SERS-Active Substrate with Collective Amplification Design for Trace Analysis of Pesticides
Abstract
1. Introduction
2. Materials and Methods
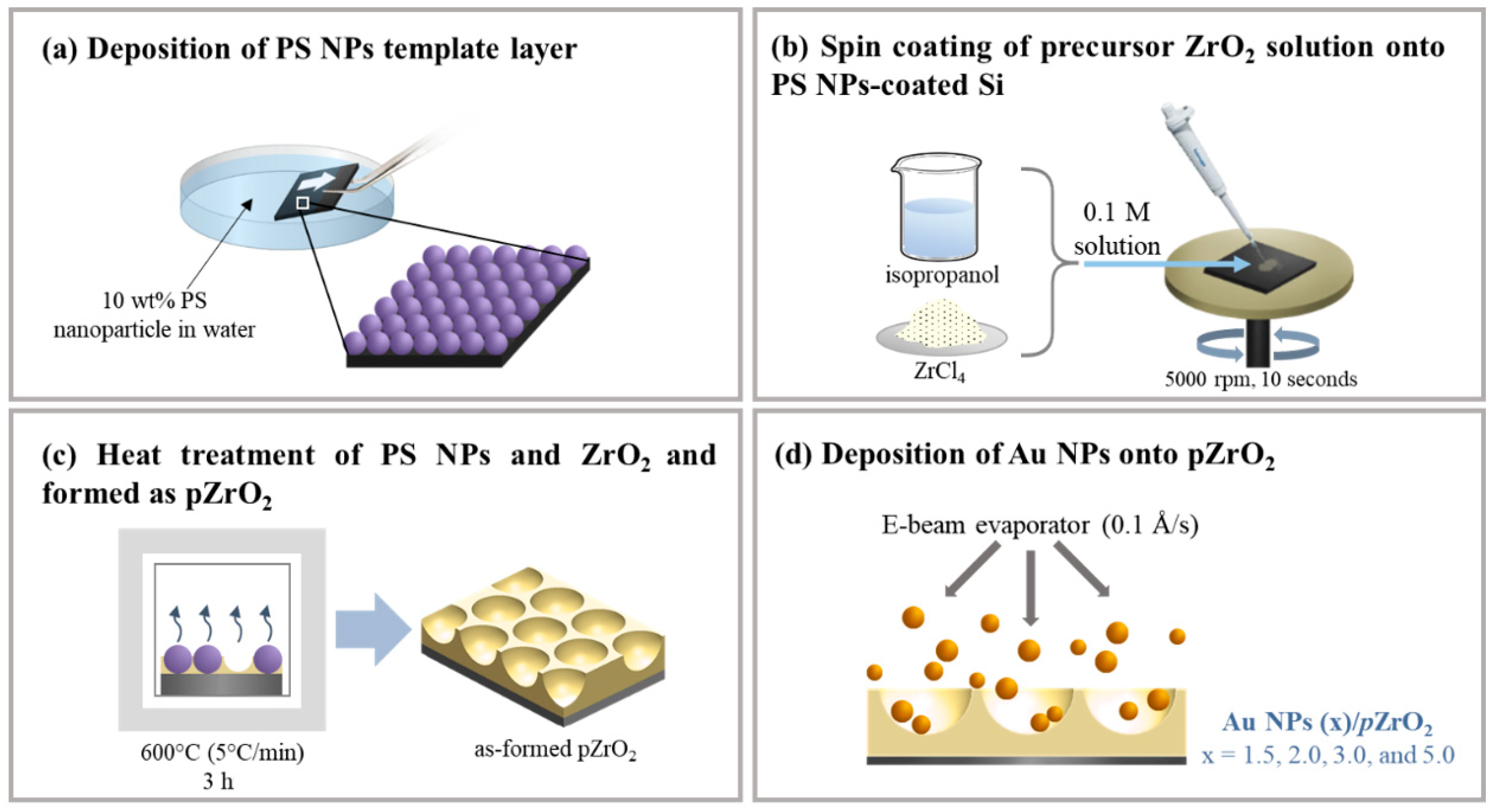
2.1. Fabrication of Au NPs (x)/pZrO2
2.2. Physical Characterization of Au NPs (x)/pZrO2
2.3. SERS Property of Au NPs (x)/pZrO2
2.4. Trace Analysis of Pesticide(s) upon Au NPs (x)/pZrO2
3. Results and Discussion
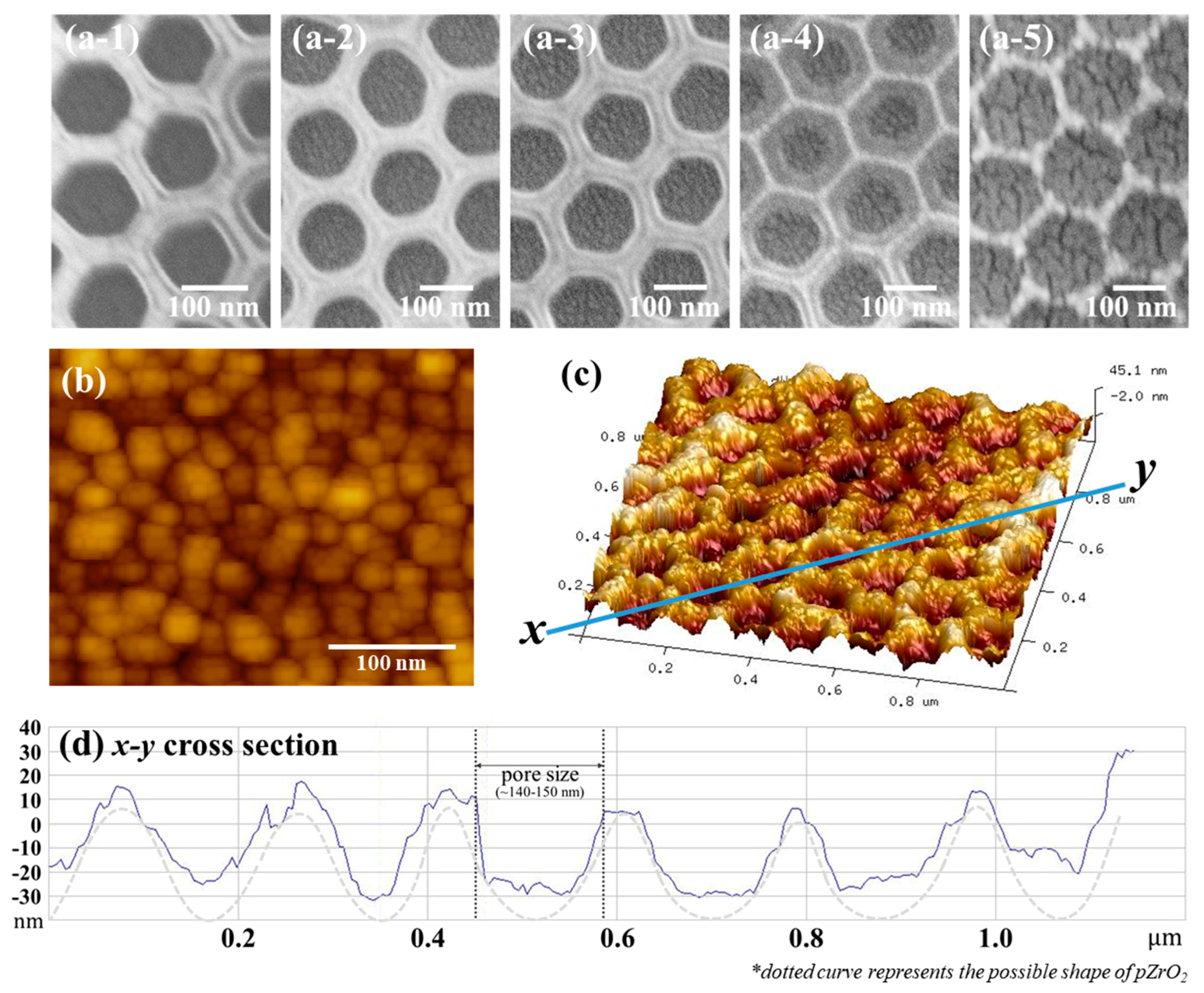
3.1. Size and Dimension of Au NPs (x)/pZrO2
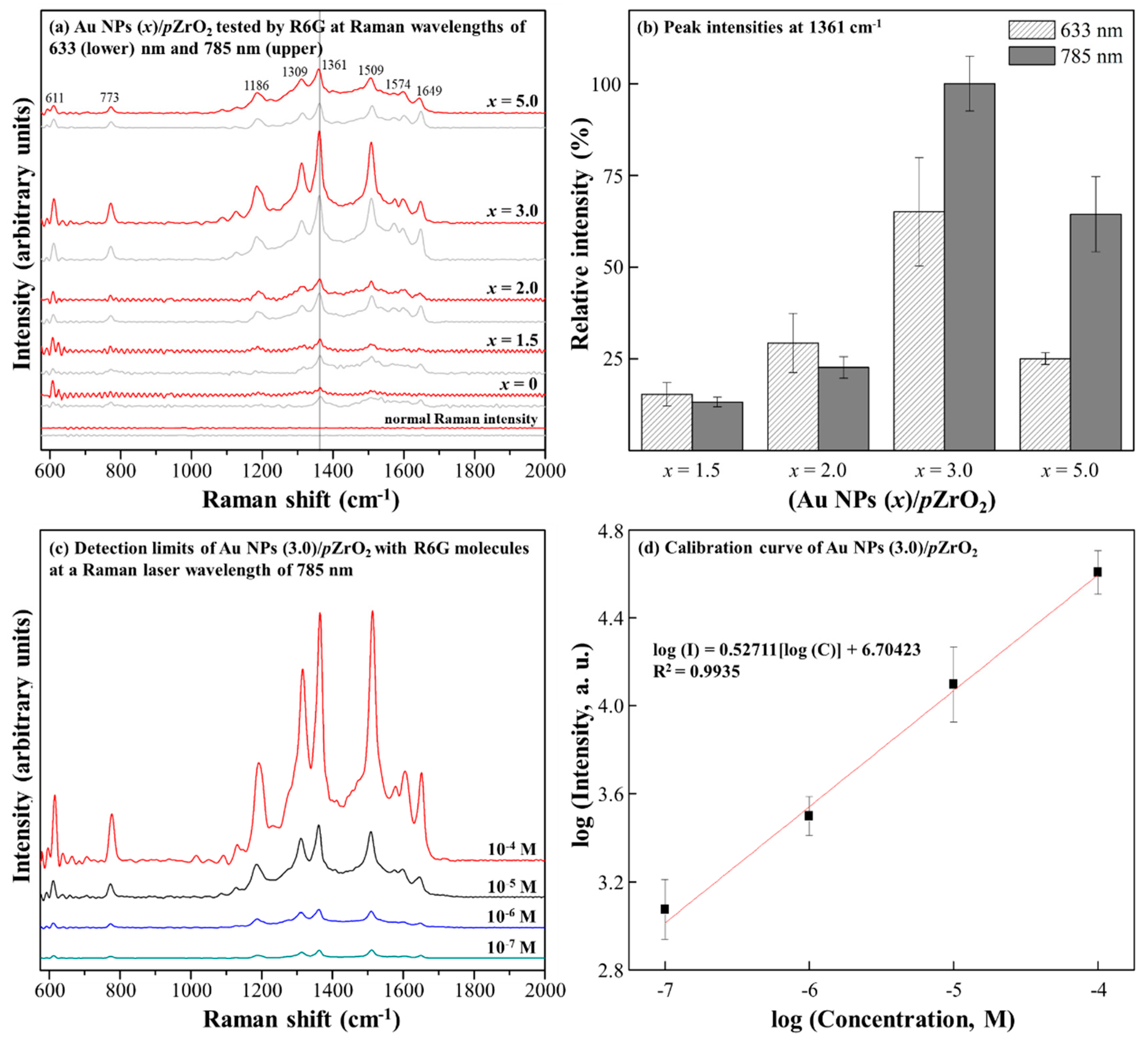
3.2. SERS Property of Au NPs (3.0)/pZrO2
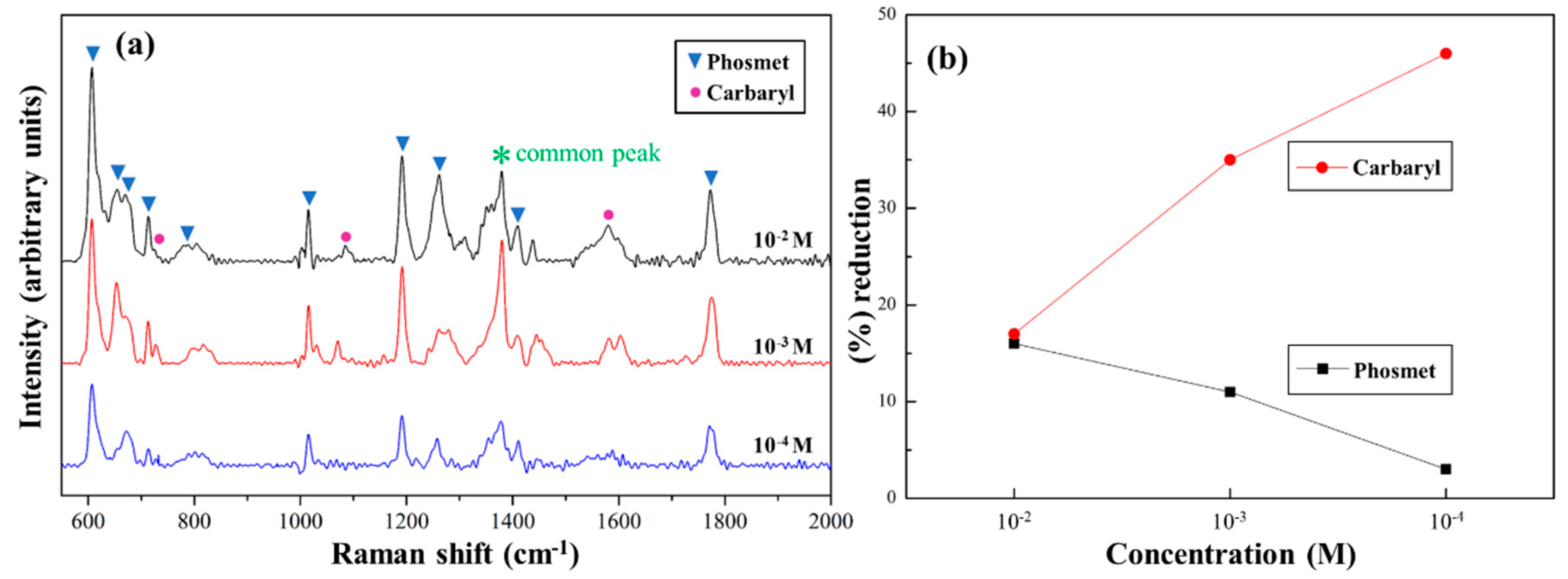
3.3. SERS Properties of Au NPs (x)/pZrO2 to Pesticides Detection
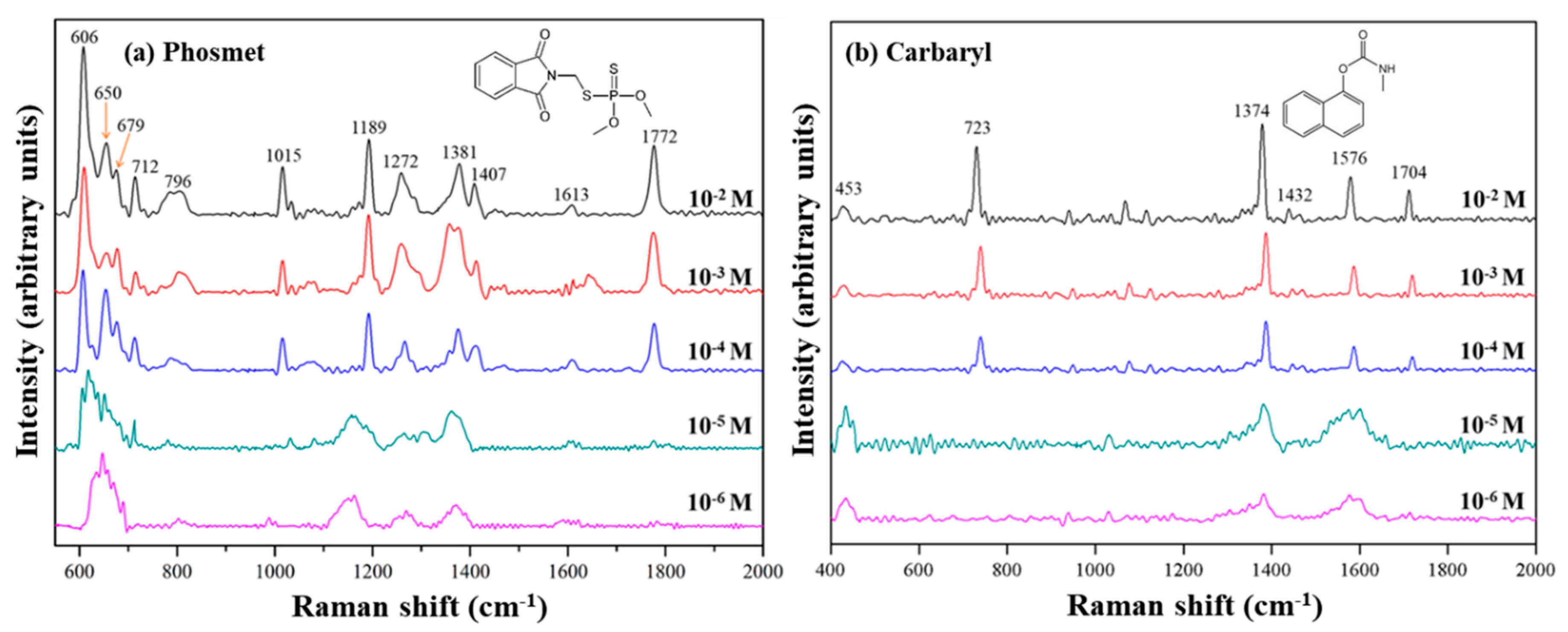
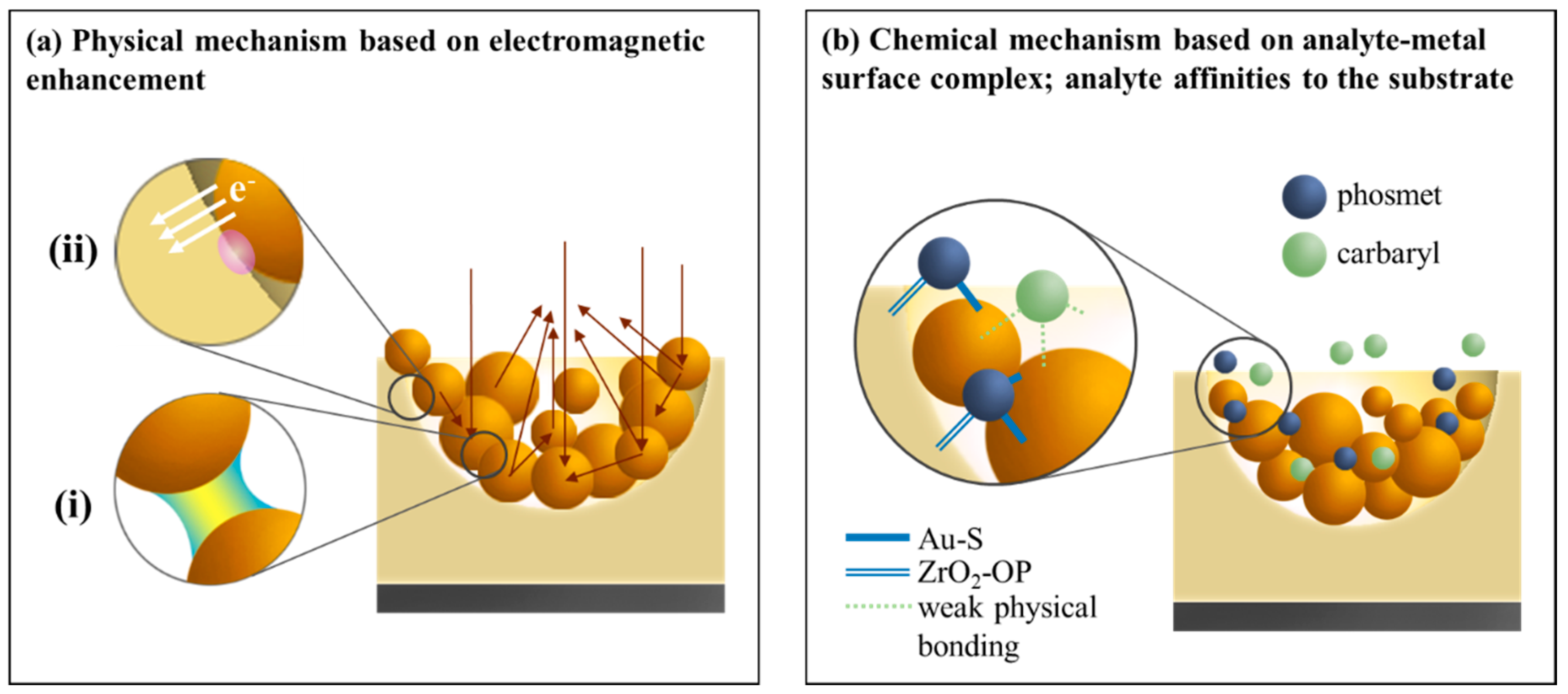
3.4. Particular Two Pesticides Detection Using Au NPs (3.0)/pZrO2
3.5. Particular Two Pesticides Detection Using Au NPs (3.0)/pZrO2
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lutz, B.; Dentinger, C.; Sun, L.; Nguyen, L.; Zhang, J.; Chmura, A.; Allen, A.; Chan, S.; Knudsen, B. Raman nanoparticle probes for antibody-based protein detection in tissues. J. Histochem. Cytochem. 2008, 56, 371–379. [Google Scholar] [CrossRef]
- Deconinck, E.; Sacré, P.Y.; Courselle, P.; De Beer, J.O. Chromatography in the detection and characterization of illegal pharmaceutical preparations. J. Chromatogr. Sci. 2013, 51, 791–806. [Google Scholar] [CrossRef]
- Chambers, M.; Maclean, B.; Burke, R. A cross-platform toolkit for mass spectrometry and proteomics. Nat. Biotechnol. 2012, 30, 918–920. [Google Scholar] [CrossRef]
- Liu, B.; Zhou, P.; Liu, X.; Sun, X.; Li, H.; Lin, M. Detection of Pesticides in Fruits by Surface-Enhanced Raman Spectroscopy Coupled with Gold Nanostructures. Food Bioprocess Technol. 2013, 6, 710–718. [Google Scholar] [CrossRef]
- Shiohara, A.; Wang, Y.; Liz-Marzán, L.M. Recent approaches toward creation of hot spots for SERS detection. J. Photochem. Photobiol. C Photochem. Rev. 2014, 21, 2–25. [Google Scholar] [CrossRef]
- Granger, J.H.; Granger, M.C.; Firpo, M.A.; Mulvihill, S.J.; Porter, M.D. Toward development of a surface-enhanced Raman scattering (SERS)-based cancer diagnostic immunoassay panel. Analyst 2013, 138, 410–416. [Google Scholar] [CrossRef]
- Kneipp, K.; Kneipp, H.; Kneipp, J. Surface-enhanced raman scattering in local optical fields of silver and gold nanoaggregates—From single-molecule raman spectroscopy to ultrasensitive probing in live cells. Acc. Chem. Res. 2006, 39, 443–450. [Google Scholar] [CrossRef]
- Guerrini, L.; Graham, D. Molecularly-mediated assemblies of plasmonic nanoparticles for Surface-Enhanced Raman Spectroscopy applications. Chem. Soc. Rev. 2012, 41, 7085–7107. [Google Scholar] [CrossRef] [PubMed]
- Tarakeshwar, P.; Finkelstein-Shapiro, D.; Hurst, S.J.; Rajh, T.; Mujica, V. Surface-enhanced Raman scattering on semiconducting oxide nanoparticles: Oxide nature, size, solvent, and pH effects. J. Phys. Chem. C 2011, 115, 8994–9004. [Google Scholar] [CrossRef]
- Knight, M.W.; Wu, Y.; Lassiter, J.B.; Nordlander, P.; Halas, N.J. Substrates Matter Influence of an Adjacent Dielectric on an Individual Plasmonic Nanoparticle. Nano Lett. 2009, 9, 2188–2192. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Chen, J.; Mcbride, J.R.; Lian, T. Efficient hot-electron transfer by a plasmon-induced interfacial charge-transfer transition. Science 2015, 349, 632–635. [Google Scholar] [CrossRef]
- Li, X.; Jia, C.; Ma, B.; Wang, W.; Fang, Z.; Zhang, G.; Guo, X. Substrate-induced interfacial plasmonics for photovoltaic conversion. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jia, C.; Li, X.; Xin, N.; Gong, Y.; Guan, J.; Meng, L.; Meng, S.; Guo, X. Interface-Engineered Plasmonics in Metal/Semiconductor Heterostructures. Adv. Energy Mater. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Luo, S.C.; Sivashanmugan, K.; Liao, J.D.; Yao, C.K.; Peng, H.C. Nanofabricated SERS-active substrates for single-molecule to virus detection in vitro: A review. Biosens. Bioelectron. 2014, 61, 232–240. [Google Scholar] [CrossRef]
- Wolosiuk, A.; Tognalli, G.; Mart, E.D.; Granada, M.; Fuertes, M.C.; Troiani, H.; Bilmes, S.A.; Fainstein, A.; Soler-illia, G.J.A.A. Silver Nanoparticle-Mesoporous Oxide Nanocomposite Thin Films: A Platform for Spatially Homogeneous SERS-Active Substrates with Enhanced Stability. ACS Appl. Mater. Interfaces 2014, 6, 5263–5272. [Google Scholar] [CrossRef] [PubMed]
- Pisarek, M.; Roguska, A.; Kudelski, A.; Holdynski, M.; Janik-Czachor, M.; Hnida, K.; Sulka, G.D. Ag/ZrO2-NT/Zr hybrid material: A new platform for SERS measurements. Vib. Spectrosc. 2014, 71, 85–90. [Google Scholar] [CrossRef]
- Liu, G.; Lin, Y. Electrochemical sensor for organophosphate pesticides and nerve agents using zirconia nanoparticles as selective sorbents. Anal. Chem. 2005, 77, 5894–5901. [Google Scholar] [CrossRef]
- Zhang, D.; Liang, P.; Yu, Z.; Huang, J.; Ni, D.; Shu, H.; Dong, Q.-M. The effect of solvent environment toward optimization of SERS sensors for pesticides detection from chemical enhancement aspects. Sens. Actuators B Chem. 2018, 256, 721–728. [Google Scholar] [CrossRef]
- Luo, H.; Huang, Y.; Lai, K.; Rasco, B.A.; Fan, Y. Surface-enhanced Raman spectroscopy coupled with gold nanoparticles for rapid detection of phosmet and thiabendazole residues in apples. Food Control 2016, 68, 229–235. [Google Scholar] [CrossRef]
- Bassi, B.; Albini, B.; D’Agostino, A.; Dacarro, G.; Pallavicini, P.; Galinetto, P.; Taglietti, A. Robust, reproducible, recyclable SERS substrates: Monolayers of gold nanostars grafted on glass and coated with thin silica layer. Nanotechnology 2019, 30, 025302. [Google Scholar] [CrossRef]
- Lee, M.; Oh, K.; Choi, H.-K.; Lee, S.G.; Youn, H.J.; Lee, H.K.; Jeong, D.H. Subnanomolar Sensitivity of Filter Paper-Based SERS Sensor for Pesticide Detection by Hydrophobicity Change of Paper Surface. ACS Sens. 2018, 3, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Guselnikova, O.; Postnikov, P.; Elashnikov, R.; Miliutina, E.; Svorcik, V.; Lyutakov, O. Metal-organic framework (MOF-5) coated SERS active gold gratings: A platform for the selective detection of organic contaminants in soil. Anal. Chim. Acta 2019. [Google Scholar] [CrossRef]
- Gingery, D.; Bühlmann, P. Formation of gold nanoparticles on multiwalled carbon nanotubes by thermal evaporation. Carbon 2008, 46, 1966–1972. [Google Scholar] [CrossRef]
- Keyes, T.E.; Forster, R.J. Spectroelectrochemistry. In Handbook of Electrochemistry; Zoski, C.G., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 591–635. [Google Scholar]
- Kahraman, M.; Wachsmann-Hogiu, S. Label-free and direct protein detection on 3D plasmonic nanovoid structures using surface-enhanced Raman scattering. Anal. Chim. Acta 2015, 856, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Sui, C.; Wang, K.; Wang, S.; Ren, J.; Bai, X.; Bai, J. SERS activity with tenfold detection limit optimization on a type of nanoporous AAO-based complex multilayer substrate. Nanoscale 2016, 8, 5920–5927. [Google Scholar] [CrossRef]
- Grochowska, K.; Siuzdak, K.; Sokołowski, M.; Karczewski, J.; Szkoda, M.; Śliwiński, G. Properties of ordered titanium templates covered with Au thin films for SERS applications. Appl. Surf. Sci. 2016, 388, 716–722. [Google Scholar] [CrossRef]
- Lee, H.; Liao, J.; Sivashanmugan, K.; Liu, B.H.; Chen, C.; Chen, G.D.; Juang, Y. Gold Nanoparticle-Coated ZrO2-Nanofiber Surface as a SERS-Active Substrate for Trace Detection of Pesticide Residue. Nanomaterials 2018, 8, 402. [Google Scholar] [CrossRef]
- Singh, D.K.; Ganbold, E.O.; Cho, E.M.; Lee, C.M.; Yang, S.I.; Joo, S.W. Tautomerism of a thiabendazole fungicide on Ag and Au nanoparticles investigated by Raman spectroscopy and density functional theory calculations. J. Mol. Struct. 2013, 1049, 464–472. [Google Scholar] [CrossRef]
- Kudelski, A.; Pisarek, M.; Roguska, A.; Hoałdýski, M.; Janik-Czachor, M. Surface-enhanced Raman scattering investigations on silver nanoparticles deposited on alumina and titania nanotubes: Influence of the substrate material on surface-enhanced Raman scattering activity of Ag nanoparticles. J. Raman Spectrosc. 2012, 43, 1360–1366. [Google Scholar] [CrossRef]
- Fan, Y.; Lai, K.; Rasco, B.A.; Huang, Y. Analyses of phosmet residues in apples with surface-enhanced Raman spectroscopy. Food Control 2014, 37, 153–157. [Google Scholar] [CrossRef]
- Liu, Y.; He, B.; Zhang, Y.; Wang, H.; Ye, B. Detection of Phosmet Residues on Navel Orange Skin by Surface-enhanced Raman Spectroscopy. Intell. Autom. Soft Comput. 2015, 21, 423–432. [Google Scholar] [CrossRef]
- Fan, Y.; Lai, K.; Rasco, B.A.; Huang, Y. Determination of carbaryl pesticide in Fuji apples using surface-enhanced Raman spectroscopy coupled with multivariate analysis. LWT-Food Sci. Technol. 2015, 60, 352–357. [Google Scholar] [CrossRef]
- Altun, A.O.; Bond, T.; Pronk, W.; Park, H.G. Sensitive Detection of Competitive Molecular Adsorption by Surface-Enhanced Raman Spectroscopy. Langmuir 2017, 33, 6999–7006. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sitjar, J.; Liao, J.-D.; Lee, H.; Liu, B.H.; Fu, W.-e. SERS-Active Substrate with Collective Amplification Design for Trace Analysis of Pesticides. Nanomaterials 2019, 9, 664. https://doi.org/10.3390/nano9050664
Sitjar J, Liao J-D, Lee H, Liu BH, Fu W-e. SERS-Active Substrate with Collective Amplification Design for Trace Analysis of Pesticides. Nanomaterials. 2019; 9(5):664. https://doi.org/10.3390/nano9050664
Chicago/Turabian StyleSitjar, Jaya, Jiunn-Der Liao, Han Lee, Bernard Haochih Liu, and Wei-en Fu. 2019. "SERS-Active Substrate with Collective Amplification Design for Trace Analysis of Pesticides" Nanomaterials 9, no. 5: 664. https://doi.org/10.3390/nano9050664
APA StyleSitjar, J., Liao, J.-D., Lee, H., Liu, B. H., & Fu, W.-e. (2019). SERS-Active Substrate with Collective Amplification Design for Trace Analysis of Pesticides. Nanomaterials, 9(5), 664. https://doi.org/10.3390/nano9050664






