Abstract
Solid oxide electrolysis cells (SOECs) are considered one of the most promising technologies for carbon neutralization, as they can efficiently convert CO2 into CO fuel. Sr2Fe1.5Mo0.5O6−δ (SFM) double perovskite is a potential cathode material, but its catalytic activity for CO2 reduction needs further improvement. In this study, Cu ions were introduced to partially replace Mo ions in SFM to adjust the electrochemical performance of the cathode, and the role of the Cu atom was revealed. The results show Cu substitution induced lattice expansion and restrained impurity in the electrode. The particle size of the Sr2Fe1.5Mo0.4Cu0.1O6−δ (SFMC0.1) electrode was about 500 nm, and the crystallite size obtained from the Williamson–Hall plot was 75 nm. Moreover, Cu doping increased the concentration of oxygen vacancies, creating abundant electrochemical active sites, and led to a reduction in the oxidation states of Fe and Mo ions. Compared with other electrodes, the SFMC0.1 electrode exhibited the highest current density and the lowest polarization resistance. The current density of SFMC0.1 reached 202.20 mA cm−2 at 800 °C and 1.8 V, which was 12.8% and 102.8% higher than the SFM electrodes with and without an isolation layer, respectively. Electrochemical impedance spectroscopy (EIS) analysis demonstrated that Cu doping not only promoted CO2 adsorption, dissociation and diffusion processes, but improved the charge transfer and oxygen ion migration. Theory calculations confirm that Cu doping lowered the surface and lattice oxygen vacancy formation energy of the material, thereby providing more CO2 active sites and facilitating oxygen ion transfer.
1. Introduction
The extensive use of fossil fuels has caused a dramatic increase in CO2 emissions. To achieve carbon neutrality, it is crucial to develop effective technologies to reduce and utilize CO2. Various CO2 reduction and conversion technologies have been developed, including photocatalysis [1,2], thermocatalysis [3,4], and electrocatalysis [5].
Photocatalysis is a technology that uses light energy to drive the reduction reaction of CO2. Its core principle is that photosensitizers or semiconductor materials absorb photons, generating electron–hole pairs, which then catalyze the conversion of CO2 into hydrocarbons or other high-value-added chemicals [6]. Current research focuses on strategies to enhance photocatalytic efficiency, including heterojunction construction, defect engineering, single-atom catalysts, and cocatalyst loading. Photocatalysis offers advantages such as direct solar energy utilization, mild reaction conditions, and environmental friendliness. However, its limitations include low quantum efficiency, poor product selectivity, and insufficient catalyst stability [7,8]. Thermocatalysis primarily employs high temperatures and catalysts (Cu/ZnO, Fe-based, Co-based) to hydrogenate CO2 into high-value chemicals such as CH4, methanol, and light olefins. The fundamental principle involves the adsorption and activation of CO2 and H2 on catalyst active sites, followed by hydrogenation reactions that cleave C=O bonds and form C-H bonds [9]. Contemporary research efforts are primarily directed toward sophisticated catalyst engineering strategies, aiming to enhance both catalytic activity and product distribution control. Thermocatalysis offers rapid reaction rates and high product yields, making it suitable for large-scale industrial applications. However, it requires harsh conditions (high temperature and pressure) and suffers from catalyst deactivation (coking or sintering) [10,11]. Electrocatalysis employs electrical energy to drive catalysts for converting CO2 into value-added chemicals such as CO, formate, ethylene, and ethanol. The core mechanism involves electrochemical reactions in which CO2 molecules accept electrons at the cathode surface, undergoing a series of intermediate steps to form target products [12]. Electrocatalytic CO2 reduction offers significant advantages, including tunable reaction pathways via applied voltage and temperature control, compatibility with renewable energy sources (wind, solar, hydro, and geothermal power), and the potential to establish a carbon-neutral energy cycle [13]. Moreover, it provides an effective means of storing intermittent renewable energy in chemical form.
Solid oxide electrolysis cells (SOECs) are an effective CO2 reduction technology and energy conversion device [14]. With external electrical power, SOECs can convert CO2 into CO, which can be used as an industrial feedstock. More importantly, they can directly use renewable energy as power, offering the potential to close the CO2 loop and achieve carbon neutrality [13]. The cell structure consists of three components: the porous cathode, anode, and sandwiched solid electrolyte. During operation, CO2 gas is adsorbed at the cathode and reduced by receiving electrons to generate CO and O2−. The O2− ions migrate through the solid electrolyte to the anode, where they release electrons to generate O2. In these reactions, the reduction of CO2 at the cathode is the key rate-limiting step. Thus, developing high-performance cathode materials is indispensable for the efficient operation of SOECs [15].
Nickel-yttria-stabilized zirconia (Ni-YSZ) is a widely studied and used traditional cathode material for SOECs due to its excellent electrocatalytic activity and relatively low cost [16]. However, Ni-YSZ still faces numerous challenges when electrolyzing pure CO2 at high temperatures. For instance, its redox stability is poor, as it can be oxidized to NiO, which lowers the conductivity of the electrode [17]. Additionally, carbon deposition in pure CO2 atmospheres can block active reaction sites [18], and Ni particles within the composite tend to agglomerate and coarsen [19]. As a result, the development of nickel-free, high-performance cathode materials has become a significant research focus. Perovskite materials, due to their high ionic conductivity, stable redox properties, and good resistance to carbon deposition, have received extensive attention in recent years and are considered one of the most promising candidates for SOEC cathodes [13]. For example, Sr2Fe1.5Mo0.5O6−δ (SFM) [15], La0.75Sr0.25Cr0.5Mn0.5O3−δ [20], La0.67Sr0.33Fe0.67Ti0.33O3−δ [21], and Pr0.4Sr0.6Fe0.9Mo0.1O3 [22] have been reported as cathode materials for CO2 electrolysis in SOECs. Among these perovskite materials, SFM has attracted significant interest because of its high conductivity in both cathode and anode atmospheres, making it a promising candidate for symmetric electrodes in SOECs and offering a potential for reducing fabrication costs [19]. However, the catalytic activity of traditional SFM materials for CO2 reduction still requires further improvement.
In recent years, various strategies have been developed to enhance the catalytic activity of SFM for CO2 electrolysis, including impregnation [23,24], in situ dissolution [24,25], and doping. Doping other ions into the A-site, B-site, or even the O-site of the material can regulate the oxygen vacancy content and is an effective strategy to enhance cathode performance. For example, Yang et al. (2023) found that doping Bi into SFM improved CO2 adsorption on the electrode surface and enhanced the electron conductivity within the electrode, thereby improving the CO2 electrolysis performance [15]. Sun et al. (2022) discovered that doping La into SFM promoted the surface exchange and bulk-phase diffusion of oxygen species [26]. Li et al. (2019) doped F ions into SFM, finding that F ions nearly doubled the CO2 adsorption capacity and increased the concentration of bulk-phase oxygen vacancies by 35–37%, thus enhancing the surface CO2 reduction reaction rate and ion diffusion in the bulk phase [27]. Liu et al. (2025) doped Sc into the B-site of SFM, which increased the concentration of oxygen vacancies and improved CO2 adsorption ability, thereby increasing the current density and reducing the polarization resistance of SOECs [28]. Xi et al. (2021) reported Mg-doped SFM materials, finding that an appropriate amount of Mg doping not only improved redox stability but also lowered the oxygen vacancy formation energy, thereby promoting CO2 electrolysis [17].
Cu is a popular transition metal in the field of catalysis, and as a dopant, it can enhance various properties of perovskite materials. For instance, Lim et al. (2024) doped Cu ions into Ba0.5Sr0.5FeO3−δ for use as a cathode material in solid oxide fuel cells and found that Cu doping increased the oxygen vacancy concentration and improved surface exchange and bulk-phase transport of oxygen ions [29]. In our previous work, Cu-doped La0.8Sr0.2MnO3 was constructed for the electrochemical reduction of NOx, and it was found that Cu doping introduced more oxygen vacancies and decreased the particle size, thus increasing the NOx removal efficiency [30]. Tailor et al. (2023) studied the effect of Cu doping in lead-free halide perovskites on photocatalytic CO2 reduction performance, finding that Cu doping slowed the thermal carrier relaxation and extended the carrier decay lifetime, thus enhancing the charge migration efficiency and improving CO2 reduction performance [31]. Berger et al. (2024) investigated Cu-doped perovskite-type oxides (Nd0.6Ca0.4Fe1−xCuxO3 and Pr0.6Ca0.4Fe1−xCuxO3) as catalysts for Methanol Steam Reforming. The results demonstrated that Cu-doped perovskite-type oxides with tailored A-site and B-site compositions promote the formation of Cu nanoparticles, highlighting the importance of composition optimization for designing efficient catalytic systems [32]. Derakhshi et al. (2024) investigated the effect of Cu substitution for Fe in LaFe1−xCuxO3 perovskite nanostructures for detecting volatile organic compounds (VOCs). The results show significant improvements in gas-sensing properties, with enhanced responses to ethanol, decreased nanoparticle sizes, and increased electrical conductivity [33]. Zhang et al. (2022) developed a Cu-doped perovskite oxide, CaFe0.9Cu0.1O3, as a cathode electrocatalyst for microbial fuel cells (MFCs). The results show that this catalyst outperformed Pt/C in terms of lower overpotential, better stability, and superior power density, making it an efficient and cost-effective alternative for MFC applications [34]. Xu et al. (2019) replaced part of the Fe ions in SFM with Cu ions and found that Cu substitution enhanced the CO2 adsorption capacity of material at high temperatures and reduced the electrode interface polarization resistance [35]. However, to our best knowledge, there is no study on the effect of Cu ion substitution for Mo in SFM, nor has the micro-mechanism of Cu doping on the electrochemical CO2 reduction performance of the electrode been thoroughly investigated. This study aims to develop a novel high-performance SOEC cathode material by substituting Mo sites in SFM perovskite with Cu. The work will elucidate the regulation mechanism of Cu doping on electrocatalytic activity, providing a new strategy for designing efficient and stable cathodes for solid oxide electrolysis cells.
In this work, for the first time, we employed Cu ions to replace Mo in SFM as a symmetric electrode for CO2 electrolysis and examined how Cu doping concentration influences the electrochemical performance of SFM electrodes. In addition, we investigated the regulatory mechanism of Cu ions on the electrode performance. The crystal composition, microstructure, and surface elemental valence states of the materials and electrodes were studied by systematic characterization. Electrochemical tests were performed to evaluate the current density and electrochemical impedance spectra (EIS) at different voltages of the electrodes, and CO2 reduction processes were identified through distributed relaxation time (DRT) analysis. Combined with density functional theory (DFT) calculations, the impact of Cu ion doping on the material properties was further analyzed, thus revealing the fundamental role of Cu ions in performance improvement at the atomic level. This study systematically elucidates the influence mechanism of Cu doping on electrode material performance, providing important theoretical foundations and methodological references for the design of high-performance SOEC electrodes, thereby further enriching the research framework in this field.
2. Experiment
2.1. Powders Preparation
The Sr2Fe1.5Mo0.5−xCuxO6−δ (SFMC) powders (with x = 0, 0.1, and 0.3, denoted as SFM, SFMC0.1, and SFMC0.3, respectively) were synthesized using the citric acid-EDTA method [36]. Briefly, stoichiometric amounts of Sr(NO3)2, Fe(NO3)3·9H2O, (NH4)7Mo7O24·4H2O, and Cu(NO3)2·3H2O were dissolved in a small amount of deionized water, and then citric acid and EDTA were added. The molar ratio of the total metal ions, EDTA and citric acid was controlled at 1: 1: 1.5. The solution above was stirred in a water bath at 80 °C, while ammonia solution was slowly added to adjust the pH to around 7. As the solution evaporated, a brownish wet gel formed. The wet gel was then transferred to a muffle furnace and dried at 160 °C for 7 h, resulting in a foamy black dried gel. This dried gel was calcined in a muffle furnace at 1050 °C for 5 h to obtain a fluffy, flocculent product, which was then ground to obtain the SFMC powders. The ionic conductor Ce0.8Sm0.2O1.9 (SDC) powder was also prepared using a similar citric acid-EDTA method, but the nitrates used were Ce(NO3)3·6H2O and Sm(NO3)3·6H2O, and the calcination temperature was 900 °C. Figure 1a shows the schematic diagram of SFMC powder synthesis.
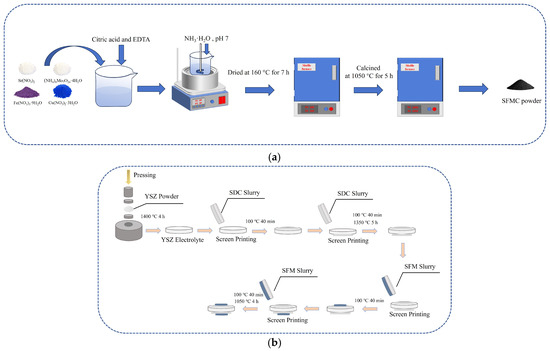
Figure 1.
Schematic diagram of (a) SFMC powder synthesis and (b) cell fabrication..
2.2. Cell Fabrication
Preparation of YSZ electrolyte: 200 mg of YSZ powder was placed into a 13 mm diameter die and pressed at 200 MPa to form a pellet-shaped green body for the electrolyte. The green body was sintered in a high-temperature furnace at 1400 °C for 4 h to obtain a dense YSZ electrolyte substrate. The thickness of the sintered YSZ electrolyte substrate was approximately 400 μm.
Preparation of SDC isolation layer: To prevent potential chemical reactions between the electrode materials and YSZ, an SDC isolation layer was applied to both sides of the YSZ electrolyte. Briefly, SDC powder was mixed with turpentine in a 1: 1.5 mass ratio, ground to a viscous slurry, and then the slurry was screen-printed onto both sides of the YSZ electrolyte. The coated electrolyte was then sintered at 1350 °C for 5 h.
Preparation of SFMC/SDC electrode layer: SFMC powder was mixed with SDC powder and turpentine in a 65:35:150 mass ratio and thoroughly ground to obtain a composite electrode slurry. The slurry was then screen-printed onto both sides of the YSZ electrolyte, which had the SDC isolation layer. The electrodes were sintered at 1050 °C for 4 h. After these steps, a single cell was assembled with YSZ as the electrolyte, SDC as the isolation layer, and SFMC/SDC as the composite symmetric electrode. To verify the role of the SDC isolation layer, another cell using SFM without the SDC isolation layer was prepared, denoted as SFM’. Figure 1b shows the schematic diagram of the cell fabrication.
2.3. Physical and Chemical Characterization
The crystal structure of the synthesized powders and cells was characterized by X-ray diffraction (XRD, D8 Advance, Bruker, Germany). The 2θ range was from 5° to 90°, and the scanning speed was 10° min−1. The microstructure of the electrodes and cells was observed using a field emission scanning electron microscope (SEM, JEOL-S4800, Tokyo, Japan and ZEISS-300, Oberkochen, Germany) and transmission electron microscope (TEM, JEOL JEM-F200, Tokyo, Japan). Elemental distribution analysis was performed using energy-dispersive spectroscopy (EDS) attached to the SEM. X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha, Waltham, MA, USA) was used to analyze the chemical states of elements on the surface of the electrode powders. To eliminate charge effects and instrument drift, the C 1s peak was calibrated to 284.8 eV.
2.4. Electrochemical Performance Testing
Silver wires were connected to the electrodes using conductive silver paste, and the cell was sealed at one end of an alumina tube with high-temperature adhesive. The alumina tube was then placed in a custom-made tube furnace for testing, where the cathode was exposed to a pure CO2 atmosphere while the anode was exposed to air. The CO2 gas flow rate was set to 50 mL min−1 using a mass flow meter. EIS plots of the cell were measured in the temperature range of 650–800 °C using an electrochemical workstation (CHI 660E, Austin, TX, USA). The frequency range for EIS measurement was 0.1–106 Hz, with a voltage amplitude of 10 mV. The obtained EIS spectra were analyzed using DRT to identify and understand different electrochemical processes. The current–voltage (I-V) characteristics of the cell were measured using linear sweep voltammetry (LSV). The short-term stability of the cell was tested using a chronoamperometry method. The CO production rate at the outlet was measured using a gas analyzer (TESTO 330, Lenzkirch, Germany), and the Faradaic efficiency (FE) of the cell was calculated using the following formula:
where R′CO is the experimentally measured CO production rate, and RCO is the theoretical CO production rate, which can be calculated using the formula [37]:
where n is the number of moles of CO produced, I is the current density, t is the electrolysis time, F is Faraday’s constant (96,485 C mol−1), z is the number of electrons transferred in CO2 reduction, and Vm is the molar volume of the gas.
FE = R′CO/RCO
RCO = nVm = (ItVm)/(Fz)
2.5. Computational Details
The DFT calculation was conducted based on CASTEP code. The generalized gradient approximation (GGA) with the Perdew–Burke–Ernzerhof (PBE) exchange correlation density functional was used, and all the calculations were spin-polarized. The simplified cell unit of Sr2FeMoO6 with 40 atoms and a cubic structure was used for basic bulk calculation. Based on the optimized structure of bulk, a slab containing five-layer atoms (52 atoms) with a 15 Å vacuum layer was built for surface calculation. The top three layers were fully relaxed and the remaining two layers were constrained for geometry optimization. Since it is widely accepted that B-site atoms dominate active molecules, only the B-O terminated structure was investigated. To model the Cu-doped SFM, one surface Mo atom was substituted by a Cu atom. The cutoff energy of 571.4 eV and SCF tolerance of 10−6 eV atom−1 was used for calculation. To better describe the structure properties, the Hubbard correlation (U) of 4.0 eV was applied in an Fe atom. The k-point grids of 2 × 2 × 2 and 2 × 2 × 1 were used for bulk and slab calculations, respectively. The geometry optimization was finished as the energy, force and displacement were simultaneously converged to a 10−5 eV atom−1, 0.03 eV Å−1 and 0.001 Å, respectively. The oxygen vacancy formation energy (Ef) was calculated according to the following formula [38]:
where Edef and Eper are the total energies of the defect structure, with one oxygen vacancy, and the perfect structure, respectively. Eoxygen is the total energy of an oxygen molecule.
Ef = Edef − Eper + 1/2Eoxygen
3. Results and Discussion
3.1. Crystal Structure and Morphology
Figure 2a shows the XRD spectra of the SFM powders with different Cu doping levels. As shown in the picture, the main peaks of all the samples match the diffraction peaks of the standard PDF card (PDF#34-0638, cubic structure, Pm-3m space group), indicating the successful synthesis of the SFM double perovskite structure. As displayed in the enlarged XRD pattern in Figure 2b, with increasing Cu content, the diffraction peaks exhibit weakened intensity and a gradual shift toward lower angles, indicating that Cu doping reduced crystallinity and induced lattice expansion. This lattice distortion weakened the Mo-O bonds and lowered the formation energy of oxygen vacancies. The direct cause of this phenomenon is that the ionic radius of Cu2+ (0.073 nm) is larger compared to Mo6+ (0.059 nm) and Mo5+ (0.061 nm) [39,40]. In addition, Cu2+ doping may also lead to a reduction in the oxidation states of Fe and Mo ions, which could further increase the crystal volume, as explained in the following XPS analysis. The Rietveld refinement results are shown in Figure S1 and Table S1 in the Supporting Information. Samples exhibit a cubic perovskite structure with the space group of Pm-3m. As shown in Figure S2, the Williamson–Hall (W-H) graph was given to determine the crystallite size. According to the results (Table S2), the crystallite size for SFM and SFMC0.1 is 98.3 and 75.4 nm, respectively, indicating that moderate Cu doping decreased the crystallite size. However, the crystallite size of SFMC0.1 obtained from the Scherrer equation was 32.5 nm, which was smaller than the size obtained from the W-H graph. This difference indicates the presence of a substantial micro-strain in the SFM perovskite, as the Scherrer equation solely attributes peak broadening to crystallite size, while the W-H method accounts for both size and strain effects, revealing that strain-induced broadening artificially reduces the Scherrer-derived size [41].

Figure 2.
(a) XRD pattern of SFM, SFMC0.1, and SFMC0.3. (b) Enlarged XRD pattern in the 2θ range of 30–35°. (c) XRD pattern of electrodes with and without the SDC isolation layer. (d) XRD spectra of SFM, SFMC0.1, and SFMC0.3 electrodes.
To investigate the chemical compatibility between the electrode material and the electrolyte, Figure 2c shows the XRD spectra of the SFM electrodes with and without the SDC isolation layer. The electrode without the SDC isolation layer (SFM’), in addition to the diffraction peaks of SFM, SDC, and YSZ, also shows impurity phases, such as SrMoO4 and Sr2ZrO4. The SrMoO4 impurity often appears during the synthesis of Mo-based perovskite oxides. According to the literature, SrMoO4 impurity likely arose from the melting of intermediate MoO3 oxides [42,43]. The Sr2ZrO4 phase is a product of the reaction between SFM and YSZ, which can significantly affect the electrode’s performance [44]. After adding the SDC isolation layer, the intensity of the Sr2ZrO4 diffraction peaks significantly decreases, confirming the necessity of adding the SDC isolation layer. Therefore, the electrodes with a SDC isolation layer are used for subsequent studies. Figure 2d shows the XRD pattern of the electrodes with different Cu doping contents. It is observed that the SrMoO4 and Sr2ZrO4 impurity phases disappear after Cu doping, and no new phases form, probably indicating that the chemical compatibility between the electrode and the electrolyte is improved.
Figure 3a,b show the cross-sectional SEM images of the cell. The images reveal a dense electrolyte and a loose, porous electrode. The SDC isolation layer is located between the electrolyte and the electrode, preventing the side reactions between the electrode material and the electrolyte. The electrode, isolation layer, and electrolyte are tightly connected, facilitating the transport of oxygen ions from the composite electrode to the electrode. Figure 3c,d show the surface SEM images of the SFMC0.1 electrode. As shown in the images, the electrode surface has a porous structure, which is beneficial for the diffusion and adsorption of gas molecules. The particles are tightly connected, facilitating the migration of electrons and O2− ions. The particle size on the surface of the SFMC0.1 electrode was approximately 500 nm. Figure 3e–g show the TEM image of SFMC0.1. The distance between the two parallel planes is 0.278 nm, corresponding to the (110) plane of SFMC0.1 perovskite. Figure 3h–o show the elemental mapping of the SFMC0.1 electrode surface. The distributions of Cu elements match those of Sr, Fe, and Mo, confirming the successful doping of Cu into the SFM. The distribution of Ce and Sm elements differs from that of the SFMC components, proving that the SDC and SFMC components are interspersed, which expands the three-phase reaction boundary. The EDS results shown in Figure 3p are in good agreement with the stoichiometry of Sr2Fe1.5Mo0.4Cu0.1O6−δ-Ce0.8Sm0.2O1.9, further confirming the successful preparation of the electrode material.
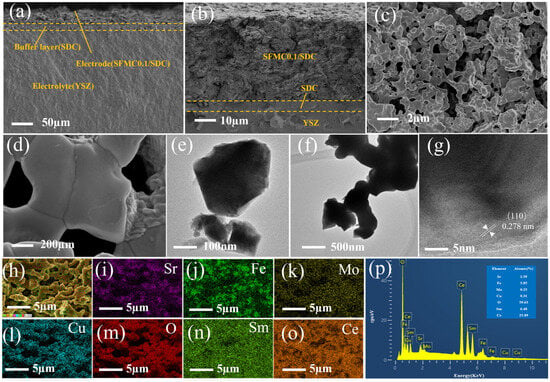
Figure 3.
(a,b) Cross-sectional SEM images, (c,d) surface SEM images, and (e–g) TEM images of the SFMC0.1 electrode. (h–o) Elemental mapping of the electrode surface, (p) EDS of the electrode surface.
3.2. XPS Analysis
As shown in Figure 4a, the XPS spectra of O 1s can be divided into two peaks. The peak at the lower binding energy (around 529.8 eV) corresponds to lattice oxygen (Olat), while the peak at the higher binding energy (around 531.0 eV) corresponds to adsorbed oxygen species [15]. It is widely believed that the content of adsorbed oxygen reflects the surface oxygen vacancy concentration [45]. According to the fitting results (Table 1), the adsorbed oxygen ratio in SFMC0.1 is 72.1%, which is significantly higher than the 63.1% in SFM. This phenomenon indicates that Cu doping increases the surface oxygen vacancies in the material. According to related literature, CO2 reduction reaction kinetics are closely related to the surface oxygen vacancy concentration of the electrode, as oxygen vacancies can serve as hosts for CO2 molecules, which is crucial for the chemical adsorption of CO2 at high temperatures [15].
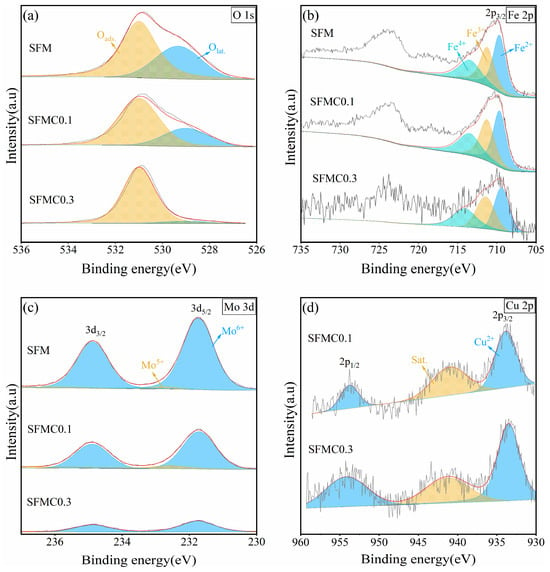
Figure 4.
XPS spectra of SFM, SFMC0.1, and SFMC0.3 powders: (a) O 1s, (b) Fe 2p, (c) Mo 3d, (d) Cu 2p.

Table 1.
Fitting results of the O 1s spectra of SFM, SFMC0.1, and SFMC0.3 powders.
Figure 4b shows the XPS spectra of Fe 2p. The Fe 2p spectrum exhibits two asymmetric peaks, with the lower binding energy region corresponding to the 2p3/2 orbital. The binding energies around 709.7 eV, 711.2 eV, and 713.5 eV correspond to Fe2+, Fe3+, and Fe4+ in the 2p3/2 orbital, respectively [38]. According to the fitting results (Table 2), Cu doping causes Fe ions to shift toward lower oxidation states, increasing the Fe2+ content while decreasing the Fe3+ and Fe4+ content.

Table 2.
Fitting results of the Fe 2p spectra of SFM, SFMC0.1, and SFMC0.3 powders.
As shown in Figure 4c, the signal intensity of Mo decreases as the Cu substitution amount increases. The Mo 3d spectrum shows two peaks, with binding energies around 232.8 eV and 231.7 eV corresponding to Mo6+ and Mo5+, respectively [38]. According to the fitting results (Table 3), Mo ions primarily exist in the Mo6+ form, and after Cu doping, the content of Mo5+ increases, indicating a slight reduction in the overall oxidation state of Mo ions. The presence of mixed valence Mo5+/Mo6+ can enhance the ionic conductivity and catalytic activity required for the CO2 electrolysis process. According to the principle of charge neutrality, higher concentrations of low-valence Fe2+ and Mo5+ ions result in increased oxygen vacancy formation. Therefore, the XPS results for Fe and Mo are generally consistent with the O 1s results.

Table 3.
Fitting results of the Mo 3d spectra of SFM, SFMC0.1, and SFMC0.3 powders.
XPS analysis demonstrates that Cu doping increases Fe2+/Fe3+ and Mo5+/Mo6+ redox couples, promoting polaron hopping and enhancing electronic conductivity. The increase in oxygen vacancy concentration (verified by the increase in oxygen defects proportion) provides a channel for O2− migration and reduces the migration activation energy of oxygen ions. Cationic defects introduced by Cu doping work synergistically with oxygen vacancy to form local charge delocalization states (such as Cu-3d/O-2p hybridization) and improve intrinsic conductivity, which can be verified by the DOS analysis [46].
Figure 4d shows the XPS spectrum of Cu 2p in the electrode material. The Cu 2p3/2 spectrum displays two peaks: the peak at 933.48 eV corresponds to Cu2+, and the peak at 941.26 eV corresponds to the satellite peak. It can be identified that Cu in SFMC is mainly in the form of Cu2+ [39].
3.3. Electrochemical Performance
Figure 5a presents the I-V curves of cells based on different electrode materials. It can be observed that the introduction of an isolation layer significantly increases the current density, and appropriate Cu doping further enhances the current density. At 1.8 V, the cell based on SFMC0.1 shows the highest current density of 202.20 mA cm−2, followed by SFM (179.26 mA cm−2), SFM’ (99.68 mA cm−2) and SFMC0.3 (67.73 mA cm−2). Figure 5b shows the EIS of different electrodes. The EIS spectra for each electrode consist of several semi-circles. In general, the high-frequency portion of the EIS spectrum and its intersection with the x-axis represent the ohmic resistance, while the low-frequency portion and its intersection with the x-axis represent the total resistance. The difference between the total resistance and the ohmic resistance represents the polarization resistance of the electrode [47]. As shown in Figure 5b, the SFMC0.1 cell exhibits the smallest total and polarization resistances, while excessive Cu doping results in a significant increase in resistance for the SFMC0.3 cell.
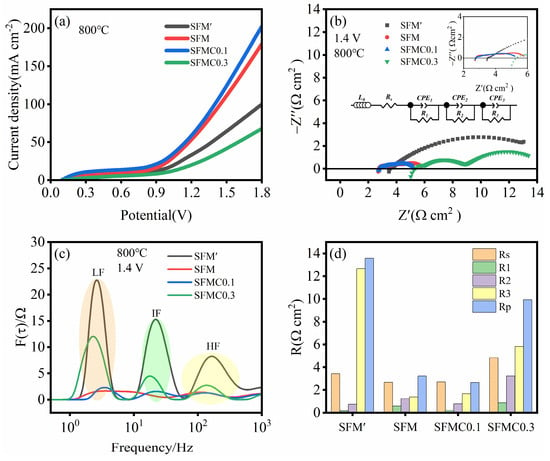
Figure 5.
Electrochemical performance of cells based on different electrode materials. (a) I-V curves, (b) EIS curves at 1.4 V, (c) corresponding DRT spectra, and (d) fitted resistance values.
To further investigate the electrochemical reaction process, the electrochemical impedance spectra were analyzed using an equivalent circuit model: L0Rs(R1-CPE1)(R2-CPE2)(R3-CPE3) via the DRT technique [26]. In the model, L represents inductance, and Rs represents the total ohmic resistance related to the interface, including electrolyte, electrode and contact resistance. R1, R2, and R3 represent the polarization resistances in the high-, medium-, and low-frequency regions, while CPE1, CPE2, and CPE3 are constant phase elements corresponding to the respective frequency regions. As shown in Figure 5c, the DRT spectrum can be divided into high-, medium- and low-frequency regions. The high-frequency region (HF > 100 Hz) is likely associated with oxygen ion transfer steps between the YSZ electrolyte and electrode, while the intermediate-frequency region (IF) may relate to charge transfer, and the low-frequency region (LF < 10 Hz) is attributed to the surface kinetics of the fuel electrode, including adsorption, activation, diffusion and dissociation processes of the active species [25]. The DRT results demonstrate that the peak areas corresponding to the HF and LF regions of the SFMC0.1 cell are reduced compared to those of the SFM cell. This indicates that Cu doping facilitates the oxygen ion transfer steps between the YSZ electrolyte and electrode, as well as charge transfer processes at the electrode–electrolyte interface, which is consistent with the analysis of the O 1s results from XPS. Figure 5d summarizes the resistances at different frequencies. In line with the trend observed in Figure 5c, R3 values are significantly higher than those of R1 and R2, indicating that the processes such as CO2 adsorption, dissociation and diffusion at the surface are rate-limiting steps. The Rp value for SFMC0.1 is the lowest at 2.66 Ω cm2, while the Rp value for SFM’ is the highest at 13.57 Ω cm2.
Figure 6a shows the I–V curves for cells with SFMC0.1 electrodes at different temperatures. The I–V curves for all temperatures exhibit a similar trend, with current density initially increasing slowly with increasing voltage and then rising sharply. Current density increases with the operating temperature, with the highest current density of 202.20 mA cm−2 at 800 °C and 1.8 V, followed by 750 °C (123.53 mA cm−2), 700 °C (64.76 mA cm−2), and 650 °C (36.94 mA cm−2). The EIS curves at open-circuit voltage (OCV) for different temperatures are shown in Figure 6b. It can be observed that, as the temperature decreases, both the ohmic resistance and polarization resistance increase significantly. This trend is consistent with the results shown in Figure 6a. The corresponding DRT curves are presented in Figure 6c. Compared to other frequencies, the peak area in the LF region decreases most significantly as the temperature increases, and there is a tendency for it to shift towards higher frequencies. This phenomenon suggests that higher temperatures significantly enhance CO2 adsorption, dissociation and diffusion at the electrode surface.
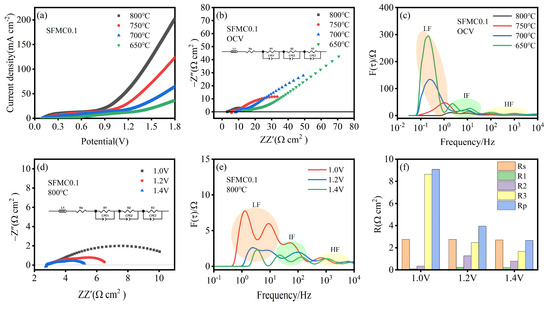
Figure 6.
Electrochemical performance of cells with SFMC0.1 electrodes at different temperatures. (a) I-V curves, (b) EIS curves at OCV, (c) corresponding DRT spectra; EIS curves at different temperatures: (d) at different voltages, (e) corresponding DRT spectra, and (f) fitted resistance values.
Figure 6d shows the EIS curves of the cell at different voltages. The polarization resistance decreases significantly with increasing voltage, while the ohmic resistance remains almost unchanged. The corresponding DRT spectra are shown in Figure 6e. It can be observed that the resistances in the low- and medium-frequency regions decrease, indicating that applying voltage significantly influences charge transfer and the surface adsorption, diffusion and dissociation processes of the active species. According to relevant reports, cathode polarization leads to the reduction of metal ions on the electrode, generating more surface oxygen vacancies and facilitating the surface reaction process [48]. Figure 6f displays the corresponding resistances at different voltages, which are consistent with the results shown in Figure 6e. The polarization resistance at 1.4 V is 2.66 Ω·cm2, much smaller than at 1.0 V (9.08 Ω·cm2) and 1.2 V (3.95 Ω·cm2). The EIS-fitted parameters of SFMC0.1 electrodes at different temperatures and voltages are shown in Table S3. Table S4 compares the CO2 electrolysis performance between this work and other previous reports. As shown in the table, our work is better than some previous reports in terms of current density, Faradaic efficiency, and polarization resistance.
Figure 7a shows the short-term stability test results for cells based on SFMC0.1 electrodes. The current density of the cell exhibits a slight degree of degradation at high voltage. Figure 7b shows the CO yield and Faradaic efficiency of the cell at various voltages. As the applied voltage increases, the CO yield gradually increases, and the corresponding Faradaic efficiency also rises significantly. The Faradaic efficiency reaches 97.1% at 1.6 V. It should be noted that current density is influenced significantly by various factors including the experimental equipment, cell assembly and sealing status. Improving these factors can further elevate the current density level. As shown in Figure 7c, SFMC0.1 demonstrates good durability over the 50 h testing period. Some slight fluctuations in current density were observed during the test.
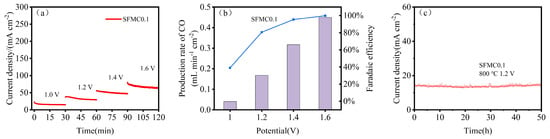
Figure 7.
Short-term stability and performance of SFMC0.1. (a) Short-term stability at 1.0–1.6 V, (b) CO conversion rate and Faradaic efficiency and (c) long-term stability of the SFMC0.1 at 800 °C.
In order to further validate the stability of the prepared SFMC0.1 electrode, Figure 8a presents the SEM image of the tested electrode. Localized particle agglomeration and coarsening are observed on its surface, indicating potential structural degradation during operation. Additionally, Figure 8b compares the XRD patterns of the SFMC0.1 electrode before and after testing. The main perovskite phase remains intact, but the weakened peak intensity may be attributed to the crystal structure at the electrode–electrolyte interface underwent changes during high-temperature testing.
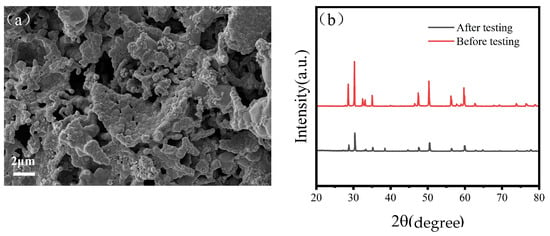
Figure 8.
(a) SEM image of SFMC0.1 electrodes after testing and (b) XRD image of SFMC0.1 electrodes before testing and after testing.
3.4. DFT Calculations
Figure 9a gives the constructed perfect slabs of SFM and SFMC. As shown in the picture, the distances between the surface Mo atom and adjacent O atoms are 1.891 and 1.849 Å, respectively, and the O-Mo-O bond angle is 95.0°. As the surface Mo atom is substituted by the Cu atom, the O-Cu bond distances are extended to 2.087 and 2.359 Å, and the O-Cu-O bond angle is decreased to 84.2°. This change indicates the lattice distortion, and the enlarged distance of Cu probably facilitates the formation of oxygen vacancies. Figure S3 displays some typical defect configurations with one oxygen vacancy for SFM and SFMC after geometry optimization, and the oxygen vacancy formation energy is summarized in Figure 9b. The surface oxygen vacancy formation energy of SFM is 3.89 eV, but it decreases significantly to 1.69 eV for SFMC. The Cu doping and the formation of oxygen vacancies may enhance the intrinsic conductivity and charge carrier concentration of the catalyst, leading to moderate adsorption energy between the catalyst and reactants/intermediates, thereby synergistically improving the catalytic activity. This result confirms that the Cu substitution promotes the formation of surface oxygen vacancy, which is in accordance with the result of O 1s XPS. In addition, the lattice oxygen vacancy formation energies are also decreased, from 3.65 and 3.82 eV to 2.15 and 2.89 eV, respectively, after Cu doping, facilitating oxygen vacancy generation and enhancing ionic conductivity via O2− migration pathways [46]. This phenomenon indicates that the formation of oxygen defects in a lattice are also improved, which is beneficial to the O2− ion transportation across the electrode material.
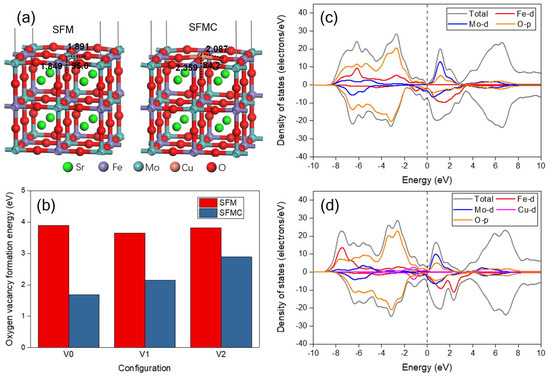
Figure 9.
(a) Optimized configurations of the perfect slab models of SFM and SFMC; (b) oxygen vacancy formation energies of SFM and SFMC; DOS plots of (c) SFM and (d) SFMC with one surface oxygen vacancy.
Figure 9c displays the DOS plots of SFM and SFMC with one surface oxygen vacancy. In Figure 9a, the undoped SFM material shows no significant upward DOS near the Fermi level, indicating a low electron density of states in this region, which suggests that the material may have a semiconductor property. The main contributions come from the Fe-d, Mo-d and O-p orbitals. In contrast, Figure 9b shows that after Cu doping, the material exhibits a noticeable upward DOS near the Fermi level, indicating that Cu doping introduces new electron states. This can significantly enhance the intrinsic electronic conductivity of the material while increasing charge supply to the active sites of the catalytic reaction, thereby optimizing charge transfer kinetics [46]. These electronic states make a substantial contribution to the total DOS in this region. The contribution of the Cu-d orbitals near the Fermi level is evident, which may alter the electronic structure of the material, making it more metallic. In addition, after Cu doping, the increase in O-p DOS near the Fermi level may contribute to enhancing the conductivity of O2− ions. These changes could have a significant impact on the material’s electrical conductivity and O2− transport properties.
4. Conclusions
In this study, Mo ions in SFM electrode were partially substituted by Cu for CO2 electrolysis in SOECs, and the role of Cu substitution was revealed by experimental and theory calculation. The results show that Cu substitution induced lattice expansion and eliminated the SrMoO4 and Sr2ZrO4 impurity phases in the electrode and thereby enhanced the compatibility between the electrode and electrolyte. XPS results indicated that Cu doping increased the concentration of oxygen vacancies and reduced the valence states of Fe and Mo. Among various samples, the SFMC0.1 cell exhibited the largest current density and the smallest polarization resistance. At 800 °C and 1.8 V, the current density of SFMC0.1 reached 202.20 mA cm−2, which is a 12.8% improvement compared to SFM. At 1.4 V, the polarization resistance was 2.66 Ω·cm2, representing a 70.7% reduction compared to that at 1.0 V. EIS analysis demonstrated that Cu doping not only promoted CO2 adsorption, dissociation and diffusion processes, but improved the charge transfer and oxygen ion migration. DFT calculations confirmed that Cu doping lowered the surface and lattice oxygen vacancy formation energy of the material, thereby providing more CO2 active sites and facilitating oxygen ion transfer. This study offers both technical and theoretical insights for the development of new CO2 electrolysis electrodes in SOECs. In the future, some other factors such as cell fabrication and sealing processes should be investigated to further improve the electrode structure and cell performance. In situ/operando XRD and TEM studies will be pursued to dynamically resolve the structural evolution of the electrode during electrolysis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano15080585/s1, Figure S1: Rietveld refinement profiles of SFMC0.1 powder; Figure S2: The Williamson–Hall plot of SFM, SFMC0.1 and SFMC0.3 powder; Figure S3: Configurations of SFM and SFMC with one oxygen vacancy; Table S1: Rietveld refinement results of SFMC0.1 powder derived from XRD patterns; Table S2: The crystallite sizes of SFM, SFMC0.1 and SFMC0.3 powder were determined using the Williamson–Hall plot and the Scherrer equation; Table S3: The EIS-fitted parameters of SFMC0.1 electrodes at different temperatures and voltages; Table S4: Comparison of literature values of polarization resistance, current density and Faradaic efficiency achieved for CO2 electrolysis in electrolyte-supported cells under 800 °C. Refs. [15,49,50,51,52,53,54,55,56] are cited in the Supplementary Materials.
Author Contributions
Conceptualization, W.L.; methodology, W.T.; formal analysis, P.H. and S.W.; investigation, T.F., H.S., S.Z. and Z.Q.; data curation, P.H.; writing—original draft preparation, W.T.; writing—review and editing, W.L.; visualization, P.H.; supervision, W.L.; funding acquisition, W.L. and Z.Q. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China (52302435) and Key Research and Development and Promotion Project of Henan Province (232102321048).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, Z.; Wang, M.; Chi, Z.; Li, W.; Yu, H.; Yang, N.; Yu, H. Internal electric field engineering step-scheme–based heterojunction using lead-free Cs3Bi2Br9 perovskite–modified In4SnS8 for selective photocatalytic CO2 reduction to CO. Appl. Catal. B-Environ. 2022, 313, 121426. [Google Scholar] [CrossRef]
- Ni, S.; Wu, W.; Yang, Z.; Zhang, M.; Yang, J. Influence of copper valence in CuOx/TiO2 catalysts on the selectivity of carbon dioxide photocatalytic reduction products. Nanomaterials 2024, 14, 1930. [Google Scholar] [CrossRef]
- Gao, J.; Choo Sze Shiong, S.; Liu, Y. Reduction of CO2 to chemicals and Fuels: Thermocatalysis versus electrocatalysis. Chem. Eng. J. 2023, 472, 145033. [Google Scholar] [CrossRef]
- Ye, R.-P.; Ding, J.; Gong, W.; Argyle, M.D.; Zhong, Q.; Wang, Y.; Russell, C.K.; Xu, Z.; Russell, A.G.; Li, Q.; et al. CO2 hydrogenation to high-value products via heterogeneous catalysis. Nat. Commun. 2019, 10, 5698. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, Z.; Bao, A.; Tang, X.; Huang, X.; Yi, H.; Zhao, S.; Sun, T.; Wang, J.; Gao, F. Recent advances on electrocatalytic CO2 reduction to resources: Target products, reaction pathways and typical catalysts. Chem. Eng. J. 2023, 453, 139663. [Google Scholar] [CrossRef]
- Sahu, R.; Patodia, T.; Juyal, S.; Fateh Singh, G.; Prasad, B.; Jain, A. Innovations and fundamentals in visible light-driven photocatalysis for CO2 reduction. Catal. Sci. Technol. 2025, 15, 988–1002. [Google Scholar] [CrossRef]
- Shen, J.; Wu, Z.; Li, C.; Zhang, C.; Genest, A.; Rupprechter, G.; He, L. Emerging applications of MXene materials in CO2 photocatalysis. FlatChem 2021, 28, 100252. [Google Scholar] [CrossRef]
- Yao, S.; He, J.; Gao, F.; Wang, H.; Lin, J.; Bai, Y.; Fang, J.; Zhu, F.; Huang, F.; Wang, M. Highly selective semiconductor photocatalysis for CO2 reduction. J. Mater. Chem. A 2023, 11, 12539–12558. [Google Scholar] [CrossRef]
- He, X.; Liu, M.; Liang, Z.; Wang, Z.; Wang, P.; Liu, Y.; Cheng, H.; Dai, Y.; Zheng, Z.; Huang, B. Photo-enhanced CO2 hydrogenation by plasmonic Cu/ZnO at atmospheric pressure. J. Solid State Chem. 2021, 298, 122113. [Google Scholar] [CrossRef]
- Dong, X.; Li, F.; Zhao, N.; Xiao, F.; Wang, J.; Tan, Y. CO2 hydrogenation to methanol over Cu/ZnO/ZrO2 catalysts prepared by precipitation-reduction method. Appl. Catal. B-Environ. 2016, 191, 8–17. [Google Scholar] [CrossRef]
- Biswas, A.N.; Winter, L.R.; Xie, Z.; Chen, J.G. Utilizing CO2 as a reactant for C3 oxygenate production via Tandem reactions. JACS Au 2023, 3, 293–305. [Google Scholar] [CrossRef]
- Zhou, Z.-Y.; Sun, S.-G. A breakthrough in electrocatalysis of CO2 conversion. Natl. Sci. Rev. 2017, 4, 155–156. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, X.; Xie, K.; Wang, G.; Bao, X. High-temperature CO2 electrolysis in solid oxide electrolysis cells: Developments, challenges, and prospects. Adv. Mater. 2019, 31, 1902033. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.-D.; Tian, N.; Hu, S.-N.; Zhou, Z.-Y.; Sun, S.-G. Recent advances of bismuth-based electrocatalysts for CO2 reduction: Strategies, mechanism and applications. Mater. Rep. Energy 2023, 3, 100191. [Google Scholar] [CrossRef]
- Yang, M.; Yao, Z.; Liu, S.; Wang, J.; Sun, A.; Xu, H.; Yang, G.; Ran, R.; Zhou, W.; Xiao, G.; et al. Bismuth doped Sr2Fe1.5Mo0.5O6−δ double perovskite as a robust fuel electrode in ceramic oxide cells for direct CO2 electrolysis. J. Mater. Sci. Technol. 2023, 164, 160–167. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, S.; Zhu, X.; Yang, W. Electrochemical reduction of CO2 in solid oxide electrolysis cells. J. Energy Chem. 2017, 26, 593–601. [Google Scholar] [CrossRef]
- Xi, X.; Liu, J.; Fan, Y.; Wang, L.; Li, J.; Li, M.; Luo, J.-L.; Fu, X.-Z. Reducing d-p band coupling to enhance CO2 electrocatalytic activity by Mg-doping in Sr2FeMoO6−δ double perovskite for high performance solid oxide electrolysis cells. Nano Energy 2021, 82, 105707. [Google Scholar] [CrossRef]
- Sharma, S.; Tiwari, P.; Basu, S.; Kumari, N. Harnessing the electrocatalytic potential of in-situ exsolution of Ni nanoparticles on lanthanum and calcium co-doped strontium titanate for CO2 reduction in solid oxide electrolysis cells. J. Alloys Compd. 2024, 996, 174831. [Google Scholar] [CrossRef]
- Wang, E.; Zhao, L.; Yang, Z.; Liu, C.; Wang, S.; Yang, R.; Jin, C. SnOx surface modified Sr2Fe1.5Mo0.5O6−δ cathode with enhanced electrocatalytic activities for direct CO2 electrolysis in solid oxide electrolysis cells. J. Colloid Interface Sci. 2025, 680, 605–612. [Google Scholar] [CrossRef]
- Qian, B.; Liu, C.; Wang, S.; Yin, B.; Zheng, Y.; Ge, L.; Chen, H.; Zhang, C. Ca-doped La0.75Sr0.25Cr0.5Mn0.5O3 cathode with enhanced CO2 electrocatalytic performance for high-temperature solid oxide electrolysis cells. Int. J. Hydrogen Energy 2021, 46, 33349–33359. [Google Scholar] [CrossRef]
- Ruan, W.; Wu, M.; Xia, Y.; Ni, J.; Ni, C. High La/Sr ratio in La0.67Sr0.33Fe0.67Ti0.33O3−δ cathode induced a controlled Fe0 exsolution for CO2 electrolysis. J. Alloys Compd. 2024, 970, 172628. [Google Scholar] [CrossRef]
- Li, P.; Yang, P.; Liu, F.; Xiao, W.; Yan, F.; Gan, T.; Zhao, K.; Fu, D. Enhancing catalytic activity of CO2 electrolysis via B-site cation doped perovskite cathode in solid oxide electrolysis cell. Ceram. Int. 2023, 49, 12980–12989. [Google Scholar] [CrossRef]
- Lv, H.; Zhou, Y.; Zhang, X.; Song, Y.; Liu, Q.; Wang, G.; Bao, X. Infiltration of Ce0.8Gd0.2O1.9 nanoparticles on Sr2Fe1.5Mo0.5O6−δ cathode for CO2 electroreduction in solid oxide electrolysis cell. J. Energy Chem. 2019, 35, 71–78. [Google Scholar] [CrossRef]
- Li, M.; Hou, J.; Fan, Y.; Xi, X.; Fu, X.-Z.; Luo, J.-L. Interface modification of Ru-CeO2 co-infiltrated SFM electrode and construction of SDC/YSZ bilayer electrolyte for direct CO2 electrolysis. Electrochim. Acta 2022, 426, 140771. [Google Scholar] [CrossRef]
- Xi, X.A.; Fan, Y.; Zhang, J.J.; Luo, J.L.; Fu, X.Z. In situ construction of hetero-structured perovskite composites with exsolved Fe and Cu metallic nanoparticles as efficient CO2 reduction electrocatalysts for high performance solid oxide electrolysis cells. J. Mater. Chem. A 2022, 10, 2509–2518. [Google Scholar] [CrossRef]
- Sun, C.; Bian, L.; Qi, J.; Yu, W.; Li, S.; Hou, Y.; Wang, L.; Peng, J.; An, S. Boosting CO2 directly electrolysis by electron doping in Sr2Fe1.5Mo0.5O6−δ double perovskite cathode. J. Power Sources 2022, 521, 230984. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Wan, Y.; Xie, Y.; Zhu, J.; Pan, H.; Zheng, X.; Xia, C. Perovskite oxyfluoride electrode enabling direct electrolyzing carbon dioxide with eExcellent electrochemical performances. Adv. Energy Mater. 2019, 9, 1803156. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, L.; Xu, C.; Wang, Z.; Qiao, J.; Sun, W.; Sun, K. Sc-doped strontium iron molybdenum cathode for high-efficiency CO2 electrolysis in solid oxide electrolysis cell. J. Fuel Chem. Technol. 2025, 53, 272–281. [Google Scholar] [CrossRef]
- Lim, T.; Jo, K.; Kim, Y.-N.; Lee, H. Valence electronic structure on oxygen reduction reaction kinetics of Cu-doped Ba0.5Sr0.5FeO3−δ for IT-SOFCs. Mater. Lett. 2024, 372, 136959. [Google Scholar] [CrossRef]
- Li, W.; Liang, K.; Wang, J.; Wen, J.; Shi, J.; Zhang, Z.; Jiang, W.; Zhang, R.; Yu, H. Effects of Cu doping on electrochemical NOx removal by La0.8Sr0.2MnO3 perovskites. Environ. Res. 2022, 210, 112955. [Google Scholar] [CrossRef]
- Kumar Tailor, N.; Singh, S.; Afroz, M.A.; Pant, K.K.; Satapathi, S. Unraveling the impact of Cu-doping in lead free halide perovskites for markedly enhancing photocatalytic CO2 reduction performance. Appl. Catal. B-Environ. 2024, 340, 123247. [Google Scholar] [CrossRef]
- Berger, T.; Drexler, H.; Ruh, T.; Lindenthal, L.; Schrenk, F.; Bock, J.; Rameshan, R.; Föttinger, K.; Irrgeher, J.; Rameshan, C. Cu-doped perovskite-type oxides: A structural deep dive and examination of their exsolution behaviour influenced by B-site doping. Catal. Today 2024, 437, 114787. [Google Scholar] [CrossRef]
- Derakhshi, Z.; Baghshahi, S.; Khodadadi, A.A.; Tamizifar, M. Cu-doped LaFe1−xCuxO3 perovskites nano-crystallites for enhanced VOCs detection. Ceram. Int. 2024, 50, 23175–23187. [Google Scholar] [CrossRef]
- Zhang, H.; Shi, H.; You, H.; Su, M.; Huang, L.; Zhou, Z.; Zhang, C.; Zuo, J.; Yan, J.; Xiao, T.; et al. Cu-doped CaFeO3 perovskite oxide as oxygen reduction catalyst in air cathode microbial fuel cells. Environ. Res. 2022, 214, 113968. [Google Scholar] [CrossRef]
- Xu, C.M.; Zhen, S.Y.; Ren, R.Z.; Chen, H.S.; Song, W.L.; Wang, Z.H.; Sun, W.; Sun, K.N. Cu-Doped Sr2Fe1.5Mo0.5O6−δ as a highly active cathode for solid oxide electrolytic cells. Chem. Commun. 2019, 55, 8009–8012. [Google Scholar] [CrossRef]
- Tan, W.; Zhao, S.; Song, H.; Hu, P.; Wang, J.; Qi, Z.; Li, W. CuO and La0.75Sr0.25Cr0.5Mn0.5O3−δ nanoparticles modified Sr2Fe1.5Mo0.5O6−δ perovskite cathodes for CO2 reduction in solid oxide electrolysis cells. J. Alloys Compd. 2025, 1014, 178705. [Google Scholar] [CrossRef]
- Wang, E.; Jin, C.; Zhao, L.; Yang, Z.; Liu, C.; Wang, S.; Lei, X.; Chao, M.; Xu, H.; Yang, R. Reinforced chemical adsorption ability for efficient CO2 electrolysis in solid oxide electrolysis cell via a dual-exsolution strategy. Chem. Eng. J. 2024, 494, 153129. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, Y.; Xia, C.; Bouwmeester, H.J.M. Sr2Fe1.4Mn0.1Mo0.5O6−δ perovskite cathode for highly efficient CO2 electrolysis. J. Mater. Chem. A 2019, 7, 22939–22949. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Gao, Y.; Yu, X.; Jiang, H.; Wei, B.; Lü, Z. In situ growth of copper-iron bimetallic nanoparticles in A-site deficient Sr2Fe1.5Mo0.5O6−δ as an active anode material for solid oxide fuel cells. J. Alloys Compd. 2022, 926, 166852. [Google Scholar] [CrossRef]
- Yang, X.; Sun, K.; Sun, W.; Ma, M.; Ren, R.; Qiao, J.; Wang, Z.; Zhen, S.; Xu, C. Surface reconstruction of defective SrTi0.7Cu0.2Mo0.1O3−δ perovskite oxide induced by in-situ copper nanoparticle exsolution for high-performance direct CO2 electrolysis. J. Eur. Ceram. Soc. 2023, 43, 3414–3420. [Google Scholar] [CrossRef]
- Rehman, A.U.; Amin, N.; Tahir, M.B.; Ajaz un Nabi, M.; Morley, N.A.; Alzaid, M.; Amami, M.; Akhtar, M.; Arshad, M.I. Evaluation of spectral, optoelectrical, dielectric, magnetic, and morphological properties of RE3+ (La3+, and Ce3+) and Co2+ co-doped Zn0.75Cu0.25Fe2O4 ferrites. Mater. Chem. Phys. 2022, 275, 125301. [Google Scholar] [CrossRef]
- Xie, Z.; Zhao, H.; Chen, T.; Zhou, X.; Du, Z. Synthesis and electrical properties of Al-doped Sr2MgMoO6−δ as an anode material for solid oxide fuel cells. Int. J. Hydrogen Energy 2011, 36, 7257–7264. [Google Scholar] [CrossRef]
- Dong, H.; Wang, M.; Liu, Y.; Han, Z. Optimized solid-state synthesis of Sr2Fe1.5Mo0.5O6−δPerovskite: Implications for efficient synthesis of Mo-containing SOFC electrodes. Crystals 2022, 12, 1533. [Google Scholar] [CrossRef]
- Feng, Q.; Lv, M.; Liu, C.; Chen, G.; Gao, P.; Li, C. Phase relations at 1573 K and 1673 K and thermodynamic assessment of ZrO2-SrO-BaO system. Calphad-Comput. Coupling Phase Diagr. Thermochem. 2024, 87, 102754. [Google Scholar] [CrossRef]
- Lu, C.; Xu, C.; Sun, W.; Ren, R.; Qiao, J.; Wang, Z.; Sun, K.; Pan, G.; Cao, Y. Enhancing catalytic activity of CO2 electrolysis by building efficient and durable heterostructure for solid oxide electrolysis cell cathode. J. Power Sources 2023, 574, 233134. [Google Scholar] [CrossRef]
- Yu, Z.; Si, C.; Sabaté, F.; LaGrow, A.P.; Tai, Z.; Diaconescu, V.M.; Simonelli, L.; Meng, L.; Sabater, M.J.; Li, B.; et al. Defective Ru-doped α-MnO2 nanorods enabling efficient hydrazine oxidation for energy-saving hydrogen production via proton exchange membranes at near-neutral pH. Chem. Eng. J. 2023, 470, 144050. [Google Scholar] [CrossRef]
- Wang, J.; Ma, L.; Tan, W.; Wang, S.; Wen, J.; Zhang, Z.; Yu, H.; Li, W. NiO and Co3O4 nanoparticles decorated La0.8Sr0.2MnO3-based electrodes for electrochemical NOx removal in solid electrolyte cells. Chem. Eng. J. 2023, 466, 143248. [Google Scholar] [CrossRef]
- Li, W.; Yu, H.; Yu, H.; Yang, N.; Zhang, S. Electrochemical reduction of NO by solid electrolyte cells with La0.8Sr0.2MnO3-Ce0.8Sm0.2O1.9 composite cathodes. Chem. Eng. J. 2019, 378, 122188. [Google Scholar] [CrossRef]
- Xu, S.; Li, S.; Yao, W.; Dong, D.; Xie, K. Direct electrolysis of CO2 using an oxygen-ion conducting solid oxide electrolyzer based on La0.75Sr0.25Cr0.5Mn0.5O3−δ electrode. J. Power Sources 2013, 230, 115–121. [Google Scholar] [CrossRef]
- Yao, W.; Duan, T.; Li, Y.; Yang, L.; Xie, K. Perovskite chromate doped with titanium for direct carbon dioxide electrolysis. New J. Chem. 2015, 39, 2956–2965. [Google Scholar] [CrossRef]
- Qi, W.; Gan, Y.; Yin, D.; Li, Z.; Wu, G.; Xie, K.; Wu, Y. Remarkable chemical adsorption of manganese-doped titanate for direct carbon dioxide electrolysis. J. Mater. Chem. A 2014, 2, 6904–6915. [Google Scholar] [CrossRef]
- Ye, L.; Zhang, M.; Huang, P.; Guo, G.; Hong, M.; Li, C.; Irvine, J.T.S.; Xie, K. Enhancing CO2 electrolysis through synergistic control of non-stoichiometry and doping to tune cathode surface structures. Nat. Commun. 2017, 8, 14785. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, K.; Wei, H.; Qin, Q.; Qi, W.; Yang, L.; Ruan, C.; Wu, Y. In situ formation of oxygen vacancy in perovskite Sr0.95Ti0.8Nb0.1M0.1O3 (M = Mn, Cr) toward efficient carbon dioxide electrolysis. Sci. Rep. 2014, 4, 7082. [Google Scholar] [CrossRef] [PubMed]
- Shan, F.; Chen, T.; Ye, L.; Xie, K. Ni–doped Pr0.7Ba0.3MnO3−δ cathodes for enhancing electrolysis of CO2 in eolid oxide electrolytic cells. Molecules 2024, 29, 4492. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Xu, Y.; Xie, K. Enhanced electrolysis of CO2 with Metal–oxide interfaces in perovskite cathode in solid oxide electrolysis cell. Catalysts 2022, 12, 1607. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, C.; Wang, S.; Zhan, Z. Symmetrical La3+-doped Sr2Fe1.5Ni0.1Mo0.4O6−δ electrode solid oxide fuel cells for pure CO2 electrolysis. J. Inorg. Mater. 2021, 36, 1323–1329. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).