Effect of Hexagonal Boron Nitrides Injection on the Survival of Dorsal Pedicle Skin Flap in Rats: An Experimental Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design and Rat Selection
2.2. Procedure
2.3. hBNs Nanoparticles
2.4. hBNs Synthesis
2.5. SEM and TEM Imaging
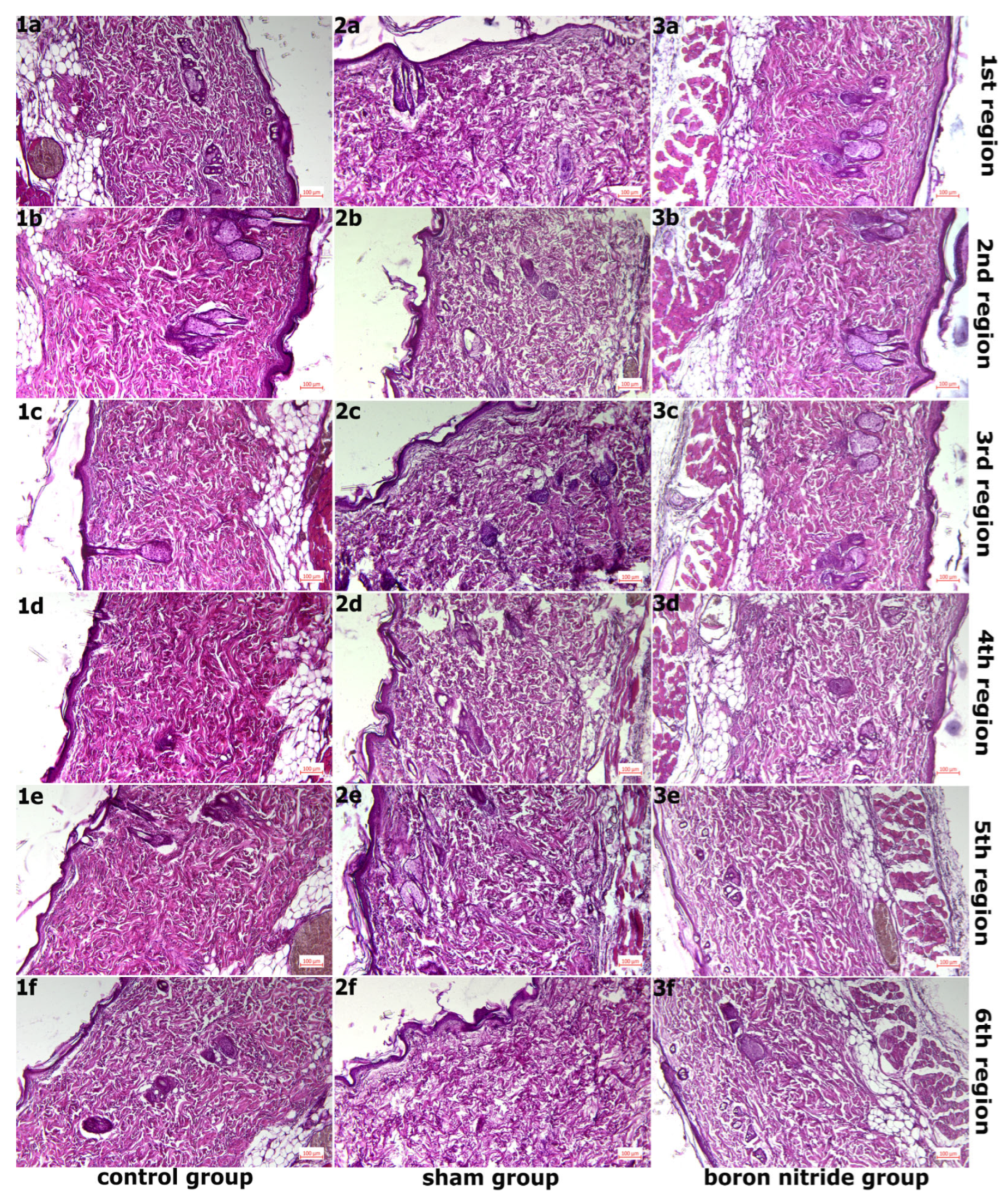
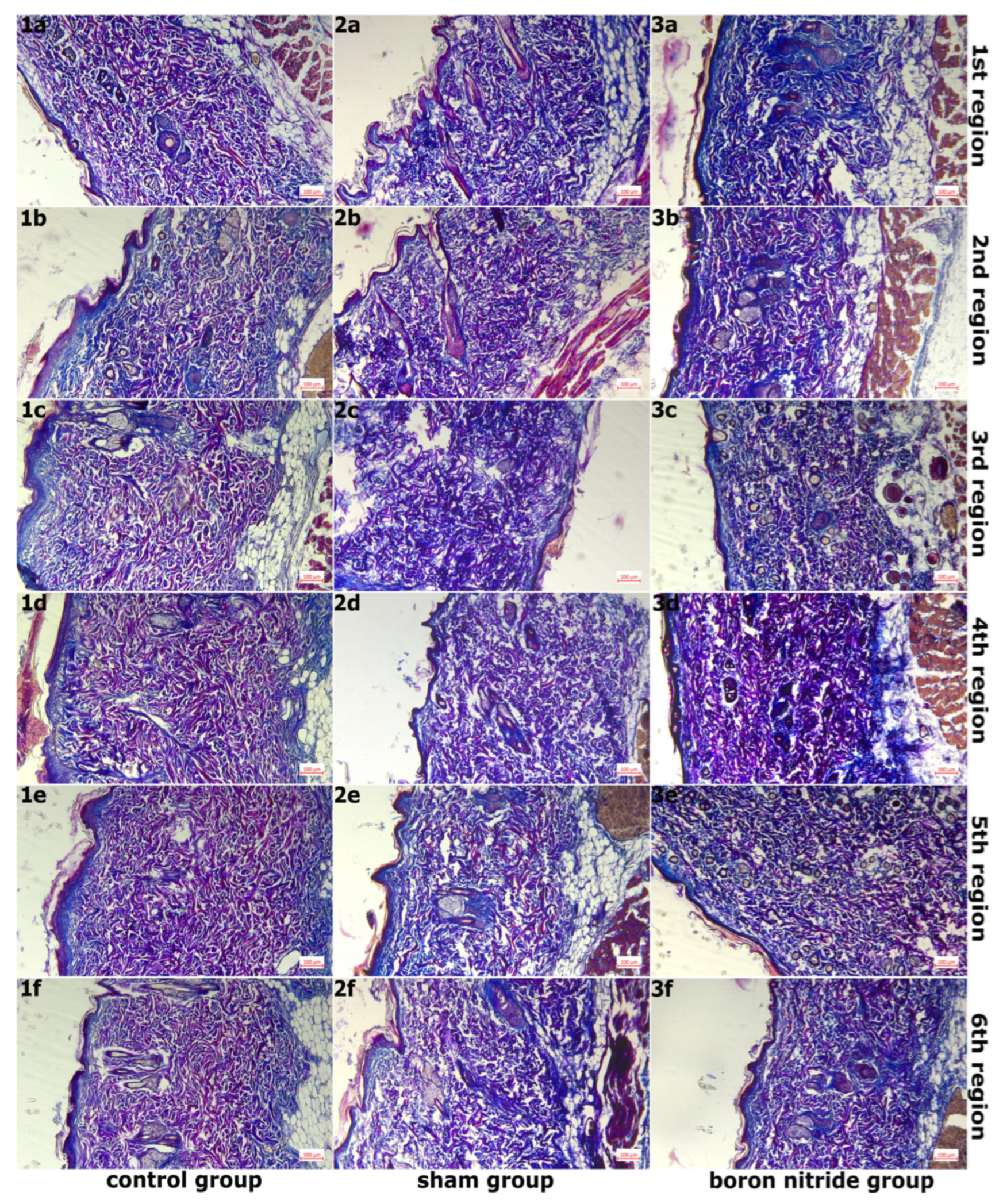
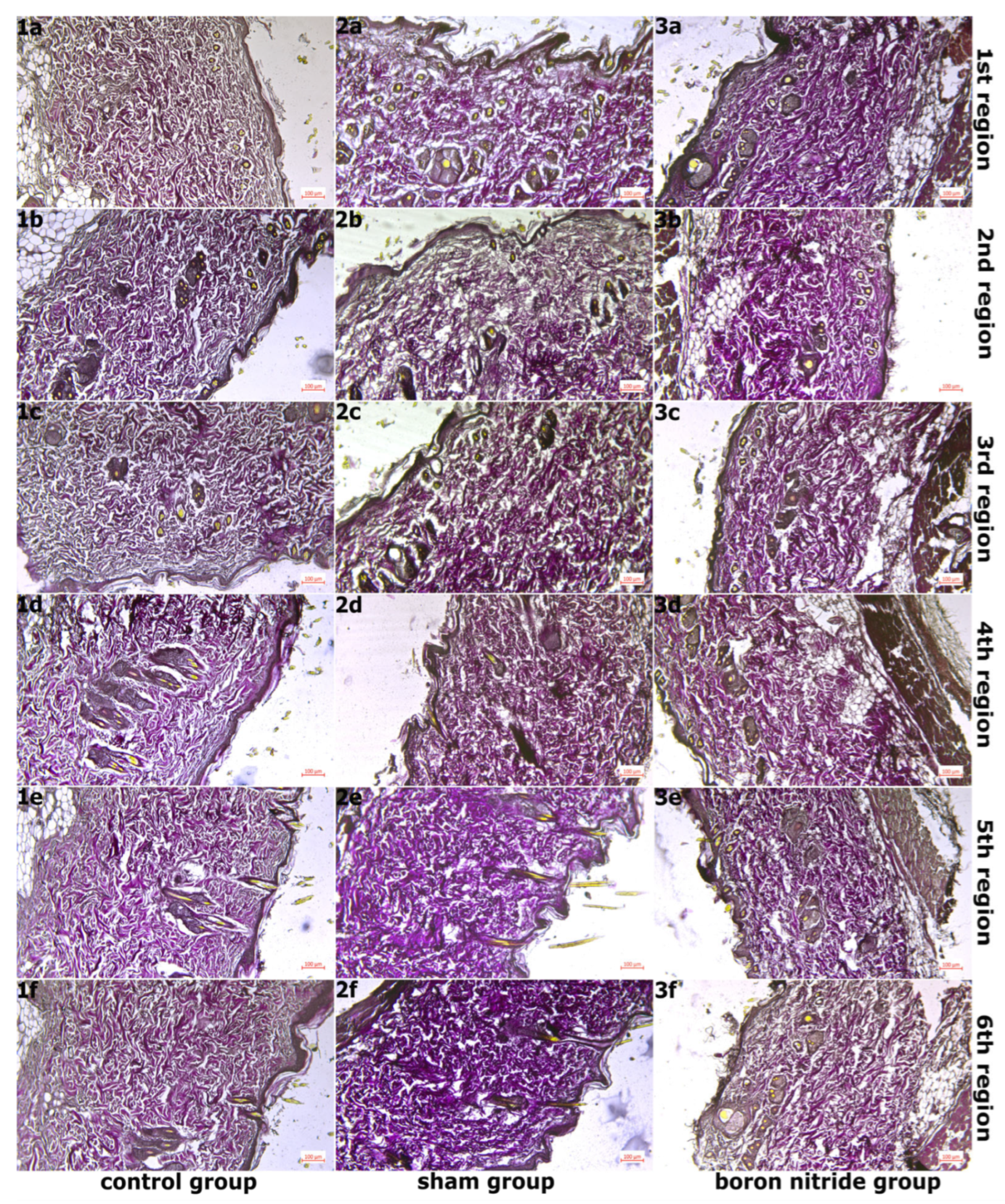
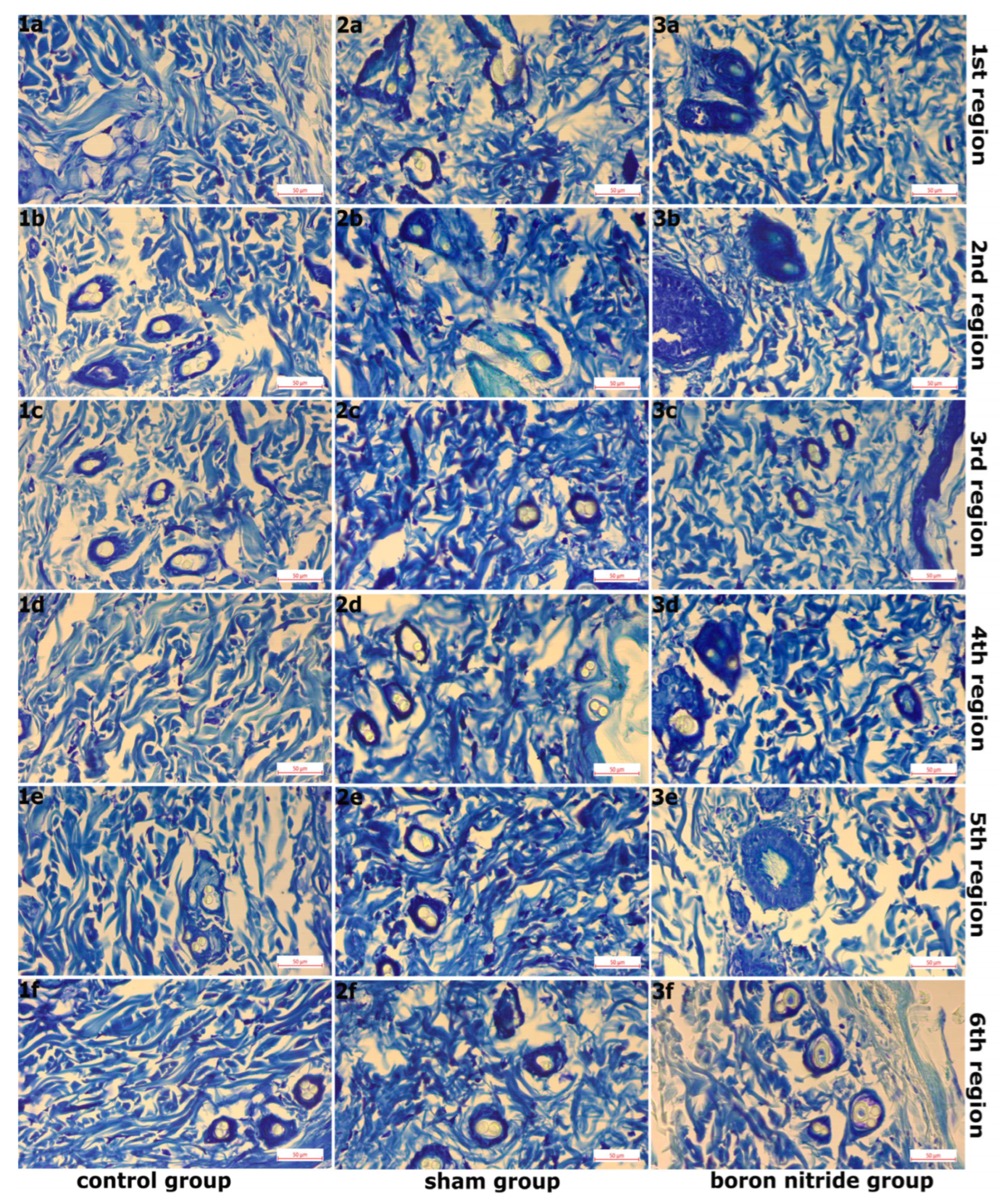
2.6. Histopathological Examination
2.7. Biochemical Analyses
2.8. Data Analysis
3. Findings
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- McFarlane, R.M.; Deyoung, G.; Henry, R.A.; McFarlane, R.M. The design of a pedicle flap in the rat to study necrosis and its prevention. Plast. Reconstr. Surg. 1965, 35, 177–182. [Google Scholar] [CrossRef]
- Myers, M.B. Investigation of skin flap necrosis. In Skin Flaps; Little, Brown: Boston, MA, USA, 1975; p. 3. [Google Scholar]
- He, J.B.; Ma, X.Y.; Li, W.J.; Liu, Y.Y.; Lin, D.S. Exenatide inhibits necrosis by enhancing angiogenesis and ameliorating ischemia/reperfusion injury in a random skin flap rat model. Int. Immunopharmacol. 2021, 90, 107192. [Google Scholar] [CrossRef]
- Wald, G.; Van, Y.V.; Towne, W.; Otterburn, D.M. The effect of topical tacrolimus on pedicled flap survival: A histological analysis. Ann. Plast. Surg. 2021, 87, S57–S59. [Google Scholar] [CrossRef]
- Kelly, C.P.; Gupta, A.; Keskin, M.; Jackson, I.T. A new design of a dorsal flap in the rat to study skin necrosis and its prevention. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 1553–1556. [Google Scholar] [CrossRef] [PubMed]
- Miyawaki, T.; Jackson, I.T.; Bier, U.C.; Andrus, L.; Williams, F.; Bradford, M. The effect of capsaicin ointment on skin for the survival of a cutaneous flap. Eur. J. Plast. Surg. 2001, 24, 28–30. [Google Scholar] [CrossRef]
- Bilgen, K.; Bilgen, E.; Cetinkunar, S.; Celep, R.B.; Isik, S. The efficacy of topical phenytoin and capsaicin on random pattern dorsal skin flaps in rats. Ann. Ital. Di Chir. 2017, 88, 87–93. [Google Scholar]
- Erçöçen, A.R.; Kono, T.; Kikuchi, Y.; Kitazawa, Y.; Nozaki, M. Efficacy of the Flashlamp-Pumped Pulsed-Dye Laser in Nonsurgical Delay of Skin Flaps. Dermatol. Surg. 2003, 29, 692–699. [Google Scholar] [PubMed]
- Yasunaga, Y.; Matsuo, K.; Tanaka, Y.; Yuzuriha, S. Near-Infrared Irradiation Increases Length of Axial Pattern Flap Survival in Rats. Eplasty 2017, 17, e26. [Google Scholar]
- Huemer, G.M.; Wechselberger, G.; Otto-Schoeller, A.; Gurunluoglu, R.; Piza-Katzer, H.; Schoeller, T. Improved dorsal random-pattern skin flap survival in rats with a topically applied combination of nonivamide and nicoboxil. Plast. Reconstr. Surg. 2003, 111, 1207–1211. [Google Scholar] [CrossRef]
- Büyük, B.; Aydeğer, C.; Adalı, Y.; Eroğlu, H.A. The effect of topically applied boric acid on the ephrin-eph pathway in wound treatment: An experimental study. Int. J. Low. Extrem. Wounds 2021, 23, 15347346211055260. [Google Scholar] [CrossRef]
- Das, B.C.; Thapa, P.; Karki, R.; Schinke, C.; Das, S.; Kambhampati, S.; Banerjee, S.K.; Van Veldhuizen, P.; Verma, A.; Weiss, L.M.; et al. Boron chemicals in diagnosis and therapeutics. Future Med. Chem. 2013, 5, 653–676. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Abd El-Hack, M.E.; Swelum, A.A.; Perillo, A.; Losacco, C. The vital roles of boron in animal health and production: A comprehensive review. J. Trace Elem. Med. Biol. 2018, 50, 296–304. [Google Scholar] [CrossRef]
- Tsubata, T. Involvement of reactive oxygen species (ROS) in BCR signaling as a second messenger. Adv. Exp. Med. Biol. 2020, 1254, 37–46. [Google Scholar] [CrossRef]
- Jackson, D.G.; Cardwell, L.A.; Oussedik, E.; Feldman, S.R. Utility of boron in dermatology. J. Dermatol. Treat. 2020, 31, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Doğan, A.; Aydın, S.; Dülger, E.Ç.; Şahin, F. Boron promotes streptozotocin-induced diabetic wound healing: Roles in cell proliferation and migration, growth factor expression, and inflammation. Mol. Cell. Biochem. 2016, 417, 119–133. [Google Scholar] [CrossRef]
- Chupakhin, O.; Khonina, T.; Kungurov, N.; Zilberberg, N.; Evstigneeva, N.; Kokhan, M.; Polishchuk, A.I.; Shadrina, E.V.; Larchenko, E.Y.; Larionov, L.P.; et al. Silicon–boron-containing glycerohydrogel having wound healing, regenerative, and antimicrobial activity. Russ. Chem. Bull. 2017, 66, 558–563. [Google Scholar] [CrossRef]
- Zhang, H.; Li, W.X.; Tang, S.; Chen, Y.; Lan, L.M.; Li, S.; Xiong, M.; Hu, X.; Liu, Y.; Sun, J.; et al. A boron-based probe driven theranostic hydrogel dressing for visual monitoring and matching chronic wound healing. Adv. Funct. Mater. 2023, 33, 2305580. [Google Scholar] [CrossRef]
- Wang, G.; Ye, J.; Wang, M.; Qi, Y.; Zhang, S.; Shi, L.; Fang, Y.; Tian, Y.; Ning, G. Copper boron–imidazolate framework incorporated chitosan membranes for bacterial infected wound healing dressing. Carbohydr. Polym. 2022, 291, 119588. [Google Scholar] [CrossRef]
- Badhan, J.; Rajput, J.K.; Dogra, S. A comprehensive review on photocatalytically active modified boron nitride materials for degradation of organic pollutants. Environ. Sci. Pollut. Res. 2025, 32, 24487–24510. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Hanagata, N.; Golberg, D. Boron nitride nanomaterials for cancer therapy: Tailor-made strategies. J. Mater. Res. 2025, 40, 2039–2055. [Google Scholar] [CrossRef]
- Adiguzel, S.; Cicek, N.; Cobandede, Z.; Misirlioglu, F.B.; Yilmaz, H.; Culha, M. Piezoelectricity of hexagonal boron nitrides improves bone tissue generation as tested on osteoblasts. Beilstein J. Nanotechnol. 2025, 16, 1068–1081. [Google Scholar] [CrossRef] [PubMed]
- Demirci, S.; Doğan, A.; Karakuş, E.; Halıcı, Z.; Topçu, A.; Demirci, E.; Sahin, F. Boron and poloxamer (F68 and F127) containing hydrogel formulation for burn wound healing. Biol. Trace Elem. Res. 2015, 168, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Şen, Ö.; Emanet, M.; Çulha, M. One-step synthesis of hexagonal boron nitrides, their crystallinity and biodegradation. Front. Bioeng. Biotechnol. 2018, 6, 83. [Google Scholar] [CrossRef]
- DuFrane, W.; Cervantes, O.; Ellsworth, G.; Kuntz, J. Consolidation of cubic and hexagonal boron nitride composites. Diam. Relat. Mater. 2016, 62, 30–41. [Google Scholar] [CrossRef]
- Bhimanapati, G.R.; Kozuch, D.; Robinson, J.A. Large-scale synthesis and functionalization of hexagonal boron nitride nanosheets. Nanoscale 2014, 6, 11671–11675. [Google Scholar] [CrossRef]
- Emanet, M.; Sen, Ö.; Çulha, M. Evaluation of boron nitride nanotubes and hexagonal boron nitrides as nanocarriers for cancer drugs. Nanomedicine 2017, 12, 797–810. [Google Scholar] [CrossRef]
- Lee, J.H.; You, H.J.; Lee, T.Y.; Kang, H.J. Current status of experimental animal skin flap models: Ischemic preconditioning and molecular factors. Int. J. Mol. Sci. 2022, 23, 5234. [Google Scholar] [CrossRef] [PubMed]
- Hom, D.B.; Ostrander, B.T. Reducing risks for local skin flap failure. Facial Plast. Surg. Clin. 2023, 31, 275–287. [Google Scholar] [CrossRef]
- de Oliveira, J.A.V.; de Santana, E.S.; da Silva, L.A.; Fernandes, F.H.P.; Lira, E.C.; Vieira, J.R.C. Ischemic Skin Flaps: What To Use To Save Them? A Narrative Review. Rev. Foco 2023, 16, e728. [Google Scholar] [CrossRef]
- Han, H.H.; Lim, Y.M.; Park, S.W.; Lee, S.J.; Rhie, J.W.; Lee, J.H. Improved skin flap survival in venous ischemia-reperfusion injury with the use of adipose-derived stem cells. Microsurgery 2015, 35, 645–652. [Google Scholar] [CrossRef]
- Chehelcheraghi, F.; Eimani, H.; Sadraie, S.H.; Torkaman, G.; Amini, A.; Shemshadi, H.; Majd, H.A. Improved viability of random pattern skin flaps with the use of bone marrow mesenchymal-derived stem cells and chicken embryo extract. Iran. J. Basic Med. Sci. 2015, 18, 764–772. [Google Scholar]
- Liang, F.; Kang, N.; Liu, X.; Yang, J.; Li, Z.; Tan, J.W. Effect of HMGB1/NF-κB in hyperbaric oxygen treatment on decreasing injury caused by skin flap grafts in rats. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 2010–2018. [Google Scholar]
- Fan, W.; Liu, Z.; Chen, J.; Liu, S.; Chen, T.; Li, Z.; Lin, D. Effect of memantine on the survival of an ischemic random skin flap and the underlying mechanism. Biomed. Pharmacother. 2021, 143, 112163. [Google Scholar] [CrossRef]
- Huang, T.; Shi, J.; Sang, K.; Yu, C.; Xie, Y.; Chen, H.; Jin, Z.; Yan, H.; Zhao, B. The effect of different modes of microneedling technique on random flap survival in rats. J. Plast. Reconstr. Aesthet. Surg. 2021, 74, 2768–2775. [Google Scholar] [CrossRef]
- Li, W.J.; Liu, Y.Y.; He, J.B.; Ma, X.Y.; Lin, Y.; Zheng, P.; Lin, D.S. Effect of paeoniflorin on distal survival of random flaps. Int. Immunopharmacol. 2022, 105, 108562. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.S.; Moon, S.Y.; Lee, Y.E.; Kang, H.J. Therapeutic Effects against Tissue Necrosis of Remote Ischemic Preconditioning Combined with Human Adipose-Derived Stem Cells in Random-Pattern Skin Flap Rat Models. J. Investig. Surg. 2021, 34, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Hu, E.C.; Topp, S.; Lei, M.; Chen, W.; Lineaweaver, W.C. Proinflammatory cytokines gene expression in skin flaps with arterial and venous ischemia in rats. J. Reconstr. Microsurg. 2006, 22, 641–647. [Google Scholar] [CrossRef]
- Chen, T.; Chen, H.; Fu, Y.; Liu, X.; Huang, H.; Li, Z.; Li, S. The eNOS-Induced Leonurine’s New Role in Improving the Survival of RandomSkin Flap. Int. Immunopharmacol. 2023, 124, 111037. [Google Scholar] [CrossRef] [PubMed]
- Ballestín, A.; Casado, J.G.; Abellán, E.; Vela, F.J.; Álvarez, V.; Usón, A.; López, E.; Marinaro, F.; Blázquez, R.; Sánchez-Margallo, F.M. Ischemia-Reperfusion Injury in a Rat Microvascular Skin Free Flap Model: A Histological, Genetic, and Blood Flow Study. PLoS ONE 2018, 13, e0209624. [Google Scholar] [CrossRef]
- Wang, C.; Cai, Y.; Zhang, Y.; Xiong, Z.; Li, G.; Cui, L. Local Injection of Deferoxamine Improves Neovascularization in Ischemic Diabetic Random Flap by Increasing HIF-1α and VEGF Expression. PLoS ONE 2014, 9, e100818. [Google Scholar] [CrossRef]
- Durick, K.; Tomita, M.; Santoro, T.; Hunt, C.; Bradley, D. Evidence that boron down-regulates inflammation through the Nf-(Kappa) b pathway. J. Fed. Am. Soc. Exp. Biol. 2005, 19, A1705. [Google Scholar]
- Yuan, W.; Varga, J. Transforming growth factor-β repression of matrix metalloproteinase-1 in dermal fibroblasts involves Smad3. J. Biol. Chem. 2001, 276, 38502–38510. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, M.; Dong, X.; Liu, Y.; Hao, Y.; Wang, Y. Necrostatin-1 Protects against Ischemia/Reperfusion Injury by Inhibiting Receptor-Interacting Protein 1 in a Rat Flap Model. J. Plast. Reconstr. Aesthetic Surg. 2019, 72, 194–202. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, X.; Wang, K.; Lin, Y.; Meng, Z.; Lan, Q.; Jiang, Z.; Chen, J.; Lin, Y.; Liu, X.; et al. Activation of Aldehyde Dehydrogenase-2 Improves Ischemic Random Skin Flap Survival in Rats. Front. Immunol. 2023, 14, 1127610. [Google Scholar] [CrossRef] [PubMed]
- Rouwkema, J.; Khademhosseini, A. Vascularization and Angiogenesis in Tissue Engineering: Beyond Creating Static Networks. Trends Biotechnol. 2016, 34, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Emanet Ciofani, M.; Sen, O.; Çulha, M. Hexagonal boron nitride nanoparticles for prostate cancer treatment. ACS Appl. Nano Mater. 2020, 3, 2364–2372. [Google Scholar] [CrossRef]
- Şen, O.; Emanet, M.; Çulha, M. Stimulatory effect of hexagonal boron nitrides in wound healing. ACS Appl. Bio Mater. 2019, 2, 5582–5596. [Google Scholar] [CrossRef]
- Konca, M.; Korkmaz, M. Comparison of effects of administration of oral or topical boron on wound healing and oxidative stress in rats. Kocatepe Vet. J. 2020, 13, 11–18. [Google Scholar] [CrossRef]
- Smoum, R.; Rubinstein, A.; Dembitsky, V.M.; Srebnik, M. Boron containing compounds as protease inhibitors. Chem. Rev. 2012, 112, 4156–4220. [Google Scholar] [CrossRef]
- De Seta, F.; Schmidt, M.; Vu, B.; Essmann, M.; Larsen, B. Antifungal mechanisms supporting boric acid therapy of Candida vaginitis. J. Antimicrob. Chemother. 2009, 63, 325–336. [Google Scholar] [CrossRef]
- Wang, G.; Wang, L.; Xiong, Z.Y.; Mao, B.; Li, T.Q. Compound salvia pellet, a traditional Chinese medicine, for the treatment of chronic stable angina pectoris compared with nitrates: A meta-analysis. Med. Sci. Monit. 2005, 12, SR1–SR7. [Google Scholar] [PubMed]
- Horváthová, E.; Slameňová, D.; Maršálková, L.; Šramková, M.; Wsólová, L. Effects of borneol on the level of DNA damage induced in primary rat hepatocytes and testicular cells by hydrogen peroxide. Food Chem. Toxicol. 2009, 47, 1318–1323. [Google Scholar] [CrossRef]
- Sedighi-Pirsaraei, N.; Tamimi, A.; Khamaneh, F.S.; Dadras-Jeddi, S.; Javaheri, N. Boron in Wound Healing: A Comprehensive Investigation of Its Diverse Mechanisms. Front. Bioeng. Biotechnol. 2024, 12, 1475584. [Google Scholar] [CrossRef]
- Kakarla, A.B.; Kong, I.; Nguyen, T.H.; Kong, C.; Irving, H. Boron Nitride Nanotubes Reinforced Gelatin Hydrogel-Based Ink for Bioprinting and Tissue Engineering Applications. Biomater. Adv. 2022, 141, 213103. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, C.; Bal Albayrak, M.G.; Yanar, S.; Kayir, N.; Yozgat, A.H.; Aydin, S.; Şahin, F. A Boron-Based Topical Strategy for Enhancing Flap Survival: Mechanistic Insights Through Proteomic Analysis. Biomimetics 2025, 10, 741. [Google Scholar] [CrossRef] [PubMed]

| Group | Control | Sham | Bor | Test and Sig. |
|---|---|---|---|---|
| TGF-beta receptor type-1 (TGF-β1 ng/mL) | 8.99 ± 1.83 | 11.55 ± 0.76 | 10.54 ± 0.26 | F = 1.283, p = 0.543 |
| Metalloproteinase-1 (MMP-1 ng/mL) | 3.74 ± 1.52 | 4.71 ± 1.10 | 4.69 ± 1.05 | F = 1.721, p = 0.749 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.
Share and Cite
Tutak, F.N.; Balik, O.; Annac, E.; Ozdemir, A.; Bulbuloglu, S. Effect of Hexagonal Boron Nitrides Injection on the Survival of Dorsal Pedicle Skin Flap in Rats: An Experimental Study. Nanomaterials 2026, 16, 29. https://doi.org/10.3390/nano16010029
Tutak FN, Balik O, Annac E, Ozdemir A, Bulbuloglu S. Effect of Hexagonal Boron Nitrides Injection on the Survival of Dorsal Pedicle Skin Flap in Rats: An Experimental Study. Nanomaterials. 2026; 16(1):29. https://doi.org/10.3390/nano16010029
Chicago/Turabian StyleTutak, Fatma Nilay, Ozan Balik, Ebru Annac, Azimet Ozdemir, and Semra Bulbuloglu. 2026. "Effect of Hexagonal Boron Nitrides Injection on the Survival of Dorsal Pedicle Skin Flap in Rats: An Experimental Study" Nanomaterials 16, no. 1: 29. https://doi.org/10.3390/nano16010029
APA StyleTutak, F. N., Balik, O., Annac, E., Ozdemir, A., & Bulbuloglu, S. (2026). Effect of Hexagonal Boron Nitrides Injection on the Survival of Dorsal Pedicle Skin Flap in Rats: An Experimental Study. Nanomaterials, 16(1), 29. https://doi.org/10.3390/nano16010029






