Abstract
We report a novel approach to fabricating high-performance and robust quantum dot light-emitting diodes (QLEDs) utilizing sputtered SnO2 thin films as the electron transport layer (ETL). While conventional solution-processed ZnMgO NP ETLs face limitations in mass production, the sputtering process offers advantages for uniform and reproducible thin film deposition. Herein, the structural, optical, and electrical properties of SnO2 thin films were optimized by controlling the Ar/O2 ratio and substrate heating temperature during sputtering. SnO2 thin films with O2 gas improve charge balancing in QLEDs by lowering the conduction band minimum. Furthermore, it was observed that oxygen vacancies in SnO2 function as exciton quenching sites, which directly impacts the long-term stability of the device. QLEDs fabricated under optimal conditions (Ar/O2 = 35:5, 200 °C heating) achieved a peak luminance of 99,212 cd/m2 and a current efficiency of 21.17 cd/A with excellent device stability. The findings suggest that sputtered SnO2 ETLs are a highly promising technology for the commercial production of QLEDs.
1. Introduction
Colloidal quantum dots (QDs) are semiconductor nanocrystals that have emerged as highly promising materials for next-generation display technologies. Owing to the quantum confinement effect, their emission wavelength can be precisely tuned by controlling their size, exhibiting exceptional color purity with a narrow full-width at half-maximum [1,2,3,4]. These unique optoelectronic properties have propelled the development of quantum dot light-emitting diodes (QLEDs), which have demonstrated remarkable performance advancements since their first report in 1994 [5,6,7,8]. Through the optimization of QD core/shell structures and the engineering of charge transport layers, recent advancements in QLED performance, characterized by external quantum efficiencies exceeding 22%, have solidified their status as a benchmark technology for the upcoming era of lighting and displays [9].
The performance of a QLED is critically dependent on the efficient injection and transport of charge carriers into the emitting layer (EML). To this end, inorganic metal oxide nanoparticles (NPs), particularly ZnMgO, have been extensively employed as electron transport layers (ETLs), replacing conventional organic materials [10,11,12]. Solution-processed ZnMgO NP ETLs offer suitable energy level alignment with QDs and facile fabrication, which has led to significant improvements in device luminance and efficiency. However, the solution-based spin-coating method presents inherent challenges for commercial viability. Residual hydroxyl and acetate ligands on the NP surface can act as charge traps, impeding electron transport, while the aggregation of NPs in colloidal solutions often compromises the uniformity and reproducibility of the film, especially over large areas [13,14].
To overcome these limitations, SnO2 has been identified as a superior alternative ETL material to ZnMgO. SnO2 exhibits a deeper conduction band minimum, which can lower the electron injection barrier from the ETL to the QDs, and possesses comparable or even higher electron mobility, alongside greater chemical stability [15,16]. Therefore, QLEDs utilizing solution-processed SnO2 NPs as an ETL have demonstrated enhanced performance, confirming the material’s intrinsic advantages [17,18]. Nevertheless, these devices are still constrained by the same fabrication issues associated with the solution-based deposition of NP films.
An ideal approach to resolve these issues is to replace the solution-based process with a vacuum deposition technique. Sputtering, a well-established physical vapor deposition method, is renowned for its ability to deposit highly uniform, dense, and reproducible thin films over large-area substrates, making it highly compatible with mass production. While there have been reports on sputtered ZnO or ZnMgO ETLs for QLEDs, their device performance has often lagged behind that of their best solution-processed counterparts [19,20]. To date, however, the application of sputtered SnO2 as an ETL in QLEDs has not been reported yet.
Herein, we demonstrate high-performance and durable QLEDs by employing a sputtered SnO2 thin film as the ETL, thereby synergistically combining the material advantages of SnO2 with the process advantages of sputtering. We systematically investigated the structural, optical, and electrical properties of the sputtered SnO2 films by varying Ar/O2 ratio and substrate heating temperature. QLEDs using SnO2 ETL deposited under an optimized Ar/O2 ratio of 35:5 reached a maximum luminance of 99,212 cd/m2 and current efficiency of 21.17 cd/A after heating at 200 °C, representing one of the highest performances for QLEDs employing SnO2 as the ETL. The optimized SnO2 ETL enabled efficient electron injection and transport, leading to QLEDs with significantly improved luminance, current efficiency, and operational stability. This work highlights the potential of sputtered SnO2 as a robust and effective ETL for the fabrication of commercially viable QLEDs.
2. Materials and Methods
2.1. Synthesis of Green QDs
To synthesize green CdZnSeS/ZnS QDs, CdO (0.14 mmol) and ZnO (3.41 mmol) were combined with 7 mL of oleic acid (OA) at 150 °C under N2 atmosphere. After adding 15 mL of 1-octadecene (ODE), the temperature was raised to 310 °C. The composition-gradient CdZnSeS cores were synthesized by a swift injection of a Se + S stock solution (2.2 mmol of Se and S in 2.2 mL of trioctylphosphine), maintaining the reaction for 10 min. An additional 1.6 mmol of S in 2.4 mL ODE was then added for a 12 min reaction. The ZnS shell process was initiated by the rapid introduction of Zn acetate dihydrate (2.86 mmol) dissolved in a mixture of OA (1 mL) and ODE (4 mL), and the reaction was maintained at 270 °C for 10 min. To facilitate ZnS formation, 9.65 mmol of S dissolved in 5 mL was added dropwise and reacted for 20 min. The resulting green CdZnSeS/ZnS QDs were isolated and purified through successive centrifugation using a combination of solvent and nonsolvent. The resulting purified QDs were subsequently redispersed in hexane for further analysis and EML fabrication.
2.2. Deposition of SnO2 Layer by the Sputtering Method
SnO2 ETLs were deposited onto substrates using the radio frequency (RF) magnetron sputtering. With a base pressure maintained below 1.5 × 10−5 Torr, the sputtering plasma was sustained at 100 W RF power with a 10 mTorr working pressure. The layers were optimized for QLED application by adjusting the Ar/O2 gas flow ratio. A pre-sputtering treatment was applied for 5 min to remove potential contaminants.
2.3. Fabrication of the Inverted QLEDs
The architecture of the inverted QLEDs is schematically presented in Figure 1, with devices constructed on indium-tin-oxide (ITO) patterned glass. These substrates underwent a sequential cleaning process using isopropyl alcohol followed by deionized water. Prior to the deposition of SnO2 layers via RF sputtering, a 15 min ultraviolet-ozone treatment was applied to the ITO surfaces. For the EML, a QD dispersion was spin-coated onto the ITO/SnO2 at 5000 rpm for 20 s. To complete the device, 4,4′-bis (carbazol-9-yl) biphenyl (CBP), MoO3, and Al were sequentially deposited via thermal evaporation without vacuum interruption. The evaporation rates were precisely maintained at 1 Å/s for CBP, 0.5 Å/s for MoO3, and 3 Å/s for Al cathode.
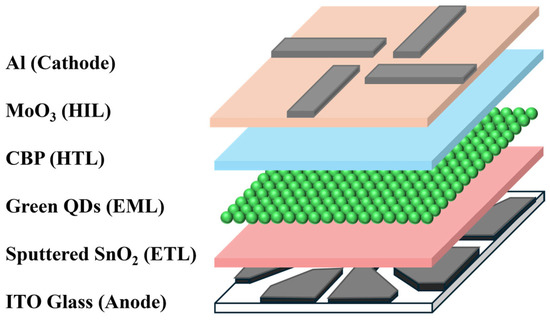
Figure 1.
A schematic of the inverted QLEDs.
2.4. Characterizations
The current density–voltage–luminance (J–V–L) parameters were evaluated using a spectroradiometer (Konica Minolta CS2000, Tokyo, Japan) coupled with a Keithley 2400 source meter (Cleveland, OH, USA) in an ambient atmosphere. These measurements allowed for a comprehensive analysis of the luminance and current efficiency profiles relative to the applied bias. By correlating the photocurrent from a silicon photodiode with the luminance measured via a spectroradiometer, we precisely determined the device’s luminance and current efficiency. The crystal phase of SnO2 was analyzed using an X-ray diffractometer (AERIS, PANalytical, Malvern, UK). X-ray photoelectron spectroscopy (XPS) (Thermo VG, Waltham, MA, USA) with Al Kα x-ray (E = 1486.6 eV) was used to analyze the actual chemical composition of SnO2. Photoluminescence (PL) characterization was performed using a PL spectrophotometer (PSI) with a fixed excitation wavelength at 365 nm. The surface morphology was measured by atomic force microscopy (AFM) (XE-100, PSIA, Suwon, Republic of Korea). The energy band structures of SnO2 were investigated by ultraviolet photoelectron spectroscopy (UPS) (ESCALAB 250, Thermo Scientific, Waltham, MA, USA). The operational lifetime of the QLEDs was measured using a multi-channel lifetime test system (M6000 Plus, McScience, Daejeon, Republic of Korea).
3. Results and Discussion
Figure 2a shows the X-ray diffraction patterns of the sputtered SnO2 layers deposited under various Ar/O2 ratios. All SnO2 films showed identical diffraction peaks at 2θ = 26.6°, 33.9°, and 51.8°, corresponding to the (110), (200) and (211) crystal planes of a rutile structure (JCPDS: 41-1445), respectively, indicating that the Ar/O2 ratio had a negligible effect on the fundamental crystal structure. The SnO2 thin films deposited in pure Ar (100%) gas exhibit an amorphous phase, which is attributed to compositional instability caused by inevitable oxygen vacancies (Vo) during the sputtering process. However, as the O2 partial pressure increases during deposition, the oxygen deficiencies within the film are compensated, resulting in enhanced crystallinity. Figure 2b presents the improvement in crystallinity after the substrate heating at 200 °C. However, the temperature dependence of crystal growth diminishes with increasing oxygen partial pressure. This is attributed to the formation of compositionally stable and more ordered SnO2 structure at higher O2 partial pressures [21].
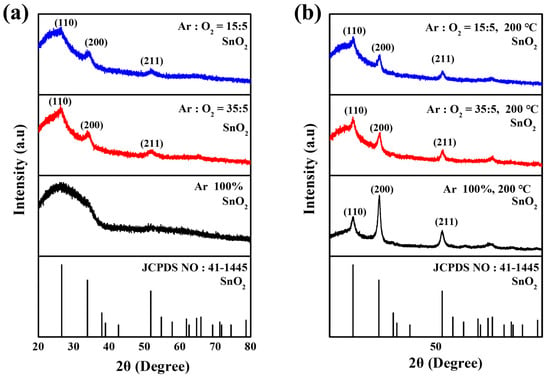
Figure 2.
XRD patterns of sputtered SnO2 layer (a) deposited under various Ar/O2 ratios and (b) after substrate heating at 200 °C.
The chemical bonding states of oxygen in sputtered SnO2 films under different Ar/O2 ratios were characterized by XPS, and the resulting O 1s spectra are shown in Figure 3. The O 1s core-level spectra were resolved into two distinct components: the lower binding energy peak at 530.4 ± 0.2 eV is assigned to the Sn–O lattice bonds, while the higher peak at 531.9 ± 0.2 eV is attributed to oxygen vacancies [17]. The relative ratio of oxygen vacancies in the sputtered SnO2 films effectively decreased from 13.43% (Ar/O2 ratio = 35:5) to 8.21% (Ar/O2 ratio = 15:5) by increasing O2 partial pressure. Owing to that, oxygen vacancies serve as exciton quenching sites in QLEDs, the SnO2 films deposited under Ar/O2 ratio of 15:5, which exhibit the lowest oxygen vacancy concentration, are considered favorable as an ETL [22].
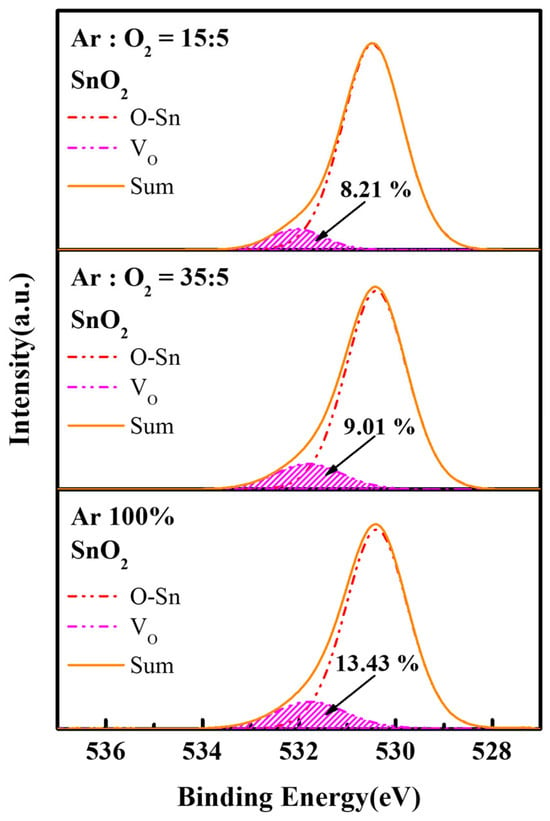
Figure 3.
The O 1s XPS spectra of sputtered SnO2 films under various Ar/O2 ratios.
Figure 4a illustrates the PL emission profiles of glass/SnO2/QD structures as a function of the Ar/O2 gas ratio used during SnO2 deposition. The reduced PL intensity in the glass/SnO2/QDs structure was attributed to exciton quenching at surface defect sites. However, PL intensities of SnO2 films deposited with O2 gas were significantly enhanced, indicating that the quenching was effectively suppressed due to reduced oxygen vacancies, as shown in Figure 3. Figure 4b presents the optical transmittance data of the SnO2 films under different Ar/O2 ratios. In a bottom-emission configuration, high optical transparency is essential because the underlying layers can unintentionally attenuate the light emitted from the QDs through absorption. The SnO2 films sputtered under 100% Ar gas exhibited low transmittance, whereas increasing the O2 partial pressure led to improved transparency due to the decreased oxygen vacancy concentration.
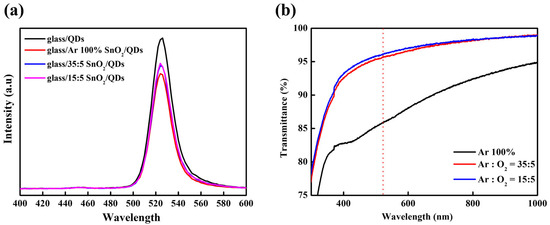
Figure 4.
(a) PL spectra of QDs on various substrates: glass/QDs and glass/SnO2 (Ar/O2 ratio = Ar 100%, 35:5, 15:5)/QDs and (b) transmittance of sputtered SnO2 films under various Ar/O2 ratios.
Figure 5a shows the absorption spectra of SnO2 films deposited under different Ar/O2 ratios, presented as the plots of (αhν)2 vs. hν (photon energy), where α is the absorbance. The bandgaps (Eg) were estimated from the intercept of the linear portion of the absorption edge; the Eg values for SnO2 films with Ar 100%, Ar/O2 ratio = 35:5 and Ar/O2 ratio = 15:5 were 4.35, 4.19, and 4.15 eV, respectively. These data indicate bandgap narrowing upon increasing the O2 partial pressure, which can modulate electron injection to the EML. The energy band structures of SnO2 films were further investigated by UPS; the resulting secondary-electron cutoff and valence-band regions are shown in Figure S1a,b, respectively. The valence band maximum (VBM) was calculated by the incident photon energy (21.21 eV), the high-binding energy cutoff (Ecutoff), and the onset energy in the valence-band region (Eonset) according to the equation VBM = 21.21 − (Ecutoff − Eonset) (see Table S1 for detailed values). Figure 5b presents a schematic energy band diagram of inverted QLEDs with various SnO2 films. Based on the calculation, the VBM positions of SnO2 films with Ar 100%, Ar/O2 ratio = 35:5 and Ar/O2 ratio = 15:5 were calculated to be 7.58, 7.65, and 7.71 eV below the vacuum level, respectively, exhibiting a large downshift of the VBM level with increasing O2 partial pressure. Based on the Eg and VBM values, the conduction band minimum (CBM) levels were estimated to be 3.23, 3.46, and 3.56 eV below the vacuum level for SnO2 films with Ar 100%, Ar/O2 ratio = 35:5 and Ar/O2 ratio = 15:5, respectively.

Figure 5.
(a) (αhv)2 vs. hv plots converted from the absorption spectra of sputtered SnO2 films under various Ar/O2 ratios and (b) the energy band diagram of the inverted QLEDs with SnO2 films under various Ar/O2 ratios.
Figure 6 shows the voltage-dependent characteristics in luminance, current density, and current efficiency of QLEDs with various SnO2 films. Using the SnO2 films (Ar/O2 ratio = 35:5) as an ETL, the QLEDs exhibited a maximum luminance of 58,149 cd/m2 at 8.5 V and current efficiency of 13.72 cd/A at 8 V. The CBM level (−3.46 eV) of SnO2 films (Ar/O2 ratio = 35:5), which is comparable to that of green QDs, facilitates efficient electron injection and transport from the ITO into the EML, while its deep-lying VBM level (−7.65 eV) effectively blocks holes at the EML/ETL interface. We attribute enhanced performance to the downward shift of the CBM in the optimized SnO2 films, which facilitates electron transport by lowering the injection barrier from the electrode to the ETL, thereby improving the charge balance within the EML. The unsmooth curves in J-V graphs may originate from surface defects or oxygen vacancies of SnO2 thin film during the high-energy sputtering process. The sputtered thin films are denser than solution-processed ones, but these plasma-induced surface states are inevitable. Interestingly, we observed that these fluctuations were suppressed following substrate heating at 200 °C.
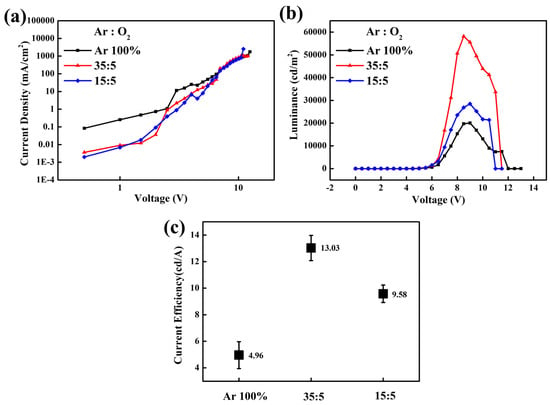
Figure 6.
Voltage-dependent characteristics of (a) current density and (b) luminance of the inverted QLEDs with sputtered SnO2 films under different Ar/O2 ratios. Statistical data of (c) current efficiency for 5 devices based on various SnO2 films as ETL.
While the optimization of the Ar/O2 ratio during the sputtering process successfully controlled the stoichiometry and oxygen vacancy of the SnO2 films, the surface morphology is another critical factor determining the performance of QLEDs [23,24]. Sputtered films deposited at room temperature often exhibit rough surfaces, which can lead to poor interfacial contact with the QD EML. The surface profiles of various SnO2 films were measured using AFM to observe morphological changes induced by different Ar/O2 ratios and substrate heating at 200 °C. As shown in Figure 7, the surface roughness decreased with increasing oxygen partial pressure and substrate heating, which is mainly attributed to the formation of a dense and uniform surface facilitated by improved adatom mobility [25]. Consequently, the QLEDs with SnO2 films (Ar/O2 ratio = 35:5) with substrate heating at 200 °C exhibited improved device performance, as shown in Figure 8, achieving a peak luminance of 99,212 cd/m2 at 10 V and current efficiency of 21.17 cd/A at 8.5 V.
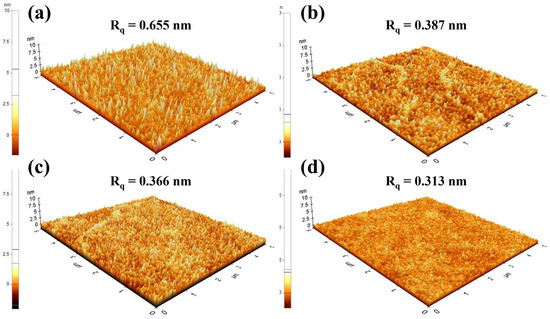
Figure 7.
AFM images (5 μm × 5 μm) of sputtered SnO2 films deposited under various conditions. (a) Ar 100%, (b) Ar 100% at 200 °C, (c) Ar:O2 = 35:5, and (d) Ar:O2 = 35:5 at 200 °C.
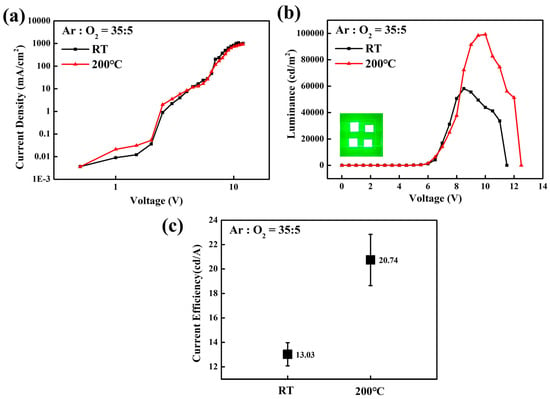
Figure 8.
Voltage-dependent characteristics of (a) current density and (b) luminance of the inverted QLEDs with sputtered SnO2 films after substrate heating at 200 °C. The inset shows the green emission from 2.5 mm × 2.5 mm pixels. Statistical data of (c) current efficiency for 5 devices based on substrate heating at 200 °C.
Interestingly, although the maximum luminance and current efficiency of the QLEDs with SnO2 films (Ar/O2 ratio = 15:5) were lower than those of the QLEDs with SnO2 films (Ar/O2 ratio = 35:5), they exhibited excellent operational stability. As shown in Figure 9, the predicted T50 at 100 cd/m2 was 4480 h, based on the T50 at 1000 cd/m2 with an acceleration factor of 1.9. Excitons are easily quenched at defect sites via charge transport at the SnO2/QD interface; this quenching process is effectively suppressed by the reduction of oxygen vacancies, as confirmed in Figure 3.
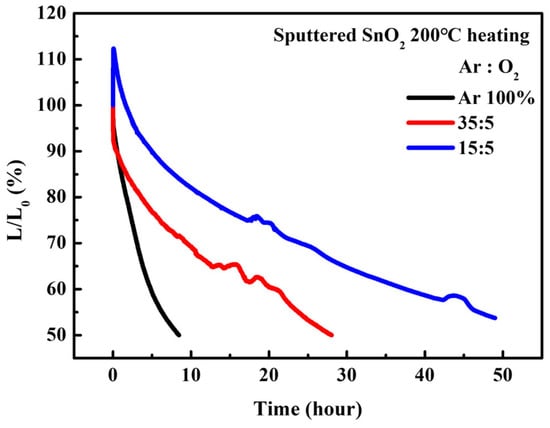
Figure 9.
Operational lifetime characteristics of QLEDs with various sputtered SnO2 ETLs.
Although spin-coating is commonly employed for inorganic ETL fabrication, RF sputtering facilitates superior control over physical and chemical properties of deposited films. Our study reveals that the device efficiency is highly sensitive to the CBM position, which dictates the charge injection balance. Additionally, the reduction of oxygen vacancies was identified as a fundamental mechanism for enhancing long-term stability through the prevention of exciton quenching. Although substrate heating suppressed the J–V fluctuations, the luminance drop at high current densities persisted. This suggests that while structural defects were partially removed, the device roll-off phenomenon is likely governed by charge imbalance. Future works involving interfacial modification or high-mobility hole transport materials could further improve the charge balance and minimize exciton quenching, leading to more stable luminance under high-current operation.
4. Conclusions
This study demonstrates the successful application of SnO2 thin films as ETLs in QLEDs, with properties tailored by Ar/O2 ratios. The analysis confirmed that increasing O2 content optimizes the CBM position, thereby enhancing electron transport from the cathode. QLEDs with SnO2 films (Ar/O2 ratio = 35:5) afforded a maximum luminance of 99,212 cd/m2 and current efficiency of 21.17 cd/A after heating at 200 °C. Additionally, the stability of devices was improved by reducing the oxygen vacancies of SnO2. Based on the improved device characteristics, the optimized SnO2 films are highly effective inorganic ETL materials, offering significant advantages for stable and efficient QLEDs.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/nano16010031/s1, Table S1: Energy levels of sputtered SnO2 films under various Ar/O2 ratios; Figure S1: UPS spectra of (a) the secondary-electron cutoff and (b) valence-band edge regions of sputtered SnO2 films under various Ar/O2 ratios.
Author Contributions
Methodology, J.C.; formal analysis, J.C.; investigation, J.K.; writing—original draft preparation, J.C.; writing—review and editing, J.K.; supervision, J.K.; funding acquisition, J.K. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Kyonggi University Research Grant 2025.
Data Availability Statement
The data presented in this study is available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sun, Q.; Wang, Y.A.; Li, L.S.; Wang, D.; Zhu, T.; Xu, J.; Yang, C.; Li, Y. Bright, multicoloured light-emitting diodes based on quantum dots. Nat. Photonics 2007, 1, 717–722. [Google Scholar] [CrossRef]
- Dabbousi, B.O.; Rodriguez-Viejo, J.; Mikulec, F.V.; Heine, J.R.; Mattoussi, H.; Ober, R.; Jensen, K.F.; Bawendi, M.G. (CdSe)ZnS core–shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites. J. Phys. Chem. B 1997, 101, 9463–9475. [Google Scholar] [CrossRef]
- Cao, W.; Xiang, C.; Yang, Y.; Chen, Q.; Chen, L.; Yan, X.; Qian, L. Highly stable QLEDs with improved hole injection via quantum dot structure tailoring. Nat. Commun. 2018, 9, 2608. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.S.; Lee, E.K.; Joo, W.J.; Jang, E.; Kim, T.H.; Lee, S.J.; Kwon, S.J.; Han, J.Y.; Kim, B.K.; Choi, B.L.; et al. High-performance crosslinked colloidal quantum-dot light-emitting diodes. Nat. Photonics 2009, 3, 341–345. [Google Scholar] [CrossRef]
- Shirasaki, Y.; Supran, G.J.; Bawendi, M.G.; Bulović, V. Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photonics 2013, 7, 13–23. [Google Scholar] [CrossRef]
- Tu, N.; Lee, S. Large-emitting-area quantum dot light-emitting diodes fabricated by an all-solution process. Int. J. Mol. Sci. 2023, 24, 14350. [Google Scholar] [CrossRef]
- Tian, D.; Ma, H.; Huang, G.; Gao, M.; Cai, F.; Fang, Y.; Li, C.; Jiang, X.; Wang, A.; Wang, S.; et al. A review on quantum dot light-emitting diodes: From materials to applications. Adv. Opt. Mater. 2023, 11, 2201965. [Google Scholar] [CrossRef]
- Talapin, D.; Steckel, J. Quantum dot light-emitting devices. MRS Bull. 2013, 38, 685–691. [Google Scholar] [CrossRef]
- Bai, R.; Wang, C.; Ramzan, M.; Chang, S.; Zhong, H. Progress in blue colloidal quantum dots for display applications. J. Inf. Disp. 2025, 26, 95–107. [Google Scholar] [CrossRef]
- Alexandrov, A.; Zvaigzne, M.; Lypenko, D.; Nabiev, I.; Samokhvalov, P. Al-, Ga-, Mg-, or Li-doped zinc oxide nanoparticles as electron transport layers for quantum dot light-emitting diodes. Sci. Rep. 2020, 10, 7496. [Google Scholar] [CrossRef]
- Chrzanowski, M.; Kuchowicz, M.; Szukiewicz, R.; Sitarek, P.; Misiewicz, J.; Podhorodecki, A. Enhanced efficiency of quantum dot light-emitting diode by sol–gel derived Zn1−xMgxO electron transport layer. Org. Electron. 2020, 80, 105656. [Google Scholar] [CrossRef]
- Chrzanowski, M.; Zatryb, G.; Sitarek, P.; Podhorodecki, A. Effect of air exposure of ZnMgO nanoparticle electron transport layer on efficiency of quantum-dot light-emitting diodes. ACS Appl. Mater. Interfaces 2021, 13, 20305–20312. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Lee, M.; Seo, H.; Shin, D.; Hahm, D.; Bae, W.K.; Kim, J.; Kwak, J. Efficient and stable InP quantum-dot light-emitting diodes formed by premixing 2-hydroxyethyl methacrylate into ZnMgO. J. Mater. Chem. C 2024, 12, 7270–7277. [Google Scholar] [CrossRef]
- Oh, S.; Choi, J.; Park, J.; Choi, Y.K.; Park, T.; Ali, A.; Ahn, J.; Kim, J.; Oh, S.J. Surface passivation engineering for stable optoelectronic devices via hydroxyl-free ZnMgO nanoparticles. Nano Converg. 2025, 12, 28. [Google Scholar] [CrossRef]
- Chen, M.; Chen, X.; Ma, W.; Sun, X.; Wu, L.; Lin, X.; Yang, Y.; Li, R.; Shen, D.; Chen, Y.; et al. Highly stable SnO2-based quantum-dot light-emitting diodes with the conventional device structure. ACS Nano 2022, 16, 9631–9639. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, S. Efficient and stable quantum-dot light-emitting diodes enabled by tin oxide multifunctional electron transport layer. Adv. Opt. Mater. 2022, 10, 2102404. [Google Scholar] [CrossRef]
- Zhao, J.; Man, Z.; Wang, S.; Hao, C.; Yu, Z.; Li, X.; Tang, A. Enhanced performance of quantum dot light-emitting diodes enabled by zirconium doped SnO2 as electron transport layers. Opt. Lett. 2024, 49, 1896–1899. [Google Scholar] [CrossRef]
- Gao, J.; Liu, M.; Shi, X.; Pan, D. Ligand-assisted solvothermal precipitation synthesis of quantum-sized SnO2 nanoparticles and their application in quantum dot light-emitting diodes. New J. Chem. 2024, 48, 8631–8637. [Google Scholar] [CrossRef]
- Lin, K.R.; Lin, B.-Y.; Ding, W.-C.; Lee, C.-Y.; Chen, C.-H.; Kuo, Y.-P.; Chen, P.-Y.; Lu, H.-H.; Chiu, T.-L.; Lee, J.-H. Inverted green quantum-dot light-emitting diode with sputtered ZnO as the electron transporting layer. In Proceedings of the Advances in Display Technologies XI, Online, 6–11 March 2021; SPIE: Bellingham, WA, USA, 2021; Volume 11708, p. 117080R. [Google Scholar]
- Kim, B.; Kim, J. Large-area quantum dot light-emitting diodes employing sputtered Zn0.85Mg0.15O electron transport material. Electron. Mater. Lett. 2024, 20, 140–149. [Google Scholar] [CrossRef]
- Gago, R.; Prucnal, S.; Azpeitia, J.; Esteban-Mendoza, D.; Jiménez, I. Soft X-ray absorption study of sputtered tin oxide films. J. Alloys Compd. 2022, 902, 163768. [Google Scholar] [CrossRef]
- Polydorou, E.; Zeniou, A.; Tsikritzis, D.; Soultati, A.; Sakellis, I.; Gardelis, S.; Papadopoulos, T.A.; Briscoe, J.; Palilis, L.C.; Kennou, S.; et al. Surface passivation effect by fluorine plasma treatment on ZnO for efficiency and lifetime improvement of inverted polymer solar cells. J. Mater. Chem. A 2016, 4, 11844–11858. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Ji, W. Efficient quantum-dot light-emitting diodes based on solvent-annealed SnO2 electron-transport layers. ACS Appl. Electron. Mater. 2023, 5, 537–543. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Zhao, Y.; Chen, X.; Li, Z.; Li, H.; Wang, H.; Gong, X. Quantum dot light-emitting diodes with external quantum efficiency exceeding 30% enabled by a crown ether-modified electron transport layer. ACS Appl. Mater. Interfaces 2025, 17, 48483–48491. [Google Scholar] [CrossRef]
- Cheemadan, S.; Kumar, M.S. Effect of substrate temperature and oxygen partial pressure on RF sputtered NiO thin films. Mater. Res. Express 2018, 5, 046401. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.