Abstract
Hierarchical CeO2 particles were synthesized by a hydrothermal method based on the reaction between CeCl3·7H2O and PVP at 270 °C. The flower-like CeO2 with an average diameter of about 1 μm is composed of compact nanosheets with thicknesses of about 15 nm and have a surface area of 36.8 m2/g, a large pore volume of 0.109 cm3/g, and a narrow pore size distribution (14.9 nm in diameter). The possible formation mechanism of the hierarchical CeO2 nanoparticles has been illustrated. The 3D hierarchical structured CeO2 exhibited a higher catalytic activity toward CO oxidation compared with commercial CeO2.
1. Introduction
CeO2 is playing important roles in various fields such as promoters for three-way catalysts [1], fuel cells [2], hydrogen storage materials [3], and oxygen sensors [4]. Although the utilization of ceria is based on its intrinsic chemical properties, the structures and morphologies of CeO2 also have a significant influence on its properties and applications [5,6].
So far, CeO2 with different sizes and morphologies such as nanorods [7], nanospheres [8], nanotubes [9], and nanocubes [10] have been synthesized in the last decade. It was proved that CeO2 nanoparticles with different sizes and morphologies have better properties than general CeO2 does. CeO2 nanoparticles afford more active sites because of their high specific surface areas and novel structures [11].
Preparation of CeO2 with different structures and morphologies provides the basic groundwork for its advanced applications. Hierarchical structured CeO2 with unique properties and novel functionalities has attracted the attention of many researchers in recent years.
Zhong et al. synthesized a three-dimensional (3D) flower-like CeO2 micro/nanocomposite structure using cerium chloride as a reactant by a simple and economical route based on an ethylene glycol-mediated process [12]. Li et al. synthesized mesoporous Ce(OH)CO3 microspheres with flower-like 3D hierarchical structures via different hydrothermal systems, including glucose/acylic acid, fructose/acrylic acid, glucose/propanoic acid, and glucose/n-butylamine systems. Calcination of the Ce(OH)CO3 microspheres yielded mesoporous CeO2 microspheres with the same flower-like morphology as that of Ce(OH)CO3 microspheres [13]. Ouyang et al. reported a facile electrochemical method to prepare hierarchical porous CeO2 nanospheres and applied them as highly efficient absorbents to remove organic dyes [14]. However, 3D hierarchical structured CeO2 is commonly synthesized with relatively miscellaneous process, which limited the extensive usage of the prepared ceria. In this paper, we report a facile one-pot hydrothermal route to synthesize 3D hierarchical structured CeO2. The present hydrothermal route is low cost and can be easily scaled-up. The fabricated 3D hierarchical structured CeO2 could be used as a catalyst for CO oxidation and a support for noble metal catalysts.
2. Materials and Methods
2.1. Preparation of Hierarchical Structured CeO2
Cerium (III) chloride heptahydrate (CeCl3·7H2O), polyvinyl pyrrolidone (PVP), and ethanol were purchased from Beijing Yili Chemical Reagent Co. Ltd. (Beijing, China). All materials were used without any further purification. In a typical synthetic procedure of the hierarchical structured CeO2, 0.5 mmol CeCl3·7H2O was dissolved in 30 mL deionized water, and then 1 mmol PVP and 20 mL deionized water were added to the solution. After 15 min of magnetic stirring, the homogenous solution was transferred into the Teflon vessel of a hydrothermal bomb, which was then placed in an oven and maintained at 270 °C for 24 h. Then, the solution was cooled to room temperature, and the products were separated by centrifugation and washed with absolute ethanol and distilled water.
2.2. Characterization Techniques
The crystal phases of the products were characterized by X-ray diffraction (XRD) using Philips X’pert PRO analyzer (Philips, Amsterdam, The Netherlands) equipped with a Cu Kα radiation source (λ = 0.154187 nm) and operated at an X-ray tube (Philips, Amsterdam, The Netherlands) voltage and current of 40 KV and 30 mA, respectively. The morphology of the products was examined by scanning electron microscopy (SEM) using a JEOL JSM 67OOF system (JEOL, Tokyo, Japan) and transmission electron microscopy (TEM) using a JEM-2100 system (JEOL, Tokyo, Japan) operated at 200 kV. Surface composition was determined by X-ray photoelectron spectroscopy (XPS) using an ESCALab220i-XL electron spectrometer (VG Scientific, Waltham, MA, USA) with monochromatic Al Kα radiation. Nitrogen adsorption-desorption isotherms were analyzed using an automatic adsorption system (Autosorb-1, Quantachrome, Boynton Beach, FL, USA) at the temperature of liquid nitrogen.
3. Results
3.1. 3D Hierarchical Structured CeO2 Prepared via Hydrothermal Method
The powder XRD pattern of the as-prepared sample is shown in Figure 1. As can be seen, the as-prepared sample can be indexed to the cubic phase of CeO2 (JCPDS No. 34-0394). The average crystallite size calculated by the Scherrer equation is 26.8 nm.
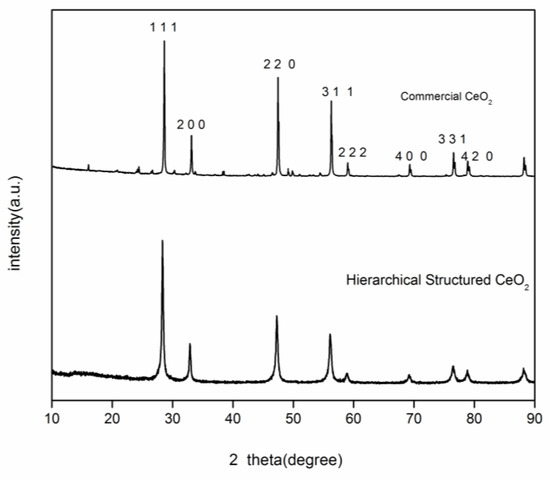
Figure 1.
XRD pattern of as-prepared 3D hierarchical structured CeO2.
The SEM images of the as-synthesized CeO2 particles are shown in Figure 2. It can be seen from Figure 2a that the as-synthesized CeO2 microspheres have diameters of about 1 μm. These CeO2 microspheres consist of many nanosheets with thicknesses of about 15 nm. The mesopores with about 20-nm pore sizes are spread over the nanosheets. The lattice fringes in the high-resolution TEM (HRTEM) image (Figure 2c) show a spacing of 0.31 nm, corresponding to the (1 1 1) plane of cubic CeO2. The selected area electron diffraction (SAED) pattern (Figure 2d) indicates that the microspheres are composed of low-crystalline CeO2 nanocrystals.
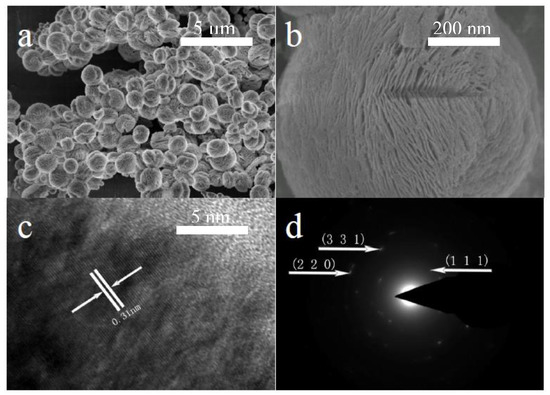
Figure 2.
(a,b) SEM images, (c) HRTEM image, and (d) SAED pattern of the 3D hierarchical structured CeO2.
The nitrogen adsorption and desorption isotherms of the as-prepared samples and the corresponding pore size distribution curve calculated by the Barret-Joyner-Halenda (BJH) method are shown in Figure 3. The nitrogen adsorption and desorption isotherms exhibit a slim hysteresis loop at a relative pressure of >0.2, which is the type-II curve. The calculated Brunauer-Emmett-Teller (BET) surface area of the as-synthesized CeO2 is about 36.8 m2g–1. The average pore size calculated by the BJH method is 14.9 nm.
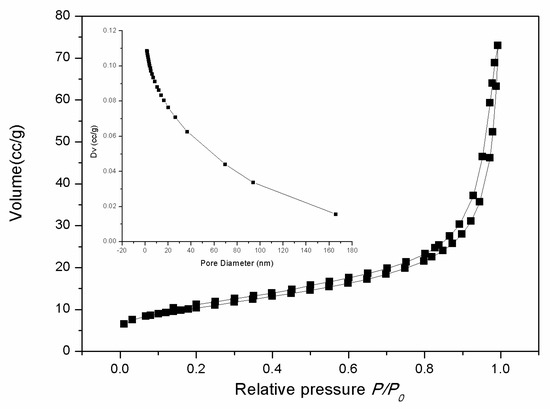
Figure 3.
Nitrogen adsorption-desorption isotherm of 3D hierarchical structured CeO2. The inset shows the pore size distribution curve obtained from the desorption data.
3.2. Effects of Synthesis Conditions on the Formation of 3D Hierarchical Structured CeO2 and the Possible Formation Mechanism
To investigate the evolution of flower-like CeO2 particles, the samples obtained after different reaction times were characterized by SEM (Figure 4). The reaction temperature and the dosages of CeCl3·7H2O and PVP were kept constant (270 °C, 0.01 M, and 0.02 M, respectively). As we can see in Figure 4a, spherical particles were obtained in the early stage. After 12 h of hydrothermal treatment, the sample (Figure 4b) evolved into spheres on which many scrappy grains grew. We speculate that PVP at the surface of the spheres decomposed gradually at such a high temperature and pressure, and simultaneously, tiny nanoparticles on the surface of the spheres began to grow into nanosheets. As seen in Figure 4c, all spheres have transformed into flower-like CeO2 particles. Based on these observations, the possible formation mechanism of the 3D hierarchical structured CeO2 can be speculated. The schematic mechanism for the 3D hierarchical structured CeO2 obtained during different hydrothermal stages is illustrated in Figure 5. At an early stage, Ce3+ ions were oxidized gradually by O2 present in the aqueous solution to form small CeO2 nanocrystals. Then, the small CeO2 nanocrystals interacted with PVP and self-assembled as building blocks into spherical particles. As the temperature of the hydrothermal system increased, the PVP at the surface of the spherical particles began to decompose and small nanoparticles began to grow into nanosheets via Ostwald ripening. Due to Ostwald ripening, more were nanosheets formed, and after 24 h of hydrothermal treatment, the PVP completely decomposed and 3D hierarchical structured CeO2 particles were formed.

Figure 4.
SEM images of CeO2 samples prepared at 270 °C for different reaction times: (a) 6 h; (b) 12 h; and (c) 24 h.
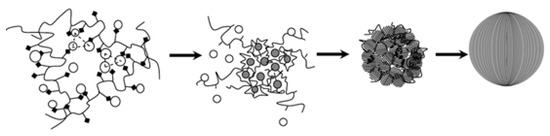
Figure 5.
Schematic illustrating the formation of 3D hierarchical structured CeO2.
3.3. Catalytic Performance of 3D Hierarchical Structured CeO2 for CO Combustion
Catalytic application is an important direction for CeO2 researches because the oxygen storage capacity of CeO2 is associated with its ability to undergo a facile conversion between Ce(III) and Ce(IV). Therefore, the catalytic activity of the as-prepared 3D hierarchical structured CeO2 was tested by CO oxidation. As shown in Figure 6, the 3D hierarchical structured CeO2 exhibits better activity toward CO oxidation than commercial CeO2 (purchased from Beijing Yili Chemical Reagent Co. Ltd., Beijing, China) does. The 50% conversion temperature of the 3D hierarchical structured CeO2 is about 320 °C, while that of the commercial CeO2 is higher than 380 °C.
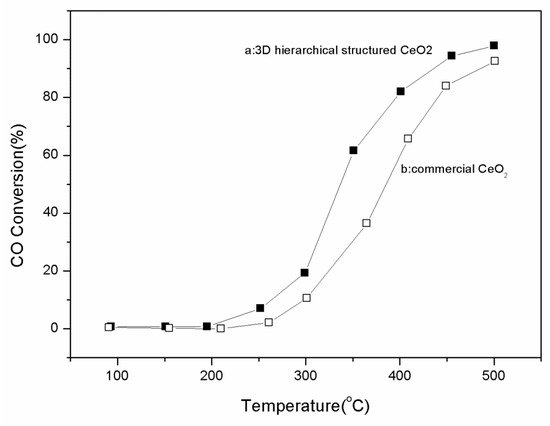
Figure 6.
CO conversion rate in the presence of (a) as-prepared 3D hierarchical structured CeO2, and (b) commercial CeO2.
The sample was further characterized by XPS and the Ce 3d electron core level spectra are shown in Figure 7. The four main 3d5/2 features at 882.7, 885.2, 888.5, and 898.3 eV correspond to V, V’, V’’, and V’’’ components, respectively. The 3d3/2 features at 901.3, 903.4, 907.3, and 916.9 eV correspond to U, U’, U’’, and U’’’ components [15], respectively. The signals V’ and U’ are characteristic of Ce(III) [16]. According to the ratio of the area for Ce3+ peaks to the whole peak area in Ce 3d region, the amount of Ce3+ of 3D hierarchical structured CeO2 is 51.8%. The amount of Ce3+ of commercial CeO2 is 13.2%. The 3D hierarchical structured CeO2 has a much higher Ce3+ concentration, which implies a much higher concentration of oxygen defects compared with commercial CeO2. A large amount of oxygen defects enhances the conversion between Ce(III) and Ce(IV), thereby supplying more reactive oxygen. Thus, the special structure of 3D hierarchical structured CeO2 provides more active sites for CO oxidation.
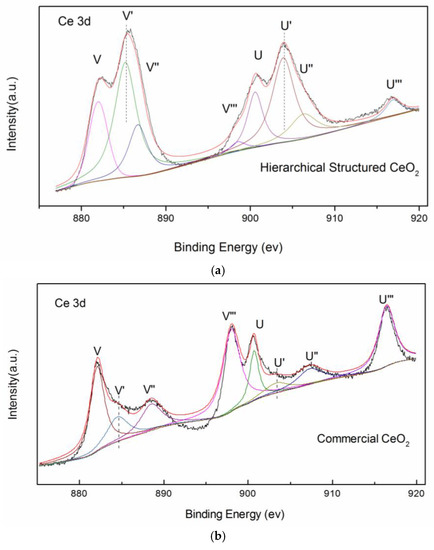
Figure 7.
X-ray photoelectron spectra of Ce 3D regions of 3D (a) hierarchical structured CeO2 and (b) commercial CeO2.
4. Conclusions
In summary, a simple and economical hydrothermal route was presented to synthesize 3D hierarchical structured CeO2 using CeCl3·7H2O and PVP. The 3D hierarchical structured CeO2 has a beautiful flower-like structure, which consists of many nanosheets. A two-stage growth process was identified for the formation of 3D hierarchical structured CeO2, and Ostwald ripening was found to play an important role in the transformation of the nanoparticles into nanosheets. The 3D hierarchical structured CeO2 exhibited a higher catalytic activity toward CO oxidation compared with commercial CeO2.
Author Contributions
Conceptualization: G.S. and Q.W.; methodology: G.S.; software: Z.W.; validation: G.S., Q.W.; formal analysis: G.S.; investigation: G.S.; resources: Q.W.; data curation: G.S.; writing—original draft preparation: G.S.; writing—review and editing: M.L.; visualization, M.L.; supervision: Q.W.; project administration: G.S.; funding acquisition: G.S.
Funding
This work was supported financially by the National Natural Science Foundation of China (no. 51402062).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cai, L.; Chen, S.H.; Zhao, M.; Gong, M.C.; Shi, Z.H.; Chen, Y.Q. Pd supported three-way catalyst: Preparation of CeO2-ZrO2-BaO support and catalytic performance. Chin. J. Inorg. Chem. 2009, 25, 474–479. [Google Scholar]
- Shimazu, M.; Isobe, T.; Ando, S.; Hiwatashi, K.; Ueno, A.; Yamaji, K.; Kishimoto, H.; Yokokawa, H.; Nakajima, A.; Okada, K. Stability of Sc2O3 and CeO2 co-doped ZrO2 electrolyte during the operation of solid oxide fuel cells. Solid State Ion. 2011, 182, 120–126. [Google Scholar] [CrossRef]
- Lee, D.H.; Cha, K.S.; Lee, Y.S.; Kang, K.S.; Park, C.S.; Kim, Y.H. Effects of CeO2 additive on redox characteristics of Fe-based mixed oxide mediums for storage and production of hydrogen. Int. J. Hydrogen Energy 2009, 34, 1417–1422. [Google Scholar] [CrossRef]
- Sanghavi, R.; Nandasiri, M.; Kuchibhatla, S.; Jiang, W.L.; Varga, T.; Nachimuthu, P.; Engelhard, M.H.; Shutthanandan, V.; Thevuthasan, S.; Kayani, A.; et al. Thickness dependency of thin-film samaria-doped ceria for oxygen sensing. IEEE Sens. J. 2011, 11, 217–224. [Google Scholar] [CrossRef]
- Lykaki, M.; Pachatouridou, E.; Iliopoulou, E.; Carabineiro, S.A.C.; Konsolakis, M. Impact of the synthesis parameters on the solid state properties and the CO oxidation performance of ceria nanoparticles. RSC Adv. 2017, 7, 6160–6169. [Google Scholar] [CrossRef]
- Lykaki, M.; Pachatouridou, E.; Carabineiro, S.A.C.; Iliopoulou, E.; Andriopoulou, C.; Kallithrakas-Kontos, N.; Boghosian, S.; Konsolakis, M. Ceria nanoparticles shape effects on the structural defects and surface chemistry: Implications in CO oxidation by Cu/CeO2 catalysts. Appl. Catal. B Environ. 2018, 230, 18–28. [Google Scholar] [CrossRef]
- Meng, F.M.; Lu, F.; Wang, L.N.; Cui, J.B.; Lü, J.G. Novel fabrication and synthetic mechanism of CeO2 nanorods by a chloride-assisted hydrothermal method. Sci. Adv. Mater. 2012, 4, 1018–1023. [Google Scholar] [CrossRef]
- Deus, R.C.; Cilense, M.; Foschini, C.R.; Ramirez, M.A.; Longo, E.; Simões, A.Z. Influence of mineralizer agents on the growth of crystalline CeO2 nanospheres by the microwave-hydrothermal method. J. Alloys Compd. 2013, 550, 245–251. [Google Scholar] [CrossRef]
- Zhao, X.B.; You, J.; Lu, X.W.; Chen, Z.G. Hydrothermal synthesis, characterization and property of CeO2 nanotube. J. Inorg. Mater. 2011, 26, 159–164. [Google Scholar] [CrossRef]
- He, L.A.; Yu, Y.B.; Zhang, C.B.; He, H. Complete catalytic oxidation of o-xylene over CeO2 nanocubes. J. Environ. Sci. China 2011, 23, 60–165. [Google Scholar] [CrossRef]
- Cao, C.Y.; Cui, Z.M.; Chen, C.Q.; Song, W.G.; Cai, W. Ceria hollow nanospheres produced by a template-free microwave-assisted hydrothermal method for heavy metal ion removal and catalysis. J. Phys. Chem. C 2010, 114, 9865–9870. [Google Scholar] [CrossRef]
- Zhong, L.S.; Hu, J.S.; Cao, A.M.; Liu, Q.; Song, W.G.; Wan, L.J. 3D flowerlike ceria micro/nanocomposite structure and its application for water treatment and CO removal. Chem. Mater. 2007, 19, 1648–1655. [Google Scholar] [CrossRef]
- Li, H.F.; Lu, G.Z.; Dai, Q.G.; Wang, Y.Q.; Guo, Y.; Guo, Y.L. Hierarchical organization and catalytic activity of high-surface-area mesoporous ceria microspheres prepared via hydrothermal routes. ACS Appl. Mater. Interfaces 2010, 2, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, X.; Li, W.; Xie, S.; Zhai, T.; Yu, M.; Gan, J.; Lu, X. Hierarchical CeO2 nanospheres as highly-efficient adsorbents for dye removal. New J. Chem. 2013, 37, 585–588. [Google Scholar] [CrossRef]
- Bêche, E.; Charvin, P.; Perarnau, D.; Abanades, S.; Flamant, G. Ce 3d XPS investigation of cerium oxides and mixed cerium oxide (CexTiyOz). Surf. Interface Anal. 2008, 40, 264–267. [Google Scholar] [CrossRef]
- Natile, M.M.; Glisenti, A. CoOx/CeO2 nanocomposite powders: Synthesis, characterization, and reactivity. Chem. Mater. 2005, 17, 3403–3414. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).