Abstract
Metal-organic frameworks (MOFs) are often used as carriers in the preparation of electrochemiluminescent (ECL) materials, and ECL materials stabilized in the aqueous phase can be prepared by encapsulating chromophores inside MOFs by an in situ growth method. In this study, nanocomposites MIL-88B(Fe)-NH2@Ru(py)32+ with excellent ECL response were prepared by encapsulating Tris(2,2′-bipyridine)ruthenium dichloride (Ru(py)32+) inside MIL-88B(Fe)-NH2 using the one-step hydrothermal method. MIL-88B(Fe)-NH2 possesses abundant amino groups, which can accelerate the catalytic activation process of K2S2O8, and its abundant pores are also conducive to the enhancement of the transmission rate of co-reactant agents, ions, and electrons, which effectively improves the ECL efficiency. In order to obtain more excellent ECL signals, we prepared aminated biochar (NH2-biochar) using Pu-erh tea dregs as precursor and loaded gold nanoparticles (Au NPs) on its surface as substrate material for modified electrodes. Both NH2-biochar and Au NPs can also be used as a co-reactant promoter to catalyze the activation process of co-reactant K2S2O8. Therefore, a sandwich-type ECL immunosensor was prepared based on a dual signal-enhanced strategy for the highly sensitive and selective detection of aflatoxin B1 (AFB1). Under the optimal experimental conditions, the sensitive detection of AFB1 was achieved in the range of 1 pg·mL−1~100 ng·mL−1 with a detection limit of 209 fg·mL−1. The proposed dual signal-enhanced ECL immunosensor can provide a simple, convenient, and efficient method for the sensitive detection of AFB1 in food and agricultural products.
1. Introduction
Food safety is always a major livelihood issue and a priority concern in every country. Generally, food safety involves all segments of food production, processing, packaging, transportation, and storage [1,2]. Aflatoxin B1 (AFB1), as one of the most popular and toxic contaminants, may appear in every segment, especially for grain crops such as corn, peanuts, oils crops, or their processed products [3,4,5]. Thus, maximum admissible levels for AFB1 in food and feed have been set by many countries [6,7]. A variety of sensors have been applied to the sensitive detection of pollutants and biomarkers [8,9], in which an electrochemiluminescence (ECL) immunosensor method attract more and more attention in the fields of contaminant and biomarker detection, as there are many exclusive merits for the ECL immunosensor, such as simplified operation, high sensitivity, and rapid optical response [10,11,12]. Therefore, to meet the great requirements of trace, rapid and high throughout detection of AFB1, the ECL immunosensing method would be a competitive analytical assay.
In recent years, more and more research teams have developed various assays with good performance for fabricating ECL immunosensors to detect AFB1 [13,14,15,16,17]. According the published works, the detection process could be roughly divided into three categories: signal enhancement [18], signal quenching [19], and signal masking [20]. Among them, the signal enhancement type attracted more attention due to the fact that it can provide a greater signal and, thus, higher sensitivity [21]. To achieve this, not only functional materials with excellent performance but also assembly methods still have a long way to go. Up to now, much work has been done and good results have been obtained [22]. For example, the Ru(bpy)32+ (bpy = 2,2′-bipyridine)-based ECL system offers high luminescence efficiency and chemical stability. However, the distance of electronic transmission between the Ru(bpy)32+ and its co-reactant will directly affect the ECL signal. Recently, Li et al. reported the ECL switching of Ru(bpy)32+ by tuning its distance from Ferrocene (Fc) allows quenching and enhancement of the ECL signal, which proves that the distance of electronic transmission between Ru(bpy)32+ and the catalysts or the quenchers will affect the enhancement of the ECL signal [15]. Another interfering factor for the ECL signal is the stable immobilization of Ru(bpy)32+. A recent work published by Zhang et al. reported the ECL properties of Ru(bpy)32+ encapsulated multifunctional metal organic frameworks (Ru-MOFs) [23]. In this study, Ru(bpy)32+ was encapsulated in internal pores by in situ-grown MOFs, allowing Ru(bpy)32+ to produce a stable ECL response in an aqueous solution. Therefore, both preparing substrate materials with superior catalytic property for catalyzing co-reactant and larger specific surface area for immobilizing antibodies and preparing labelling materials with excellent ECL property are highly demanded for the development of ECL immunosensors.
To get great ECL signals, we focused our research on metal organic framework (MOF) materials since it might provide a large specific surface area for stably loading large amounts of luminophores and good catalytic actives for catalyzing the co-reactant in order to greatly improve the ECL signal [24]. MOFs have been gradually introduced in the fabrication of ECL (bio)sensors in recent years, although they are already popular in the research fields of catalysts and energy. Their merits for enhancing ECL signals are being discovered and attracting more and more focus. As a recent good example, Yuan and Zhuo et al. prepared an isoreticular metal organic framework-3 (IRMOF-3) with 2-amino terephthalic acid (NH2-BDC) for fabricating a signal enhancing ECL immunosensor [25]. In their work, IRMOF-3 was used for loading a large amount of CdTe via the encapsulating effect and internal/external decoration. Furthermore, it was used as a novel co-reactant accelerator, which could promote the conversion of co-reactant S2O82– to SO4•− (i.e., sulfate radical anion), thus boosting the ECL emission of CdTe quantum dots (QDs).
Furthermore, based on our previous investigation and summarization [26], we found that porous activated biochar with the merits of good chemical stability and large surface area was appropriately chosen as the support material [27]. Essentially, the biochar obtained from renewable biomass through pyrolysis or carbonization processes under a low-concentration oxygen atmosphere is porous, eco-friendly, and low-cost [28,29]. Especially, after the biochar is chemically activated, the activated biochar (AB) shows superior properties of a highly functionalized surface, promoting high adsorptivity and interaction capacity [30]. Inspired by this, we proposed using AB obtained from the biomass of Pu-erh tea produced in the Yunnan Province in China as the platform for the development of ECL immunosensor.
From the above analysis, a signal enhancement type of ECL immunosensor for the detection of AFB1 was developed in this work. As shown in Scheme 1, firstly, ammonia activated biochar particles (NH2-Biochar) were prepared from Pu-erh tea biomass by low temperature pyrolysis and successively grounded via a ball-milling process. To improve its conductivity and loading capacity, Au nanoparticles (Au NPs) were in-situ grown on it to obtain the compound material NH2-Biochar (NH2-Biochar-Au). Following that, the AFB1 primary antibodies (Ab1) was loaded on the NH2-Biochar-Au via NHS-EDC connection (NH2-Biochar-Au-Ab1). Secondly, due to the ammonia iron-based metal-organic framework (MIL-88B(Fe)-NH2), nanoparticles can be easily synthesized with controlled size and peroxidase-like catalytic activity [31], and MIL-88B(Fe)-NH2 used as the co-reactant promoter and carrier to load Ru(bpy)32+ were prepared and named MIL-88B(Fe)-NH2@Ru(bpy)32+. Following that, the MIL-88B(Fe)-NH2@Ru(bpy)32+ was used as the signal probe to label AFB1 secondary antibodies (Ab2). Lastly, the sandwich type of ECL immunosensor was fabricated and investigated.
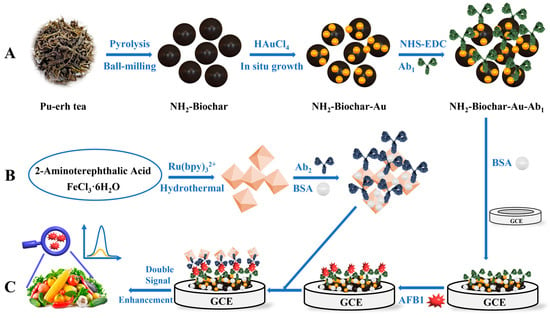
Scheme 1.
Fabrication process of the ECL immunosensor: (A) the preparation of NH2-Biochar-Au-Ab1, (B) the preparation of MIL-88B(Fe)-NH2@Ru(bpy)32+-Ab2-BSA, and (C) the detection of AFB1.
2. Results and Discussion
2.1. Characterization of MIL-88B(Fe)-NH2@Ru(bpy)32+ and NH2-Biochar-Au
Figure 1A shows the SEM characterization image of MIL-88B(Fe)-NH2@Ru(bpy)32+, which is a classical octahedral structure with a particle size of about 400 nm. The structure is not significantly changed compared with that of MIL-88B(Fe)-NH2, indicating that the encapsulated Ru(bpy)32+ in MIL-88B(Fe)-NH2 was successfully prepared [32]. To investigate the functional group species of MIL-88B(Fe)-NH2@Ru(bpy)32+, we characterized them using the FT-IR spectroscopy method, and the results are shown in Figure 1C. The two characteristic peaks near 3300–3500 cm−1 are generated by the primary amine N-H stretching vibration, and the characteristic peak at 1154 cm−1 is generated by the C-N stretching vibration, indicating that MIL-88B(Fe)-NH2 possesses an amino group. The characteristic peaks of C-O at 1382 cm−1 and C=O at 1652 cm−1 indicate that MIL-88B(Fe)-NH2 also possesses a carboxyl group, suggesting that MIL-88B(Fe)-NH2 can be attached to the antibody through an amide bond [33]. As shown in Figure 1D–F, the XPS profiles of MIL-88B(Fe)-NH2@Ru(bpy)32+, the C 1s profile and the Fe 2p profile are shown, respectively. As shown in Figure 1D, the presence of C, N, Ru, O, and Fe, and the characteristic peak of Ru 3p near 484 eV all indicate the successful preparation of MIL-88B(Fe)-NH2@Ru(bpy)32+ [19]. The characteristic peaks at 709.7 and 723.5 eV are two orbital peaks of Fe 2p, indicating that the elemental iron in MIL-88B(Fe)-NH2@Ru(bpy)32+ is in the form of Fe3+. The characteristic peaks at 284.8, 287.3 and 288.9 eV are those of C-C, C-O and O-C=O, respectively, indicating the presence of carboxyl groups in MIL-88B(Fe)-NH2@Ru(bpy)32+. The result is consistent with the information in the FT-IR spectrum [34,35]. NH2-Biochar-Au in this work was prepared using a general in situ growth process. As shown in Figure S1 and Figure 1B, Au NPs with uniform size are well composited onto the NH2-Biochar.
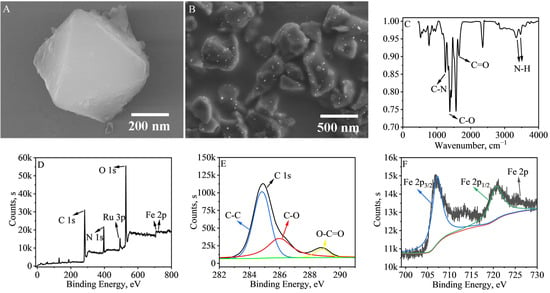
Figure 1.
SEM images of (A) MIL-88B(Fe)-NH2@Ru(bpy)32+, and (B) NH2-Biochar-Au. (C) FT-IR spectrum of MIL-88B(Fe)-NH2@Ru(bpy)32+. (D) XPS spectra of MIL-88B(Fe)-NH2@Ru(bpy)32+, (E) C 1s and (F) Fe 2p.
2.2. Mechanism of the ECL Immunosensor
Curves a, b, c, and d in Figure 2A show the ECL response curves of GCE, MIL-88B(Fe)-NH2@Ru(bpy)32+, NH2-Biochar/MIL-88B(Fe)-NH2@Ru(bpy)32+, and NH2-Biochar-Au/MIL-88B(Fe)-NH2@Ru(bpy)32+ in PBS containing 90 mmol·L−1 K2S2O8. In the figure we can find almost no ECL response in GCE (curve a). When only MIL-88B(Fe)-NH2@Ru(bpy)32+ was coated on the surface of GCE, there was a smaller ECL response (curve b). When NH2-Biochar/MIL-88B(Fe)-NH2@Ru(bpy)32+ was coated on the surface of GCE, the ECL response (curve c) doubled in size compared to when only MIL-88B(Fe)-NH2@Ru(bpy)32+ was coated. The ECL response obtained when coated with NH2-Biochar-Au/MIL-88B(Fe)-NH2@Ru(bpy)32+ was again enhanced. The synergistic catalytic effect of NH2-Biochar and Au NPs resulted in the final obtained ECL response (curve d) being about 2.6 times higher than that obtained when coated with MIL-88B(Fe)-NH2@Ru(bpy)32+. Curves e, f in Figure 2A show the ECL response curves of MIL-88B(Fe)-NH2 and NH2-Biochar-Au in PBS, respectively. We can see that MIL-88B(Fe)-NH2 and NH2-Biochar-Au have almost no ECL response in PBS. Therefore, it can be inferred that the signal enhancement effect is due to the catalytic activation of K2S2O8 by MIL-88B(Fe)-NH2 and NH2-Biochar-Au.
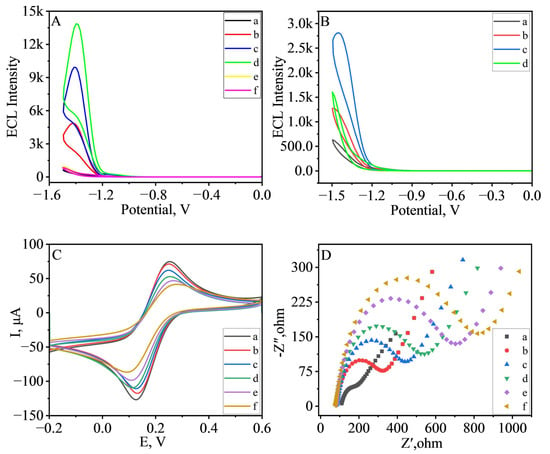
Figure 2.
(A) ECL responses of (a) GCE, (b) GCE/MIL-88B(Fe)-NH2@Ru(bpy)32+, (c) GCE/NH2-Biochar/MIL-88B(Fe)-NH2@Ru(bpy)32+, and (d) GCE/NH2-Biochar-Au/MIL-88B(Fe)-NH2@Ru(bpy)32+ (in PBS containing 90 mmol·L−1 K2S2O8). ECL responses of (e) GCE/MIL-88B(Fe)-NH2, (f) GCE/NH2-Biochar-Au (in PBS). (B) ECL responses of (a) GCE, (b) GCE/NH2-Biochar, (c) GCE/NH2-Biochar-Au, and (d) GCE/MIL-88B(Fe)-NH2 (in PBS containing 90 mmol·L−1 K2S2O8). (C) Cyclic Voltammetry Results (in 5 mM Fe(CN)63− and 0.1 M KNO3) and (D) Electrochemical Impedance Spectroscopy Results (in 2.5 mM Fe(CN)63−/4− and 0.1 M KCl) of (a) bare GCE, (b) GCE/NH2-Biochar-Au, (c) GCE/NH2-Biochar-Au/Ab1, (d) GCE/NH2-Biochar-Au/Ab1/BSA, (e) GCE/NH2-Biochar-Au/Ab1/BSA/AFB1, and (f) GCE/NH2-Biochar-Au/Ab1/BSA/AFB1/MIL-88B(Fe)-NH2@Ru(bpy)32+-Ab2-BSA. The concentration of AFB1 used for the test was 50 ng·mL−1.
To investigate the catalytic ability of NH2-Biochar, NH2-Biochar-Au, and MIL-88B(Fe)-NH2 on the co-reactant K2S2O8, we obtained their ECL responses by simultaneous CV scanning. The results are shown in Figure 2B, where the bare electrode has only a low ECL response (Figure 2B, curve a), and when NH2-Biochar is coated on the electrode surface, the ECL response is enhanced by a factor of two compared to the GCE (Figure 2B, curve b). The ECL response was about 4.5 times higher than that of GCE when NH2-Biochar@Au was coated on the electrode surface (Figure 2B, curve c). Not only that, when MIL-88B(Fe)-NH2 was coated on the electrode surface, the ECL response was about 2.7 times higher than that of GCE (Figure 2B, curve d). The above results indicate that MIL-88B(Fe)-NH2 can promote the reduction of S2O82− in the NH2-Biochar-Au/MIL-88B(Fe)-NH2@Ru(bpy)32+ system. Likewise, NH2-Biochar-Au showed the same effect. In conclusion, NH2-Biochar-Au and MIL-88B(Fe)-NH2 can be used as co-reactant promoters to further enhance the ECL reaction of Ru(bpy)32+ when S2O82− is used as co-reactant. The ECL reaction mechanism of MIL-88B(Fe)-NH2@Ru(bpy)32+ can be summarized as follows [36,37,38]:
Ru(bpy)32+ + e− → Ru(bpy)3+
S2O82− + e− → SO42− + SO4•−
Ru(bpy)3+ + SO4•− → Ru(bpy)32+* + SO42−
Ru(bpy)32+* → Ru(bpy)32+ + hv
2.3. Characterization of Layer Modification Process of ECL Immunosensor
To verify the successful construction of the ECL immunosensor, the techniques of Cyclic Voltammetry (CV) and Electrochemical Impedance Spectroscopy (EIS) were used to characterize the layer modification process in the construction of the immunosensor, and the results are shown in Figure 2C,D. It can be observed from Figure 2C that the bare GCE has the highest peak current. As NH2-Biochar-Au-Ab1, BSA, AFB1, and MIL-88B(Fe)-NH2@Ru(bpy)32+-Ab2-BSA, which contain macromolecular proteins, are modified to the electrode surface, the peak current decreases continuously. Similarly in Figure 2D, as the electrode is modified layer by layer, its electron transfer resistance increases. This is because AFB1, Ab1, Ab2, and BSA are all large molecules with poor electrical conductivity, and the results indicate that the immunosensor was successfully constructed.
2.4. Selection of Measurement Condition
Experimental condition is an important factor affecting the excellent performance of ECL immunosensors in use, and the best performance can be obtained by testing under optimal experiment conditions. A controlled variable approach was used to select optimal parameters for the immunosensor during the detection.
The value of pH is an important factor affecting the signal of the immunosensor, and we first selected the pH of the substrate and tested the performance of the immunosensor in PBS containing 90 mmol·L−1 K2S2O8 at different pH. The results are shown in Figure 3A. The ECL signal of the immunosensor increased as the pH value increased, and the best ECL signal was obtained when pH = 8.0, and the signal gradually decreased when the pH value continually increased, so we chose pH = 8.0 of PBS for the subsequent tests.
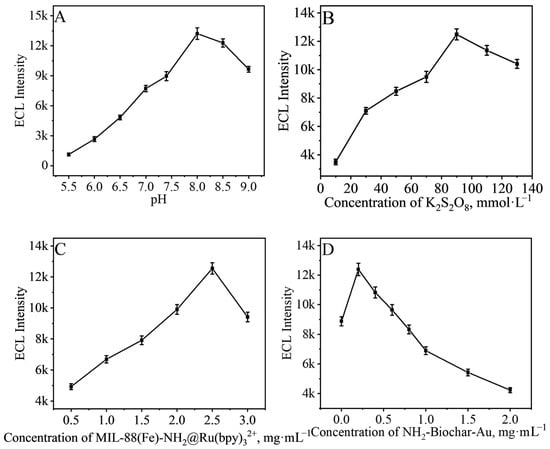
Figure 3.
Effects of (A) pH, (B) concentration of K2S2O8, (C) concentration of MIL-88B(Fe)-NH2@Ru(bpy)32+, and (D) concentration of NH2-Biochar-Au on the ECL response.
In this study, K2S2O8 was used as a co-reactant, and an excess of K2S2O8 would inhibit its own activation process. Therefore, the performance of the immunosensor in PBS containing different concentrations of K2S2O8 at pH = 8.0 was tested. The results are shown in Figure 3B, where the immunosensor obtained the best ECL signal at the K2S2O8 concentration of 90 mmol·L−1.
Moreover, the concentrations of MIL-88B(Fe)-NH2@Ru(bpy)32+ and NH2-Biochar-Au for the construction of the immunosensor were also selected. As shown in Figure 3C, when the concentration of MIL-88B(Fe)-NH2@Ru(bpy)32+ was at 2.5 mg·mL−1, the best ECL signal was obtained by the immunosensor. However, the signal decreased with continued increase of the concentration of MIL-88B(Fe)-NH2@Ru(bpy)32+. This might be because of the masking of self-luminescence caused by excess MIL-88B(Fe)-NH2@Ru(bpy)32+. The concentration of NH2-Biochar-Au reached the best ECL signal at 0.2 mg·mL−1, and, thus, 0.2 mg·mL−1 of NH2-Biochar-Au was selected for the construction of the immunosensor.
2.5. Performance of the ECL Immunosensor
To verify the performance of the designed immunosensor, the analytical performance of the immunosensor was firstly investigated under the above optimal experimental conditions. The results are shown in Figure 4A. The obtained ECL signal was positively correlated with the logarithm of AFB1 concentration and was able to achieve sensitive detection of AFB1 in the range of 1 pg·mL−1~100 ng·mL−1. The regression equation of the obtained fitted curve was I = 9241 + 1696 log c, where I is the ECL intensity, c is the concentration of AFB1, and R2 = 0.9967. We also prepared a batch of signal probes without AFB1, and the detection limit of the immunosensor was calculated to be 209 fg·mL−1 after testing.
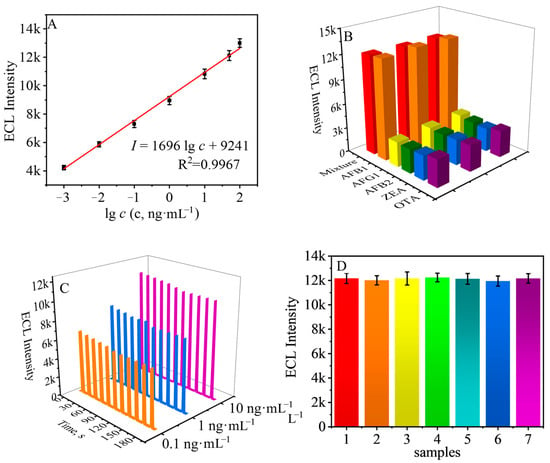
Figure 4.
(A) Working curves of the designed ECL immunosensor in the linear range (0.001, 0.01, 0.1, 1, 10, 50, and 100 ng·mL−1). (B) Selectivity of the designed ECL immunosensor in the detection of AFB1 (50 ng·mL−1 for AFB1 and other interferents). (C) Stability of the designed ECL immunosensor in detecting different concentrations of AFB1. (D) Reproducibility of the designed ECL immunosensor. Error bars = SD (n = 3).
2.6. Other Parameters of the ECL Immunosensor
As shown in Figure 4B, when the detection objects were Ochratoxin A (OTA), Zearalenone (ZEA), Aflatoxin B2 (AFB2), and Aflatoxin G1 (AFG1), the ECL signals were all much smaller than that of detecting AFB1 and the mixture, indicating that the prepared ECL immunosensor has good selectivity and can accurately detect AFB1 in complicate samples.
The stability of the designed immunosensors was evaluated by testing different concentrations of AFB1. Specifically, three batches were constructed using AFB1 at concentrations of 0.1, 1, and 10 ng·mL−1. The results are shown in Figure 4C, and the constructed immunosensors showed stable ECL signals. Additionally, we also prepared seven batches of immunosensors using 50 ng·mL−1 of AFB1 under the same experimental conditions, and as the results show in Figure 4D, the prepared seven batches of immunosensors showed good reproducibility with an RSD value of only 2.71%.
In order to verify the performance of our constructed ECL immunosensor in detecting real samples, we conducted experiments using the standard addition method [39]. Firstly, the corn grains were mixed with deionized water and broken with a wall breaker, and the filtrate was obtained by filtration using a 0.22 μm syringe filter membrane, and the filtrate and deionized water were prepared into a dilute solution at a ratio of 1:100. Six sets of mixed solutions were obtained by adding 1 mL of 0.1, 0.5, 1, 2, 5, and 10 ng/mL AFB1 to 1 mL dilute solution and mixing thoroughly. Seven ECL sensors were fabricated and tested under the same experimental conditions using each group of mixed solutions, and the obtained ECL signals were combined with the working curve to calculate the concentrations. The results are shown in Table S1. The relative standard deviation (RSD) was less than 3.8% and the recoveries were between 96.0 and 106%, indicating that our prepared ECL immunosensors can be used for the detection of real samples. As shown in Table S2, we compared the constructed ECL immunosensor with other previously reported methods for the detection of AFB1, and the results showed that the ECL immunoassay prepared in this study has excellent performance in detecting AFB1.
3. Materials and Methods
3.1. Preparation of NH2-Biochar
The NH2-Biochar materials were obtained by a two-step process using the Pu-erh tea produced in Yunnan Province in China as the carbon precursor. Typically, Pu-erh tea was first washed with deionized water to remove unwanted impurities. After being dried at 60 °C, the precursors were put into a tubular furnace and carbonized at 800 °C under an N2 gas for 2 h (heating rate 5 °C·min−1). Following that, the black products were cooled down to room temperature and ground up in a Guge AD-G858 blender just as the previous work [40]. The black products were sieved to biochar powders with the diameter between 100 and 250 μm range. The prepared biochar was then polished into particles of uniform particle size using a ball milling technique [41,42,43]. Specifically, the biochar and agate balls (φ = 6 mm) were mixed well in a 500 mL agate container with a mass ratio of 3:200 and then placed in the agate container, and polished on a semi-circular planetary ball mill at 300 rpm for 5 h after adding an appropriate amount of ammonia. After the mixture was washed with DI water followed by isopropanol until neutrality, the NH2-Biochar was obtained through a process of successive oven drying.
3.2. Preparation of MIL-88B(Fe)-NH2@Ru(bpy)32+
MIL-88B(Fe)-NH2@Ru(bpy)32+ was prepared according to a previous study with slight modifications. NH2-BDC (0.25 g) and FeCl3·6H2O (0.38 g) were first added to 30 mL DMF and sonicated to dissolve them well. Then, Ru(bpy)32+ (50 mg) was added to the mixed solution with stirring for five minutes and transferred to a 50 mL autoclave containing Teflon liner and was heated at 120 °C for 24 h. The product was collected by centrifugation and washed with DMF and ethanol alternately, and was finally dried at 60 °C overnight to obtain MIL-88B(Fe)-NH2@Ru(bpy)32+.
3.3. Preparation of NH2-Biochar-Au, NH2-Biochar-Au-Ab1, and the Label of MIL-88B(Fe)-NH2@Ru(bpy)32+-Ab2-BSA
The preparation processes are as shown in Scheme 1A,B. The specific experimental steps are provided in the Supporting Information.
3.4. Preparation Process of ECL Immunosensor
The preparation process of the ECL immunosensor for the detection of AFB1 is as shown in Scheme 1C. Specifically, at first, 6 μL of NH2-Biochar-Au-Ab1 was dropped onto the GCE and stored at 4 °C until slightly wetted. Then, 6 μL of 1% BSA solution was dropped on the surface of the NH2-Biochar-Au-Ab1 modified electrode and incubated at 4 °C for 2 h to block the non-specific active site on the surface of the modified electrode. The excess BSA on the electrode surface was washed off by rinsing the electrode in PBS (pH = 7.4). To enable the AFB1 to immune attach on the surface of the above electrode, 6 μL different concentrations of AFB1 were dropped onto the uppermost layer of the electrode and it was incubated for 2 h at 4 °C. Following that, the unfixed AFB1 was washed away with PBS (pH = 7.4). Finally, 10 μL 2.5 mg·mL−1 MIL-88B(Fe)-NH2@Ru(bpy)32+-Ab2-BSA was dropped onto the topmost layer of the above-modified electrode to specifically bind to the AFB1 and the electrode was washed by means of PBS to completely eliminate the unattached bioconjugate.
4. Conclusions
In summary, we successfully prepared MIL-88B(Fe)-NH2@Ru(bpy)32+ as ECL signal probe using a one-step hydrothermal method. The amino-rich MIL-88B(Fe)-NH2 cannot only be used as carrier to load Ru(bpy)32+, but also as a co-reactor promoter to catalyze the activation process of the K2S2O8. MIL-88B(Fe)-NH2 possesses abundant pores that can enrich the SO4•− produced by the catalytic activation of K2S2O8, which shortens the material transfer distance and energy transfer distance between Ru(bpy)32+ and SO4•−, and effectively improves the cathodoluminescence efficiency of MIL-88B(Fe)-NH2@Ru(bpy)32+. In addition, we used Pu-erh tea dregs as precursor to obtain biochar by calcination in a high-temperature and oxygen-free environment. The obtained biochar was ball-milled and aminated to prepare NH2-Biochar, and Au NPs were grown in situ on its surface to prepare NH2-Biochar-Au used as substrate material. The Au NPs could not only improve the conductivity of NH2-Biochar, but also provide active sites for antibody immobilization. Not only that, both NH2-Biochar and Au NPs can be used as co-reactant promoters to accelerate the catalytic activation process of K2S2O8. Through the co-catalysis between MIL-88B(Fe)-NH2 and NH2-Biochar-Au, our ECL immunosensor prepared based on dual signal-enhanced strategy was capable of efficient and sensitive detection of AFB1 in the range of 1 pg·mL−1~100 ng·mL−1, with a detection limit of 209 fg·mL−1. It provides a simple and efficient method for the rapid and sensitive detection of AFB1 in food and agricultural products. Compared with other methods for the detection of AFB1, our ECL immunosensor constructed based on the dual signal amplification strategy has a lower detection limit, but the construction process is more cumbersome requiring multiple washes. Therefore, we hope to develop more convenient wash-free sensors for the sensitive detection of AFB1.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/bios13090846/s1, Materials and reagents; Apparatus; Measurement Procedure; Preparation Procedure; Table S1: Detection results of AFB1-spiked corn samples; Table S2: Comparison of our work and other methods; Figure S1: Characterization of Au NPs. References [44,45,46,47,48,49,50] are cited in the supplementary materials.
Author Contributions
L.T.: Investigation, Conceptualization, Methodology, Writing—original draft. Y.S. (Yuying Shi): Investigation, Software, Editing. Y.S. (Yanan Song): Software, Methodology. H.G.: Funding acquisition. Y.L.: Conceptualization, Data curation. R.X.: Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
The authors appreciate the support from the National Natural Science Foundation of China (22264025), the Yunnan Province Education Department Scientific Research Foundation Project (2022J0136), the Applied Basic Research Foundation of Yunnan Province (202201AS070020, 202201AU070061), Yunnan Provincial Observation and Research Station of Soil Degradation and Restoration for Cultivating Plateau Traditional Chinese Medicinal Plants (202105AM07003), and the Major Science and Technology Special Project of Yunnan Province (202202AE090025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yu, L.; Chang, J.; Zhuang, X.; Li, H.; Hou, T.; Li, F. Two-Dimensional Cobalt-Doped Ti3c2 Mxene Nanozyme-Mediated Homogeneous Electrochemical Strategy for Pesticides Assay Based on in Situ Generation of Electroactive Substances. Anal. Chem. 2022, 94, 3669–3676. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Du, J.; Luo, J.; Chen, S.; Yuan, R. Coreactant-Free Electrochemiluminescence Biosensor for the Determination of Organophosphorus Pesticides. Biosens. Bioelectron. 2020, 150, 111898. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Sun, Y.; Duan, J.; Wang, M.; Ai, S.; Hou, J. A Novel Immunocolorimetric Probe for Aflatoxin B1 Based on Multifunctional Metal−Organic Frameworks. Sens. Actuators B Chem. 2022, 369, 132362. [Google Scholar] [CrossRef]
- Li, J.; Wang, Q.; Xiong, C.; Deng, Q.; Zhang, X.; Wang, S.; Chen, M.-M. An Ultrasensitive Ch3nh3pbbr3 Quantum Dots@Sio2-Based Electrochemiluminescence Sensing Platform Using an Organic Electrolyte for Aflatoxin B1 Detection in Corn Oil. Food Chem. 2022, 390, 133200. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Yang, L.; Yao, L.; Xu, J.; Yao, B.; Liu, G.; Cheng, L.; Chen, W. Ingenious Electrochemiluminescence Bioaptasensor Based on Synergistic Effects and Enzyme-Driven Programmable 3d DNA Nanoflowers for Ultrasensitive Detection of Aflatoxin B1. Anal. Chem. 2020, 92, 14122–14129. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.; Zhao, Q. A Signal-on Electrochemical Aptasensor for Rapid Detection of Aflatoxin B1 Based on Competition with Complementary DNA. Biosens. Bioelectron. 2019, 144, 111641. [Google Scholar] [CrossRef]
- Yue, Q.; Li, X.; Fang, J.; Li, M.; Zhang, J.; Zhao, G.; Cao, W.; Wei, Q. Oxygen Free Radical Scavenger Ptpd@Pda as a Dual-Mode Quencher of Electrochemiluminescence Immunosensor for the Detection of Afb1. Anal. Chem. 2022, 94, 11476–11482. [Google Scholar] [CrossRef]
- Pirsa, S.; Alizadeh, N. Nanoporous Conducting Polypyrrole Gas Sensor Coupled to a Gas Chromatograph for Determination of Aromatic Hydrocarbons Using Dispersive Liquid–Liquid Microextraction Method. IEEE Sens. J. 2011, 11, 3400–3405. [Google Scholar] [CrossRef]
- Zhang, H.; Li, B.; Wang, R.; Miao, Q.; Cui, X.; Shang, L.; Ma, R.; Jia, L.; Li, C.; Li, F.; et al. Perylene Derivative and Persulfate as Highly Efficient Electrochemical System for Constructing Sensitive Amperometric Aptasensor. Talanta 2023, 259, 124489. [Google Scholar] [CrossRef]
- Zhuo, Y.; Wang, H.-J.; Lei, Y.-M.; Zhang, P.; Liu, J.-L.; Chai, Y.-Q.; Yuan, R. Electrochemiluminescence Biosensing Based on Different Modes of Switching Signals. Analyst 2018, 143, 3230–3248. [Google Scholar] [CrossRef]
- Wei, X.; Zhu, M.; Yan, H.; Lu, C.; Xu, J. Recent Advances in Aggregation-Induced Electrochemiluminescence. Chem. A Eur. J. 2019, 25, 12671–12683. [Google Scholar] [CrossRef]
- Lu, H.-J.; Xu, J.-J.; Zhou, H.; Chen, H.-Y. Recent Advances in Electrochemiluminescence Resonance Energy Transfer for Bioanalysis: Fundamentals and Applications. Trac-Trends Anal. Chem. 2020, 122, 115746. [Google Scholar] [CrossRef]
- Xia, M.; Yang, X.; Jiao, T.; Oyama, M.; Chen, Q.; Chen, X. Self-Enhanced Electrochemiluminescence of Luminol Induced by Palladium-Graphene Oxide for Ultrasensitive Detection of Aflatoxin B-1 in Food Samples. Food Chem. 2022, 381, 132276. [Google Scholar] [CrossRef]
- Lv, X.; Tan, F.; Miao, T.; Zhang, J.; Zhang, Z.; Cui, B.; Fang, Y. Potential-Resolved Differential Electrochemiluminescence Immunosensor Based on Novel Designed Ibphf for Self-Correctable Detection of Afb(1). Microchem. J. 2022, 181, 107845. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Meng, S.; Zhang, J.; Li, L.; You, T. Regulation of Ru(Bpy)32+ Electrochemiluminescence Based on Distance-Dependent Electron Transfer of Ferrocene for Dual-Signal Readout Detection of Aflatoxin B1 with High Sensitivity. Anal. Chem. 2022, 94, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Song, Y.; Wang, Y.; Gong, Y.; Shang, L.; Ma, R.; Jia, L.; Xue, Q.; Du, Y.; He, S.; et al. Perylene Dianhydride and Perylene Diimide Luminophores Integrated with Gold Nanoparticles for Dual-Potential Electrochemiluminescence Ratiometric Immunosensors. ACS Appl. Nano Mater. 2021, 4, 683–690. [Google Scholar] [CrossRef]
- Chen, Y.; Lin, J.; Zhang, R.; He, S.; Ding, Z.; Ding, L. Electrochemiluminescence of Water-Dispersed Nitrogen and Sulfur Doped Carbon Dots Synthesized from Amino Acids. Analyst 2021, 146, 5287–5293. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Wang, X.; Xiang, G.; Lac, K.; Wang, C.; Wang, S.; Ding, Z. Enhanced Electrochemiluminescence of a Macrocyclic Tetradentate Chelate Pt(Ii) Molecule through Its Collisional Interactions with the Electrode. Chem. Asian J. 2022, 17, e202200727. [Google Scholar] [CrossRef]
- Dong, X.; Zhao, G.; Li, Y.; Zeng, Q.; Ma, H.; Wu, D.; Ren, X.; Wei, Q.; Ju, H. Dual-Mechanism Quenching of Electrochemiluminescence Immunosensor Based on a Novel Ecl Emitter Polyoxomolybdate-Zirconia for 17β-Estradiol Detection. Anal. Chem. 2022, 94, 12742–12749. [Google Scholar] [CrossRef]
- Cheng, R.; Ding, Y.; Wang, Y.; Wang, H.; Zhang, Y.; Wei, Q. A Novel Molecularly Imprinted Electrochemiluminescence Sensor Based on Cobalt Nitride Nanoarray Electrode for the Sensitive Detection of Bisphenol S. RSC Adv. 2021, 11, 11011–11019. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, W.; He, S.; Shang, L.; Ma, R.; Jia, L.; Wang, H. Perylene Diimide and Luminol as Potential-Resolved Electrochemiluminescence Nanoprobes for Dual Targets Immunoassay at Low Potential. ACS Appl. Mater. Interfaces 2019, 11, 33676–33683. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Song, Y.; Wang, Y.; He, S.; Shang, L.; Ma, R.; Jia, L.; Wang, H. A Perylenetetracarboxylic Dianhydride and Aniline-Assembled Supramolecular Nanomaterial with Multi-Color Electrochemiluminescence for a Highly Sensitive Label-Free Immunoassay. J. Mater. Chem. B 2020, 8, 3676–3682. [Google Scholar] [CrossRef] [PubMed]
- Xiong, C.; Huang, J.; Liu, H.; Chen, M.-M.; Wen, W.; Zhang, X.; Wang, S. Ruthenium(Ii) Complex Encapsulated Multifunctional Metal Organic Frameworks Based Electrochemiluminescence Sensor for Sensitive Detection of Hydrogen Sulfide. Talanta 2022, 249, 123602. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Li, Y.; Zhang, Y.; Zhao, G.; Wang, Y.; Wang, H.; Wang, H.; Xu, R.; Wei, Q. Signal-Enhanced Electrochemiluminescence Strategy Using Iron-Based Metal-Organic Frameworks Modified with Carboxylated Ru(Ii) Complexes for Neuron-Specific Enolase Detection. Biosens. Bioelectron. 2022, 215, 114605. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.Q.; Peng, L.Z.; Lei, Y.M.; Chai, Y.Q.; Yuan, R.; Zhuo, Y. Strong Electrochemiluminescence from Mof Accelerator Enriched Quantum Dots for Enhanced Sensing of Trace Ctni. Anal. Chem. 2018, 90, 3995–4002. [Google Scholar] [CrossRef]
- Li, Y.; Xu, R.; Wang, H.; Xu, W.; Tian, L.; Huang, J.; Liang, C.; Zhang, Y. Recent Advances of Biochar-Based Electrochemical Sensors and Biosensors. Biosensors 2022, 12, 508. [Google Scholar] [CrossRef]
- Zou, J.; Yu, Q.; Gao, Y.; Chen, S.; Huang, X.; Hu, D.; Liu, S.; Lu, L.-M. Bismuth Nanoclusters/Porous Carbon Composite: A Facile Ratiometric Electrochemical Sensing Platform for Pb2+ Detection with High Sensitivity and Selectivity. Acs Omega 2021, 7, 1132–1138. [Google Scholar] [CrossRef]
- Zou, J.; Qian, W.; Li, Y.; Yu, Q.; Yu, Y.; Chen, S.; Qu, F.; Gao, Y.; Lu, L. Multilayer Activated Biochar/Uio-66-Nh2 Film as Intelligent Sensing Platform for Ultra-Sensitive Electrochemical Detection of Pb2+ and Hg2+. Appl. Surf. Sci. 2021, 569, 151006. [Google Scholar] [CrossRef]
- Chu, K.; Adsetts, J.R.; He, S.; Zhan, Z.; Yang, L.; Wong, J.M.; Love, D.A.; Ding, Z. Cover Feature: Electrogenerated Chemiluminescence and Electroluminescence of N-Doped Graphene Quantum Dots Fabricated from an Electrochemical Exfoliation Process in Nitrogen-Containing Electrolytes (Chem. Eur. J. 68/2020). Chem. A Eur. J. 2020, 26, 15756. [Google Scholar] [CrossRef]
- Lv, X.; Tan, F.; Miao, T.; Cui, B.; Zhang, J.; Fang, Y.; Shen, Y. In Situ Generated Ptnps to Enhance Electrochemiluminescence of Multifunctional Nanoreactor Cop T4vtp6 for Afb1 Detection. Food Chem. 2023, 399, 134002. [Google Scholar] [CrossRef]
- Fu, X.; Yang, Y.; Wang, N.; Chen, S. The Electrochemiluminescence Resonance Energy Transfer between Fe-Mil-88 Metal-Organic Framework and 3,4,9,10-Perylenetetracar-Boxylic Acid for Dopamine Sensing. Sens. Actuators B-Chem. 2017, 250, 584–590. [Google Scholar] [CrossRef]
- Duan, S.; Huang, Y. Electrochemical sensor using NH2-MIL-88(Fe)-rGO composite for trace Cd2+, Pb2+, and Cu2+ detection. J. Electroanal. Chem. 2017, 807, 253–260. [Google Scholar] [CrossRef]
- Kim, S.; Park, C.G.; Huh, B.K.; Lee, S.H.; Min, C.H.; Lee, Y.Y.; Kim, Y.K.; Park, K.H.; Choy, Y.B. Metal-organic frameworks, NH2-MIL-88(Fe), as carriers for ophthalmic delivery of brimonidine. Acta Biomater. 2018, 79, 344–353. [Google Scholar] [CrossRef]
- Zango, Z.U.; Jumbri, K.; Sambudi, N.S.; Hanif Abu Bakar, N.H.; Fathihah Abdullah, N.A.; Basheer, C.; Saad, B. Removal of anthracene in water by MIL-88(Fe), NH2-MIL-88(Fe), and mixed-MIL-88(Fe) metal-organic frameworks. RSC Adv. 2019, 9, 41490–41501. [Google Scholar] [CrossRef] [PubMed]
- Xie, D.; Ma, Y.; Gu, Y.; Zhou, H.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. Bifunctional NH2-MIL-88(Fe) metal–organic framework nanooctahedra for highly sensitive detection and efficient removal of arsenate in aqueous media. J. Mater. Chem. A 2017, 5, 23794–23804. [Google Scholar] [CrossRef]
- Han, S.; Gao, Y.; Li, L.; Lu, B.; Zou, Y.; Zhang, L.; Zhang, J. Synergistic Enhancement Effects of Carbon Quantum Dots and Au Nanoclusters for Cathodic ECL and Non-enzyme Detections of Glucose. Electroanalysis 2020, 32, 1155–1159. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, Q.; Hong, Z.; Lin, Y.; Dai, H. A bifunctional catalyst based ECL immunosensor for a cardiac biomarker regulated by oxygen evolution reaction. Electrochim. Acta 2016, 215, 326–333. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, H.; Chai, Y.; Shi, W.; Yuan, R. High-Efficiency CNNS@NH2-MIL(Fe) Electrochemiluminescence Emitters Coupled with Ti3C2 Nanosheets as a Matrix for a Highly Sensitive Cardiac Troponin I Assay. Anal. Chem. 2020, 92, 8992–9000. [Google Scholar] [CrossRef]
- Zhang, W.; Song, Y.; He, S.; Shang, L.; Ma, R.; Jia, L.; Wang, H. Perylene Diimide as a Cathodic Electrochemiluminescence Luminophore for Immunoassays at Low Potentials. Nanoscale 2019, 11, 20910–20916. [Google Scholar] [CrossRef]
- Lee, J.T.E.; Lim, E.Y.; Zhang, L.; Tsui, T.H.; Tian, H.; Yan, M.; Lim, S.; Abdul Majid, M.B.; Jong, M.C.; Zhang, J.; et al. Methanosarcina Thermophila Bioaugmentation and Its Synergy with Biochar Growth Support Particles Versus Polypropylene Microplastics in Thermophilic Food Waste Anaerobic Digestion. Bioresour. Technol. 2022, 360, 127531. [Google Scholar] [CrossRef]
- Yang, G.X.; Jiang, H. Amino Modification of Biochar for Enhanced Adsorption of Copper Ions from Synthetic Wastewater. Water Res. 2014, 48, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yue, X.; Xu, W.; Zhang, H.; Li, F. Amino Modification of Rice Straw-Derived Biochar for Enhancing Its Cadmium (Ii) Ions Adsorption from Water. J. Hazard. Mater. 2019, 379, 120783. [Google Scholar] [CrossRef]
- Qi, G.; Pan, Z.; Zhang, X.; Miao, X.; Xiang, W.; Gao, B. Effect of Ball Milling with Hydrogen Peroxide or Ammonia Hydroxide on Sorption Performance of Volatile Organic Compounds by Biochar from Different Pyrolysis Temperatures. Chem. Eng. J. 2022, 450, 138027. [Google Scholar] [CrossRef]
- Ou, G.; Zhao, A.; Liao, H.; Zhang, Z.; Xiao, F. Au nanopartics decorated urchin-like Bi2S3 on graphene wrapped carbon fiber microelectrode: Towards electrochemical immunosensor for sensitive determination of aflatoxin B1. J. Electroanal. Chem. 2023, 929, 117124. [Google Scholar] [CrossRef]
- Chen, W.; Zhu, M.; Liu, Q.; Guo, Y.; Wang, H.; Wang, K. Fabricating photoelectrochemical aptasensor for sensitive detection of aflatoxin B1 with visible-light-driven BiOBr/nitrogen-doped graphene nanoribbons. J. Electroanal. Chem. 2019, 840, 67–73. [Google Scholar] [CrossRef]
- Wu, J.; Xie, Z.; Li, M.; Lin, Y.; Tan, X.; Huang, K. Molecularly imprinted photoelectrochemical sensing supported by Bi2S3/Bi2O2CO3 direct Z-scheme heterojunction for aflatoxin B1 detection. Sens. Actuators B Chem. 2023, 378, 133143. [Google Scholar] [CrossRef]
- Tian, D.; Wang, J.; Zhuang, Q.; Wu, S.; Yu, Y.; Ding, K. An electrochemiluminescence biosensor based on Graphitic carbon nitride luminescence quenching for detection of AFB1. Food Chem. 2023, 404, 134183. [Google Scholar] [CrossRef]
- Zhang, J.; Xia, Y.-K.; Chen, M.; Wu, D.-Z.; Cai, S.-X.; Liu, M.-M.; He, W.-H.; Chen, J.-H. A fluorescent aptasensor based on DNA-scaffolded silver nanoclusters coupling with Zn(II)-ion signal-enhancement for simultaneous detection of OTA and AFB1. Sens. Actuators B Chem. 2016, 235, 79–85. [Google Scholar] [CrossRef]
- Jia, Y.; Zhou, G.; Wang, X.; Zhang, Y.; Li, Z.; Liu, P.; Yu, B.; Zhang, J. A metal-organic framework/aptamer system as a fluorescent biosensor for determination of aflatoxin B1 in food samples. Talanta 2020, 219, 121342. [Google Scholar] [CrossRef]
- Lerdsri, J.; Chananchana, W.; Upan, J.; Sridara, T.; Jakmunee, J. Label-free colorimetric aptasensor for rapid detection of aflatoxin B1 by utilizing cationic perylene probe and localized surface plasmon resonance of gold nanoparticles. Sens. Actuators B Chem. 2020, 320, 128356. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).