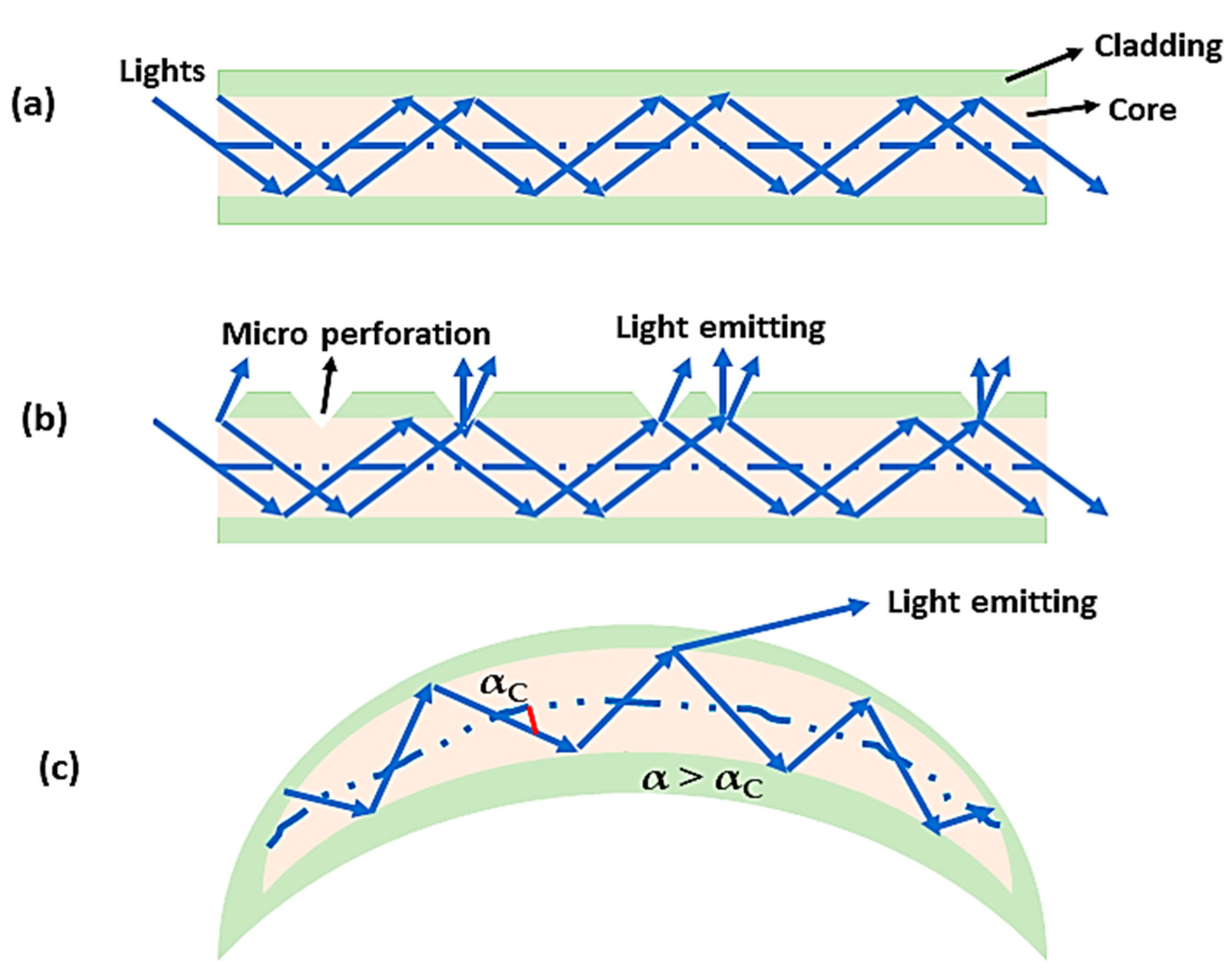
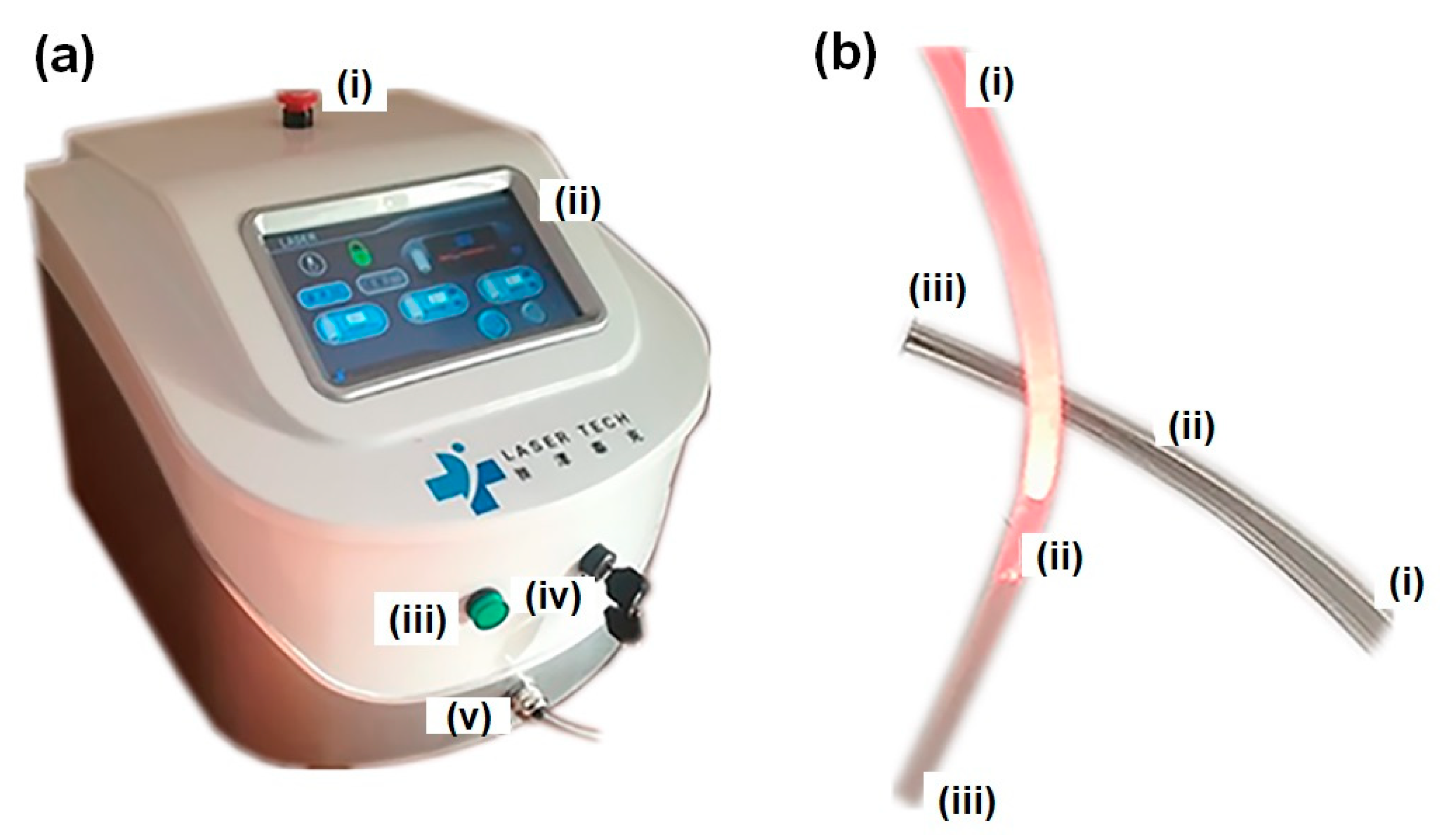
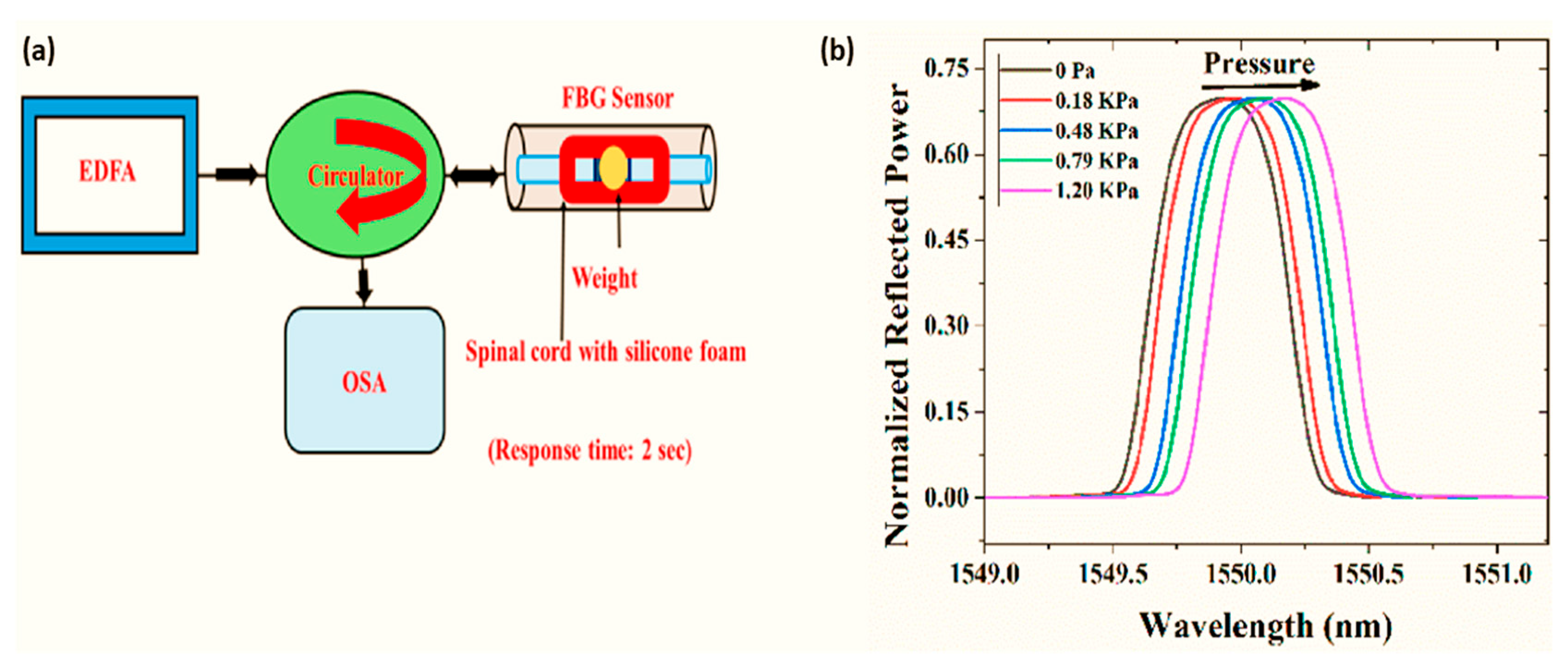
6. SCI Treatment with Optical Fiber-Based Devices
7. Photoacoustic Imaging through Plasmonic Nanoparticle
8. Future Perspectives and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- La Torre, D. Spinal cord injury: Review of basic and applied research. Spine 1981, 6, 315–335. [Google Scholar] [CrossRef]
- Venkatesan, D.; Elangovan, A.; Winster, H.; Pasha, M.Y.; Abraham, K.S.; Satheeshkumar, J.; Sivaprakash, P.; Niraikulam, A.; Gopalakrishnan, A.V.; Narayanasamy, A.; et al. Diagnostic and therapeutic approach of artificial intelligence in neuro-oncological diseases. Biosens. Bioelectron. X 2022, 11, 100188. [Google Scholar] [CrossRef]
- Budd, M.A.; Gater, D.R.; Channell, I., Jr. Psychosocial consequences of spinal cord injury: A narrative review. J. Pers. Med. 2022, 12, 1178. [Google Scholar] [CrossRef] [PubMed]
- Manohararaj, N.; Oey, N.E.; Zaw, E.M.; Jing, C.; Prasanna, K.V. Prasanna, Spinal Cord Injury: Extent of Disability, Complications, and Management of Patients in The Community–An Overview for Primary Care Physicians. J. Phys. Med. Rehabil. Stud. 2023, 165, 2–7. [Google Scholar]
- Dalle, D.U.; Sriram, S.; Bandyopadhyay, S.; Egiz, A.; Kotecha, J.; Kanmounye, U.S.; Higginbotham, G.; Ooi, S.Z.Y.; Bankole, N.D.A. Management and outcomes of traumatic pediatric spinal cord injuries in low-and middle-income countries: A scoping review. World Neurosurg. 2022, 165, 180–187.e3. [Google Scholar] [CrossRef] [PubMed]
- Malekzadeh, H.; Golpayegani, M.; Ghodsi, Z.; Sadeghi-Naini, M.; Asgardoon, M.; Baigi, V.; Vaccaro, A.R.; Rahimi-Movaghar, V. Direct cost of illness for spinal cord injury: A systematic review. Glob. Spine J. 2022, 12, 1267–1281. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Hu, S.; Wang, P.; Kang, H.; Peng, R.; Dong, Y.; Li, F. Spinal cord injury: The global incidence; prevalence, and disability from the global burden of disease study 2019. Spine 2022, 47, 1532. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, S.; Tsuji, O.; Shinozaki, M.; Shibata, T.; Yoshida, T.; Tomioka, Y.; Unai, K.; Kondo, T.; Itakura, G.; Kobayashi, Y.; et al. Current progress of rehabilitative strategies in stem cell therapy for spinal cord injury: A review. npj Regen. Med. 2021, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, K.; Kawabori, M.; Seki, T.; Houkin, K. Clinical Trials of Stem Cell Treatment for Spinal Cord Injury. Int. J. Mol. Sci. 2020, 21, 3994. [Google Scholar] [CrossRef]
- Flack, J.A.; Sharma, K.D.; Xie, J.Y. Delving into the recent advancements of spinal cord injury treatment: A review of recent progress. Neural Regen. Res. 2022, 17, 283. [Google Scholar]
- Dorrian, R.M.; Berryman, C.F.; Lauto, A.; Leonard, A.V. Electrical stimulation for the treatment of spinal cord injuries: A review of the cellular and molecular mechanisms that drive functional improvements. Front. Cell. Neurosci. 2023, 17, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Karamian, B.A.; Siegel, N.; Nourie, B.; Serruya, M.D.; Heary, R.F.; Harrop, J.S.; Vaccaro, A.R. The role of electrical stimulation for rehabilitation and regeneration after spinal cord injury. J. Orthop. Traumatol. 2022, 23, 2. [Google Scholar] [CrossRef] [PubMed]
- Matter, L.; Harland, B.; Raos, B.; Svirskis, D.; Asplund, M. Generation of direct current electrical fields as regenerative therapy for spinal cord injury: A review. APL Bioeng. 2023, 7, 031505. [Google Scholar] [CrossRef] [PubMed]
- Thuret, S.; Moon, L.D.; Gage, F.H. Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 2006, 7, 628–643. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Peer, D.; Petersen, B. Molecular and Cellular Therapies: New challenges and opportunities. Mol. Cell. Ther. 2013, 1, 1. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Guan, J.; Xu, P.; Zhao, J.; Zhang, C.; Zhang, B.; Mao, Y.; Cui, W. Cell therapeutic strategies for spinal cord injury. Adv. Wound Care 2019, 8, 585–605. [Google Scholar] [CrossRef] [PubMed]
- De Vivo, M.; Richards, J.; Stover, S.; Go, B. Spinal cord injury. Rehabil. Adds Life Years. West. J. Med. 1991, 154, 602. [Google Scholar]
- Burns, A.S.; Marino, R.J.; Flanders, A.E.; Flett, H. Clinical diagnosis and prognosis following spinal cord injury. Handb. Clin. Neurol. 2012, 109, 47–62. [Google Scholar] [PubMed]
- Tjardes, T.; Shafizadeh, S.; Rixen, D.; Paffrath, T.; Bouillon, B.; Steinhausen, E.S.; Baethis, H. Image-guided spine surgery: State of the art and future directions. Eur. Spine J. 2010, 19, 25–45. [Google Scholar] [CrossRef]
- Barker, R.; Fareedi, S.; Thompson, D.; Saunders, D. The use of CT angiography in the preoperative planning of cervical spine surgery in children. Child’s Nerv. Syst. 2009, 25, 955–959. [Google Scholar] [CrossRef]
- Sammon, P.M.; Gibson, R.; Fouyas, I.; Hughes, M.A. Intra-operative localisation of spinal level using pre-operative CT-guided placement of a flexible hook-wire marker. Br. J. Neurosurg. 2011, 25, 778–779. [Google Scholar] [CrossRef]
- Ou, P.; Schmit, P.; Layouss, W.; Sidi, D.; Bonnet, D.; Brunelle, F. CT angiography of the artery of Adamkiewicz with 64-section technology: First experience in children. Am. J. Neuroradiol. 2007, 28, 216–219. [Google Scholar]
- Stroman, P.W. Magnetic resonance imaging of neuronal function in the spinal cord: Spinal FMRI. Clin. Med. Res. 2005, 3, 146–156. [Google Scholar] [CrossRef] [PubMed]
- Braun, I.; Raghavendra, B.; Kricheff, I. Spinal cord imaging using real-time high-resolution ultrasound. Radiology 1983, 147, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Moseley, M.E.; Cohen, Y.; Kucharczyk, J.; Mintorovitch, J.; Asgari, H.; Wendland, M.; Tsuruda, J.; Norman, D. Diffusion-weighted MR imaging of anisotropic water diffusion in cat central nervous system. Radiology 1990, 176, 439–445. [Google Scholar] [CrossRef]
- Mcafee, P.C.; Bohlman, H.H.; Han, J.S.; Salvagno, R.T. Comparison of nuclear magnetic resonance imaging and computed tomography in the diagnosis of upper cervical spinal cord compression. Spine 1986, 11, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Giardini, M.E.; Zippo, A.G.; Valente, M.; Krstajic, N.; Biella, G.E.M. Electrophysiological and Anatomical Correlates of Spinal Cord Optical Coherence Tomography. PLoS ONE 2016, 11, e0152539. [Google Scholar] [CrossRef]
- Xia, Y.; Hu, J.; Zhao, S.; Tao, L.; Li, Z.; Yue, T.; Kong, J. Build-in sensors and analysis algorithms aided smartphone-based sensors for point-of-care tests. Biosens. Bioelectron. X 2022, 11, 100195. [Google Scholar] [CrossRef]
- Silbergleit, R.; Mehta, B.A.; Sanders, W.P.; Talati, S.J. Imaging-guided injection techniques with fluoroscopy and CT for spinal pain management. Radiographics 2001, 21, 927–939. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, S.-H. Comparison of clinical effectiveness of cervical transforaminal steroid injection according to different radiological guidances (C-arm fluoroscopy vs. computed tomography fluoroscopy). Spine J. 2011, 11, 416–423. [Google Scholar] [CrossRef]
- Betz, C.; Volgger, V.; Silverman, S.; Rubinstein, M.; Kraft, M.; Arens, C.; Wong, B. Clinical optical coherence tomography in head and neck oncology: Overview and outlook. Head Neck Oncol. 2013, 5, 35. [Google Scholar]
- Desjardins, A.E.; Van der Voort, M.; Roggeveen, S.; Lucassen, G.; Bierhoff, W.; Hendriks, B.H.; Brynolf, M.; Holmström, B. Needle stylet with integrated optical fibers for spectroscopic contrast during peripheral nerve blocks. J. Biomed. Opt. 2011, 16, 077004. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A. Optical coherence tomography. Science 1991, 5035, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.J.; Marks, D.L.; Ralston, T.S.; Carney, P.S.; Boppart, S.A. Interferometric synthetic aperture microscopy: Computed imaging for scanned coherent microscopy. Sensors 2008, 8, 3903–3931. [Google Scholar] [CrossRef] [PubMed]
- Mokbul, M.I. Optical coherence tomography: Basic concepts and applications in neuroscience research. J. Med. Eng. 2017, 2017, 3409327. [Google Scholar] [PubMed]
- Pasarikovski, C.R.; Ku, J.C.; Ramjist, J.; Dobashi, Y.; Priola, S.M.; da Costa, L.; Kumar, A.; Yang, V.X. Minimally invasive intrathecal spinal cord imaging with optical coherence tomography. J. Biomed. Opt. 2021, 26, 056002. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Tang, Q.; Liang, C.-P.; Wu, K.; Sandlerc, A.; Li, H.; Chen, Y. Imaging spinal structures with polarization-sensitive optical coherence tomography. IEEE Photonics J. 2016, 8, 1–8. [Google Scholar] [CrossRef]
- Al-Qaisi, M.K.; Akkin, T. Swept-source polarization-sensitive optical coherence tomography based on polarization-maintaining fiber. Opt. Express 2010, 18, 3392–3403. [Google Scholar] [CrossRef] [PubMed]
- Yamanari, M.; Makita, S.; Yasuno, Y. Polarization-sensitive swept-source optical coherence tomography with continuous source polarization modulation. Opt. Express 2008, 16, 5892–5906. [Google Scholar] [CrossRef]
- Dray, C.; Rougon, G.; Debarbieux, F. Quantitative analysis by in vivo imaging of the dynamics of vascular and axonal networks in injured mouse spinal cord. Proc. Natl. Acad. Sci. USA 2009, 106, 9459–9464. [Google Scholar] [CrossRef]
- Fetcho, J.R.; O’Malley, D.M. Visualization of active neural circuitry in the spinal cord of intact zebrafish. J. Neurophysiol. 1995, 73, 399–406. [Google Scholar] [CrossRef]
- Tang, P.; Kirby, M.A.; Le, N.; Li, Y.; Zeinstra, N.; Lu, G.N.; Murry, C.E.; Zheng, Y.; Wang, R.K. Polarization sensitive optical coherence tomography with single input for imaging depth-resolved collagen organizations. Light Sci. Appl. 2021, 10, 237. [Google Scholar] [CrossRef]
- Brakenhoff, G.; Van der Voort, H.; Van Spronsen, E.; Linnemans, W.; Nanninga, N. Three-dimensional chromatin distribution in neuroblastoma nuclei shown by confocal scanning laser microscopy. Nature 1985, 317, 748–749. [Google Scholar] [CrossRef] [PubMed]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef]
- Centonze, V.E.; White, J.G. Multiphoton excitation provides optical sections from deeper within scattering specimens than confocal imaging. Biophys. J. 1998, 75, 2015–2024. [Google Scholar] [CrossRef] [PubMed]
- Czlonkowska, A.; Kurkowska-Jastrzebska, I.; Czlonkowski, A.; Peter, D.; Stefano, G.B. Immune processes in the pathogenesis of Parkinson’s disease-a potential role for microglia and nitric oxide. Med. Sci. Monit. 2002, 8, RA165–RA177. [Google Scholar] [PubMed]
- Gibbs-Strauss, S.L.; Vooght, C.; Fish, K.M.; Nasr, K.A.; Siclovan, T.M.; Barnhardt, N.E.; Hehir, C.A.T.; Frangioni, J.V. Molecular imaging agents specific for the annulus fibrosus of the intervertebral disk. Mol. Imaging 2010, 9, 7290. [Google Scholar] [CrossRef]
- Craig, S.E.; Wright, J.; Sloan, A.E.; Brady-Kalnay, S.M. Fluorescent-guided surgical resection of glioma with targeted molecular imaging agents: A literature review. World Neurosurg. 2016, 90, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Gu, R.; Zhu, Q.; Xiao, C.; Huang, L.; Zhuang, X.; Zhang, J.; Liu, L.; Ma, B.; Yang, H. Rapid fluorescence imaging of spinal cord following epidural administration of a nerve-highlighting fluorophore. Theranostics 2017, 7, 1863. [Google Scholar] [CrossRef]
- Stokes, C.; White, E.F.; Toddes, S.; Bens, N.; Kulkarni, P.; Ferris, C.F. Whole CNS 3D Cryo-Fluorescence Tomography Shows CSF Clearance along Nasal Lymphatics, Spinal Nerves, and Lumbar/Sacral Lymph Nodes. J. Imaging 2023, 9, 45. [Google Scholar] [CrossRef]
- Yun, S.H.; Kwok, S.J. Light in diagnosis; therapy. surgery. Nat. Biomed. Eng. 2017, 1, 8. [Google Scholar] [CrossRef] [PubMed]
- Gauvreau, B.; Guo, N.; Schicker, K.; Stoeffler, K.; Boismenu, F.; Ajji, A.; Wingfield, R.; Dubois, C.; Skorobogatiy, M. Color-changing and color-tunable photonic bandgap fiber textiles. Opt. Express 2008, 16, 15677–15693. [Google Scholar] [CrossRef]
- Koncar, V. Optical fiber fabric displays. Opt. Photonics News 2005, 16, 40–44. [Google Scholar] [CrossRef]
- Chapman, J.R.; Norvell, D.C.; Hermsmeyer, J.T.; Bransford, R.J.; DeVine, J.; McGirt, M.J.; Lee, M.J. Evaluating common outcomes for measuring treatment success for chronic low back pain. Spine 2011, 36, S54–S68. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.C.; Patrick, D.L.; Busse, J.W.; Schünemann, H.J.; Agarwal, A.; Guyatt, G.H. Patient-reported outcomes in meta-analyses–part 1: Assessing risk of bias and combining outcomes. Health Qual. Life Outcomes 2013, 11, 109. [Google Scholar] [CrossRef] [PubMed]
- Pryce, R.; Johnson, M.; Goytan, M.; Passmore, S.; Berrington, N.; Kriellaars, D. Relationship between ambulatory performance and self-rated disability in patients with lumbar spinal stenosis. Spine 2012, 37, 1316–1323. [Google Scholar] [CrossRef]
- Dutta, T.; Chaturvedi, P.; Llamas-Garro, I.; Velázquez-González, J.S.; Dubey, R.; Mishra, S.K. Smart materials for flexible electronics and devices: Hydrogel. RSC Adv. 2024, 14, 12984–13004. [Google Scholar] [CrossRef]
- Phan, K.; Mobbs, R.J. Long-term objective physical activity measurements using a wireless accelerometer following minimally invasive transforaminal interbody fusion surgery. Asian Spine J. 2016, 10, 366. [Google Scholar] [CrossRef] [PubMed]
- Triantafyllou, A.; Papagiannis, G.; Stasi, S.; Bakalidou, D.; Kyriakidou, M.; Papathanasiou, G.; Papadopoulos, E.C.; Papagelopoulos, P.J.; Koulouvaris, P. Application of wearable sensors technology for lumbar spine kinematic measurements during daily activities following microdiscectomy due to severe sciatica. Biology 2022, 11, 398. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Kusaka, T.; Tanaka, T.; Matsuo, Y.; Oda, M.; Sasaki, T.; Kamishima, T.; Yamanaka, M. Calibration method for lumbosacral dimensions in wearable sensor system of lumbar alignment. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 3909–3912. [Google Scholar]
- Uludağ, K.; Roebroeck, A. General overview on the merits of multimodal neuroimaging data fusion. Neuroimage 2014, 102, 3–10. [Google Scholar] [CrossRef]
- Stroman, P.W.; Wheeler-Kingshott, C.; Bacon, M.; Schwab, J.; Bosma, R.; Brooks, J.; Cadotte, D.; Carlstedt, T.; Ciccarelli, O.; Cohen-Adad, J. The current state-of-the-art of spinal cord imaging: Methods. Neuroimage 2014, 84, 1070–1081. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.R.; Aleksanderek, I.; Cohen-Adad, J.; Tarmohamed, Z.; Tetreault, L.; Smith, N.; Cadotte, D.W.; Crawley, A.; Ginsberg, H.; Mikulis, D.J. Translating state-of-the-art spinal cord MRI techniques to clinical use: A systematic review of clinical studies utilizing DTI, MT, MWF, MRS, and fMRI. NeuroImage Clin. 2016, 10, 192–238. [Google Scholar] [CrossRef] [PubMed]
- Koletar, M.M.; Dorr, A.; Brown, M.E.; McLaurin, J.; Stefanovic, B. Refinement of a chronic cranial window implant in the rat for longitudinal in vivo two–photon fluorescence microscopy of neurovascular function. Sci. Rep. 2019, 9, 5499. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bixel, M.G. Intravital multiphoton imaging of the bone and bone marrow environment. Cytom. Part A 2020, 97, 496–503. [Google Scholar] [CrossRef]
- Marker, D.F.; Tremblay, M.-E.; Lu, S.-M.; Majewska, A.K.; Gelbard, H.A. A thin-skull window technique for chronic two-photon in vivo imaging of murine microglia in models of neuroinflammation. JoVE (J. Vis. Exp.) 2010, 43, e2059. [Google Scholar]
- Xie, Y.; Chan, A.W.; McGirr, A.; Xue, S.; Xiao, D.; Zeng, H.; Murphy, T.H. Resolution of high-frequency mesoscale intracortical maps using the genetically encoded glutamate sensor iGluSnFR. J. Neurosci. 2016, 36, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.H.; Mohajerani, M.H.; LeDue, J.; Boyd, J.; Chen, S.; Murphy, T.H. In vivo large-scale cortical mapping using channelrhodopsin-2 stimulation in transgenic mice reveals asymmetric and reciprocal relationships between cortical areas. Front. Neural Circuits 2012, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, J.; Desjardins, M. Movement correction method for laser speckle contrast imaging of cerebral blood flow in cranial windows in rodents. J. Biophotonics 2022, 15, e202100218. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.M.; Liu, Y.; Mac, K.D.; Kim, M.; Safi, A.M.; Chung, E.J.O. Quantitative blood flow estimation in vivo by optical speckle image velocimetry. Optica 2021, 8, 1092–1101. [Google Scholar] [CrossRef]
- Qureshi, M.M.; Brake, J.; Jeon, H.-J.; Ruan, H.; Liu, Y.; Safi, A.M.; Eom, T.J.; Yang, C.; Chung, E.J.B.O.E. In vivo study of optical speckle decorrelation time across depths in the mouse brain. Biomed. Opt. Express 2017, 8, 4855–4864. [Google Scholar] [CrossRef]
- Heo, C.; Lee, S.Y.; Jo, A.; Jung, S.; Suh, M.; Lee, Y.H. Flexible; transparent, and noncytotoxic graphene electric field stimulator for effective cerebral blood volume enhancement. ACS Nano 2013, 7, 4869–4878. [Google Scholar] [CrossRef] [PubMed]
- Mohammadzadeh, L.; Latifi, H.; Khaksar, S.; Feiz, M.-S.; Motamedi, F.; Asadollahi, A.; Ezzatpour, M. Measuring the frequency-specific functional connectivity using wavelet coherence analysis in stroke rats based on intrinsic signals. Sci. Rep. 2020, 10, 9429. [Google Scholar] [CrossRef] [PubMed]
- Shabir, O.; Pendry, B.; Lee, L.; Eyre, B.; Sharp, P.S.; Rebollar, M.A.; Drew, D.; Howarth, C.; Heath, P.R.; Wharton, S.B. Assessment of neurovascular coupling and cortical spreading depression in mixed mouse models of atherosclerosis and Alzheimer’s disease. eLife 2022, 11, e68242. [Google Scholar] [CrossRef] [PubMed]
- Provansal, M.; Labernède, G.; Joffrois, C.; Rizkallah, A.; Goulet, R.; Valet, M.; Deschamps, W.; Ferrari, U.; Chaffiol, A.; Dalkara, D. Functional ultrasound imaging of the spreading activity following optogenetic stimulation of the rat visual cortex. Sci. Rep. 2021, 11, 12603. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, Y.; Chen, Y.; Chen, C.; Yin, L.; Yu, T.; He, W.; Ma, C. A skull-removed chronic cranial window for ultrasound and photoacoustic imaging of the rodent brain. Front. Neurosci. 2021, 15, 673740. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.; Wu, R.; Li, M.; Hu, Y.; Li, Y.; Li, J.; Rong, H.; Wu, H.; Xu, Y.; Lu, Y. Fast high-resolution miniature two-photon microscopy for brain imaging in freely behaving mice. Nat. Methods 2017, 14, 713–719. [Google Scholar] [CrossRef] [PubMed]
- Homma, R.; Baker, B.J.; Jin, L.; Garaschuk, O.; Konnerth, A.; Cohen, L.B.; Bleau, C.X.; Canepari, M.; Djurisic, M.; Zecevic, D. Wide-field and two-photon imaging of brain activity with voltage and calcium-sensitive dyes. In Dynamic Brain Imaging: Multi-Modal Methods In Vivo Applications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 43–79. [Google Scholar]
- Rubart, M. Two-photon microscopy of cells and tissue. Circ. Res. 2004, 95, 1154–1166. [Google Scholar] [CrossRef] [PubMed]
- White, B.R.; Bauer, A.Q.; Snyder, A.Z.; Schlaggar, B.L.; Lee, J.-M.; Culver, J.P. Imaging of functional connectivity in the mouse brain. PLoS ONE 2011, 6, e16322. [Google Scholar] [CrossRef] [PubMed]
- Vanni, M.P.; Chan, A.W.; Balbi, M.; Silasi, G.; Murphy, T.H. Mesoscale mapping of mouse cortex reveals frequency-dependent cycling between distinct macroscale functional modules. J. Neurosci. 2017, 37, 7513–7533. [Google Scholar] [CrossRef]
- Guevara, E.; Sadekova, N.; Girouard, H.; Lesage, F. Optical imaging of resting-state functional connectivity in a novel arterial stiffness model. Biomed. Opt. Express 2013, 4, 2332–2346. [Google Scholar] [CrossRef]
- Vincis, R.; Lagier, S.; Van De Ville, D.; Rodriguez, I.; Carleton, A. Sensory-evoked intrinsic imaging signals in the olfactory bulb are independent of neurovascular coupling. Cell Rep. 2015, 12, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Devor, A.; Sakadžić, S.; Srinivasan, V.J.; Yaseen, M.A.; Nizar, K.; Saisan, P.A.; Tian, P.; Dale, A.M.; Vinogradov, S.A.; Franceschini, M.A. Frontiers in optical imaging of cerebral blood flow and metabolism. J. Cereb. Blood Flow Metab. 2012, 32, 1259–1276. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.D.; Chen, G.; Cai, J.; Roe, A.W. Intrinsic signal optical imaging of visual brain activity: Tracking of fast cortical dynamics. NeuroImage 2017, 148, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Senarathna, J.; Rege, A.; Li, N.; Thakor, N.V. Laser speckle contrast imaging: Theory, instrumentation and applications. IEEE Rev. Biomed. Eng. 2013, 6, 99–110. [Google Scholar] [CrossRef]
- Briers, D.; Duncan, D.D.; Hirst, E.; Kirkpatrick, S.J.; Larsson, M.; Steenbergen, W.; Stromberg, T.; Thompson, O.B. Laser speckle contrast imaging: Theoretical and practical limitations. J. Biomed. Opt. 2013, 18, 066018. [Google Scholar] [CrossRef] [PubMed]
- Grinvald, A.; Omer, D.; Sharon, D.; Vanzetta, I.; Hildesheim, R. Voltage-Sensitive Dye Imaging of Neocortical Activity. Cold Spring Harb. Protoc. 2016, 2016, 13–29. [Google Scholar] [CrossRef] [PubMed]
- Takashima, I.; Kajiwara, R. Voltage-Sensitive Dye versus Intrinsic Signal Optical Imaging: Comparison of Tactile Responses in Primary and Secondary Somatosensory Cortices of Rats. Brain Sci. 2021, 11, 1294. [Google Scholar] [CrossRef] [PubMed]
- Fan, B.; Wei, Z.; Yao, X.; Shi, G.; Cheng, X.; Zhou, X.; Zhou, H.; Ning, G.; Kong, X.; Feng, S. Microenvironment imbalance of spinal cord injury. Cell Transplant. 2018, 27, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery 2017, 80, S9–S22. [Google Scholar] [CrossRef]
- Wu, Q.; Li, Y.; Ning, G.; Feng, S.-Q.; Chu, T.; Li, Y.; Hao, Y.; Wu, Q. Epidemiology of traumatic cervical spinal cord injury in Tianjin, China. Spinal Cord 2012, 50, 740–744. [Google Scholar] [CrossRef]
- Hawthorne, A.L.; Popovich, P.G. Emerging concepts in myeloid cell biology after spinal cord injury. Neurotherapeutics 2011, 8, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Gensel, J.C.; Zhang, B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015, 1619, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vafaei-Nezhad, S.; Hassan, M.P.; Noroozian, M.; Aliaghaei, A.; Tehrani, A.S.; Abbaszadeh, H.A.; Khoshsirat, S. A review of low-level laser therapy for spinal cord injury: Challenges and safety. J. Lasers Med. Sci. 2020, 11, 363. [Google Scholar] [CrossRef] [PubMed]
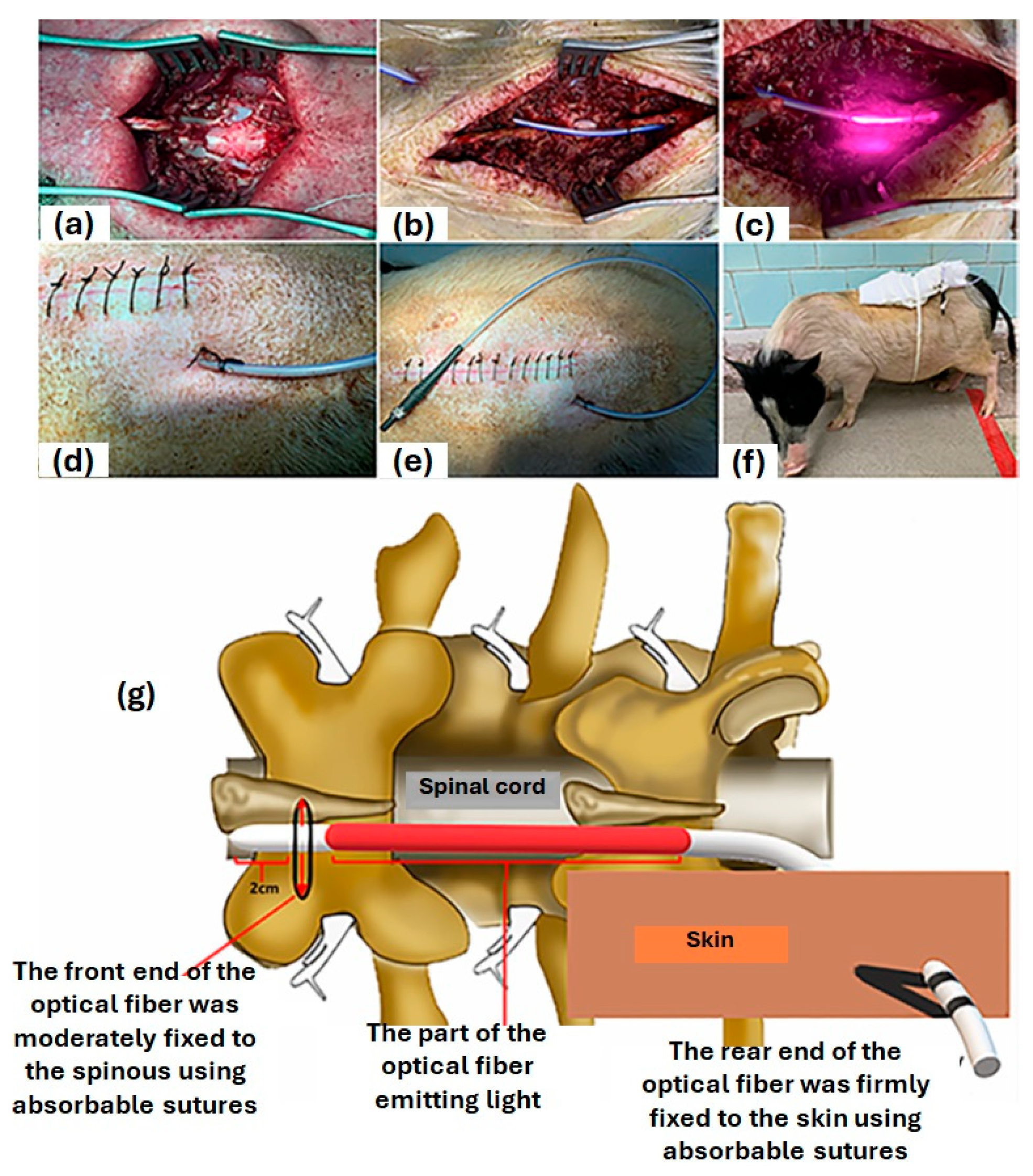
- Liang, Z.; Lei, T.; Wang, S.; Zuo, X.; Li, K.; Song, J.; Sun, J.; Zhang, J.; Zheng, Q.; Kang, X. Photobiomodulation by diffusing optical fiber on spinal cord: A feasibility study in piglet model. J. Biophotonics 2020, 13, e201960022. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Yam, S.S.; Loock, H.-P. Single-mode fiber refractive index sensor based on core-offset attenuators. IEEE Photonics Technol. Lett. 2008, 20, 1387–1389. [Google Scholar] [CrossRef]
- Tian, Z.; Yam, S.S.-H. In-line single-mode optical fiber interferometric refractive index sensors. J. Light. Technol. 2009, 27, 2296–2306. [Google Scholar] [CrossRef]
- Qian, Y.; Zhao, Y.; Wu, Q.-L.; Yang, Y. Review of salinity measurement technology based on optical fiber sensor. Sens. Actuators B Chem. 2018, 260, 86–105. [Google Scholar] [CrossRef]
- Fukano, H.; Watanabe, D.; Taue, S. Sensitivity characteristics of multimode-interference optical-fiber temperature-sensor with solid cladding material. IEEE Sens. J. 2016, 16, 8921–8927. [Google Scholar] [CrossRef]
- Li, L.; Xia, L.; Xie, Z.; Hao, L.; Shuai, B.; Liu, D. In-line fiber Mach–Zehnder interferometer for simultaneous measurement of refractive index and temperature based on thinned fiber. Sens. Actuators A Phys. 2012, 180, 19–24. [Google Scholar] [CrossRef]
- Qian, W.; Zhao, C.-L.; He, S.; Dong, X.; Zhang, S.; Zhang, Z.; Jin, S.; Guo, J.; Wei, H. High-sensitivity temperature sensor based on an alcohol-filled photonic crystal fiber loop mirror. Opt. Lett. 2011, 36, 1548–1550. [Google Scholar] [CrossRef]
- Sun, G.; Moon, D.S.; Chung, Y. Simultaneous temperature and strain measurement using two types of high-birefringence fibers in Sagnac loop mirror. IEEE Photonics Technol. Lett. 2007, 19, 2027–2029. [Google Scholar] [CrossRef]
- Fernandes, L.A.; Becker, M.; Frazao, O.; Schuster, K.; Kobelke, J.; Rothhardt, M.; Bartelt, H.; Santos, J.L.; Marques, P.V. Temperature and strain sensing with femtosecond laser written Bragg gratings in defect and nondefect suspended-silica-core fibers. IEEE Photonics Technol. Lett. 2012, 24, 554–556. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, M.-Q.; Lv, R.-Q.; Xia, F. In-fiber rectangular air fabry-perot strain sensor based on high-precision fiber cutting platform. Opt. Commun. 2017, 384, 107–110. [Google Scholar] [CrossRef]
- Ren, K.; Ren, L.; Liang, J.; Kong, X.; Ju, H.; Wu, Z. Highly strain and bending sensitive microtapered long-period fiber gratings. IEEE Photonics Technol. Lett. 2017, 29, 1085–1088. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, W. Bent fiber interferometer. J. Light. Technol. 2015, 33, 3351–3356. [Google Scholar] [CrossRef]
- Bas, J.; Dutta, T.; Garro, I.L.; Velázquez-González, J.S.; Dubey, R.; Mishra, S.K. Embedded Sensors with 3D Printing Technology: Review. Sensors 2024, 24, 1955. [Google Scholar] [CrossRef] [PubMed]
- Campanella, C.E.; Cuccovillo, A.; Campanella, C.; Yurt, A.; Passaro, V.M. Fibre Bragg grating based strain sensors: Review of technology and applications. Sensors 2018, 18, 3115. [Google Scholar] [CrossRef] [PubMed]
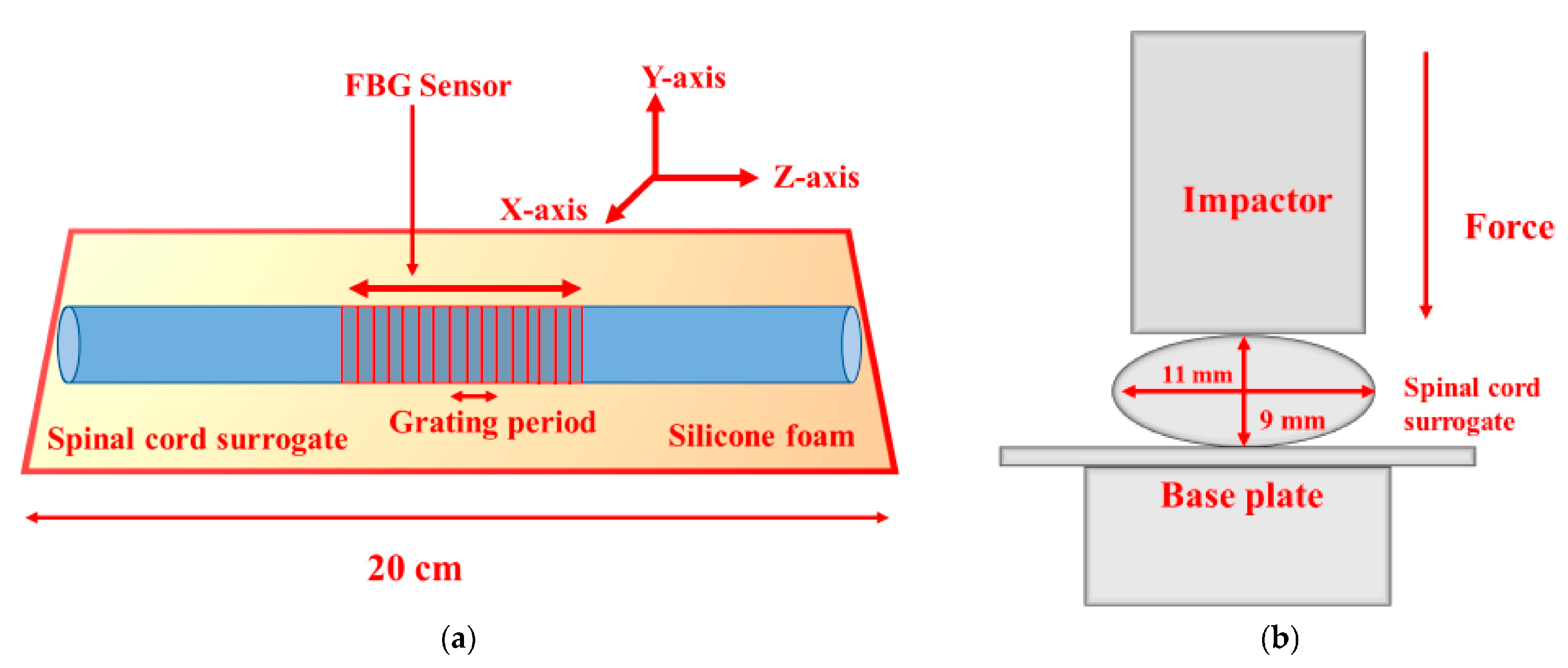
- Mishra, S.K.; Mac-Thiong, J.-M.; Wagnac, É.; Petit, Y.; Ung, B. A sensitive and fast fiber Bragg grating-based investigation of the biomechanical dynamics of in vitro spinal cord injuries. Sensors 2021, 21, 1671. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, E.M.; Lamanna, J.; Boulis, N.M. Therapy, Stem cell therapy for the spinal cord. Stem Cell Res. Ther. 2012, 3, 24. [Google Scholar] [CrossRef]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise review: Multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef]
- Lamanna, J.J.; Miller, J.H.; Riley, J.P.; Hurtig, C.V.; Boulis, N.M. Cellular therapeutics delivery to the spinal cord: Technical considerations for clinical application. Ther. Deliv. 2013, 4, 1397–1410. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Boulis, N.M.; Hur, J.; Johe, K.; Rutkove, S.B.; Federici, T.; Polak, M.; Bordeau, J.; Sakowski, S.A.; Glass, J.D. Intraspinal neural stem cell transplantation in amyotrophic lateral sclerosis: Phase 1 trial outcomes. Ann. Neurol. 2014, 75, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.; Glass, J.; Feldman, E.L.; Polak, M.; Bordeau, J.; Federici, T.; Johe, K.; Boulis, N.M. Intraspinal stem cell transplantation in amyotrophic lateral sclerosis: A phase I trial, cervical microinjection, and final surgical safety outcomes. Neurosurgery 2014, 74, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Bartus, R.T.; Johnson, E.M., Jr. Clinical tests of neurotrophic factors for human neurodegenerative diseases, part 2: Where do we stand and where must we go next? Neurobiol. Dis. 2017, 97, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Lee, A.; Wu, J.C. Long term non-invasive imaging of embryonic stem cells using reporter genes. Nat. Protoc. 2009, 4, 1192–1201. [Google Scholar] [CrossRef] [PubMed]
- Boddington, S.E.; Henning, T.D.; Jha, P.; Schlieve, C.R.; Mandrussow, L.; DeNardo, D.; Bernstein, H.S.; Ritner, C.; Golovko, D.; Lu, Y. Labeling human embryonic stem cell-derived cardiomyocytes with indocyanine green for noninvasive tracking with optical imaging: An FDA-compatible alternative to firefly luciferase. Cell Transplant. 2010, 19, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Lamanna, J.J.; Gutierrez, J.; Urquia, L.N.; Hurtig, C.V.; Amador, E.; Grin, N.; Svendsen, C.N.; Federici, T.; Oshinski, J.N.; Boulis, N.M. Ferumoxytol labeling of human neural progenitor cells for diagnostic cellular tracking in the porcine spinal cord with magnetic resonance imaging. Stem Cells Transl. Med. 2017, 6, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Emelianov, S.Y.; Li, P.-C.; O’Donnell, M. Photoacoustics for molecular imaging and therapy. Phys. Today 2009, 62, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Mallidi, S.; Luke, G.P.; Emelianov, S. Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance. Trends Biotechnol. 2011, 29, 213–221. [Google Scholar] [CrossRef]
- Luke, G.P.; Yeager, D.; Emelianov, S.Y. Biomedical applications of photoacoustic imaging with exogenous contrast agents. Ann. Biomed. Eng. 2012, 40, 422–437. [Google Scholar] [CrossRef]
- Ricles, L.M.; Nam, S.Y.; Sokolov, K.; Emelianov, S.Y.; Suggs, L.J. Function of mesenchymal stem cells following loading of gold nanotracers. Int. J. Nanomed. 2011, 6, 407–416. [Google Scholar] [CrossRef]
- Nam, S.Y.; Ricles, L.M.; Suggs, L.J.; Emelianov, S.Y. In vivo ultrasound and photoacoustic monitoring of mesenchymal stem cells labeled with gold nanotracers. PLoS ONE 2012, 7, e37267. [Google Scholar] [CrossRef] [PubMed]
- Chung, E.; Nam, S.Y.; Ricles, L.M.; Emelianov, S.Y.; Suggs, L.J. Evaluation of gold nanotracers to track adipose-derived stem cells in a PEGylated fibrin gel for dermal tissue engineering applications. Int. J. Nanomed. 2013, 8, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Betzer, O.; Meir, R.; Dreifuss, T.; Shamalov, K.; Motiei, M.; Shwartz, A.; Baranes, K.; Cohen, C.J.; Shraga-Heled, N.; Ofir, R. In-vitro optimization of nanoparticle-cell labeling protocols for in-vivo cell tracking applications. Sci. Rep. 2015, 5, 15400. [Google Scholar] [CrossRef] [PubMed]
- Wan, D.; Chen, D.; Li, K.; Qu, Y.; Sun, K.; Tao, K.; Dai, K.; Ai, S. Gold nanoparticles as a potential cellular probe for tracking of stem cells in bone regeneration using dual-energy computed tomography. ACS Appl. Mater. Interfaces 2016, 8, 32241–32249. [Google Scholar] [CrossRef] [PubMed]
- Nold, P.; Hartmann, R.; Feliu, N.; Kantner, K.; Gamal, M.; Pelaz, B.; Hühn, J.; Sun, X.; Jungebluth, P.; Del Pino, P. Optimizing conditions for labeling of mesenchymal stromal cells (MSCs) with gold nanoparticles: A prerequisite for in vivo tracking of MSCs. J. Nanobiotechnol. 2017, 15, 24. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, E.M.; Kubelick, K.P.; Dumani, D.S.; Emelianov, S.Y. Photoacoustic image-guided delivery of plasmonic-nanoparticle-labeled mesenchymal stem cells to the spinal cord. Nano Lett. 2018, 18, 6625–6632. [Google Scholar] [CrossRef]
- Deng, W.-W.; Wu, G.-Y.; Min, L.-X.; Feng, Z.; Chen, H.; Tan, M.-L.; Sui, J.-F.; Liu, H.-L.; Hou, J.-M. Optogenetic neuronal stimulation promotes functional recovery after spinal cord injury. Front. Neurosci. 2021, 15, 640255. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; He, S.; Wu, J.; Chen, C.; Li, X.; Liu, K.; Qu, J.Y. Long-term in vivo imaging of mouse spinal cord through an optically cleared intervertebral window. Nat. Commun. 2022, 13, 1959. [Google Scholar] [CrossRef]
- Fang, X.; Huang, H.; Zhang, H.; Yang, Z.; Lyu, Z.; Yang, H.; Li, N.; Zhao, T.; Yu, X.; Zhang, L. High resolution terahertz ATR frequency-domain spectroscopy for monitoring spinal cord injury in rats. Biomed. Opt. Express 2024, 15, 479–490. [Google Scholar] [CrossRef]
- Hernandez, M.; Ramon-Julvez, U.; Vilades, E.; Cordon, B.; Mayordomo, E.; Garcia-Martin, E. Explainable artificial intelligence toward usable and trustworthy computer-aided diagnosis of multiple sclerosis from Optical Coherence Tomography. PLoS ONE 2023, 18, e0289495. [Google Scholar] [CrossRef] [PubMed]
- Tavares, C.; Domingues, M.F.; Paixão, T.; Alberto, N.; Silva, H.; Antunes, P. Wheelchair pressure ulcer prevention using FBG based sensing devices. Sensors 2019, 20, 212. [Google Scholar] [CrossRef] [PubMed]



| S.No. | Methods | Critical Parameters | References |
|---|---|---|---|
| 1 | Optical coherence tomography (OCT) | Spatial resolution of about 10 to 15 μm and penetration depth of about 3 mm. | [33] |
| 2 | Fluorescence imaging | Capability to highlight deep tissue. | [48,49,50] |
| 3 | Wearable optical technology | Flexible, easy to wear, precision. | [54] |
| 4 | Neuroimaging with optical techniques | Sub-micrometer spatial resolution and 500 μm~1 mm depth of view (2PE), spatial and temporal resolutions of around 100 μm and 2 s, respectively (OISI), time resolution of 10 msec to 10 sec and a spatial resolution of 10 μm (LSCI). | [72,73,74,76,77,78,81,82] |
| 5 | SCI treatment with optical fiber-based devices | Flexibility, biocompatibility, minimal loss of light energy, and ability to measure the stress on the spinal cord post-injury. | [93,94] |
| 6 | Photoacoustic imaging through plasmonic nanoparticles | Radiationless targeted imaging. | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, S.; Kalyani, N.; Dutta, T.; Velázquez-González, J.S.; Llamas-Garro, I.; Ung, B.; Bas, J.; Dubey, R.; Mishra, S.K. Optical Devices for the Diagnosis and Management of Spinal Cord Injuries: A Review. Biosensors 2024, 14, 296. https://doi.org/10.3390/bios14060296
Sharma S, Kalyani N, Dutta T, Velázquez-González JS, Llamas-Garro I, Ung B, Bas J, Dubey R, Mishra SK. Optical Devices for the Diagnosis and Management of Spinal Cord Injuries: A Review. Biosensors. 2024; 14(6):296. https://doi.org/10.3390/bios14060296
Chicago/Turabian StyleSharma, Sonika, Neeti Kalyani, Taposhree Dutta, Jesús Salvador Velázquez-González, Ignacio Llamas-Garro, Bora Ung, Joan Bas, Rakesh Dubey, and Satyendra K. Mishra. 2024. "Optical Devices for the Diagnosis and Management of Spinal Cord Injuries: A Review" Biosensors 14, no. 6: 296. https://doi.org/10.3390/bios14060296
APA StyleSharma, S., Kalyani, N., Dutta, T., Velázquez-González, J. S., Llamas-Garro, I., Ung, B., Bas, J., Dubey, R., & Mishra, S. K. (2024). Optical Devices for the Diagnosis and Management of Spinal Cord Injuries: A Review. Biosensors, 14(6), 296. https://doi.org/10.3390/bios14060296







