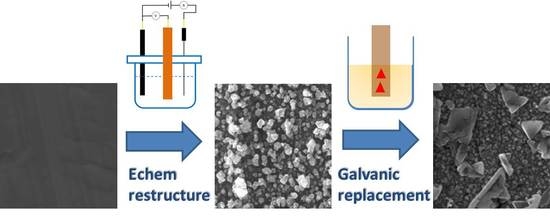
Galvanic Replacement of Electrochemically Restructured Copper Electrodes with Gold and Its Electrocatalytic Activity for Nitrate Ion Reduction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Preparation
2.3. Electrochemical Measurements
2.4. Surface Characterization
3. Results and Discussion
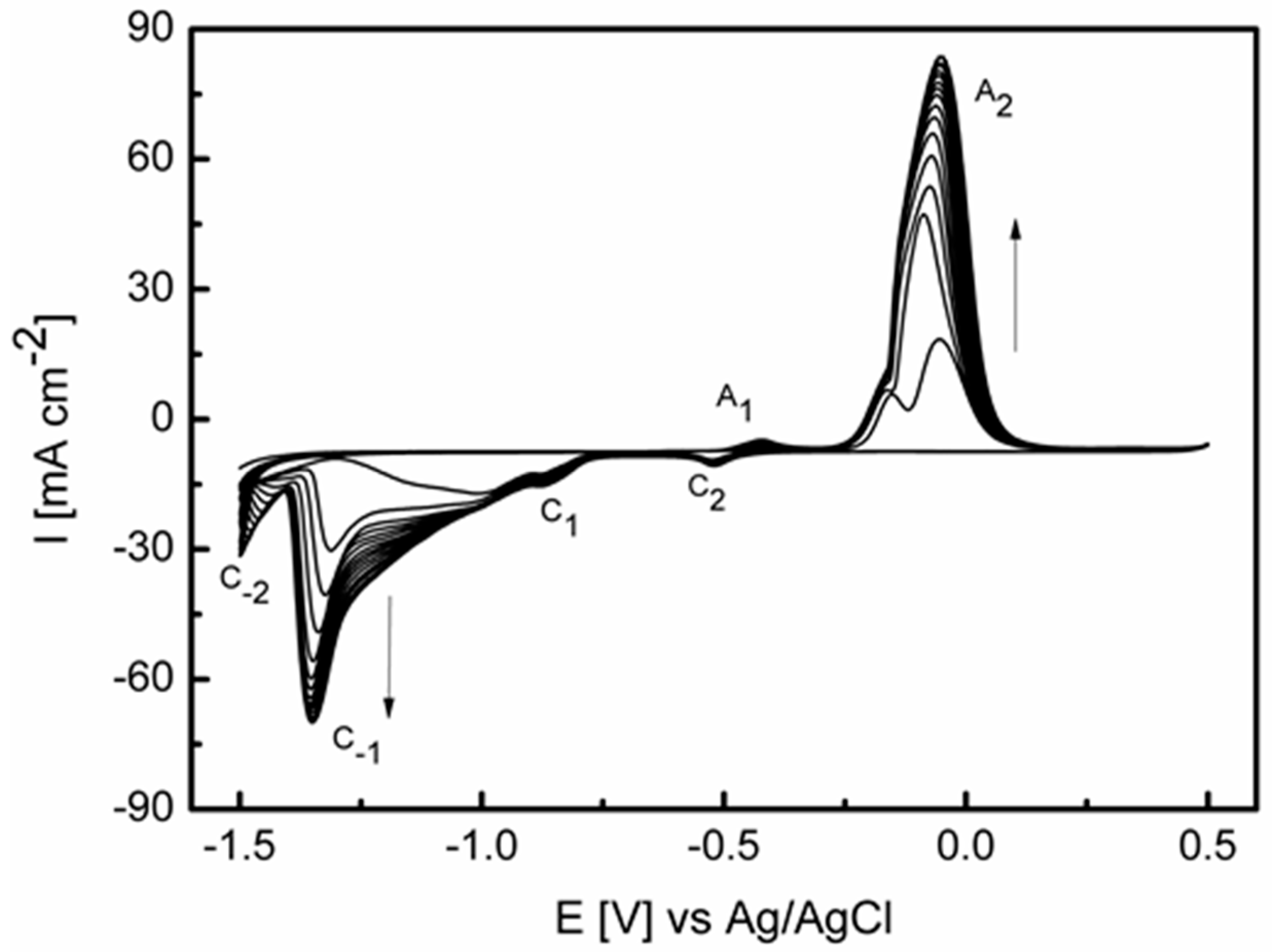
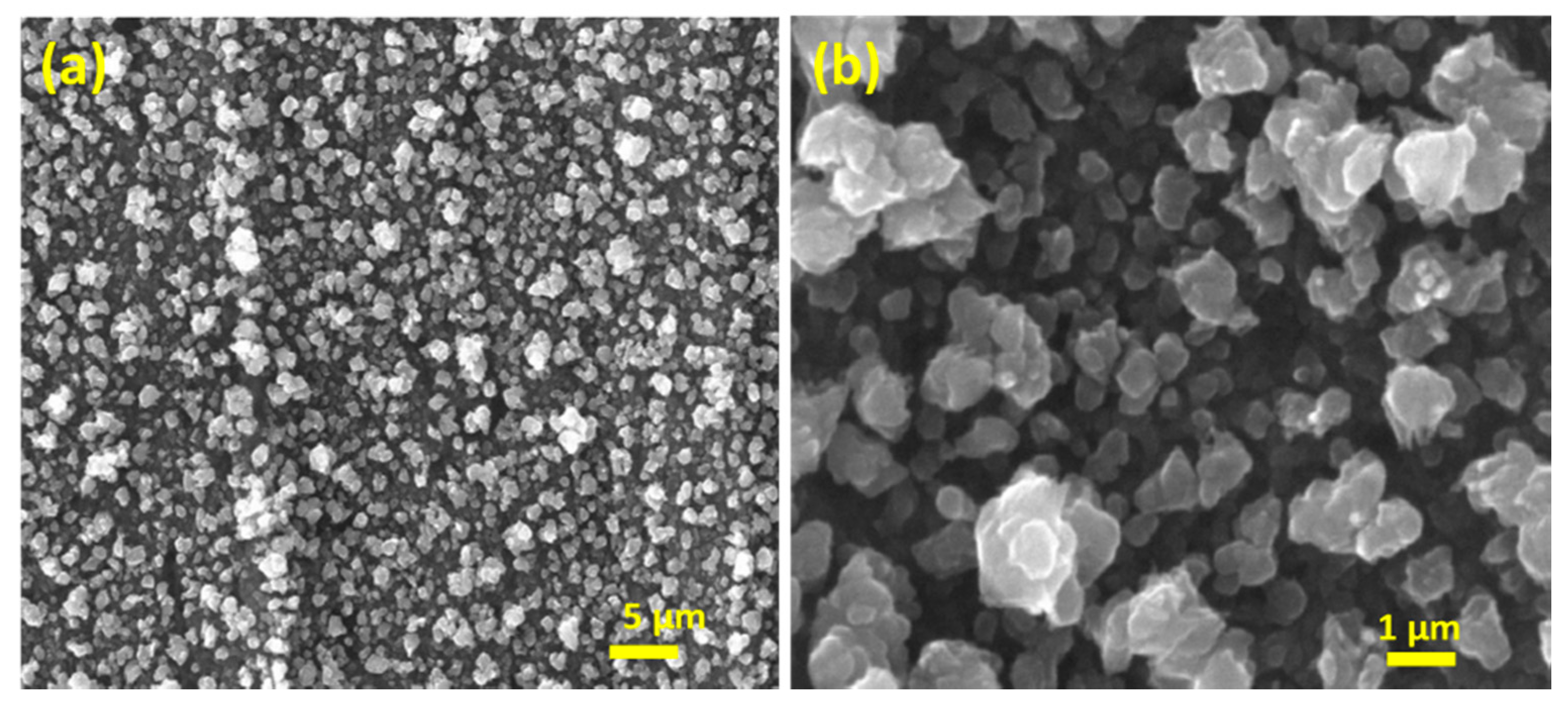
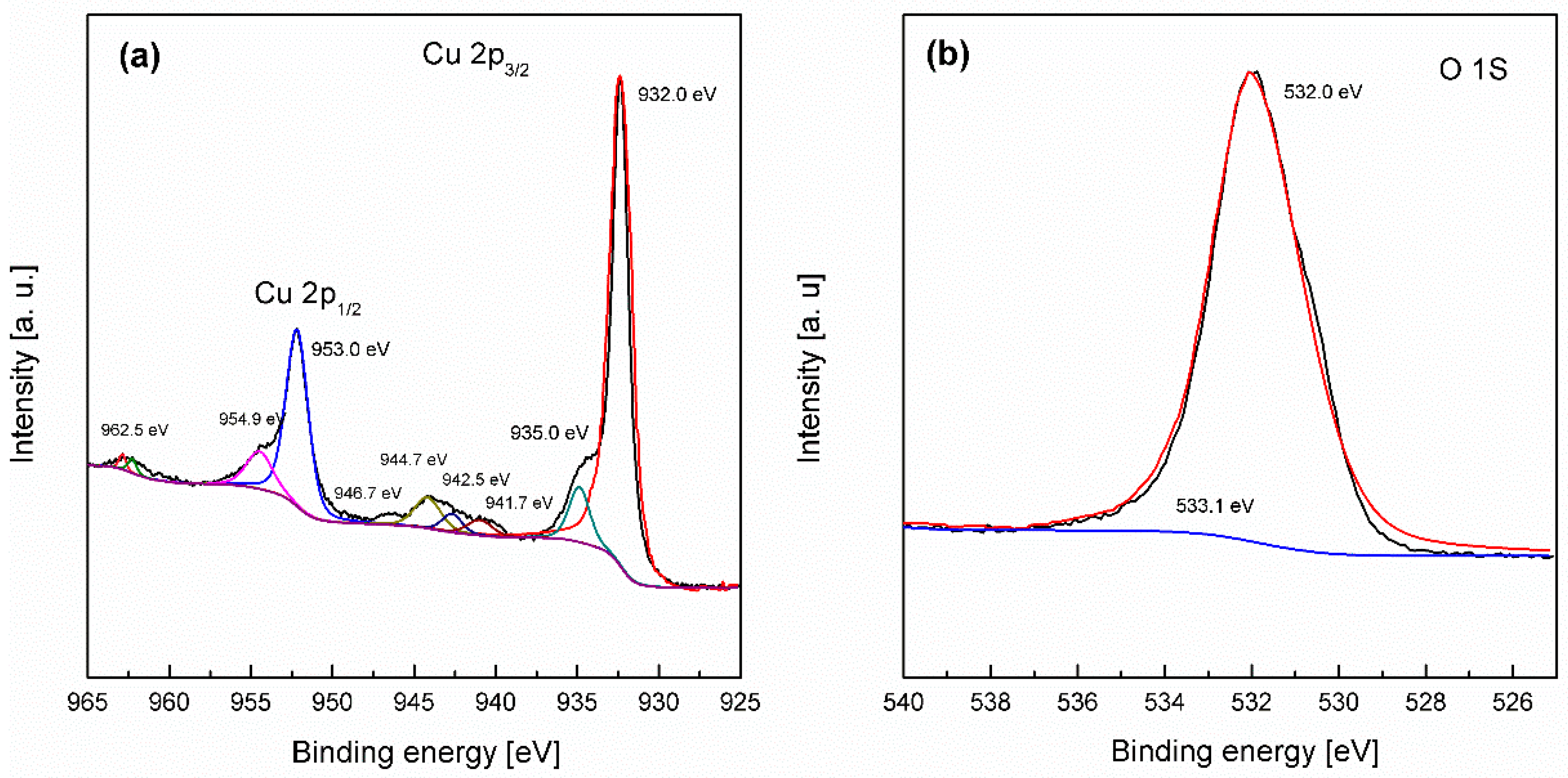
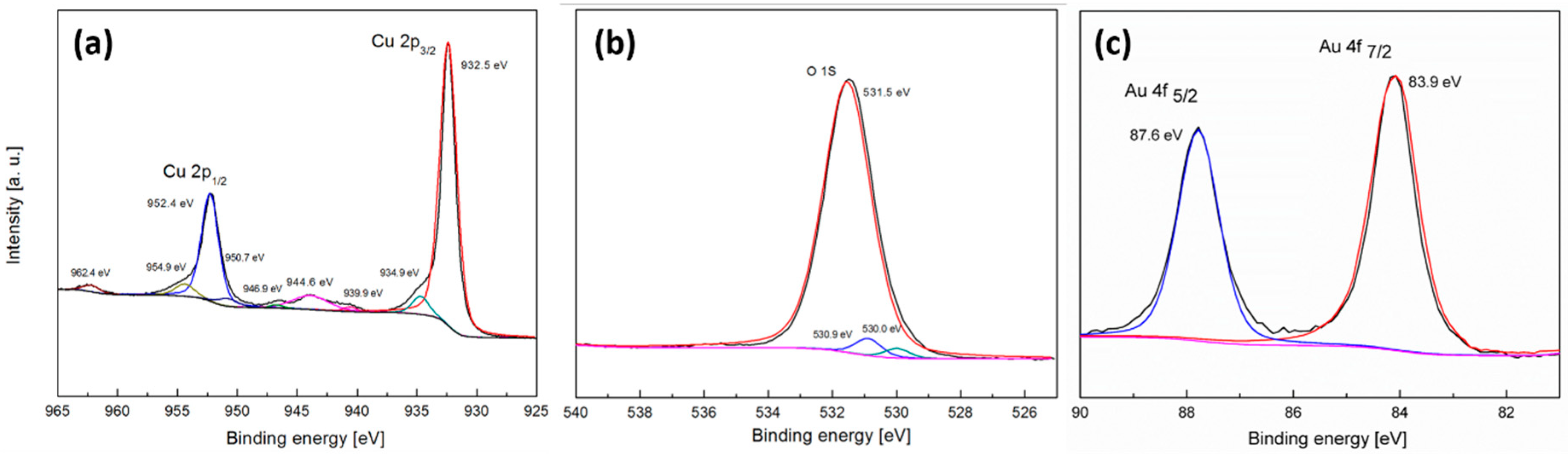
3.1. Preparation and Characterization of the Activated Electrode
3.2. Nitritate Ion Electrocatalysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Sun, I.-W.; Chang, J.-K. Electrodeposition of nanomaterials. In Springer Handbook of Electrochemical Energy; Breitkopf, C., Swider-Lyons, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 835–895. [Google Scholar]
- Davydov, A.D.; Volgin, V.M. Template electrodeposition of metals. Review. Russ. J. Electrochem. 2016, 52, 806–831. [Google Scholar] [CrossRef]
- Braun, T.M.; Schwartz, D.T. The emerging role of electrodeposition in additive manufacturing. Electrochem. Soc. Interface 2016, 25, 69–73. [Google Scholar] [CrossRef]
- Plowman, B.J.; Jones, L.A.; Bhargava, S.K. Building with bubbles: The formation of high surface area honeycomb-like films via hydrogen bubble templated electrodeposition. Chem. Commun. 2015, 51, 4331–4346. [Google Scholar] [CrossRef] [PubMed]
- Paul, S. Nanomaterials synthesis by electrodeposition techniques for high-energetic electrodes in fuel cell. Nanomater. Energy 2015, 4, 80–89. [Google Scholar] [CrossRef]
- Plowman, B.J.; Bhargava, S.K.; O’Mullane, A.P. Electrochemical fabrication of metallic nanostructured electrodes for electroanalytical applications. Analyst 2011, 136, 5107–5119. [Google Scholar] [CrossRef] [PubMed]
- Gamburg, Y.D.; Zangari, G. Theory and Practice of Metal Electrodeposition; Springer: New York, NY, USA, 2011. [Google Scholar]
- Plowman, B.J.; O’Mullane, A.P.; Bhargava, S.K. The active site behaviour of electrochemically synthesised gold nanomaterials. Faraday Discuss. 2011, 152, 43–62. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Zhou, Z.-Y.; Sun, S.-G.; Ding, Y.; Wang, Z.L. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity. Science 2007, 316, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Shao-Horn, Y.; Sheng, W.C.; Chen, S.; Ferreira, P.J.; Holby, E.F.; Morgan, D. Instability of supported platinum nanoparticles in low-temperature fuel cells. Top. Catal. 2007, 46, 285–305. [Google Scholar] [CrossRef]
- Shim, J.H.; Kim, J.; Lee, C.; Lee, Y. Electrocatalytic activity of gold and gold nanoparticles improved by electrochemical pretreatment. J. Phys. Chem. C 2011, 115, 305–309. [Google Scholar] [CrossRef]
- Huang, W.; Wang, M.; Zheng, J.; Li, Z. Facile fabrication of multifunctional three-dimensional hierarchical porous gold films via surface rebuilding. J. Phys. Chem. C 2009, 113, 1800–1805. [Google Scholar] [CrossRef]
- Balkis, A.; O’Mullane, A.P. Electrochemical restructuring of copper surfaces using organic additives and its effect on the electrocatalytic reduction of nitrate ions. Aust. J. Chem. 2015, 68, 1213–1220. [Google Scholar] [CrossRef]
- Kondo, K.; Akolkar, R.N.; Barkey, D.P. Copper Electrodeposition for Nanofabrication of Electronics Devices; Springer: New York, NY, USA, 2014. [Google Scholar]
- Shiddiky, M.J.A.; O’Mullane, A.P.; Zhang, J.; Burke, L.D.; Bond, A.M. Large amplitude fourier transformed ac voltammetric investigation of the active state electrochemistry of a copper/aqueous base interface and implications for electrocatalysis. Langmuir 2011, 27, 10302–10311. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jia, H.; Sun, Z.; Chen, H.; Xu, P.; Du, P. Nanostructured copper oxide electrodeposited from copper(ii) complexes as an active catalyst for electrocatalytic oxygen evolution reaction. Electrochem. Commun. 2014, 46, 1–4. [Google Scholar] [CrossRef]
- Garza, A.J.; Bell, A.T.; Head-Gordon, M. Mechanism of CO2 reduction at copper surfaces: Pathways to C2 products. ACS Catal. 2018, 8, 1490–1499. [Google Scholar] [CrossRef]
- Raciti, D.; Wang, C. Recent advances in CO2 reduction electrocatalysis on copper. ACS Energy Lett. 2018, 3, 1545–1556. [Google Scholar] [CrossRef]
- Abdallah, R.; Geneste, F.; Labasque, T.; Djelal, H.; Fourcade, F.; Amrane, A.; Taha, S.; Floner, D. Selective and quantitative nitrate electroreduction to ammonium using a porous copper electrode in an electrochemical flow cell. J. Electroanal. Chem. 2014, 727, 148–153. [Google Scholar] [CrossRef]
- Li, Q.; Xu, P.; Zhang, B.; Tsai, H.; Zheng, S.; Wu, G.; Wang, H.-L. Structure-dependent electrocatalytic properties of Cu2O nanocrystals for oxygen reduction reaction. J. Phys. Chem. C 2013, 117, 13872–13878. [Google Scholar] [CrossRef]
- Huan, T.N.; Rousse, G.; Zanna, S.; Lucas, I.T.; Xu, X.; Menguy, N.; Mougel, V.; Fontecave, M. A dendritic nanostructured copper oxide electrocatalyst for the oxygen evolution reaction. Angew. Chem. Int. Ed. 2017, 56, 4792–4796. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.; Wang, D.; Su, D.S.; Prati, L. New challenges in gold catalysis: Bimetallic systems. Catal. Sci. Technol. 2015, 5, 55–68. [Google Scholar] [CrossRef]
- Porter, N.S.; Wu, H.; Quan, Z.; Fang, J. Shape-control and electrocatalytic activity-enhancement of pt-based bimetallic nanocrystals. Acc. Chem. Res. 2013, 46, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Plowman, B.J.; Najdovski, I.; Pearson, A.; O’Mullane, A.P. Decoration of active sites to create bimetallic surfaces and its implication for electrochemical processes. Faraday Discuss. 2013, 164, 199. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Plowman, B.J.; Field, M.R.; Bhargava, S.K.; O’Mullane, A.P. Exploiting the facile oxidation of evaporated gold films to drive electroless silver deposition for the creation of bimetallic Au/Ag surfaces. ChemElectroChem 2013, 1, 76–82. [Google Scholar] [CrossRef]
- Yu, W.; Porosoff, M.D.; Chen, J.G. Review of pt-based bimetallic catalysis: From model surfaces to supported catalysts. Chem. Rev. 2012, 112, 5780–5817. [Google Scholar] [CrossRef] [PubMed]
- Balkis, A.; O’Mullane, A.P. Direct electrochemical formation of alloyed AuPt nanostructured electrocatalysts for the oxidation of formic acid. Mater. Chem. Phys. 2014, 143, 747–753. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, Y.; Vasileff, A.; Jiao, Y.; Chen, S.; Song, L.; Zheng, B.; Zheng, Y.; Qiao, S.-Z. Strain effect in bimetallic electrocatalysts in the hydrogen evolution reaction. ACS Energy Lett. 2018, 3, 1198–1204. [Google Scholar] [CrossRef]
- Najdovski, I.; Selvakannan, P.R.; O’Mullane, A.P. Electrochemical formation of Cu/Ag surfaces and their applicability as heterogeneous catalysts. RSC Adv. 2014, 4, 7207–7215. [Google Scholar] [CrossRef]
- Najdovski, I.; Selvakannan, P.R.; O’Mullane, A.P.; Bhargava, S.K. Rapid synthesis of porous honeycomb Cu/Pd through a hydrogen-bubble templating method. Chem. Eur. J. 2011, 17, 10058–10063. [Google Scholar] [CrossRef] [PubMed]
- Castegnaro, M.V.; Paschoalino, W.J.; Fernandes, M.R.; Balke, B.; MAlves, M.C.; Ticianelli, E.A.; Morais, J. Pd–m/c (m = Pd, Cu, Pt) electrocatalysts for oxygen reduction reaction in alkaline medium: Correlating the electronic structure with activity. Langmuir 2017, 33, 2734–2743. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Li, D.; Ding, Y.; Zhu, W.; Guo, S.; Wang, Z.L.; Sun, S. Core/shell Au/CuPt nanoparticles and their dual electrocatalysis for both reduction and oxidation reactions. J. Am. Chem. Soc. 2014, 136, 5745–5749. [Google Scholar] [CrossRef] [PubMed]
- Najdovski, I.; Selvakannan, P.R.; Bhargava, S.K.; O’Mullane, A.P. Formation of nanostructured porous Cu-Au surfaces: The influence of cationic sites on (electro)-catalysis. Nanoscale 2012, 4, 6298–6306. [Google Scholar] [CrossRef] [PubMed]
- Hasnat, M.A.; Ishibashi, I.; Sato, K.; Agui, R.; Yamaguchi, T.; Ikeue, K.; Machida, M. Electrocatalytic reduction of nitrate using Cu-Pd and Cu-Pt cathodes/H+-conducting solid polymer electrolyte membrane assemblies. Bull. Chem. Soc. Jpn. 2008, 81, 1675–1680. [Google Scholar] [CrossRef]
- Sun, X.; Huang, B.; Cui, X.; Bin, E.; Feng, Y.; Huang, X. Platinum–copper rhombic dodecahedral nanoframes with tunable channels as efficient bifunctional electrocatalysts for fuel-cell reactions. ChemCatChem 2018, 10, 931–935. [Google Scholar] [CrossRef]
- Papaderakis, A.; Mintsouli, I.; Georgieva, J.; Sotiropoulos, S. Electrocatalysts prepared by galvanic replacement. Catalysts 2017, 7, 80. [Google Scholar] [CrossRef]
- Xia, X.; Wang, Y.; Ruditskiy, A.; Xia, Y. 25th anniversary article: Galvanic replacement: A simple and versatile route to hollow nanostructures with tunable and well-controlled properties. Adv. Mater. 2013, 25, 6313–6333. [Google Scholar] [CrossRef] [PubMed]
- Niu, K.-Y.; Kulinich, S.A.; Yang, J.; Zhu, A.L.; Du, X.-W. Galvanic replacement reactions of active-metal nanoparticles. Chem. Eur. J. 2012, 18, 4234–4241. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Chen, J.; Skrabalak, S.E.; Xia, Y. Galvanic replacement reaction: A simple and powerful route to hollow and porous metal nanostructures. J. Nanoeng. Nanosyst. 2007, 221, 1–16. [Google Scholar] [CrossRef]
- Hoshyargar, F.; Crawford, J.; O’Mullane, A.P. Galvanic replacement of the liquid metal galinstan. J. Am. Chem. Soc. 2017, 139, 1464–1471. [Google Scholar] [CrossRef] [PubMed]
- Giorgetti, E.; Marsili, P.; Canton, P.; Muniz-Miranda, M.; Caporali, S.; Giammanco, F. Cu/Ag-based bifunctional nanoparticles obtained by one-pot laser-assisted galvanic replacement. J. Nanopart. Res. 2012, 15, 1–12. [Google Scholar] [CrossRef]
- O’Mullane, A.P.; Ippolito, S.J.; Bond, A.M.; Bhargava, S.K. A study of localised galvanic replacement of copper and silver films with gold using scanning electrochemical microscopy. Electrochem. Commun. 2010, 12, 611. [Google Scholar] [CrossRef]
- Lu, X.; McKiernan, M.; Peng, Z.; Lee, E.P.; Yang, H.; Xia, Y. Noble-metal nanotubes prepared via a galvanic replacement reaction between Cu nanowires and aqueous HAuCl4, H2PtCl6, or Na2PdCl4. Sci. Adv. Mater. 2010, 2, 413–420. [Google Scholar] [CrossRef]
- Bansal, V.; Jani, H.; Du Plessis, J.; Coloe, P.J.; Bhargava, S.K. Galvanic replacement reaction on metal films: A one-step approach to create nanoporous surfaces for catalysis. Adv. Mater. 2008, 20, 717–723. [Google Scholar] [CrossRef]
- Bakthavatsalam, R.; Kundu, J. A galvanic replacement-based Cu2O self-templating strategy for the synthesis and application of Cu2O-Ag heterostructures and monometallic (Ag) and bimetallic (Au-Ag) hollow mesocages. CrystEngComm 2017, 19, 1669–1679. [Google Scholar] [CrossRef]
- Zhang, Z.; Song, R.; Cao, T.; Huang, W. Morphology-dependent structures and catalytic performances of au nanostructures on Cu2O nanocrystals synthesized by galvanic replacement reaction. J. Energy Chem. 2016, 25, 1086–1091. [Google Scholar] [CrossRef]
- Xiong, L.; Li, S.; Zhang, B.; Du, Y.; Miao, P.; Ma, Y.; Han, Y.; Zhao, H.; Xu, P. Galvanic replacement-mediated synthesis of hollow Cu2O -Au nanocomposites and au nanocages for catalytic and SERS applications. RSC Adv. 2015, 5, 76101–76106. [Google Scholar] [CrossRef]
- Susman, M.D.; Popovitz-Biro, R.; Vaskevich, A.; Rubinstein, I. pH-dependent galvanic replacement of supported and colloidal Cu2O nanocrystals with gold and palladium. Small 2015, 11, 3942–3953. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.H.; Yu, T.; Yu, S.-H.; Lim, B.; Ko, K.-T.; Willinger, M.-G.; Seo, D.-H.; Kim, B.H.; Cho, M.G.; Park, J.-H.; et al. Galvanic replacement reactions in metal oxide nanocrystals. Science 2013, 340, 964–968. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Xu, P.; Zhang, B.; Wu, G.; Zhao, H.; Fu, E.; Wang, H.-L. Self-supported Pt nanoclusters via galvanic replacement from Cu2O nanocubes as efficient electrocatalysts. Nanoscale 2013, 5, 7397–7402. [Google Scholar] [CrossRef] [PubMed]
- Badea, G.E. Electrocatalytic reduction of nitrate on copper electrode in alkaline solution. Electrochim. Acta 2009, 54, 996–1001. [Google Scholar] [CrossRef]
- Duca, M.; Koper, M.T.M. Powering denitrification: The perspectives of electrocatalytic nitrate reduction. Energy Environ. Sci. 2012, 5, 9726–9742. [Google Scholar] [CrossRef]
- Davis, J.; Moorcroft, M.J.; Wilkins, S.J.; Compton, R.G.; Cardosi, M.F. Electrochemical detection of nitrate and nitrite at a copper modified electrode. Analyst 2000, 125, 737–742. [Google Scholar] [CrossRef]
- Stortini, A.M.; Moretto, L.M.; Mardegan, A.; Ongaro, M.; Ugo, P. Arrays of copper nanowire electrodes: Preparation, characterization and application as nitrate sensor. Sens. Actuators B 2015, 207, 186–192. [Google Scholar] [CrossRef]
- Mattarozzi, L.; Cattarin, S.; Comisso, N.; Guerriero, P.; Musiani, M.; Vázquez-Gómez, L.; Verlato, E. Electrochemical reduction of nitrate and nitrite in alkaline media at cuni alloy electrodes. Electrochim. Acta 2013, 89, 488–496. [Google Scholar] [CrossRef]
- Burke, D.; O’Mullane, A.; Lodge, V.; Mooney, M. Auto-inhibition of hydrogen gas evolution on gold in aqueous acid solution. J. Solid State Electrochem. 2001, 5, 319–327. [Google Scholar] [CrossRef]
- Burke, L.D.; Collins, J.A. Role of surface defects in the electrocatalytic behaviour of copper in base. J. Appl. Electrochem. 1999, 29, 1427–1438. [Google Scholar] [CrossRef]
- Burke, L.D.; Ryan, T.G. The participation of interfacial hydrous oxide species in some anodic reactions at copper electrodes in base. J. Electrochem. Soc. 1990, 137, 1358–1364. [Google Scholar] [CrossRef]
- Burke, L.D.; Ahern, M.J.G.; Ryan, T.G. An investigation of the anodic behavior of copper and its anodically produced oxides in aqueous solutions of high pH. J. Electrochem. Soc. 1990, 137, 553–561. [Google Scholar] [CrossRef]
- Burke, L.D.; Kinsella, L.M.; O’Connell, A.M. Importance of metastable states in electrocatalytic processes at metal surfaces in aqueous media. Russ. J. Electrochem. 2004, 40, 1105–1114. [Google Scholar] [CrossRef]
- Yu, Z.-J.; Dai, Y.; Chen, W. Electrochemical deposited nanoflakes Co(OH)2 porous films for electrochemical capacitors. J. Chin. Chem. Soc. 2010, 57, 423–428. [Google Scholar] [CrossRef]
- Balram, A.; Zhang, H.; Santhanagopalan, S. Enhanced oxygen evolution reaction electrocatalysis via electrodeposited amorphous α-phase nickel-cobalt hydroxide nanodendrite forests. ACS Appl. Mater. Interfaces 2017, 9, 28355–28365. [Google Scholar] [CrossRef] [PubMed]
- Cuya Huaman, J.L.; Sato, K.; Kurita, S.; Matsumoto, T.; Jeyadevan, B. Copper nanoparticles synthesized by hydroxyl ion assisted alcohol reduction for conducting ink. J. Mater. Chem. 2011, 21, 7062–7069. [Google Scholar] [CrossRef]
- Reyter, D.; Odziemkowski, M.; Belanger, D.; Roue, L. Electrochemically activated copper electrodes. Surface characterization, electrochemical behavior, and properties for electroreduction of nitrate. J. Electrochem. Soc. 2007, 154, K36–K44. [Google Scholar] [CrossRef]
- Shankar, S.S.; Bhargava, S.; Sastry, M. Synthesis of gold nanospheres and nanotriangles by the turkevich approach. J. Nanosci. Nanotechnol. 2005, 5, 1721–1727. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.J. Chloride complexes of copper(i) chloride in aqueous solution. J. Phys. Chem. 1980, 84, 2241–2246. [Google Scholar] [CrossRef]
- Herranz, T.; Deng, X.; Cabot, A.; Alivisatos, P.; Liu, Z.; Soler-Illia, G.; Salmeron, M. Reactivity of Au nanoparticles supported over SiO2 and TiO2 studied by ambient pressure photoelectron spectroscopy. Catal. Today 2009, 143, 158–166. [Google Scholar] [CrossRef]
- Guo, X.H.; Ma, J.Q.; Ge, H.G. Synthesis and characterization of Cu2O/Au and its application in catalytic reduction of 4-nitrophenol. Russ. J. Phys. Chem. A 2015, 89, 1374–1380. [Google Scholar] [CrossRef]
- Bracey, C.L.; Ellis, P.R.; Hutchings, G.J. Application of copper-gold alloys in catalysis: Current status and future perspectives. Chem. Soc. Rev. 2009, 38, 2231–2243. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, D.R.; Alfonso, D.R.; Tafen, D.N.; Wang, C.; Zhou, Y.; Yu, Y.; Lekse, J.W.; Deng, X.; Espinoza, V.; Trindell, J.; et al. Selective electrocatalytic reduction of CO2 into CO at small, thiol-capped Au/Cu nanoparticles. J. Phys. Chem. C 2018. [Google Scholar] [CrossRef]
- Monzó, J.; Malewski, Y.; Kortlever, R.; Vidal-Iglesias, F.J.; Solla-Gullón, J.; Koper, M.T.M.; Rodriguez, P. Enhanced electrocatalytic activity of Au@Cu core@shell nanoparticles towards CO2 reduction. J. Mater. Chem. A 2015, 3, 23690–23698. [Google Scholar] [CrossRef]
- Tamašauskaitė-Tamašiūnaitė, L.; Balčiūnaitė, A.; Zabielaitė, A.; Stankevičienė, I.; Kepenienė, V.; Selskis, A.; Juškėnas, R.; Norkus, E. Investigation of electrocatalytic activity of the nanostructured Au–Cu catalyst deposited on the titanium surface towards borohydride oxidation. J. Electroanal. Chem. 2013, 700, 1–7. [Google Scholar] [CrossRef]
- Collins, J.A. The IHOAM Model of Electrocatalysis: With Particular Reference to Copper; NUI, Department of Chemistry, UCC: Cork, Ireland, 1999. [Google Scholar]
- Burke, L.D.; Casey, J.K.; Morrissey, J.A.; Murphy, M.M. Incipient hydrous oxide/adatom mediator (IHOAM) model of electrocatalysis. Bull. Electrochem. 1991, 7, 506–511. [Google Scholar]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balkis, A.; Crawford, J.; O’Mullane, A.P. Galvanic Replacement of Electrochemically Restructured Copper Electrodes with Gold and Its Electrocatalytic Activity for Nitrate Ion Reduction. Nanomaterials 2018, 8, 756. https://doi.org/10.3390/nano8100756
Balkis A, Crawford J, O’Mullane AP. Galvanic Replacement of Electrochemically Restructured Copper Electrodes with Gold and Its Electrocatalytic Activity for Nitrate Ion Reduction. Nanomaterials. 2018; 8(10):756. https://doi.org/10.3390/nano8100756
Chicago/Turabian StyleBalkis, Ali, Jessica Crawford, and Anthony P. O’Mullane. 2018. "Galvanic Replacement of Electrochemically Restructured Copper Electrodes with Gold and Its Electrocatalytic Activity for Nitrate Ion Reduction" Nanomaterials 8, no. 10: 756. https://doi.org/10.3390/nano8100756
APA StyleBalkis, A., Crawford, J., & O’Mullane, A. P. (2018). Galvanic Replacement of Electrochemically Restructured Copper Electrodes with Gold and Its Electrocatalytic Activity for Nitrate Ion Reduction. Nanomaterials, 8(10), 756. https://doi.org/10.3390/nano8100756