Flux-Type versus Concentration-Type Sensors in Transdermal Measurements
Abstract
1. Introduction
2. Methods
Sensors Types and Response Time
3. Results
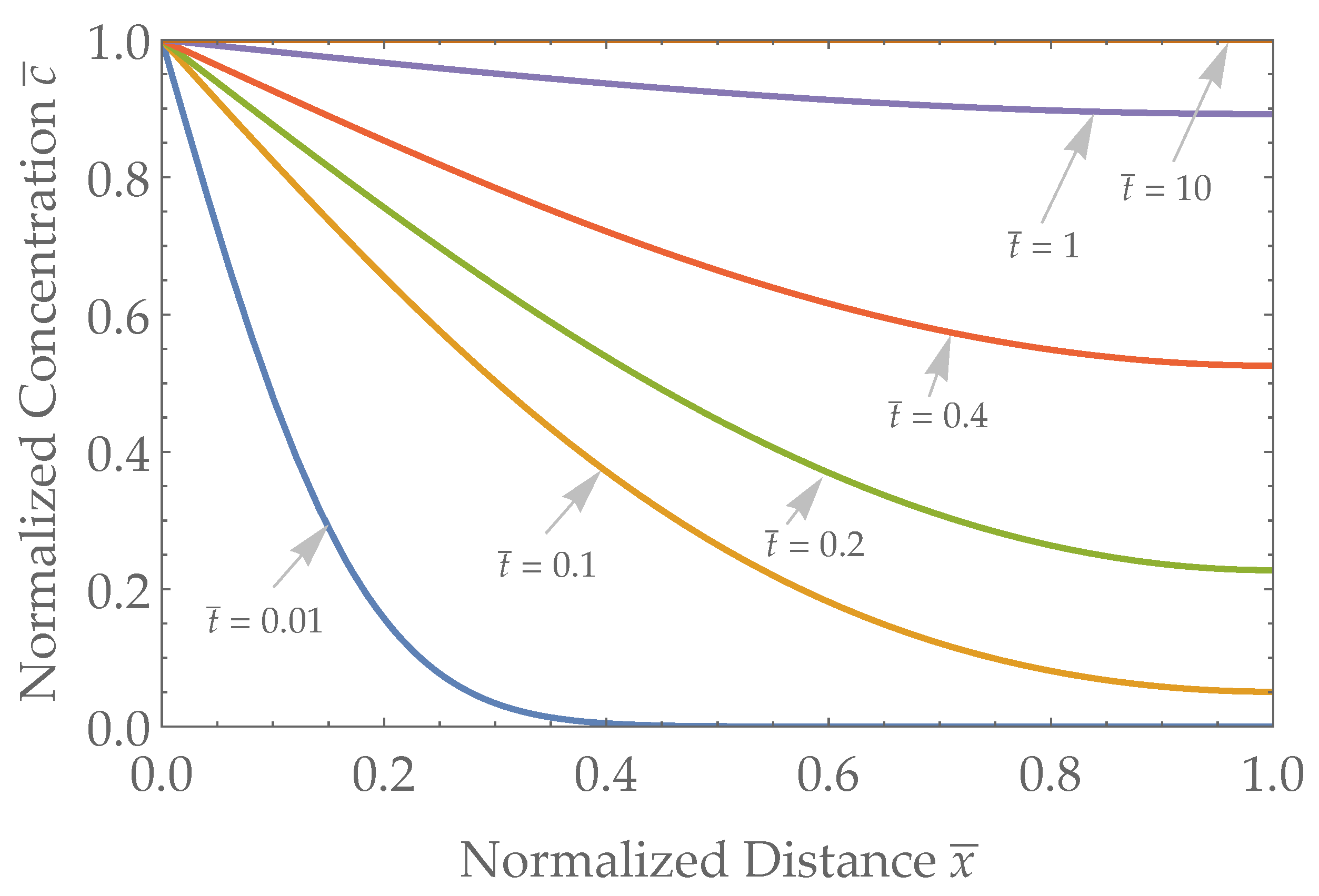
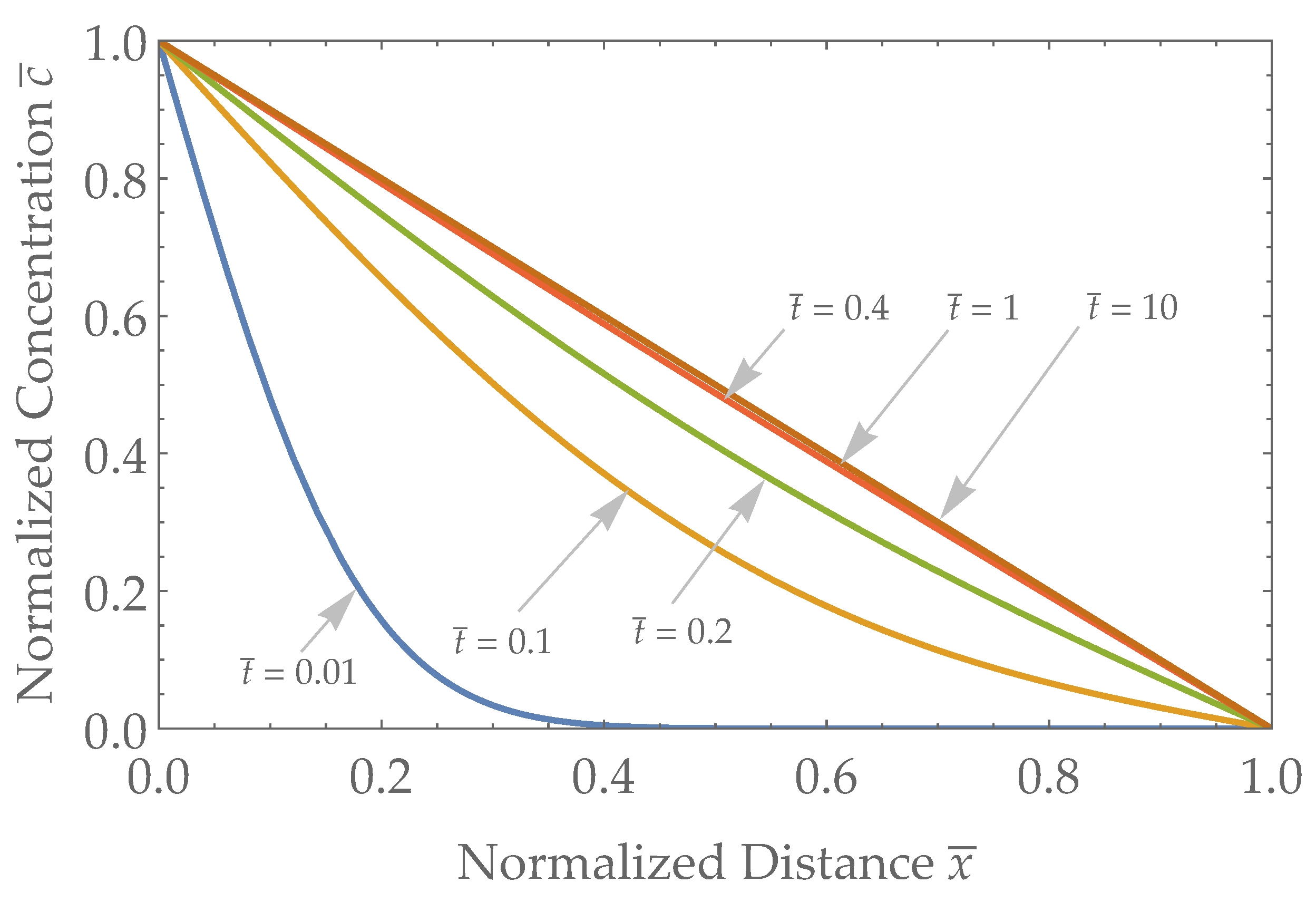
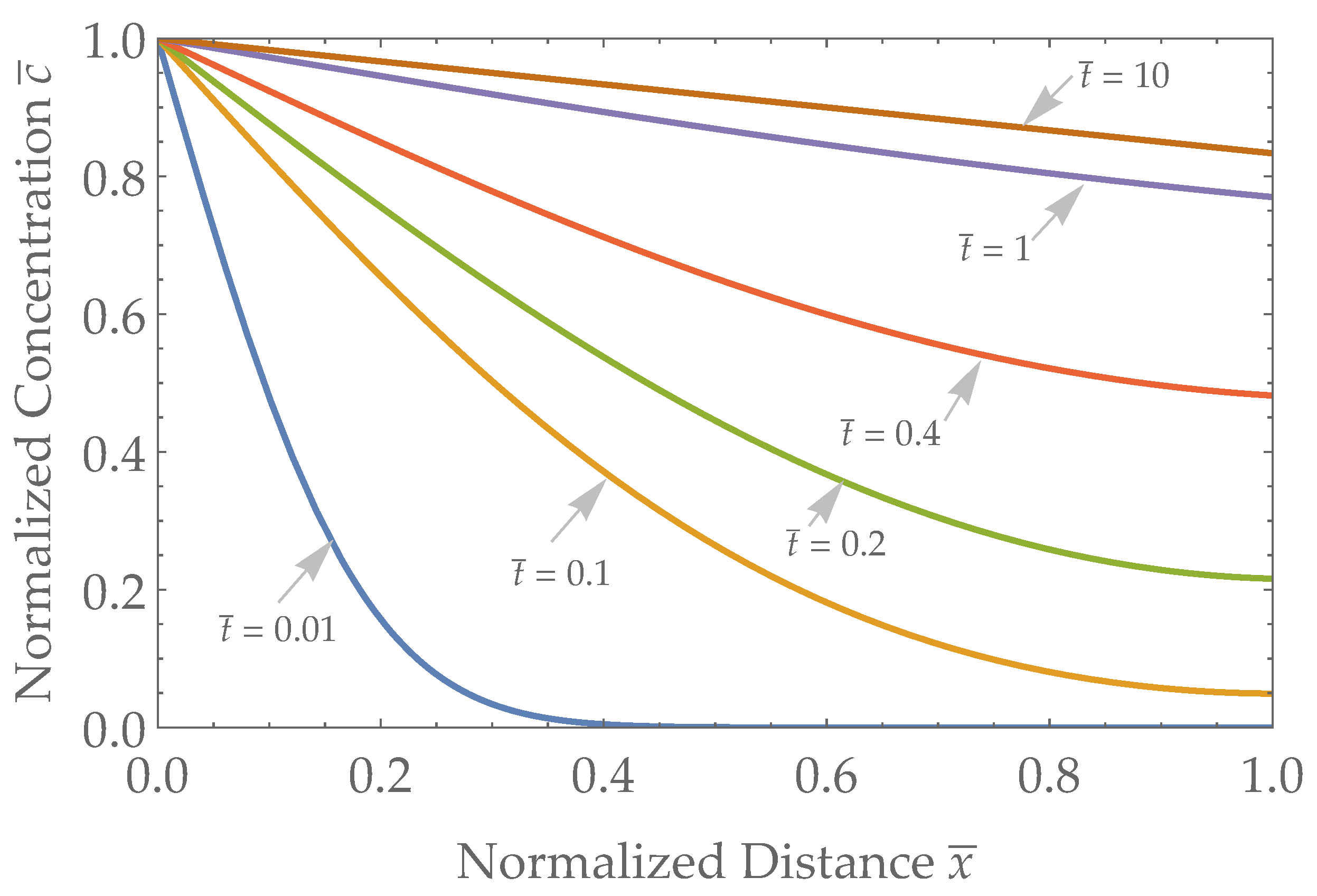
3.1. Spatial Results
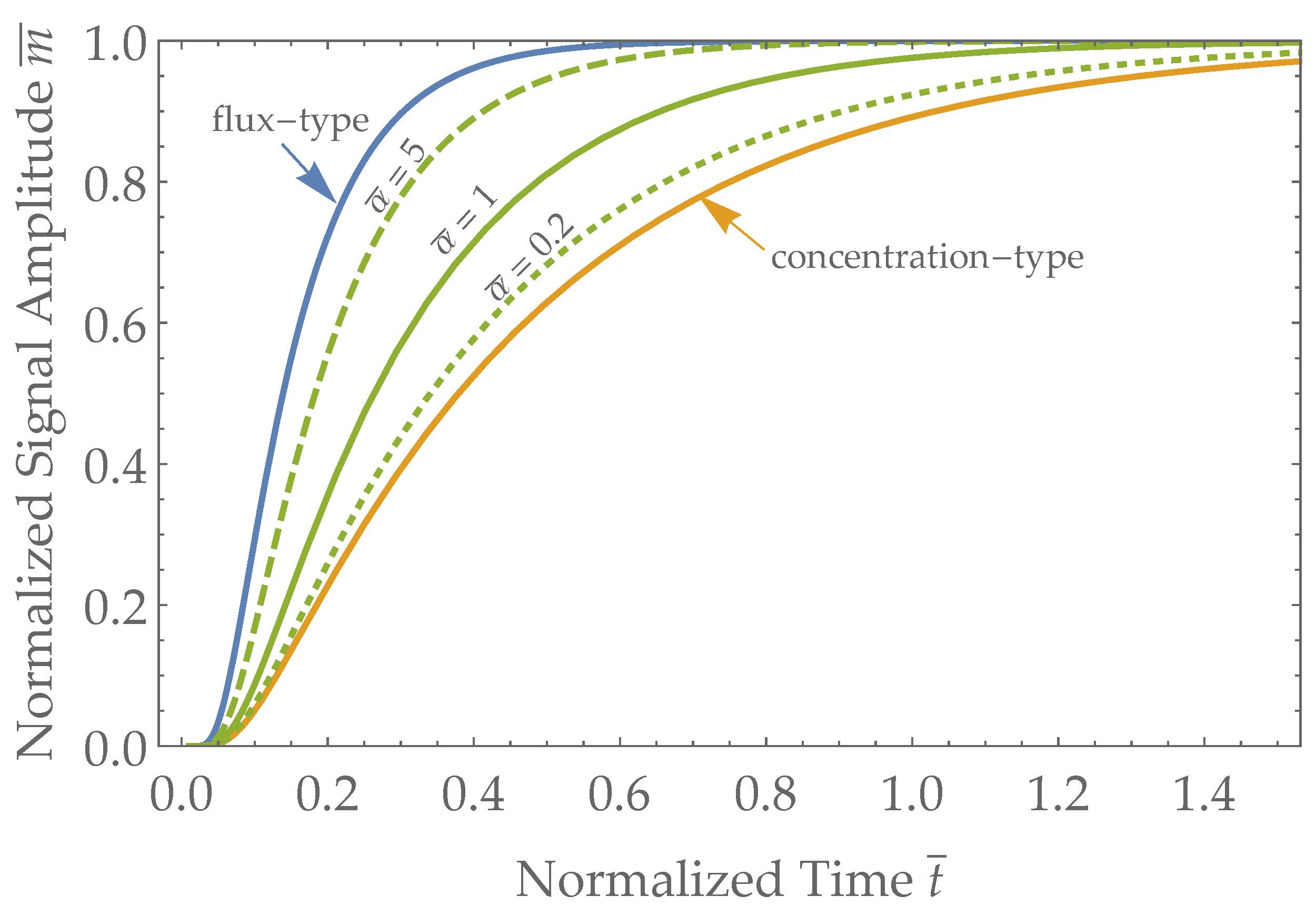
3.2. Sensor Response Times
4. Discussion
4.1. Robin Sensor in Extreme Limits
4.2. Sensor Types and Expected Response Times
4.3. Amplitude of Flux Type Sensors
4.4. Alcohol Sensors as a Case Study
Recommendations for Alcohol Research
4.5. Examples of Sensor Types and Applicability
4.6. Sensor Design Guide
4.7. Future Work and Recommendations
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FDA | Food and Drug Administration |
| TDD | Transdermal drug delivery |
| BAC | Blood Alcohol Concentration |
| TAC | Transdermal Alcohol Concentration |
| TAF | Transdermal Alcohol Flux |
| TAD | Transdermal Alcohol Detector |
Appendix A
Appendix A.1. Transformation of Variables
Appendix A.2. Steady-State Solution
Appendix A.3. Homogenizing Boundary Conditions
Appendix A.4. Non-Dimensional Equations
Appendix A.5. Solving the Diffusion Equation with Separation of Variables
Appendix A.6. The Solution
Appendix A.6.1. Case 1: k = 0
Appendix A.6.2. Case 2: k = μ2 > 0
Appendix A.6.3. Case 3: k = −μ2 < 0
- We calculate the Fourier coefficients using (A23) up to the desired precision.
- We calculate .
- We calculate .
- We calculate .
References
- Vashist, S.K. Continuous glucose monitoring systems: A review. Diagnostics 2013, 3, 385–412. [Google Scholar] [CrossRef] [PubMed]
- Tasca, F.; Tortolini, C.; Bollella, P.; Antiochia, R. Microneedle-based electrochemical devices for transdermal biosensing: A review. Curr. Opin. Electrochem. 2019, 16, 42–49. [Google Scholar] [CrossRef]
- Food, U.; Administration, D. Regulatory Considerations for Microneedling Products—Guidance for Industry and Food and Drug Administration Staff. 2017. Available online: https://www.fda.gov/media/107708/download (accessed on 17 March 2023).
- Giri, T.K.; Chakrabarty, S.; Ghosh, B. Transdermal reverse iontophoresis A novel technique for therapeutic drug monitoring. J. Control. Release 2017, 246, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Chan, N.; Hurel, S. Potential impact of a new blood glucose monitoring device: The GlucoWatch® Biographer. Pract. Diabetes Int. 2002, 19, 97–100. [Google Scholar] [CrossRef]
- Children Network (DirecNet) Study Group. A randomized multicenter trial comparing the GlucoWatch Biographer with standard glucose monitoring in children with type 1 diabetes. Diabetes Care 2005, 28, 1101–1106. [Google Scholar] [CrossRef][Green Version]
- Wimmer, N.J.; Robbins, S.; Ssemaganda, H.; Yang, E.; Normand, S.L.; Matheny, M.E.; Herz, N.; Rising, J.; Resnic, F.S. Assessing the cost burden of United States FDA-mandated post-approval studies for medical devices. J. Health Care Financ. 2016, 2016. [Google Scholar]
- Cohen, A.; Wadsworth, N. A light emitting diode skin reflectance oximeter. Med. Biol. Eng. 1972, 10, 385–391. [Google Scholar] [CrossRef]
- Yoshiya, I.; Shimada, Y.; Tanaka, K. Spectrophotometric monitoring of arterial oxygen saturation in the fingertip. Med. Biol. Eng. Comput. 1980, 18, 27–32. [Google Scholar] [CrossRef]
- Ridder, T.D.; Steeg, B.J.V.; Laaksonen, B.D. Comparison of spectroscopically measured tissue alcohol concentration to blood and breath alcohol measurements. J. Biomed. Opt. 2009, 14, 054039. [Google Scholar] [CrossRef]
- Bariya, M.; Nyein, H.Y.Y.; Javey, A. Wearable sweat sensors. Nat. Electron. 2018, 1, 160–171. [Google Scholar] [CrossRef]
- Pontrelli, G.; de Monte, F. A two-phase two-layer model for transdermal drug delivery and percutaneous absorption. Math. Biosci. 2014, 257, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Mitragotri, S.; Anissimov, Y.G.; Bunge, A.L.; Frasch, H.F.; Guy, R.H.; Hadgraft, J.; Kasting, G.B.; Lane, M.E.; Roberts, M.S. Mathematical models of skin permeability: An overview. Int. J. Pharm. 2011, 418, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Potts, R.O.; Francoeur, M.L. The influence of stratum corneum morphology on water permeability. J. Investig. Dermatol. 1991, 96, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.F.; Kasting, G.B.; Nitsche, J.M. A Multiphase Microscopic Diffusion Model for Stratum Corneum permeability. I. Formulation, Solution, and Illustrative Results for Representative Compounds. J. Pharm. Sci. 2006, 95, 620–648. [Google Scholar] [CrossRef] [PubMed]
- Giles, H.; Meggiorini, S.; Renaud, G.; Thiessen, J.; Vidins, E.; Compton, K.; Saldivia, V.; Orrego, H.; Israel, Y. Ethanol vapor above skin: Determination by a gas sensor instrument and relationship with plasma concentration. Alcohol. Clin. Exp. Res. 1987, 11, 249–253. [Google Scholar] [CrossRef]
- Webster, G.D.; Gabler, H.C. Feasibility of transdermal ethanol sensing for the detection of intoxicated drivers. Ann. Adv. Automot. Med. 2007, 51, 449. [Google Scholar]
- Anderson, J.C.; Hlastala, M.P. The kinetics of transdermal ethanol exchange. J. Appl. Physiol. 2006, 100, 649–655. [Google Scholar] [CrossRef][Green Version]
- Rosen, I.G.; Luczak, S.E.; Hu, W.; Hankin, M. Discrete-time blind deconvolution for distributed parameter systems with dirichlet boundary input and unbounded output with application to a transdermal alcohol biosensor. In Proceedings of the 2013 Conference on Control and Its Applications, SIAM, San Diego, CA, USA, 8–10 July 2013; pp. 160–167. [Google Scholar] [CrossRef]
- Dai, Z.; Rosen, I.G.; Wang, C.; Barnett, N.; Luczak, S.E. Using drinking data and pharmacokinetic modeling to calibrate transport model and blind deconvolution based data analysis software for transdermal alcohol biosensors. Math. Biosci. Eng. MBE 2016, 13, 911. [Google Scholar] [CrossRef]
- Li, B.; Downen, R.S.; Dong, Q.; Tran, N.; LeSaux, M.; Meltzer, A.C.; Li, Z. A discreet wearable iot sensor for continuous transdermal alcohol monitoring—Challenges and opportunities. IEEE Sens. J. 2020, 21, 5322–5330. [Google Scholar] [CrossRef]
- Li, B. Perspiration Compensated Transdermal Alcohol Sensor for Personalized Medicine. PhD Thesis, The George Washington University, Washington, DC, USA, 2021. [Google Scholar]
- Fairbairn, C.E.; Kang, D.; Bosch, N. Using machine learning for real-time BAC estimation from a new-generation transdermal biosensor in the laboratory. Drug Alcohol Depend. 2020, 216, 108205. [Google Scholar] [CrossRef]
- Fridberg, D.J.; Wang, Y.; Porges, E. Examining features of transdermal alcohol biosensor readings: A promising approach with implications for research and intervention. Alcohol. Clin. Exp. Res. 2022, 46, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Ash, G.I.; Gueorguieva, R.; Barnett, N.P.; Wang, W.; Robledo, D.S.; DeMartini, K.S.; Pittman, B.; Redeker, N.S.; O’Malley, S.S.; Fucito, L.M. Sensitivity, specificity, and tolerability of the BACTrack Skyn compared to other alcohol monitoring approaches among young adults in a field-based setting. Alcohol. Clin. Exp. Res. 2022, 46, 783–796. [Google Scholar] [CrossRef] [PubMed]
- Carter, M.; Findlay, M.; Peaslee, D.; Ploense, L.; Meulendyk, B.; Devine, E.; Stetter, J.R. Transdermal Alcohol Monitoring with a Printed Amperometric Sensor. In Electrochemical Society Meeting Abstracts 239; The Electrochemical Society, Inc.: Penningtonm, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Boyce, W.E.; DiPrima, R.C.; Meade, D.B. Elementary Differential Equations and Boundary Value Problems, 7th ed.; Wiley: Hoboken, NJ, USA, 2001; pp. 573–590. [Google Scholar]
- Daileda, R.C. Partial Differential Equations Lecture Notes. 2017. Available online: http://ramanujan.math.trinity.edu/rdaileda/teach/s12/m3357/lectures/lecture_2_28_short.pdf (accessed on 4 April 2023).
- Crank, J. The Mathematics of Diffusion; Oxford University Press: New York, NY, USA, 1979. [Google Scholar]
- Scheuplein, R.J.; Blank, I.H. Permeability of the skin. Physiol. Rev. 1971, 51, 702–747. [Google Scholar] [CrossRef] [PubMed]
- Karns-Wright, T.E.; Roache, J.D.; Hill-Kapturczak, N.; Liang, Y.; Mullen, J.; Dougherty, D.M. Time delays in transdermal alcohol concentrations relative to breath alcohol concentrations. Alcohol Alcohol. 2017, 52, 35–41. [Google Scholar] [CrossRef]
- Lansdorp, B.; Ramsay, W.; Hamid, R.; Strenk, E. Wearable enzymatic alcohol biosensor. Sensors 2019, 19, 2380. [Google Scholar] [CrossRef]
- Swette, L.L.; Griffith, A.E.; LaConti, A.B. Potential and Diffusion Controlled Solid Electrolyte Sensor for Continuous Measurement of Very Low Levels of Transdermal Alcohol. U.S. Patent 5,944,661, 16 April 1999. [Google Scholar]
- Marques, P.R.; McKnight, A.S. Evaluating Transdermal Alcohol Measuring Devices; Technical report; National Highway Traffic Safety Administration: Washington, DC, USA, 2007. [CrossRef]
- Hill-Kapturczak, N.; Roache, J.D.; Liang, Y.; Karns, T.E.; Cates, S.E.; Dougherty, D.M. Accounting for sex-related differences in the estimation of breath alcohol concentrations using transdermal alcohol monitoring. Psychopharmacology 2015, 232, 115–123. [Google Scholar] [CrossRef][Green Version]
- Fairbairn, C.E.; Kang, D. Temporal dynamics of transdermal alcohol concentration measured via new-generation wrist-worn biosensor. Alcohol. Clin. Exp. Res. 2019, 43, 2060–2069. [Google Scholar] [CrossRef]
- van Egmond, K.; Wright, C.J.; Livingston, M.; Kuntsche, E. Wearable transdermal alcohol monitors: A systematic review of detection validity, and relationship between transdermal and breath alcohol concentration and influencing factors. Alcohol. Clin. Exp. Res. 2020, 44, 1918–1932. [Google Scholar] [CrossRef]
- Yu, J.; Fairbairn, C.E.; Gurrieri, L.; Caumiant, E.P. Validating transdermal alcohol biosensors: A meta-analysis of associations between blood/breath-based measures and transdermal alcohol sensor output. Addiction 2022, 117, 2805–2815. [Google Scholar] [CrossRef]
- Lawson, B.; Aguir, K.; Fiorido, T.; Martini-Laithier, V.; Bouchakour, R.; Burtey, S.; Reynard-Carette, C.; Bendahan, M. Skin alcohol perspiration measurements using MOX sensors. Sens. Actuators Chem. 2019, 280, 306–312. [Google Scholar] [CrossRef]
- van Egmond, K.; Riordan, B.; Wright, C.J.; Livingston, M.; Kuntsche, E. Measurement of Transdermal Alcohol Concentration using a Wrist Worn Enzymatic Transdermal Monitor. Alcohol 2023, 110, 33–40. [Google Scholar] [CrossRef]
- Wang, Y.; Fridberg, D.J.; Shortell, D.D.; Leeman, R.F.; Barnett, N.P.; Cook, R.L.; Porges, E.C. Wrist-worn alcohol biosensors: Applications and usability in behavioral research. Alcohol 2021, 92, 25–34. [Google Scholar] [CrossRef]
- Yao, M.; Luczak, S.E.; Rosen, I.G. Tracking and blind deconvolution of blood alcohol concentration from transdermal alcohol biosensor data: A population model-based LQG approach in Hilbert space. Automatica 2023, 147, 110699. [Google Scholar] [CrossRef]
- Hawekotte, K.; Luczak, S.E.; Rosen, I. Deconvolving breath alcohol concentration from biosensor measured transdermal alcohol level under uncertainty: A Bayesian approach. Math. Biosci. Eng. MBE 2021, 18, 6739. [Google Scholar] [CrossRef] [PubMed]
- Ozoemena, K.I.; Musa, S.; Modise, R.; Ipadeola, A.K.; Gaolatlhe, L.; Peteni, S.; Kabongo, G. Fuel cell-based breath-alcohol sensors: Innovation-hungry old electrochemistry. Curr. Opin. Electrochem. 2018, 10, 82–87. [Google Scholar] [CrossRef]
- Sharifpour-Boushehri, S.; Hosseini-Golgoo, S.M.; Sheikhi, M.H. A low cost and reliable fiber optic ethanol sensor based on nano-sized SnO2. Opt. Fiber Technol. 2015, 24, 93–99. [Google Scholar] [CrossRef]
- Kim, D.M.; Moon, J.M.; Lee, W.C.; Yoon, J.H.; Choi, C.S.; Shim, Y.B. A potentiometric non-enzymatic glucose sensor using a molecularly imprinted layer bonded on a conducting polymer. Biosens. Bioelectron. 2017, 91, 276–283. [Google Scholar] [CrossRef]
- Bobacka, J.; Ivaska, A.; Lewenstam, A. Potentiometric ion sensors. Chem. Rev. 2008, 108, 329–351. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, M.; Li, J. Solution-gated graphene transistors for chemical and biological sensors. Adv. Healthc. Mater. 2014, 3, 313–331. [Google Scholar] [CrossRef]
- Song, S.; Wang, L.; Li, J.; Fan, C.; Zhao, J. Aptamer-based biosensors. TrAC Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- North, J.R. Immunosensors: Antibody-based biosensors. Trends Biotechnol. 1985, 3, 180–186. [Google Scholar] [CrossRef]
- Ullah, S.; Hamade, F.; Bubniene, U.; Engblom, J.; Ramanavicius, A.; Ramanaviciene, A.; Ruzgas, T. In-vitro model for assessing glucose diffusion through skin. Biosens. Bioelectron. 2018, 110, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Conner, D.P.; Millora, E.; Zamani, K.; Nix, D.; Almirez, R.G.; Rhyne-Kirsch, P.; Peck, C.C. Transcutaneous chemical collection of caffeine in normal subjects: Relationship to area under the plasma concentration-time curve and sweat production. J. Investig. Dermatol. 1991, 96, 186–190. [Google Scholar] [CrossRef]
- Alsaqr, A.; Rasoully, M.; Musteata, F.M. Investigating transdermal delivery of vitamin D 3. AAPS PharmSciTech 2015, 16, 963–972. [Google Scholar] [CrossRef] [PubMed]
- Cone, E.J.; Hillsgrove, M.J.; Jenkins, A.J.; Keenan, R.M.; Darwin, W.D. Sweat testing for heroin, cocaine, and metabolites. J. Anal. Toxicol. 1994, 18, 298–305. [Google Scholar] [CrossRef]
- De Giovanni, N.; Fucci, N. The current status of sweat testing for drugs of abuse: A review. Curr. Med. Chem. 2013, 20, 545–561. [Google Scholar] [CrossRef]
- Degim, I.T.; Pugh, W.J.; Hadgraft, J. Skin permeability data: Anomalous results. Int. J. Pharm. 1998, 170, 129–133. [Google Scholar] [CrossRef]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® synthetic membrane: Permeability comparison to human cadaver skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef]
- Dannecker, J.R., Jr.; Shaskan, E.G.; Phillips, M. A new highly sensitive assay for breath acetaldehyde: Detection of endogenous levels in humans. Anal. Biochem. 1981, 114, 1–7. [Google Scholar] [CrossRef]
- Yamada, Y.I.; Yamada, G.; Otsuka, M.; Nishikiori, H.; Ikeda, K.; Umeda, Y.; Ohnishi, H.; Kuronuma, K.; Chiba, H.; Baumbach, J.I.; et al. Volatile organic compounds in exhaled breath of idiopathic pulmonary fibrosis for discrimination from healthy subjects. Lung 2017, 195, 247–254. [Google Scholar] [CrossRef]
- Kushner IV, J.; Deen, W.; Blankschtein, D.; Langer, R. First-principles, structure-based transdermal transport model to evaluate lipid partition and diffusion coefficients of hydrophobic permeants solely from stratum corneum permeation experiments. J. Pharm. Sci. 2007, 96, 3236–3251. [Google Scholar] [CrossRef] [PubMed]
- Potts, R.O.; Guy, R.H. Predicting skin permeability. Pharm. Res. 1992, 9, 663–669. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lansdorp, B.M. Flux-Type versus Concentration-Type Sensors in Transdermal Measurements. Biosensors 2023, 13, 845. https://doi.org/10.3390/bios13090845
Lansdorp BM. Flux-Type versus Concentration-Type Sensors in Transdermal Measurements. Biosensors. 2023; 13(9):845. https://doi.org/10.3390/bios13090845
Chicago/Turabian StyleLansdorp, Bob M. 2023. "Flux-Type versus Concentration-Type Sensors in Transdermal Measurements" Biosensors 13, no. 9: 845. https://doi.org/10.3390/bios13090845
APA StyleLansdorp, B. M. (2023). Flux-Type versus Concentration-Type Sensors in Transdermal Measurements. Biosensors, 13(9), 845. https://doi.org/10.3390/bios13090845




