Abstract
Magnetite (Fe3O4) nanoparticles are biocompatible, non-toxic, and easily functionalized. Coating them with mesoporous silica (mSiO2) offers high surface area, pore volume, and tunable surface chemistry for drug loading. In this study, Fe3O4 magnetic nanoparticles were synthesized and coated with mSiO2 shells enriched with calcium ions (Ca2+), aiming to enhance bioactivity for bone regeneration and tissue engineering. Different synthesis routes were tested to optimize shell formation Their characterization confirmed the presence of a crystalline Fe3O4 core with partial conversion to maghemite (Fe2O3) post-coating. The silica shell was mostly amorphous and the optimized samples exhibited mesoporous structure (type IVb). Calcium incorporation slightly altered the magnetic properties without significantly affecting core crystallinity or particle size (11.68–13.56 nm). VSM analysis displayed symmetric hysteresis loops and decreased saturation magnetization after coating and Ca2+ addition. TEM showed spherical morphology with some agglomeration. MTT assays confirmed overall non-toxicity, except for mild cytotoxicity at high concentrations in the Ca2+-enriched sample synthesized by a modified Stöber method. Their capacity to induce human periodontal ligament cell osteogenic differentiation, further supports the potential of Fe3O4/mSiO2/Ca2+ core–shell nanoparticles as promising candidates for bone-related biomedical applications due to their favorable magnetic, structural, and biological properties.
1. Introduction
Magnetic nanoparticles (MNPs) have attracted considerable attention in biomedical research due to their controllable size, magnetic responsiveness, and versatile surface functionalization [1]. These properties enable applications in drug delivery, imaging, hyperthermia, and tissue engineering [2,3,4,5]. However, bare MNPs often face challenges such as aggregation and structural instability under physiological conditions, which limit their practical use.
To address these limitations, core–shell architectures, in which magnetic cores are coated with a protective shell such as mesoporous silica (mSiO2), have been developed (Figure 1). The shell not only preserves magnetic properties and enhances stability, but also provides a platform for surface functionalization and incorporation of therapeutic ions, opening avenues for multifunctional biomedical applications [6]. Core–shell nanoparticles are typically synthesized via bottom-up approaches, which allow precise control over particle size and uniform shell coating, and can be composed of various organic and inorganic materials [6,7]. The shell additionally protects the magnetic core from oxidation, improves biocompatibility, enables controlled circulation and drug release, and ensures optimal nanoparticle dispersion, making these systems suitable for advanced biomedical applications such as magnetic hyperthermia, bone regeneration, and drug delivery [7,8,9,10,11,12].
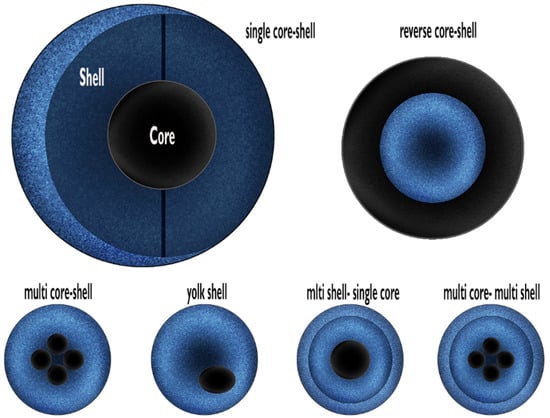
Figure 1.
Classification of core–shell nanostructures. Redrawing from Singh R et al. [8].
Mesoporous silica core–shell MNPs combine the advantages of high surface area and pore size of the mesoporous shell structure, and the biocompatibility of silica, with their magnetic properties from the core, leading to versatile nanoparticles. One profound benefit of these NPs is that the shell silica network can incorporate various therapeutic ions, such as zinc, copper, magnesium, etc., that can provide them with additional features and functionalities. For example, calcium is a fundamental component of bone mineral hydroxyapatite (Ca10(PO4)6(OH)2). By incorporating calcium ions into the mesoporous silica network, these NPs can release them in the surrounding environment upon degradation, promoting osteogenic differentiation, bone regeneration [13], and modulation of intracellular calcium levels, which are crucial in signaling pathways that drive these processes. Calcium ions can trigger the deposition of apatite (a component of bone) in physiological conditions, making these NPs suitable for use in bone repair and orthopedic applications. They can also enhance the adsorption of bioactive proteins or growth factors onto the surface of the shell, which can further stimulate cellular responses like osteogenesis or angiogenesis. In addition, they may enhance the biodegradability of the NPs, enabling faster and more robust calcium release into the surrounding tissues [13,14].
Although numerous Fe3O4/SiO2 core–shell magnetic nanoparticles have been explored for applications such as drug delivery and imaging, only a few studies have attempted to integrate magnetic targeting with ion-mediated biological activity in a single nanostructure [15,16]. Building upon an established mesoporous silica platform [8,14,15,16], we propose a multifunctional Fe3O4 mesoporous-SiO2 system enriched with Ca2+ ions. The design concept combines the high surface area, tunable porosity, and loading capacity of the mesoporous silica shell with the incorporation of calcium ions into the silica matrix to introduce additional osteogenic functionality through controlled ion release. As a key constituent involved in bone physiology, calcium plays a critical role in supporting osteogenic differentiation, guiding bone regeneration, and regulating intracellular signaling associated with these processes [12,13], while its incorporation into the mesoporous structure can enhance protein adsorption and overall bioactivity, thereby further contributing to regenerative outcomes [12,13,14].
The novelty of this study lies in uniting magnetic responsiveness, mesoporous-mediated therapeutic loading, and calcium-induced osteogenic potential within a single core–shell architecture, an approach that remains insufficiently addressed in the current literature. However, achieving a stable, bioactive Fe3O4/mSiO2/Ca2+ architecture requires careful optimization of synthesis routes to balance magnetic, structural, and biological performance. Therefore, this study aimed to synthesize and assess Ca2+-enriched Fe3O4/mSiO2 core–shell nanoparticles produced through different fabrication approaches. Comprehensive characterization, including structural, magnetic, morphological, cytocompatibility analyses and osteogenic differentiation, was conducted to determine how synthetic variations influence nanoparticle properties relevant to bone tissue engineering applications [8,12,13,14].
2. Materials and Methods
2.1. Chemicals
The materials used for the synthesis of magnetic Fe3O4 cores were ferric chloride hexahydrate (FeCl3•6H2O, 98%), ferrous chloride tetrahydrate (FeSO4•7H2O, 98%), and ammonium hydroxide (NH4OH, 25%). For the shell’s synthesis, tetraethyl orthosilicate (TEOS), cetyltrimethyl ammonium bromide (CTAB), ammonium hydroxide (25 wt%), ethanol (CH3CH2OH), calcium nitrate tetrahydrate (Ca(NO3)2•4H2O), triethanolamine (C6H15NO3), and chlorobenzene (C6H5Cl) are used across the different procedures. All reagents were from Merck, Darmstadt, Germany.
2.2. Synthesis of Fe3O4 (Core)
For the synthesis of the magnetic core, NPs of Fe3O4 were selected, which, together with Fe2O3, are biocompatible, and the chemical co-precipitation method was used [17]. FeCl3 (7.0003 g) and Fe3SO4 (3.6000 g) were dissolved in 50 mL of double-distilled water (ddH2O). After dissolution, the two aqueous solutions were mixed within a three-necked flask, and the sample was heated for 15 min at 60 °C under a flow of inert argon gas (Ar) and a magnetic stirring speed of 450 rpm. pH measurements, up to this stage, showed a value of pHbefore = 1.6. Subsequently, a gradual (drop-by-drop) addition of ammonia (NH3) (50 mL) solution followed, which raised the pH value to 10. The solution was subsequently subjected to 90 min of stirring and parallel magnetic stirring at 450 rpm and 60 °C, to enhance nucleation and achieve a narrower size distribution. During nucleation, Ar flow was controlled through viscous glycerol. At the end of the nucleation (deep black-colored solution), the stirring and heating stopped, and the magnetic rod was removed from the flask [17]. The black solution was placed over a strong permanent magnet (NdFeB) to rapidly and effectively separate the precipitate from the solution, completing the submersion process. The following chemical equation presents the possible reaction for the Fe3O4 NPs’ formation [17]:
2FeCl3⋅6H2O + FeSO4⋅7H2O + 8NH4OH → Fe3O4 + 6NH4Cl + (NH4)2SO4 + 23H2O,
Τhe sediment was subjected to ten washes with ddH2O, while re-using the same strong magnet, until the strong ammonia odor disappeared. Measurements of pH during the 4th and 10th washing showed values of 9.7 and 8.91, respectively. The repeated washes were performed to reduce the amount of salt coming from other reactions that probably took place in the solution [17]. The washed solution was centrifuged, transferred to Falcon tubes, and dried overnight in ceramic containers in an oven at 70–80 °C The dried material was finally ground using a mortar and pestle to produce fine Fe3O4 powder.
2.3. Synthesis of mSiO2 (Shell Composition)
Different experimental procedures were followed to synthesize the shell. In the first approach, a modified Stöber method was used (sample Fe3O4/mSi1), while the second approach employed a surfactant-templated sol–gel synthesis (sample Fe3O4/mSi2). For calcium enrichment, a calcium-doped variant of the modified Stöber synthesis was performed with different TEOS:Ca ratios (60:40 and 90:10) for samples Fe3O4/mSi/Ca_1A and Fe3O4/mSi/Ca_1B, respectively. Sample Fe3O4/mSi/Ca_1C was prepared via a wet impregnation method, while the synthesis of sample Fe3O4/mSi/Ca_2 followed a calcium-doped variant of the surfactant-templated sol–gel procedure (Figure 2).
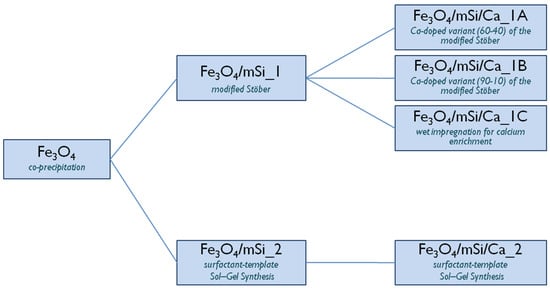
Figure 2.
Classification of the different shell and Ca-enriched shell synthesis methods.
2.3.1. Experimental Procedure 1
Sample Fe3O4/mSi_1 (Modified Stöber)
In the first case, for the synthesis of mSiO2/Fe3O4, the modified Stöber method was used [18]. More specifically, 0.1 g Fe3O4 NPs previously synthesized were added to 80 mL ddH2O, and the mixture was subjected to dispersion at 70 °C using an ultrasonic homogenizer for a duration of 10 min (10″–30″ pulse″ on/off, respectively). In the second stage, 60 mL of ethanol and 0.3 g CTAB were added, the solution was then subjected to manual stirring for 15 min, and then the ultrasonic homogenizer was used once more (10″–30″ pulse on/off). CTAB is a quaternary ammonium salt that acts as a surfactant. Then 1.12 mL, 25%wt NH3 was added into the solution under ultrasonic homogenization (10″–30″ pulse on/off). Afterwards, 0.3 mL of the silica source, TEOS was added (drop-by-drop), while the sample was subjected to mechanical, manual stirring for 15′. The final solution was placed in a shaker for 6 h at 37 °C and 250 rpm [14].
At the end of the 6 h reaction, the product was separated from the suspension by a NdFeB magnet (external magnetic field). Next, two washing steps were performed to remove nitrates, the first with ethanol and the second with ddH2O, each for 5 min at 8000 rpm. The sample was then dried overnight at 60 °C. The next day, the sample was placed in a flask and subjected to minimal pressure to relieve any residual stress, resulting in a fine powder. Finally, the material underwent heat treatment (calcination) at 350 °C for 2 h to remove the CTAB.
Sample Fe3O4/mSi/Ca_1A (Ca-Doped Variant 60:40 of the Modified Stöber)
For the enrichment of the silica shell with calcium ions, the same synthesis procedure was followed until the dropwise addition of TEOS. After 30′ of shaking, at 37 °C and 250 rpm, 0.157 g of hydrated Ca nitrate Ca(NO3)2•4H2O was added to the solution, in a chemical composition of TEOS:Ca2+ in a 60:40 ratios, respectively. The new solution was dispersed for 5′ (10″–30″ pulse on/off), stirred once again for another 5′30 h at 37 °C and 250 rpm to ensure complete reaction. After stirring, the product was separated from the suspension through a strong magnet. Two washing procedures were performed, followed by drying and calcination as described above.
Sample Fe3O4/mSi/Ca_1B (Ca-Doped Variant 90:10 of the Modified Stöber)
To explore the amount of TEOS on the properties of the shell, the same shell synthesis procedure was followed once more with a chemical composition of TEOS:Ca2+ in a 90:10 ratio. More specifically, the previously described procedure of sample Fe3O4/mSi_1 was followed up to the point of TEOS addition. Following 30 min of shaking at 37 °C and 250 rpm, 0.027 g of Ca(NO3)2•4H2O was introduced into the solution, maintaining a TEOS:Ca2+ molar ratio of 90:10. The resulting mixture was subjected to ultrasonic dispersion for 5 min (10″–30″ pulse on/off), followed by an additional stirring phase lasting 5′30 h under the same temperature and agitation conditions, to ensure complete reaction. Upon completion, the product was magnetically separated from the suspension. Subsequent washing steps with ethanol and deionized water were performed, and the sample was then dried and calcined as previously described.
Fe3O4/mSi/Ca_1C (Wet Impregnation for Calcium Enrichment)
Another experimental procedure for the Ca2+ enrichment was performed with the method of wet-impregnation [15], to receive a mesoporous silica and a Ca-doped shell. First, Fe3O4/mSi_1 sample (0.10 g) was dispersed in ddH2O (10.0 mL) via ultrasonic treatment. Then, Ca(NO3)2•4H2O (30 mg) was added to the mixture, and the resulting solution was evaporated at 80 °C and subsequently calcined at 600 °C for 3 h to convert Ca(NO3)2 into CaO.
2.3.2. Experimental Procedure 2
Sample Fe3O4/mSi_2 (Surfactant-Templated Sol–Gel Synthesis)
For this approach, 300 mg Fe3O4 NPs were mixed with 0.05 g triethanolamine and 30 mL of ddH2O [16]. This first solution was dispersed in an ultrasonic homogenizer for 15’ (10″–30″ pulse on/off). A second solution of 1.5 g CTAB and 15 mL ddH2O was magnetically stirred for 15’ at 60 °C, and then the two solutions were mixed and left to react for 1 h at 60 °C [16]. Subsequently, 14.5 mL of chlorobenzene (CB) and 1.5 mL of TEOS were added to the final solution, which was then dispersed using an ultrasonic homogenizer for 5 min (10–30 s pulse on/off). The mixture was afterwards stirred in a shaker at 37 °C and 100 rpm for 15 h. Once stirring was complete, the sample was washed twice with ethanol and dried overnight in an oven at 60 °C. The next day, the solution was calcinated for 2 h at 350 °C similar to synthesis procedure 1 [16].
Sample Fe3O4/mSi/Ca_2 (Ca-Doped Variant of the Surfactant-Templated Sol–Gel Synthesis)
Enrichment of the shell with Ca ions was also performed in the second synthesis route. The procedure followed the same steps described in the previous section for sample Fe3O4/mSi2, up to the point at which 1.5 mL of TEOS was added to the solution. After that, the solution was dispersed in an ultrasonic homogenizer for 5’ (10″–30″ pulse on/off) and then placed in a shaker for 30’ at 37 °C, 100 rpm. After half an hour of shaking, 0.785 g of Ca(NO3)2•4H2O was added to the solution which was placed once more into the shaker to complete 15 h of shaking [16]. After shaking, the sample was washed with ethanol twice, dried overnight at 60 °C and the next day calcined for 2 h at 350 °C [16].
Sample names and composition for all experimental procedures are presented in Table 1.

Table 1.
Samples names with their chemical compositions.
2.4. Materials Characterization
X-ray diffraction (XRD) patterns of the materials were recorded using a Rigaku Ultima type diffractometer (Rigaku Corporation, Tokyo, Japan), with a Cu tube mounted on it and a Ni filter for CuKa radiation (λ = 0.1542 Å). The scanning angle range 2θ was between 5 and 90°, with a step size of 0.05°, and a scanning speed of 0.05° 2θ/s. The Jade software version 6.0.3 was used for identification.
X-ray photoelectron spectroscopy (XPS) analysis was performed for Fe3O4/mSi_1 and Fe3O4/mSi/Ca_1C samples on a Kratos Analytical AXIS Ultra DLD system (Kratos Analytical, Manchester, UK) with an Al Kα X-ray source (hν = 1486.6 eV), under ultra-high vacuum conditions (~10−9 torr). By applying 105 W (7 mA/12 kV) to the X-ray source, the analyzer with a pass energy of 160 eV recorded wide-scan (survey) spectra. High-Resolution (HR) regions were recorded with a pass energy of 20 eV, applying 120 W (10 mA/15 kV) during a three-sweep scan, except for the Ca 2p regions, where a five-sweep scan was used for better signal-to-noise ratio. The C 1s peak at 284.6 ± 0.2 eV, corresponding to C–C bonds from environmental contamination, was used for the spectra calibration.
For quantitative analysis, Shirley (non-linear) baselines were used to subtract the background from the HR peaks, and the atomic percentages were calculated using the RSF factors of each element. Gaussian (70%) and Lorentzian (30%) components, as well as asymmetric curves (depending on the chemical state of each element), were used to fit the experimental curves for the chemical state analysis.
Fourier-transform infrared (FTIR) measurements were carried out using potassium bromide (KBr) pellets the background. The synthesized MNPs powder was mixed with KBr powder at a 1:100 ratio. Next, the samples were compressed in a hydraulic press under 7 tons of pressure to produce 13 mm diameter pellets. Transmittance spectra were obtained with a PerkinElmer FTIR Spectrum 1000 spectrometer, in the mid-infrared (MRI) range (4000–400 cm−1), with a resolution of 4 cm−1 and 32 scans.
The magnetic properties of the nanoparticles were characterized using a 1.2H/CF/HT Oxford Instruments Vibrating Sample Magnetometer (VSM). A magnetic sample is deposited at the center of both four small collection coils (contributing to the inductive signal) and at the center of two electromagnet poles that produce a highly homogeneous magnetic field. By means of a sinusoidal signal, emitted by the oscillator, a vertical signal—vibration—is identified by means of a transducer. The sample maintains a frequency of 60 Hz and an amplitude of 1.5 mm. The external field used remains constant for the purpose of magnetizing the sample only, thus not affecting the voltage. The maximum external magnetic field amplitude used in this VSM device is 1.2 T. The measured samples are in powder form and weigh at least 0.5 mg, to achieve the minimum required signal while reducing the signal noise [19,20]. Finally, the hysteresis loops for each type of MNPs were recorded at room temperature.
For Transmission Electron Microscopy (TEM) analysis, a small amount of each MNP sample was dispersed in ethanol and sonicated for 30 min. Drops of the ethanol-MNP samples were dispersed on a 300-mesh Cu carbon-coated grid and dried at room temperature. The TEM instrument (JEOL JEM Cold Field Emission Gun (CFEG) TEM/STEM F200, JEOL Ltd., Tokyo, Janpan) was operated at 200 kV, with 0.19 nm point-to-point resolution, equipped with a RIO GATAN CMOS 9 Mpixel bottom-mounted camera (Gatan, Inc., San Diego, CA, USA). For Fe3O4/mSi/Ca_1A, Fe3O4/mSi/Ca_1B, and Fe3O4/mSi/Ca_2 samples, an additional EDX detector (T-max 65, Oxford Instruments, Oxfordshire, UK) was employed. The analysis focused on morphology, particle size, and structure. In the case of Fe3O4/mSi/Ca_2 solution, the chemical composition of the NPs was also investigated.
TEM was employed to determine nanoparticle size statistics for more accurate average size measurements. TEM images from different regions of each MNP sample were analyzed, and the diameters of at least 300 nanoparticles per sample were measured using ImageJ version 1.8.0_172 (64-bit). The resulting size distributions were visualized as histograms in OriginLab, OriginPro 9.0 software. Smoothing of the average particle size data was performed to produce a histogram in normal or log-normal format. In the case of lognormal, the average particle size of each sample is calculated by fitting the particle size distribution histogram to the log-normal distribution function expressed by the relation:
is the average particle size, is the standard deviation [19]. From each histogram and by Origin’s library, the values of Inmean and InSd were obtained and the values of and were calculated as:
Subsequently, according to the sample measurement, a series of HRTEM images was obtained, equivalent to that of the PSA300 system’s software. Particles were separated from the background by setting the threshold function parameter [19,20].
2.5. Biological Characterization
2.5.1. Cytotoxicity
To investigate MNPs cytotoxicity, the indirect MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay was employed. Eluates were collected after 24 h incubation time with the NPs at different concentrations (C1 = 0.5 mg/mL and C2 = 0.25 mg/mL) in Dulbecco’s minimal essential medium, DMEM. In detail, eluates were performed in sterile chemically inert closed containers using aseptic techniques, and passed through 0.22 µm filters. More specifically, human gingival fibroblasts (HGFs) (1 × 103 cells per well) were seeded in 96-well plates to allow cell attachment. After 24 h of cell culture, DMEM was removed and replaced with eluates of the three tested materials. HGFs cultured in DMEM supplemented with FBS and antibiotics served as the positive control. Analysis of mitochondrial activity and thus cell proliferation was succeeded by measuring the mitochondrial dehydrogenase activity of metabolically active cells, verified by the capability of transforming the yellow tetrazolium salt into blue formazan crystals [20,21]. MTT solution was added in each well (10% of the total volume per well) and 3 h incubation at 37 °C and 5% CO2 followed. After this period, the medium containing the MTT solution was discarded, and the insoluble formazan was dissolved in dimethyl sulfoxide (DMSO) [21,22]. Cell viability was evaluated by measuring the optical density with an ELISA-spectrophotometer (Epock, Biotek, Winooski, VT, USA) at a double wavelength (570–630 nm). The experiments were performed in triplicate, and the results are presented as a % compared to the positive control.
2.5.2. Reactive Oxygen Species (ROS) Levels
Human periodontal ligament cells (hPDLCs) were seeded at a density of 4 × 104 cells/well in 48-well plates and allowed to attach for 24 h before experimentation. Reactive Oxygen Species (ROS) levels were assessed on days 1 and 3 following exposure to MNPs. After incubation with the NPs (0.25 μg/mL) for 1 and 3 days, consequently, cells were washed with PBS and incubated with DCFH-DA solution (according to manufacturer’s instructions) for 30 min at 37 °C. Fluorescence intensity (Ex/Em: 485/535 nm) was measured using a Tecan fluorometer with black 96-well microplates to quantify ROS production; their background signals were subtracted from the respective readings. Maximal emission corresponded to the fluorescence measured in a reaction containing only H2DCFDA and H2O2 at a final concentration of 3 mM, as previously performed. This concentration was chosen based on a standard curve generated with H2O2 concentrations ranging from 0.1 to 5 mM, which allowed fluorescence to be recorded without exceeding the detection limit. A final concentration of 3 mM H2O2 produced the highest fluorescence values without signal overflow.
2.5.3. Osteogenic Differentiation
Human periodontal ligament cells (hPDLCs) were seeded at a density of 4 × 104 cells/well in 48-well plates and allowed to attach for 24 h before experimentation. Fe3O4/mSi, Fe3O4/mSi/Ca_1A, Fe3O4/mSi/Ca_1B, and Fe3O4/mSi/Ca_1C MNPs were pre-treated for 1 day in DMEM under sterile conditions prior to cell exposure. MNPs were used at a final concentration of 0.25 μg/mL, and all conditions were performed in triplicate. Osteogenic differentiation was induced using an osteogenic medium (OM), prepared according to a previously described protocol [23]. The following experimental groups were established:
- (1)
- hPDLCs cultured with each MNP type in OM;
- (2)
- hPDLCs cultured in OM without NPs (positive control).
For all conditions, the culture medium was refreshed every 2 days. The endpoints for each assay were as follows: ALP activity (day 7 and day 14), and ARS staining (day 21).
2.5.4. Alkaline Phosphatase Activity
Alkaline Phosphatase (ALP) activity was analyzed on day 7 and day 14. After incubation with culture medium in the presence or absence of NPs (0.25 μg/mL), both cell lysates and culture supernatants were collected. Samples were mixed with an ALP reaction buffer (Apollo Scientific, BI4545/BIN0446, Manchester, UK), following the procedure described previously [23,24].
The enzymatic conversion of p-nitrophenyl phosphate (pNPP) was quantified by measuring the absorbance at 405 nm. ALP activity values were compared with those obtained from hPDLCs cultured without NPs under identical medium conditions.
2.5.5. Alizarine Red Staining
Calcium mineral deposition was evaluated on day 21 via Alizarin Red S (ARS) Staining. hPDLCs were seeded in 12-well plates at 4 × 104 cells/well and cultured with or without the MNPs formulations (0.25 μg/mL) in OM. Media were replaced every 2 days. Parallel wells containing MNPs without cells were included to enable subtraction of OD values originating from NPs. At the endpoint, cultures were fixed and stained with Alizarin Red S (Sigma-Aldrich, St. Louis, MO, USA). Mineralized nodules were visualized using an inverted microscope. For quantification, the bound dye was eluted using 10% (w/v) cetylpyridinium chloride for 20 min at room temperature, and absorbance was measured at 540 nm using a microplate spectrophotometer.
3. Results
3.1. XRD
Figure 3 shows the XRD patterns of Fe3O4, Fe3O4/mSi_1, Fe3O4/mSi/Ca_1A, Fe3O4/mSi/Ca_1B, Fe3O4/mSi/Ca_1C, and Fe3O4/mSi_2, Fe3O4/mSi/Ca_2 samples. Fe3O4 NPs exhibit diffraction peaks localized at specific angles 2θ and corresponding to crystal surfaces of magnetite (Fe3O4) diffraction peaks (Jade tab: #880315). The observed 2θ angles included 30°, 35.5°, 43.3°, 53.8°, 57.2°, 62.8°, 71.2°, 74.3°, 79.65°, and 87.2°, which map respectively to the (220), (311), (400), (422), (511), (440), (620), (533), (444), and (731) crystalline surfaces of magnetite [16,17].
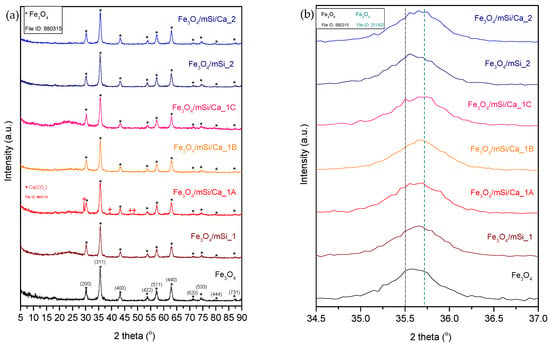
Figure 3.
(a) X-ray diffraction peak plots for all magnetic core–shell NPs synthesized. (b) Enlarged region between 34 and 37° 2θ. * = diffraction peak corresponding to Fe3O4, + = diffraction peak corresponding to Ca(CO3).
All diffraction peaks correspond to the characteristic face-centered cubic (fcc) structure, although secondary phases (peaks at 2θ ≥ 71.2°) are also observed. The crystal structure and high-intensity main diffraction peaks of the samples reveal high crystallization for the magnetite-rich sample, while the wide reflections of the sample highlight its nanocrystalline nature [6]. At 35.7 2θ, there is a tendency for a secondary peak attributed to Fe2O3. All Fe3O4 peaks are still distinct after coating the magnetic core with a SiO2 shell and after shell’s enrichment with Ca2+ in all samples. This indicates the good preservation of the magnetic core. After coating and shell-enrichment, samples do not undergo a significant change in crystallinity compared to the control (Fe3O4), except for a faint decrease in diffraction peaks’ intensity due to the addition of an amorphous shell. At the same time, during shell formation, the secondary peak of Fe2O3 is distinctly visible in the diffraction patterns. No new peaks corresponding to a calcium crystal phase were observed, with the notable exception of a strong double peak in the Fe3O4/mSi/Ca_1A sample at 2θ = 29.35° and 30.22°, along with weaker peaks at 39.35°, 47.5°, and 48.4°. This indicates that calcium ions are well dispersed within the silica shell [16,17].
3.2. XPS
The recording of the wide/survey spectrum for the Fe3O4/mSi_1 sample yielded peaks corresponding only to O and Si. Si at 0 nm is in an oxidative state (IV), in the form of SiO2 (Figure 4).
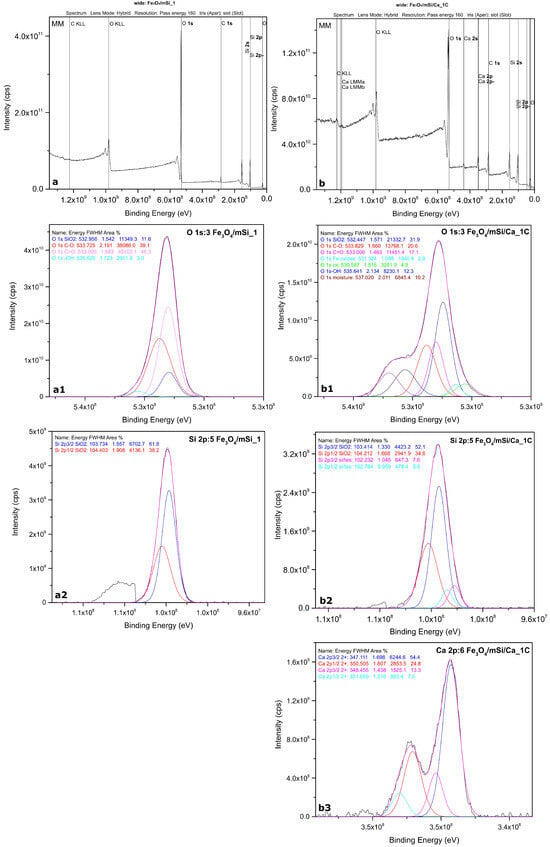
Figure 4.
XPS spectrums: (a) wide scan of Fe3O4/mSi_1 MNPs, (b) wide scan of Fe3O4/mSi/Ca_1C MNPs, (a1) O of Fe3O4/mSi_1 MNPs, (a2) Si of Fe3O4/mSi_1 MNPs, (b1) O of Fe3O4/mSi/Ca_1C MNPs, (b2) Si of Fe3O4/mSi/Ca_1C MNPs and (b3) Ca of Fe3O4/mSi/Ca_1C MNPs.
Regarding Fe3O4/mSi/Ca_1C wide-scan spectra yielded an almost identical stoichiometry, with the exception that additional Ca peaks appear in the present sample, confirming its incorporation (Figure 4). The behavior of Si is similar to that of the previous sample and Ca is present in the Ca(II) oxidation state. The fitted Ca 2p spectra are presented below. The quantification results of both samples are presented in Table 2.

Table 2.
Quantification results of Fe3O4/mSi_1 and Fe3O4/mSi/Ca_1C MNPs.
3.3. FTIR
Figure 5 shows the FTIR spectra in Transmittance mode of all tof the synthesized samples. For the Fe3O4 sample, transmittance bands appear at 562 cm−1 and, more weakly, at 440 cm−1, attributed to stretching vibrations of Fe–O bonds [16,17]. The band of 562 cm−1 corresponds to Fe3+–O at tetrahedral sites, while the band at 440 cm−1 corresponds to Fe2+–O vibrations at octahedral sites [25,26]. Weak bands appear at 818 cm−1 and 1104 cm−1, which are attributed to antisymmetric and symmetric stretching vibrations of Si–O–Si bonds, respectively [15,17]. Subsequently, the presence of carboxylic groups (C=O) is observed at 1624 cm−1 and weakly at 1364 cm−1. Finally, the peak at 3418 cm−1, corresponds to O–H stretching vibrations, due to the interaction of water (H2O) molecules with the surface of the MNPs. The presence of hydroxyl groups ion Fe3O4 NPs facilitates modification through the incorporation of additional materials [15,16,17,25,26].
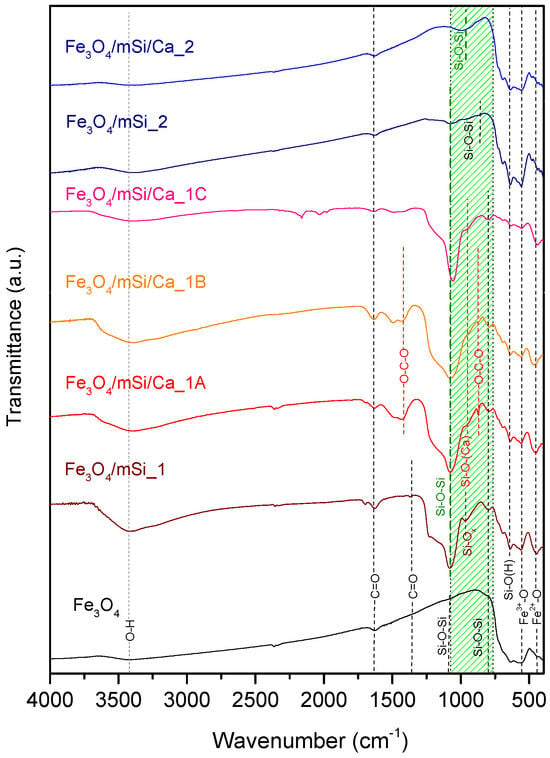
Figure 5.
FTIR spectra for Fe3O4, Fe3O4/mSi_1, Fe3O4/mSi/Ca_1A, Fe3O4/mSi/Ca_1B, Fe3O4/mSi/Ca_1C, Fe3O4/mSi_2 and Fe3O4/mSi/Ca_2 MNPs. The green highlighted area corresponds to Si–O and Si–O–X bonds.
Fe3O4/mSi_1 sample’s peak at 446 cm−1 (vibration between Fe2+–O bonds) is more pronounced after the silica coating, so it is likely that during shell coating, part of Fe3O4 was transformed into Fe2O3. A band in the 620–740 cm−1 region supports the presence of Fe2O3 phases [3,4]. The peak at 638 cm−1 refers to the symmetric stretching vibration between Si–O(H) bonds [13,15,16]. Peak at 968 cm−1 is attributed to asymmetric vibrations of Si–OH or SiOx bonds due to the formation of an open silicate network [27,28]. The characteristic peaks at 789 cm−1 and 1082 cm−1 assigned to symmetric and antisymmetric Si–O–Si stretching [14,15], confirm successful silica coating. Similarly, in the spectra of Fe3O4/mSi/Ca1A, Fe3O4/mSi/Ca1B, and Fe3O4/mSi/Ca1C, the peak near 440 cm−1 appears more pronounced, indicating an increased formation of Fe2O3 following Ca enrichment [3,4]. The open silica network formation at ~968 cm−1 decreases in intensity, consistent with Si–O–Ca bond formation. Confirming XRD’s results, additional peaks appear at 1425 cm−1 and 874 cm−1 due to out-of-plane bending/asymmetric stretching of O–C–O bonds, respectively [29,30].
The analysis of the Fe3O4/mSi2 sample was consistent with the core’s characteristic peaks due to stretching vibrations primarily reflecting Fe3+–O stretching vibrations and to a lesser extent Fe2+–O vibrations. A weaker band in the 620–740 cm−1 region again suggests minor Fe2O3 formation. A peak at 638 cm−1 corresponds to Si–O(H) stretching, while bands at 876 cm−1 (antisymmetric Si–O–Si) and 1076 cm−1 (symmetric Si–O–Si) indicate the presence of silicates, but with lower intensity compared to the first synthesis. After Ca2+ enrichment (sample Fe3O4/mSi/Ca_2), the silicate contribution is limited to a shifted band at 994 cm−1 (symmetric Si–O–Si).
3.4. VSM
Figure 6a–c presents the hysteresis loops of samples synthesized via the first (Figure 6a) and second (Figure 6b) methods. All NPs, after coating, display hysteresis loops characteristic of Fe3O4 NPs, regardless of the synthesis route, but with notably reduced magnetization. In the first synthesis procedure, a slight decrease in magnetization is observed following coating, followed by an increase in the sample Fe3O4/mSi/Ca_1A with a TEOS:Ca2+ ratio of 60:40. This trend contrasts with the magnetization decrease noted in samples Fe3O4/mSi/Ca_1B and Fe3O4/mSi/Ca_1C, prepared with a TEOS:Ca2+ ratio of 90:10 (Figure 6a). For samples synthesized with the second method (Fe3O4/mSi_2 and Fe3O4/mSi/Ca_2, Figure 6b), magnetization decreases progressively after shell addition and calcium enrichment.
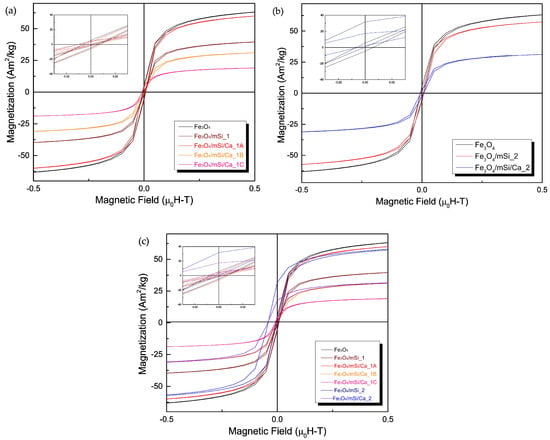
Figure 6.
Hysteresis loops of (a) Fe3O4, Fe3O4/mSi_1, Fe3O4/mSi/Ca_1, Fe3O4/mSi/Ca_1A and Fe3O4/mSi/Ca NPs, (b) Fe3O4, Fe3O4/mSi_2 and Fe3O4/mSi/Ca_2 NPs and (c) all synthesized samples.
These results indicate that both the synthesis method and TEOS:Ca2+ ratio influence the magnetic properties, potentially due to variations in shell thickness and calcium incorporation efficiency. Saturation magnetization (MS) values measured under an external magnetic field of 0.5 [μ0H–T] are summarized in Table 3.

Table 3.
Saturation magnetization values for each synthesized sample of core–shell MNPs.
3.5. TEM
3.5.1. Morphology, Structure and Composition of NPs
The microstructure of the Fe3O4 NPs, in both procedures 1 and 2, was investigated using TEM, SAED, and HRTEM imaging modes. From the bright-field TEM images (Figure 7), two types of shapes were observed in terms of Fe3O4 NPs’ morphology: the spherical (smaller NPs) with diameters up to 13 nm, and a nearly rectangular shape for larger NPs. The average size of spherical NPs was calculated to be close to 11.7 nm. SAED analysis revealed that the NPs exhibit the characteristic ring pattern of Fe3O4’s crystal structure, as shown in the insets of Figure 8. HRTEM images (Figure 8) showed an almost spherical shape of the NPs with excellent crystallinity, while the spatial frequencies visible through FFT confirmed the face-centered cubic (fcc) structure of Fe3O4. The shape, size, and crystallinity degree of the NPs did not show significant changes after coating in either of the two synthesis procedures. The particles exhibited a small size distribution, were aggregated, and were overly surrounded by a successfully formed amorphous SiO2 shell, which increased the Fe3O4 particles’ average diameter.
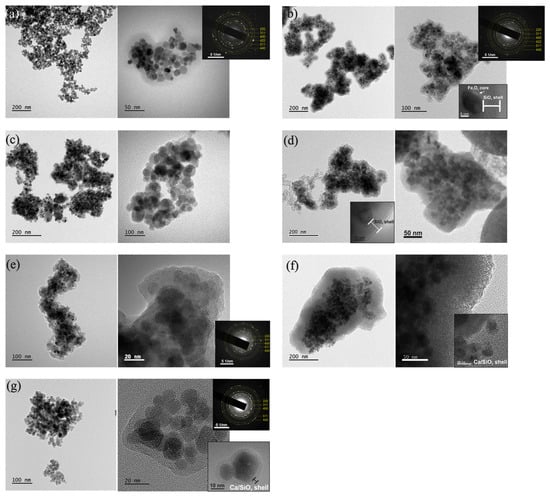
Figure 7.
TEM1: Bright-field TEM images of (a) Fe3O4 NPs and corresponding SAED ring pattern of magnetite cubic Fe3O4 structure, (b) Fe3O4/mSi_1 NPs and corresponding SAED ring pattern of magnetite cubic Fe3O4 structure. The amorphous shell around the NP is shown as an inset, (c) Fe3O4/mSi_2 NPs, (d) Fe3O4/mSi/Ca_1A NPs and the existence of a Ca/SiO2 shell as an inset, (e) Fe3O4/mSi/Ca_1B NPs and the corresponding SAED ring pattern, (f) Fe3O4/mSi/Ca_1C NPs and the existence of thick Ca/SiO2 shell and (g) Fe3O4/mSi/Ca_2 NPs and corresponding SAED ring pattern along with the existence of Ca/SiO2 shell around the NPs.
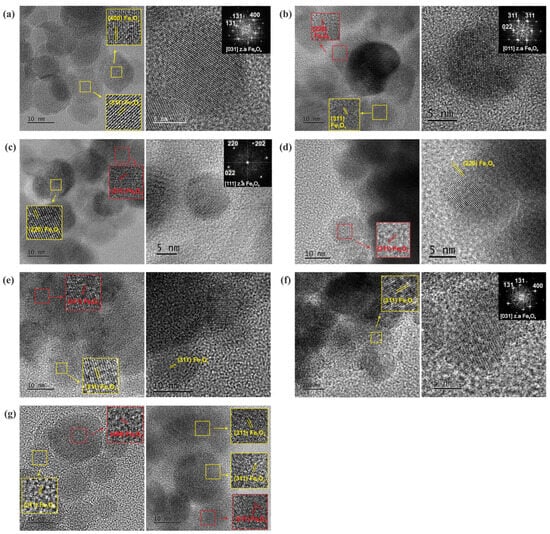
Figure 8.
TEM 2: HRTEM images of (a) nearly spherical Fe3O4 NPs with excellent crystallinity and corresponding FTT pattern, (b) Fe3O4/mSi_1 NPs and a representative NP with corresponding FTT confirming the fcc structure, (c) Fe3O4/mSi_2 NPs and a representative NP with corresponding FFT confirming the atomic structure of Fe3O4 NPs projected along the <111> zone axis, (d) Fe3O4/mSi/Ca_1A, (e) Fe3O4/mSi/Ca_1B, (f) Fe3O4/mSi/Ca_1C and (g) Fe3O4/mSi/Ca_2 NPs. The detected lattice fringes of (220), (311), and (400), highlighted in yellow rectangles, correspond to the cubic structure of magnetite (Fe3O4), while lattice fringes highlighted in red rectangles correspond to the cubic structure of maghemite (Fe2O3), indicating phase coexistence.
In particular, synthesis procedure 1 revealed a shell thickness of ~10 nm (Figure 7b). The NPs exhibited darker contrast compared to the brighter SiO2 shell, while the formed pores contributed to future dispersion of alkaline species [15,16]. The corresponding SAED ring patterns, shown as insets in Figure 7b,e, depict the Fe3O4 crystal structure. The sequence of the diffraction rings, from the inner to the outer, corresponds to the interplanar spacing of {220}, {311}, {400}, {422}, {511}, and {440} lattice planes of the Fe3O4 magnetite structure (cubic F3m). The HRTEM images in Figure 8b,d–f reveal the cubic structure of Fe3O4 along with a minority of the cubic structure of Fe2O3, indicating partial phase coexistence. FFT analysis further confirmed the face-centered cubic structure of Fe3O4, and the NPs remained single-crystalline, as illustrated in Figure 8b,f by characteristic NPs showing their atomic structure projected along the <011> and <031> zone axes respectively. HRTEM images of the enriched SiO2 shell with Ca2+ ions [Figure 8d–f] (samples Fe3O4/mSi/Ca_1A, Fe3O4/mSi/Ca_1B, Fe3O4/mSi/Ca_1C) revealed the existence of a Ca/SiO2 shell with a slightly increased thickness (~11–20 nm) [Figure 8d,f] and the coexistence of the Fe3O4–Fe2O3 phases. STEM-EDX microanalysis was employed to determine the chemical composition of the NPs. The elemental mapping [Figure 9a,b] confirms the presence of iron (Fe, red), oxygen (O, green), and silica (Si, purple) in all samples, while calcium (Ca, blue) is also detected, although in very small amounts. The corresponding EDX spectra and atomic percentage tables further verify Ca incorporation in the Fe3O4/mSi/Ca samples of procedure 1, supporting the successful introduction of Ca into the mesoporous silica shell, even at low concentrations.
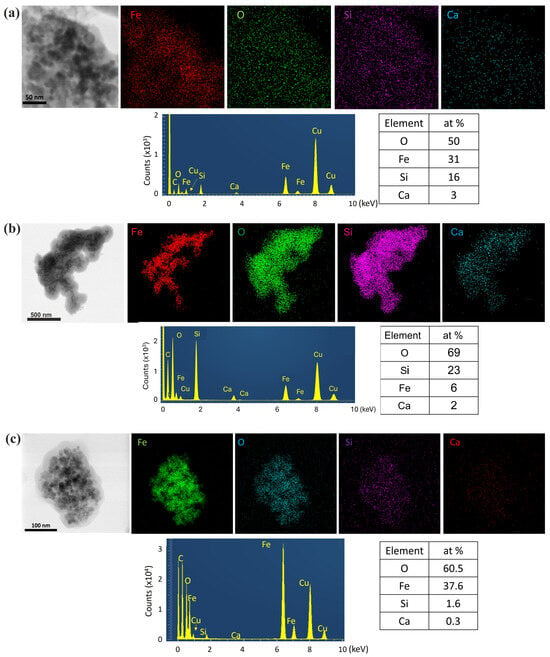
Figure 9.
TEM 3: STEM-EDX elemental mapping and corresponding EDX spectra for (a) Fe3O4/mSi/Ca_1A sample, (b) Fe3O4/mSi/Ca_1C, and (c) Fe3O4/mSi/Ca_2 samples, showing the distribution and atomic content of all detected elements. The additional C and Cu peaks originate from the carbon-coated Cu TEM grid.
In contrast, synthesis procedure 2 (Fe3O4/mSi_2 and Fe3O4/mSi/Ca_2 samples) resulted in a very thin and irregularly distributed amorphous silica shell around the NPs [Figure 7c,g]. In particular, analysis of the Fe3O4/mSi/Ca_2 sample revealed an amorphous silica shell enriched with calcium Ca/SiO2 ions of ~3 nm. HRTEM and SAED analyses, in agreement with all the previously coated samples, confirmed the FCC structure of Fe3O4 and the cubic structure of Fe2O3 [Figure 8c,g]. Particularly, the HRTEM image of Figure 8c and the corresponding FFT, given as an inset, of a spherical NP clearly reveal the cubic Fe3O4 structure projected along the <111> zone axis. Complementary STEM-EDX analysis [Figure 9c] further supported these findings. The EDX spectrum clearly displayed the Fe, O, and Si peaks, followed by a relatively faint Ca peak throughout the particles’ distribution. STEM-EDX mapping indicated that Fe (green) and O (blue) elements were confined to the particles while silicon Si and Ca elements overlapped the aggregated imaged field, confirming that the NPs are coated by a shell around their periphery. The presence of carbon (C) and copper (Cu) peaks was also observed, attributed to a carbon-coated Cu TEM grid.
According to synthesis procedure 2, it seems that the formation of a silica shell enriched with Ca ions around individual magnetite MNPs does not appear to have been successfully achieved, as compared to the first synthesis. Unlike the first synthesis, only a thin, barely visible amorphous layer was observed. While the Fe3O4 MNPs maintained their crystallinity and structural integrity, significant aggregation of the magnetic particles occurred. This aggregation explains why the mSiO2 coating of each nanoparticle was not obtained; instead, the silica shell formed around both aggregated and isolated particles. In general, magnetite and maghemite crystallize in the cubic spinel structure with very similar lattice constants α = 8.397 Å and α = 8.3515 Å, respectively. Therefore, it is quite challenging to distinguish these two phases.
3.5.2. Size Distribution
Figure 10 shows the size distribution histograms of MNPs. For a qualitative estimate of their size, the calculation was focused on the magnetic particle cores. The results confirmed the good preservation of the core dimensions (~13 nm).
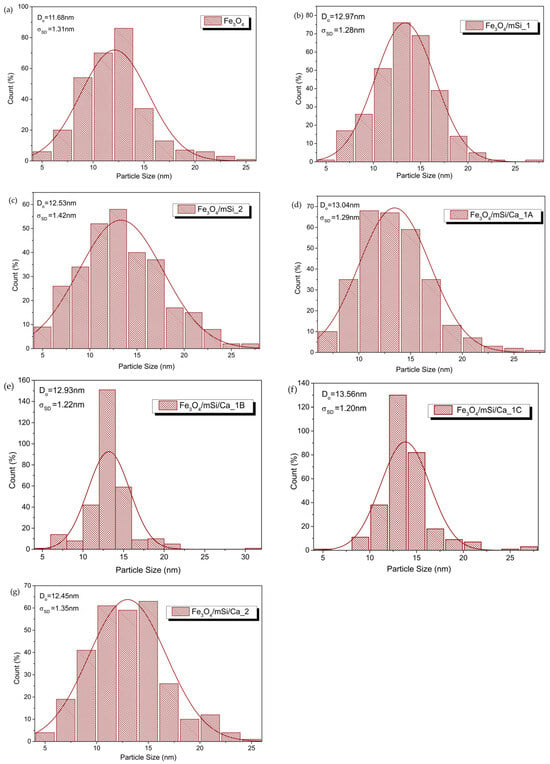
Figure 10.
Size distribution, determination of mean size and standard deviation for (a) Fe3O4, (b) Fe3O4/mSi_1, (c) Fe3O4/mSi_2, (d) Fe3O4/mSi/Ca_1A, (e) Fe3O4/mSi/Ca_1B, (f) Fe3O4/mSi/Ca_1C and (g) Fe3O4/mSi/Ca_2 NPs, with log-normal distribution function.
3.6. BET Analysis
In the porosity analysis, the N2 adsorption step of all samples exhibited a porous-like behavior, yet with significant differences that lead to the assumption of an inter-porosity nature of the synthesized NPs (Figure 11). Fe3O4 NPs did not present a hysteresis loop, had a range of pore diameters between 4 and 50 nm, while the peak of the curve in BJH analysis has a maximum at 25 nm; both low surface area and pores’ volume were low enough to assume that, unlike a classic type IV(b) isotherm of a MCM-41 material, Fe3O4 does not contain uniform pores in terms of size neither contain mesoporous structure. After coating with silica shell, the type IV(b) isotherm is formed for the Fe3O4/mSi_1 but not for the Fe3O4/mSi_2 sample. For the Ca2+ enriched samples, only the Fe3O4/mSi/Ca created a type IV(b) mesoporous isotherm, with comparable peaks to the previously synthesized Fe3O4/mSi_1 sample. Fe3O4/mSi_2, Fe3O4/mSi/Ca_1A, Fe3O4/mSi/Ca_1B and Fe3O4/mSi/Ca_2 samples did not demonstrate a mesoporous structure. However, in the case of the Fe3O4/mSi_1 and Fe3O4/mSi/Ca_1C samples, the desired mesoporous shell was developed. All data are presented in Table 4.
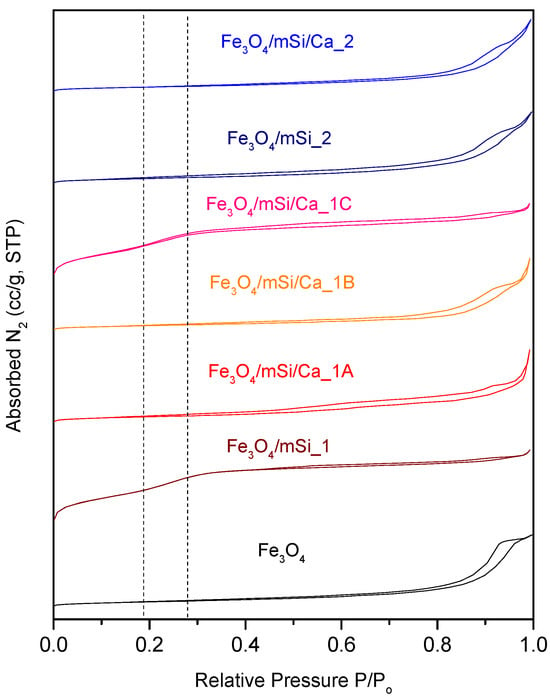
Figure 11.
Nitrogen adsorption isotherms of all synthesized NPs.

Table 4.
BET/BJH data of all samples.
3.7. Cytotoxicity
The previously discussed results indicate that the synthesis procedure 1 was more effective in producing an amorphous porous silica shell around MNPs; therefore, cytotoxicity tests were carried out for the core (Fe3O4), Fe3O4/mSi_1, Fe3O4/mSi/Ca_1A, Fe3O4/mSi/Ca_1B, and Fe3O4/mSi/Ca_1C samples. Figure 12 presents the % metabolic activity of the cells compared to the control after culture of NPs eluates with HGFs. All samples exhibited similar biocompatibility comparable to control, with the exception of the Fe3O4/mSi/Ca_1A sample, which showed mild cytotoxicity at the highest tested concentration. Notably, no dose-dependent toxicity was observed in any of the samples, indicating that the biocompatibility of these NPs is not significantly influenced by their concentration within the tested range. The mild toxicity observed in the Fe3O4/mSi/Ca_1B NPs at higher concentrations highlights the need for further optimization of surface modifications to balance functionality with biocompatibility.
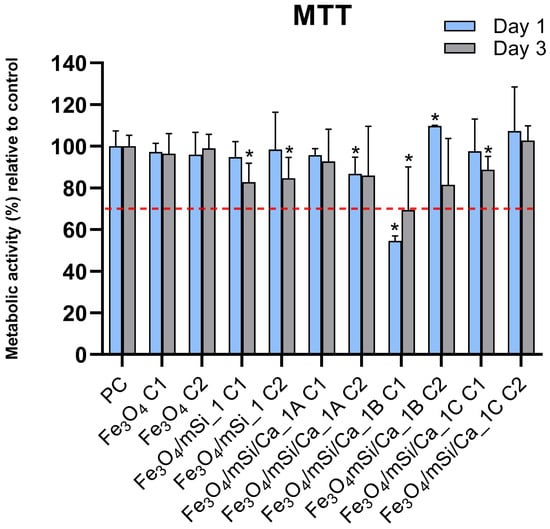
Figure 12.
Cytotoxicity test results of the tested MNPs eluate at two different concentrations (C1: 0.5 mg/mL, C2: 0.25 mg/mL).* = statistically significant (p < 0.05) compared to PC. The dashed red line indicates the cytotoxicity acceptance threshold defined in ISO 10993-5, corresponding to 70% relative cell viability.
3.8. ROS Generation Evaluation
ROS levels were measured on days 1 and 3 for all samples. The Fe3O4/mSi_1 group showed only a slight, non-significant increase at both time points compared to the control (Figure 13). In the Ca-enriched samples, ROS values were higher. The Fe3O4/mSi/Ca_1A sample showed a significant increase on day 3, while the Fe3O4/mSi/Ca_1B and Fe3O4/mSi/Ca_1C samples presented the highest ROS levels, with statistically significant differences observed on both days compared to control cells (p < 0.05).
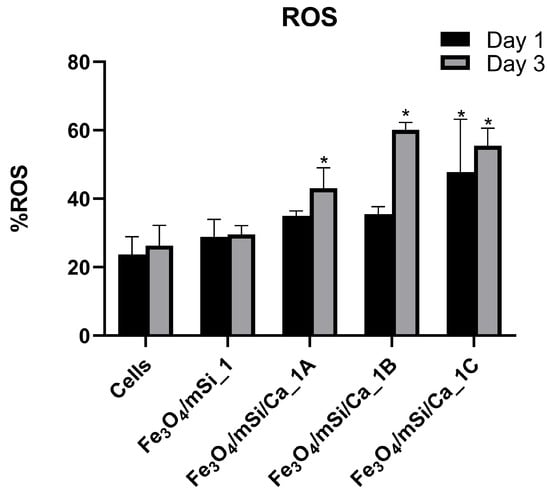
Figure 13.
ROS levels of the tested MNPs at concentration C2: 0.25 mg/mL. * = statistically significant (p < 0.05) compared to PC.
3.9. Osteogenic Differentiation
Extracellular and intracellular ALP activity was evaluated on days 7 and 14 (Figure 14). On day 7, all Ca-enriched MNPs exhibited significantly higher ALP activity compared to the cells alone, with Fe3O4/mSi/Ca_1B and Fe3O4/mSi/Ca_1A showing the highest extracellular values. By day 14, ALP levels decreased across all groups. The Fe3O4/mSi_1 sample showed only small changes in ALP activity at both time points. Alizarin Red staining was quantified on day 21 to evaluate mineral deposition. The control cells and the Fe3O4/mSi_1, Fe3O4/mSi/Ca_1A, and Fe3O4/mSi/Ca_1B samples showed similar absorbance values, with no significant differences among them. In contrast, the Fe3O4/mSi/Ca_1C sample displayed the highest absorbance, with a statistically significant increase compared with the other groups. Fe3O4/mSi/Ca_1C displayed staining around MNPs, consistent with the quantitative ARS results and confirming its ability to promote mineralization.
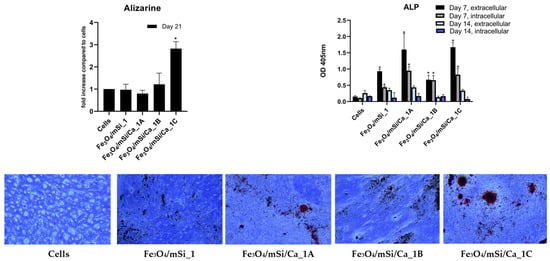
Figure 14.
ARS, ALP, and representative images after ARS staining of the tested MNPs. * = statistically significant (p < 0.05) compared to cells.
4. Discussion
Iron oxide magnetic nanoparticles (IONPs) are widely used in the biomedical field due to their low toxicity, ease of synthesis, and surface modification [4,5,16,17]. Magnetite (Fe3O4), hematite (α-Fe2O3), maghemite (γ-Fe2O3), and wustite (FeO) are classifications of iron oxide. Magnetite is the most widely studied IONP, with a size under 20 nm [26,31], attributed to the reduced relative oxygen concentration. Decreasing NPs’ size leads to a decrease in their ferromagnetic behavior and an increase in their superparamagnetic one. Therefore, Fe3O4 NPs below 20 nm have distinct superparamagnetic properties and a high surface-to-volume ratio, which is ideal for their use in biomedical applications [28,32].
Among a variety of different synthesis methods, the co-precipitation is a simple and fast preparative method with better control over composition and particle size [29,33]. Iron oxide Fe3O4 NPs tend to agglomerate and, due to their high chemical activity, oxidize easily, leading to a decrease in their magnetic properties. Indeed, during the experiments, agglomeration occurred, and the observed decrease in magnetization saturation can be attributed to the NPs’ tendency to aggregate, as indicated by TEM.
The coating on Fe3O4 NPs serves to protect the core by improving stability and preventing oxidation, which can degrade magnetic properties. It also reduces surface energy, minimizing particle agglomeration, and enhances dispersibility. Additionally, the protective shell allows for further functionalization of the nanoparticle surface, enabling versatile applications [31,34]. Mesoporous solid strong bases are easily recoverable and reusable catalysts, offering a wide range of applications and economic viability [14,15,20,22,35]. Moreover, their high surface and abundant porous channels assist adsorbate diffusion and protect active sites from deactivation [21,22,27]. A mesoporous SiO2 shell provides additional advantages, such as large pore channels and high pore volume [36,37], while maintaining the core’s magnetic properties in a stable and environment-independent manner. Finally, the shell’s enrichment with Ca ions may induce osteogenesis and promote bone regeneration [27,33,34,35,36,37]. These properties guided the synthesis of Fe3O4 nanoparticles as magnetic cores, coated with a mesoporous SiO2 shell and further enriched with Ca2+ ions, for use as drug carriers in bone tissue treatment applications.
4.1. Core–Shell Formation
Fe3O4 was successfully synthesized via the co-precipitation method, and primarily spherical-shaped NPs of an average size of ~12 nm were fabricated. The small mean size of the MNPs (below 20 nm) enhances their potential as superparamagnetic agents for biomedical applications [28,32]. As it comes to MNPs’ coating with a mesoporous silica shell, the two different procedures (modified Stöber and surfactant-templated sol–gel) were attempted to detect which could provide the most optimal properties of the synthesized MNPs.
The synthesis procedure 1 (modified Stöber), indeed, provided a satisfying amorphous silica shell with well-defined thickness on all samples (Fe3O4/mSi_1, Fe3O4/mSi/Ca_1A, Fe3O4/mSi/Ca_1B, and Fe3O4/mSi/Ca_1C). Coated MNPs were mostly aggregated, however, with good distribution. An oxidation increase after coating was detected as expected, which is translated to the co-existence of the two phases (Fe3O4–Fe2O3 [37,38]). In addition, TEM results revealed a multi-core–shell structure of the synthesized MNPs, with only a minor number of magnetic particles individually surrounded/coated by the amorphous shell. A main reason for this is the aggregation of the magnetic particles, which tend to agglomerate during synthesis, due to strong magnetic dipole–dipole attractions, leaving no space for the shell to isolate each particle. If nucleation of silica is slower than core aggregation, the forming shell grows around aggregated cores. Other factors that may contribute to multi-core clustering are the high core concentration in the reaction mixture that increases the likelihood of aggregation before coating and the slow addition of TEOS, which may give time for NPS’ aggregation before the formation of the shell. In the present study, with this synthesis approach, the MNPs were first dispersed in ddH2O, and subsequently, CTAB was added, and at a later stage, the TEOS was added drop-by-drop. As magnetic NPs tend to aggregate quickly in water, especially in the absence of a stabilizer, CTAB addition may not be able to surpass this aggregation, fully break the clusters, and isolate each MNP [38,39]. Thus, in the sol–gel synthesis procedure 2, the usage of the organic cosolvent CB was expected to decrease aggregation’s level since CB diffuses into the hydrophobic region of CTAB micelles over time, leading to the structural transformation of bilayer structures. Therefore, a large pore dendritic tail with a more detectable pore size is expected. CB’s diffusion into CTAB micelles is also faster closer to the oil phase (where tails are nucleated) rather than in aqueous solution [37,38,39]. Therefore, the shell’s structure stabilizes better and surrounds the particles more easily while preventing their tendency to aggregate. Truly, better dispersion of the particles was observed, yet the shell was barely defined, without also accomplishing the complete NPs’ isolation and coating by the mSiO2, since aggregation still occurred. This might be due to the not strong enough (or not completely built) bonds required for the silica shell’s formation and functionality. In fact, Li et al. [15] also synthesized similar core–shell nanoparticles that indeed presented aggregation yet with a higher magnetic core–particle isolation via their amorphous shell coating. The better-defined silica shell of the first synthesis method, and the undefined shell thickness detected in the 2nd method, are in agreement with the synthesis made before by Zhao et al. and Li et al. [15,16,17].
4.2. Characterization Results
XRD analysis revealed that all samples exhibited the characteristic crystal structure of Fe3O4, with a high degree of crystallinity, which was only slightly decreased after coating the magnetic core with a SiO2 shell and further enrichment of the shell with Ca2+ in all samples, in agreement with previous experiments [14,15,16,17]. This indicates the good preservation of the magnetic core and its crystal state, yet with a partial conversion of Fe3O4 to Fe2O3, which is expected due to MNPs’ ease of oxidation [37,38]. The only new peak in the Fe3O4/mSi/Ca_1A sample at angles 2θ = 29.35°, along with the weaker ones at 39.35°, 47.5°, and 48.4°, are attributed to calcium carbonate’s formation (calcite, Ca(CO3), Jade tab: #860174), indicating excess of calcium not incorporated into the silicate lattice [29,31]. This peak’s existence may be attributed to the higher amount of calcium ions in this sample, with a TEOS:Ca2+ ratio of 60:40, since the Fe3O4/mSi/Ca_1B sample (90:10 TEOS:Ca2+) revealed no such diffraction peaks. The higher Ca2+ loading may exceed silanol site availability, favoring precipitation of CaCO3 during hydrolysis and condensation [40,41]. FTIR analysis verified the presence of silicate peaks in the range of 750–1100 cm−1, assigned to the silica coating of the MNPs. The coexistence of the two SPION phases (Fe3O4 and Fe2O3), especially in the synthesis procedure 1 (stretching vibration Fe–O at ~450 cm−1), comes in agreement with the work of Zinan Zhao et al. [16], where the same two bands at ~440 cm−1 and 562 cm−1 were attributed to the co-existence of magnetite and maghemite. This was also proven before by Antarnusa G. et al. while synthesizing Fe3O4 MNPs [17]. In addition to these two studies, Alterary et al. [42,43] synthesized and characterized magnetic core–shell NPs of Fe3O4 coated with a SiO2 shell, and FTIR analysis once again revealed the second band at ~440 cm−1. It is then well assumed that the presence of these two SPION phases is nearly unavoidable.
Another interesting observation is that in experimental procedure 2 (Fe3O4/mSi_2 and Fe3O4/mSi/Ca_2), the presence of a silicate peak appeared with much lower intensity. Two factors that may have contributed to that are as follows [41,44]: a. the lower water/TEOS ratio in synthesis 1, may have slowed down TEOS hydrolysis, resulting in a thinner shell after condensation; and b. the higher concentration of magnetic NPs in the synthesis II, which may have resulted in lower available silica due to silica distribution to more cores.
4.3. Magnetic Properties
VSM measurements of all samples revealed an MS value in the range 7–22 of Am2/kg, which lies within the appropriate values for biomedical applications [45,46], and are consistent with reported values for Fe3O4/mSi magnetic core–shell nanoparticles in previous studies. For example, MS for Li X. et al. and Li T. et al. [14,15] reported values of 34.9, 27.8, and 22.5 Am2/kg for Fe3O4, Fe3O4/Si and Fe3O4/Si/Ca NPs, respectively. In another study, Ding et al. [41] found that MS values of silica-coated Fe3O4 NPs with different shell thicknesses were 30.0, 5.2, and even 1.9 Am2/kg. The lower MS on the coated samples is expected because the MS value is found to be proportional to the amount of weight for the same magnetic material [43]. The difference is due to the surface of the samples bearing an irregular magnetic spin due to interactions between Fe–O bonds and incomplete coordination between surface atoms. As for the low magnetization value of the synthesized NPs, it may be due to the oxidation of magnetite to maghemite, a phase of SPIONS with lower magnetization compared to Fe3O4, as well as disordered spins at the particle surface [17]. The satisfactory magnetization range indicates the material’s ability to separate immediately after acting in aqueous solution [15,16,17].
Moreover, in this work, all samples’ hysteresis loops are so narrow that they make coercivity and remanence difficult to determine. Consequently, both core and core–shell compositions are classified as soft materials, due to the narrow hysteresis curve they form and exhibit ideal soft magnetization at ambient temperature [15,19]. The superparamagnetic behavior of the synthesized NPs can be seen based on the morphology and shape of the hysteresis loops [17,19]. Loop’s curves and shape suggest that small NPs demonstrate high magnetic response, since the small particle of the particles’ grains leads to instability in the magnetic moment of the MNPs, which is due to the small anisotropy energy characteristic of MNPs. Thus, in the case of the application of an external magnetic field, the magnetic moment in small NPs leads to a faster response. In general, an increase in NPs’ size, with any non-magnetic additions (silica shell, calcium ions and so on), implies a decrease in their magnetization. In fact, there is a critical point (threshold) at which NPs become superparamagnetic. It is therefore expected that by adding a non-magnetic coating and enriching it with an additional element (calcium ions in this case), magnetization decreases as observed in all the samples. Moreover, experimental procedure 1 successfully resulted in the formation of an amorphous, well-defined silica shell, of higher thickness in contrast to experimental procedure 2, where the shell was thin and almost undetectable, hence MNPs of samples Fe3O4/mSi_2 and Fe3O4/mSi/Ca_2 mainly contained magnetic core particles, which explains the smaller decrease in MS. However, experimental procedure 1, despite the higher a % of decrease in MS, remained within the required range for biomedical applications. The higher magnetization saturation (MS) observed in Fe3O4/mSi/Ca_1A, compared to the expected decrease of MS in the Fe3O4/mSi/Ca_1B sample, may be due to a different rate of Ca incorporation into the silica shell. This could be related to the different TEOS:Ca ratios used during synthesis (60:40 for Fe3O4/mSi/Ca_1A vs. 90:10 for Fe3O4/mSi/Ca_1B), with the higher Ca content in Fe3O4/mSi/Ca_1A potentially contributing to a greater reduction in MS.
4.4. Mesoporous Structure
Type IV(b) is a reversible isotherm of materials with smaller mesopores. These isotherms are created due to conical and cylindrical mesopores closed at the tapered end [27,28]. Considering BET results, all coated samples revealed a poorly developed porous structure, with primarily interparticle porosity, except for the Fe3O4/mSi_1 and Fe3O4/mSi/Ca_1C samples. Regarding the Ca-enriched samples, Fe3O4/mSi/Ca_1A a and Fe3O4/mSi/Ca_1B, there is a possibility that Ca2+ ions are blocking the existing pores and CTAB cannot completely be removed. In the case of Fe3O4/mSi_1 and Fe3O4/mSi/Ca_1C, a distinct inflection point (“knee”) appears successfully on the respective isotherms, indicating the existence of a mesoporous type IV(b) amorphous mSiO2 shell, enriched with Ca in these two cases. This assumption agrees with the study of Pouroutzidou et al. [27,28], who synthesized mesoporous Mg and Sr-Doped NPs for moxifloxacin drug delivery and calcinated the samples over 550 °C [27,28].
All samples were confirmed to be porous materials. The observed variations in the isotherms reflect differences in particle aggregation, which is more realistic since isolated nanoparticles are difficult to detect due to the formation of multi-core–shell structures. Despite that the first experimental procedure led to the successful synthesis of a mesoporous type IV(b) silica shell (modified Stober method: Fe3O4/mSi_1), neither the Fe3O4/mSi/Ca_1A nor Fe3O4/mSi/Ca_B maintained this structure due to Ca2+ ions blocking the existing pores. Thus, the wet impregnation technique (Fe3O4/mSi/Ca_1C) was employed and indeed formed the desired mesoporous Ca-doped shell. Εxperimental procedure 2 did not result in a mesoporous shell, as shown by its faint presence around the particles, as mentioned before.
Lastly, among all samples, Fe3O4/mSi/Ca_1C exhibited the highest specific surface area (463.3 m2/g), which is comparable to or superior to values reported in similar mesoporous drug delivery systems. For instance, triple-porous Fe3O4/SiO2 microspheres have been reported to reach surface area of ~426 m2/g [44], while the Fe3O4/DPU/MSH hybrid structure achieved surface area of ~386 m2/g by Moorthy [46]. Given that surface area directly influences drug loading capacity, this elevated value highlights the superior potential of Fe3O4/mSi/Ca_1C as a promising candidate for further pharmaceutical applications.
As it comes to size distribution, all samples maintained a mean particle size of ~13 nm, which is acceptable for utilization in biomedical applications. However, since the synthesis approaches resulted in multi-core–shell structures, the measurements were taken for the Fe3O4 particles embedded within a major amorphous, porous, silica shell. As a result, it was practically impossible to determine the size distribution of isolated core–shell NPs, except in very limited areas, where isolated magnetite cores were fully encapsulated by the shell, as revealed by TEM imaging. This overall coating effect was also detected by Alterary S. et al. [42] in their TEM results, where they synthesized and characterized Fe3O4-SiO2 core–shell nanostructures. Their samples exhibited mainly spherical shapes, yet were also aggregated with a SiO2 shell covering larger regions containing coexisting aggregated magnetic cores.
4.5. Cytotoxicity—Osteogenic Potential and ROS Analysis
The lack of toxicity in HGFs was verified through indirect toxicity testing. Indirect tests mainly assess toxic effects caused by soluble or released components, and lack of toxicity, indicating good chemical stability and low release of harmful species and residual reagents due to the sol–gel synthesis. However, indirect tests do not capture the physical interactions between NPs and cells, thereby potentially hindering a possible harmful contact-dependent toxicity. Lu et al. and Guo et al. [44,47] carried out direct cytotoxicity tests in L929 fibroblasts for Fe3O4 and Fe3O4/mSiO2, with low particle doses or short incubation times, and showed little reduction in cell viability, while fibroblast cell viability decreased with the increase in time and concentration. Dose-dependent changes in cell morphology, viability, and apoptosis rate have been observed in other studies, as well as ROS production, challenging the protective effect of even the silica coating [48,49]. Inconclusive results also exist in terms of NPs size [50,51,52,53], highlighting the need for more thorough evaluation of cell toxicity and possible immunomodulating effects.
We selected hPDLCs to evaluate the capacity of our materials to induce osteogenic differentiation. These cells possess osteogenic potential and can readily shift toward an osteoblast-like phenotype when exposed to appropriate biochemical or biomaterial-derived stimuli [24,54]. In the present study, it was demonstrated that the Fe3O4/mSi/Ca_1C MNPs were more efficient in promoting the hPDLCs osteogenic differentiation in vitro according to higher ALP activity and increased matrix mineralization (ARS). This is probably attributed to the presence of calcium in the silica shell, as verified by XPS. ALP was increased in all samples on day 7 and decreased after 14 days of culture, a finding that was expected, as ALP is an early osteogenic marker. Although mesoporous silica-coated magnetic (Fe3O4) nanoparticles present remarkable capability in promoting the osteogenic differentiation of mesenchymal stem cells via the Wnt/β-catenin pathway in vitro [55], the long-term mineralization data (ARS, day 21) reveal differences among the MNPs. While Fe3O4/mSi_1, Fe3O4/mSi/Ca_1A, and Fe3O4/mSi/Ca_1B showed ARS values comparable to the control cells, Fe3O4/mSi/Ca_1C exhibited a significantly higher absorbance, indicating higher calcium deposition. Taken together with the viability (MTT) data, showing overall acceptable cytocompatibility when testing the MNPs eluates, these results suggest that a certain level of ROS generation by the direct contact of cells with the Ca-doped Fe3O4/mSi nanoparticles does not abolish, and may even accompany, osteogenic differentiation, provided that oxidative stress remains below a cytotoxic threshold. On the other hand, mesoporous silica-coated MNPs can promote osteogenic differentiation through a combination of bioactive ion release, favorable surface properties, and magnetic responsiveness [56]. The gradual dissolution of the silica shell releases soluble silica species known for their capacity to activate osteogenic pathways [50,53] and upregulate markers such as RUNX2, ALP, and osteocalcin, while the high surface area of the mesoporous structure enhances protein adsorption and integrin-mediated cell adhesion, further stimulating osteogenic signaling [55]. The magnetic Fe3O4 core can also contribute to mechanotransductive stimulation, which has been shown to enhance osteoblast differentiation under static or dynamic magnetic cues [53]. Together, these features create a bioactive microenvironment that supports osteogenic commitment and mineralization of progenitor cells. In our study, the Ca-doped mesoporous silica-coated magnetic nanoparticles similarly induced elevated ROS levels in direct-contact culture, especially in Ca_1B and Ca_1C, yet still Fe3O4/mSi/Ca_1C supported osteogenic differentiation. This finding supports the concept that moderate ROS levels can be pro-osteogenic, particularly when accompanied by Ca2+ and silicate ion release, which further enhances osteogenic gene expression and mineralization. However, further research is needed to capture ROS levels at different stages of differentiation, with thorough monitoring of ion species release to fully elaborate on their role in bone tissue engineering.
4.6. Comparative Analysis and Selection of the Optimum Synthesis Method
In this study, we synthesized core–shell magnetic nanoparticles enriched with calcium. Although core–shell structures were achieved in all cases, only two samples exhibited a clear mesoporous silica shell (Fe3O4/mSi_1 and Fe3O4/mSi/Ca_1C), while the others showed an interparticle porosity. Different synthesis approaches were employed to collectively combine the desired functionalities. The optimized samples Fe3O4/mSi_1 and Fe3O4/mSi/Ca_1C presented mainly spherical shape and size below 20 nm, verifying their suitability for biomedical applications. These samples retained primarily multi-core single-shell structure due to magnetic particle aggregation, retained the crystallinity of Fe3O4, and although partial oxidation of Fe3O4 into Fe2O3 may have slightly reduced magnetization, it remained within acceptable limits. Considering Ca2+ addition, the wet impregnation method for calcium enrichment yielded a mesoporous, amorphous silica shell with a uniform thin thickness coating of the magnetic cores. This process also maintained the biocompatibility of the MNPs and preserved the mesoporous type IV(b) structure of the silica shell after Ca-doping, supporting their ability to promote osteogenic differentiation. Among the tested formulations, Fe3O4/mSi/Ca_1C appears to achieve the most favorable balance between ROS induction, cell viability, early ALP upregulation, and late-stage mineralization, highlighting it as the most promising candidate for bone-regenerative applications. Thus, the aforementioned samples were selected as ideal candidates for further pharmaceutical or biomedical applications.
Achieving good dispersion to form a classic single core–shell structure while maintaining magnetic properties remains a challenge that requires further investigation. To prevent particle aggregation and enhance the stabilization and functionalization of type IV porous shells, research should focus on testing different water/TEOS, NPs/water, and TEOS:Ca2+ ratios, as well as calcination times and TEOS addition to the solution. It has been reported that for the same amount of TEOS a slow incorporation (stepwise or on different increments) and a low concentration of magnetic NPs can enhance dispersion and homogeneous coating of isolated NPs [57]. Additionally, the wet impregnation method for calcium enrichment is preferable to ensure the formation and preservation of the mesoporous shell. These approaches should allow micelles to better stabilize the mesoporous structure of the shell compared to current attempts, facilitating drug encapsulation within the pores and avoiding pore blockage by Ca2+ ions. Future research should focus on testing biocompatibility with different cell lines, the exploration of their bioactivity in terms of in vitro biological hydroxyapatite formation, their potential immunomodulatory properties, and the encapsulation of pharmaceuticals or biological molecules for controlled release under an external magnetic field.
5. Conclusions
Silica-coated, calcium-doped multi-core–shell magnetic nanoparticles were successfully synthesized using different methods. The optimized samples featured a mesoporous amorphous silica shell formed by a modified Stöber method, followed by successful calcium addition via wet impregnation. The other samples exhibited an amorphous, Ca-doped silica shell characterized primarily by interparticle porosity. The coating preserved the core’s crystallinity and magnetic properties but caused partial conversion of magnetite to maghemite, more evident in experimental procedure 1. Procedure 2 (sol–gel) resulted in reduced particle aggregation, but in thinner, less defined shells. Particle size remained around 13 nm throughout the different synthesis routes. In general, the materials were biocompatible, with minor exceptions; a slight toxicity was recorded at the highest concentration of the Fe3O4/mSi/Ca_1B sample. Their capacity to promote the osteogenic differentiation of hPDLCs further demonstrates their supportive roles in tissue engineering and biomedical applications. Future research should focus on improving particle dispersion, maintaining magnetic properties, and exploring biological functions like hydroxyapatite formation and their capacity for osteogenic differentiation and controlled drug release under static or dynamic magnetic fields.
Author Contributions
Conceptualization, D.K. and E.K.; methodology, D.K., G.K.P., N.F., I.T., K.K., D.G., G.V., M.A. and P.K.; formal analysis, D.K., P.P., E.K., G.K.P., N.F., I.T., D.G., M.A.; investigation, D.K., G.K.P., N.F., I.T., D.G., M.A.; resources, E.K.; data curation, D.K., E.K., G.K.P., N.F., I.T., D.G., M.A.; writing—original draft preparation, D.K., G.K.P., N.F., I.T., D.G.; writing—review and editing, E.K., P.P., P.K., M.A.; supervision, E.K., M.A.; funding acquisition, E.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by internal resources.
Data Availability Statement
All data are presented in figures.
Acknowledgments
The authors want to acknowledge the contribution of Dimitrios Karfaridis for the XPS measurements.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| Fe3O4 | Magnetite |
| Fe2O3 | Maghemite |
| mSiO2 | Mesoporous Silica |
| Ca2+ | Calcium Ions |
| NPs | Nanoparticles |
| MNPs | Magnetic Nanoparticles |
| MRI | Magnetic Resonance Imaging |
| Ca10(PO4)6(OH)2 | Hydroxyapatite |
| PEG | Polyethylene Glycol |
| FeCl3•6H2O | Ferric Chloride Hexahydrate |
| FeSO4•7H2O | Ferrous Chloride Tetrahydrate |
| NH4OH | Ammonium Hydroxide |
| TEOS | Tetraethyl Orthosilicate |
| CTAB | Cetyltrimethyl Ammonium Bromide |
| CH3CH2OH | Ethanol |
| Ca(NO3)2•4H2O | Calcium Nitrate Tetrahydrate |
| C6H15NO3 | Triethanolamine |
| C6H5Cl or CB | Chlorobenzene |
| dd-H2O | Double-Distilled Water |
| Ar | Argon Gas |
| NH3 | Ammonia |
| XRD | X-ray Diffraction |
| FTIR | Fourier-Transform Infrared |
| KBr | Potassium Bromide |
| VSM | Vibrating Sample Magnetometer |
| TEM | Transmission Electron Microscopy |
| HRTEM | High Resolution Transmission Electron Microscopy |
| DMEM | Dulbecco’s Minimal Essential Medium |
| HGFs | Human Gingival Fibroblasts |
| hPDLCs | Human Periodontal Ligament Cells |
| DMSO | Dimethyl Sulfoxide |
| fcc | Face-centered Cubic |
| BET | Brunauer-Emmett-Teller theory |
| BJH | Barrett–Joyner–Halenda |
| XPS | X-ray photoelectron spectroscopy |
| ARS | Alizarin Red Staining |
| ALP | Alkaline Phosphatase Activity |
| ROS | Reactive Oxygen Species |
| ART | Artemisin |
| PC | Positive Control |
References
- Wu, A.; Ou, P.; Zeng, L. Biomedical Applications of Magnetic Nanoparticles. Nano 2010, 5, 245–270. [Google Scholar] [CrossRef]
- Tran, N.; Webster, T.J. Magnetic Nanoparticles: Biomedical Applications and Challenges. J. Mater. Chem. 2010, 20, 8760–8767. [Google Scholar] [CrossRef]
- Vallabani, N.V.S.; Singh, S.; Karakoti, A.S. Magnetic Nanoparticles: Current Trends and Future Aspects in Diagnostics and Nanomedicine. Curr. Drug Metab. 2018, 20, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Bertolucci, E.; Maria, A.; Galletti, R.; Antonetti, C.; Marracci, M.; Tellini, B.; Piccinelli, F. Chemical and Magnetic Properties Characterization of Magnetic Nanoparticles. In Proceedings of the 2015 IEEE International Instrumentation and Measurement Technology Conference (I2MTC), Pisa, Italy, 11–14 May 2015. [Google Scholar]
- Fathi-Achachelouei, M.; Knopf-Marques, H.; Ribeiro da Silva, C.E.; Barthès, J.; Bat, E.; Tezcaner, A.; Vrana, N.E. Use of Nanoparticles in Tissue Engineering and Regenerative Medicine. Front. Bioeng. Biotechnol. 2019, 7, 113. [Google Scholar] [CrossRef]
- Ghosh Chaudhuri, R.; Paria, S. Core/Shell Nanoparticles: Classes, Properties, Synthesis Mechanisms, Characterization, and Applications. Chem. Rev. 2012, 112, 2373–2433. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Sarkar, S.; Jagajjanani Rao, K.; Paria, S. Core/Shell Nanoparticles in Biomedical Applications. Adv. Colloid Interface Sci. 2014, 209, 8–39. [Google Scholar] [CrossRef]
- Singh, R.; Bhateria, R. Core–Shell Nanostructures: A Simplest Two-Component System with Enhanced Properties and Multiple Applications. Environ. Geochem. Health 2021, 43, 2459–2482. [Google Scholar] [CrossRef]
- Tsamos, D.; Krestou, A.; Papagiannaki, M.; Maropoulos, S. An Overview of the Production of Magnetic Core–Shell Nanoparticles and Their Biomedical Applications. Metals 2022, 12, 605. [Google Scholar] [CrossRef]
- Manatunga, D.C.; Godakanda, V.U.; de Silva, R.M.; de Silva, K.M.N. Recent Developments in the Use of Organic–Inorganic Nanohybrids for Drug Delivery. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1605. [Google Scholar] [CrossRef]
- Mahdavi, Z.; Rezvani, H.; Keshavarz Moraveji, M. Core–Shell Nanoparticles Used in Drug Delivery-Microfluidics: A Review. RSC Adv. 2020, 10, 18280–18295. [Google Scholar] [CrossRef]
- Xu, B.; Li, S.; Shi, R.; Liu, H. Multifunctional Mesoporous Silica Nanoparticles for Biomedical Applications. Signal Transduct. Target. Ther. 2023, 8, 435. [Google Scholar] [CrossRef] [PubMed]
- Sutthavas, P.; Tahmasebi Birgani, Z.; Habibovic, P.; van Rijt, S. Calcium Phosphate-Coated and Strontium-Incorporated Mesoporous Silica Nanoparticles Can Effectively Induce Osteogenic Stem Cell Differentiation. Adv. Healthc. Mater. 2022, 11, 2101588. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, L.; Dong, X.; Liang, J.; Shi, J. Preparation of Mesoporous Calcium Doped Silica Spheres with Narrow Size Dispersion and Their Drug Loading and Degradation Behavior. Microporous Mesoporous Mater. 2007, 102, 151–158. [Google Scholar] [CrossRef]
- Li, T.T.; Liu, Y.; Qi, S.C.; Liu, X.Q.; Huang, L.; Sun, L.B. Calcium Oxide-Modified Mesoporous Silica Loaded onto Ferriferrous Oxide Core: Magnetically Responsive Mesoporous Solid Strong Base. J. Colloid Interface Sci. 2018, 526, 366–373. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, M.; Jiang, X.; Lv, R.; Mao, C. Fe3O4 Nanoparticles Coated with Mesoporous Shells for Pb(II) Removal from Blood. ACS Appl. Nano Mater. 2022, 5, 249–258. [Google Scholar] [CrossRef]
- Antarnusa, G.; Jayanti, P.D.; Denny, Y.R.; Suherman, A. Utilization of Co-Precipitation Method on Synthesis of Fe3O4/PEG with Different Concentrations of PEG for Biosensor Applications. Materialia 2022, 25, 101525. [Google Scholar] [CrossRef]
- Stober, W.; Fink, A.; Ernst Bohn, D. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Paswan, S.K.; Kumari, S.; Kar, M.; Singh, A.; Pathak, H.; Borah, J.P.; Kumar, L. Optimization of Structure-Property Relationships in Nickel Ferrite Nanoparticles Annealed at Different Temperature. J. Phys. Chem. Solids 2021, 151, 109928. [Google Scholar] [CrossRef]
- Kazeli, K.; Athanasiadou, A.; Makridis, A.; Malletzidou, L.; Vourlias, G.; Kontonasaki, E.; Lymperaki, E.; Angelakeris, M. Synthesis and characterization of a novel multifunctional magnetic bioceramic nanocomposite. Ceram. Int. 2023, 49, 24650–24659. [Google Scholar] [CrossRef]
- Kazeli, K.; Tsamesidis, I.; Theocharidou, A.; Malletzidou, L.; Rhoades, J.; Pouroutzidou, G.K.; Likotrafiti, E.; Chrissafis, K.; Lialiaris, T.; Papadopoulou, L.; et al. Synthesis and Characterization of Novel Calcium-Silicate Nanobioceramics with Magnesium: Effect of Heat Treatment on Biological, Physical and Chemical Properties. Ceramics 2021, 4, 628–651. [Google Scholar] [CrossRef]
- Tsamesidis, I.; Gkiliopoulos, D.; Pouroutzidou, G.K.; Lymperaki, E.; Papoulia, C.; Reybier, K.; Perio, P.; Paraskevopoulos, K.M.; Kontonasaki, E.; Theocharidou, A. Effect of Artemisinin-Loaded Mesoporous Cerium-Doped Calcium Silicate Nanopowder on Cell Proliferation of Human Periodontal Ligament Fibroblasts. Nanomaterials 2021, 11, 2189. [Google Scholar] [CrossRef]
- Nakiou, E.A.; Lazaridou, M.; Pouroutzidou, G.K.; Michopoulou, A.; Tsamesidis, I.; Liverani, L.; Arango-Ospina, M.; Beketova, A.; Boccaccini, A.R.; Kontonasaki, E.; et al. Poly(Glycerol Succinate) as Coating Material for 1393 Bioactive Glass Porous Scaffolds for Tissue Engineering Applications. Polymers 2022, 14, 5028. [Google Scholar] [CrossRef] [PubMed]
- Tsamesidis, I.; Theocharidou, A.; Beketova, A.; Bousnaki, M.; Chatzimentor, I.; Pouroutzidou, G.K.; Gkiliopoulos, D.; Kontonasaki, E. Artemisinin Loaded Cerium-Doped Nanopowders Improved In Vitro the Biomineralization in Human Periodontal Ligament Cells. Pharmaceutics 2023, 15, 655. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Farjami Shayesteh, S.; Salouti, M.; Boustani, K. Effect of Annealing Temperature on Magnetic Phase Transition in Fe3O4 Nanoparticles. J. Magn. Magn. Mater. 2015, 379, 305–312. [Google Scholar] [CrossRef]
- El-Dib, F.I.; Mohamed, D.E.; El-Shamy, O.A.A.; Mishrif, M.R. Study the Adsorption Properties of Magnetite Nanoparticles in the Presence of Different Synthesized Surfactants for Heavy Metal Ions Removal. Egypt. J. Pet. 2020, 29, 1–7. [Google Scholar] [CrossRef]
- Pouroutzidou, G.K.; Liverani, L.; Theocharidou, A.; Tsamesidis, I.; Lazaridou, M.; Christodoulou, E.; Beketova, A.; Pappa, C.; Triantafyllidis, K.S.; Anastasiou, A.D.; et al. Synthesis and Characterization of Mesoporous Mg-and Sr-Doped Nanoparticles for Moxifloxacin Drug Delivery in Promising Tissue Engineering Applications. Int. J. Mol. Sci. 2021, 22, 577. [Google Scholar] [CrossRef]
- Pouroutzidou, G.K.; Lazaridou, M.; Papoulia, C.; Tsamesidis, I.; Chrissafis, K.; Vourlias, G.; Paraskevopoulos, K.M.; Bikiaris, D.; Kontonasaki, E. Electrospun PLGA Membranes with Incorporated Moxifloxacin-Loaded Silica-Based Mesoporous Nanocarriers for Periodontal Regeneration. Nanomaterials 2022, 12, 850. [Google Scholar] [CrossRef]
- Taghizadeh, S.M.; Ghoshoon, M.B.; Berenjian, A.; Ghasemi, Y.; Dehshahri, A.; Ebrahiminezhad, A. Impacts of Magnetic Immobilization on the Recombinant Proteins Structure Produced in Pichia Pastoris System. Mol. Biotechnol. 2021, 63, 80–89. [Google Scholar] [CrossRef]
- Hajji, S.; Turki, T.; Boubakri, A.; Ben Amor, M.; Mzoughi, N. Study of Cadmium Adsorption onto Calcite Using Full Factorial Experiment Design. Desalin. Water Treat. 2017, 83, 222–233. [Google Scholar] [CrossRef]
- Liu, S.; Yu, B.; Wang, S.; Shen, Y.; Cong, H. Preparation, Surface Functionalization and Application of Fe3O4 Magnetic Nanoparticles. Adv. Colloid Interface Sci. 2020, 281, 102165. [Google Scholar] [CrossRef]
- Li, W.; Zhao, D. Extension of the Stöber Method to Construct Mesoporous SiO2 and TiO2 Shells for Uniform Multifunctional Core–Shell Structures. Adv. Mater. 2013, 25, 142–149. [Google Scholar] [CrossRef]
- Jin, Z.; Wu, R.; Shen, J.; Yang, X.; Shen, M.; Xu, W.; Huang, R.; Zhang, L.; Yang, G.; Gao, C.; et al. Nonstoichiometric Wollastonite Bioceramic Scaffolds with Core–Shell Pore Struts and Adjustable Mechanical and Biodegradable Properties. J. Mech. Behav. Biomed. Mater. 2018, 88, 140–149. [Google Scholar] [CrossRef]
- Du, Z.; Guo, L.; Zheng, T.; Cai, Q.; Yang, X. Formation of Core–Shell Structured Calcium Silicate Fiber via Sol–Gel Electrospinning and Controlled Calcination. Ceram. Int. 2019, 45, 23975–23983. [Google Scholar] [CrossRef]
- Beketova, A.; Pouroutzidou, G.K.; Kontonasaki, E.; Giourieva, V.; Smits, K.; Stepanova, V.; Tsamesidis, I.; Choudhary, R.; Rubenis, K.; Eiduks, T.V.; et al. Zn containing mesoporous bioglasses with enhanced textural and antibacterial properties produced by three modifications of the sol–gel method. J. Mater. Sci. Mater. Med. 2025, 36, 105. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.A.; Essien, E.R.; Kaufmann, E.E. A New Route to Sol–Gel Crystalline Wollastonite Bioceramic. J. Asian Ceram. Soc. 2018, 6, 132–138. [Google Scholar] [CrossRef]
- Son, S.; Hwang, S.H.; Kim, C.; Yun, J.Y.; Jang, J. Designed synthesis of SiO2/TiO2 core/shell structure as light scattering material for highly efficient dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2013, 5, 4815–4820. [Google Scholar] [CrossRef] [PubMed]
- Glaria, A.; Soulé, S.; Hallali, N.; Ojo, W.S.; Mirjolet, M.; Fuks, G.; Cornejo, A.; Allouche, J.; Dupin, J.C.; Martinez, H.; et al. Silica Coated Iron Nanoparticles: Synthesis, Interface Control, Magnetic and Hyperthermia Properties. RSC Adv. 2018, 8, 32146–32156. [Google Scholar] [CrossRef] [PubMed]
- Abbaraju, P.L.; Meka, A.K.; Song, H.; Yang, Y.; Jambhrunkar, M.; Zhang, J.; Xu, C.; Yu, M.; Yu, C. Asymmetric Silica Nanoparticles with Tunable Head-Tail Structures Enhance Hemocompatibility and Maturation of Immune Cells. J. Am. Chem. Soc. 2017, 139, 6321–6328. [Google Scholar] [CrossRef]
- Marciniak, P.; Sztorch, B.; Martyła, A.; Czapik, A.; Stodolny, M.; Przekop, R.E. Metallic Calcium as a Precursor for Sol–Gel Synthesis of CaCO3-SiO2 and CaO-SiO2 Systems. Ceramics 2021, 4, 278–290. [Google Scholar] [CrossRef]
- Ding, H.L.; Zhang, Y.X.; Wang, S.; Xu, J.M.; Xu, S.C.; Li, G.H. Fe3O4@SiO2 Core/Shell Nanoparticles: The Silica Coating Regulations with a Single Core for Different Core Sizes and Shell Thicknesses. Chem. Mater. 2012, 24, 4572–4580. [Google Scholar] [CrossRef]
- Alterary, S.S.; Alkhamees, A. Synthesis, Surface Modification, and Characterization of Fe3O4@SiO2core@shell Nanostructure. Green Process. Synth. 2021, 10, 384–391. [Google Scholar] [CrossRef]
- Zhang, Z.J.; Chen, X.Y.; Wang, B.N.; Shi, C.W. Hydrothermal Synthesis and Self-Assembly of Magnetite (Fe3O4) Nanoparticles with the Magnetic and Electrochemical Properties. J. Cryst. Growth 2008, 310, 5453–5457. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Q.; Wang, L.; Jiang, W.; Zhang, W.; Song, X. Multifunctional Triple-Porous Fe3O4@SiO2 Superparamagnetic Microspheres for Potential Hyperthermia and Controlled Drug Release. RSC Adv. 2017, 7, 32049–32057. [Google Scholar] [CrossRef]
- Guardia, P.; Batlle-Brugal, B.; Roca, A.G.; Iglesias, O.; Morales, M.P.; Serna, C.J.; Labarta, A.; Batlle, X. Surfactant Effects in Magnetite Nanoparticles of Controlled Size. J. Magn. Magn. Mater. 2007, 316, e756–e759. [Google Scholar] [CrossRef]
- Moorthy, M.S.; Bharathiraja, S.; Manivasagan, P.; Oh, Y.; Phan, T.T.V.; Mondal, S.; Kim, H.; Lee, K.D.; Oh, J. Synthesis of Fe3O4 Modified Mesoporous Silica Hybrid for PH-Responsive Drug Delivery and Magnetic Hyperthermia Applications. J. Porous Mater. 2018, 25, 1251–1264. [Google Scholar] [CrossRef]
- Guo, X.; Mao, F.; Wang, W.; Yang, Y.; Bai, Z. Sulfhydryl-Modified Fe3O4@SiO2 Core/Shell Nanocomposite: Synthesis and Toxicity Assessment in Vitro. ACS Appl. Mater. Interfaces 2015, 7, 14983–14991. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Kuang, H.; Zhang, W.; Aguilar, Z.P.; Xiong, Y.; Lai, W.; Xu, H.; Wei, H. Size Dependent Biodistribution and Toxicokinetics of Iron Oxide Magnetic Nanoparticles in Mice. Nanoscale 2015, 7, 625–636. [Google Scholar] [CrossRef]
- Ying, E.; Hwang, H.-M. In Vitro Evaluation of the Cytotoxicity of Iron Oxide Nanoparticles with Different Coatings and Different Sizes in A3 Human T Lymphocytes. Sci. Total Environ. 2010, 408, 4475–4481. [Google Scholar] [CrossRef]
- Li, J.; Zhang, F.; Zhang, N.; Geng, X.; Meng, C.; Wang, X.; Yang, Y. Osteogenic Capacity and Cytotherapeutic Potential of Periodontal Ligament Cells for Periodontal Regeneration in Vitro and in Vivo. PeerJ 2019, 7, e6589. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, P.; Sun, Y.; Kang, Q.; Xu, J.; Zhang, C.; Chai, Y. Regeneration of Large Bone Defects Using Mesoporous Silica Coated Magnetic Nanoparticles during Distraction Osteogenesis. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102040. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Xue, J.; Deb, S. Magnetic Nanoparticles in Bone Tissue Engineering. Nanomaterials 2022, 12, 757. [Google Scholar] [CrossRef]
- Uribe, P.; Johansson, A.; Jugdaohsingh, R.; Powell, J.J.; Magnusson, C.; Davila, M.; Westerlund, A.; Ransjö, M. Soluble Silica Stimulates Osteogenic Differentiation and Gap Junction Communication in Human Dental Follicle Cells. Sci. Rep. 2020, 10, 9923. [Google Scholar] [CrossRef] [PubMed]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.K.; Hench, L.L.; Polak, J.M. Ionic Products of Bioactive Glass Dissolution Increase Proliferation of Human Osteoblasts and Induce Insulin-like Growth Factor II MRNA Expression and Protein Synthesis. Biochem. Biophys. Res. Commun. 2000, 276, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Marycz, K.; Sobierajska, P.; Wiglusz, R.; Idczak, R.; Nedelec, J.-M.; Fal, A.; Kornicka-Garbowska, K. Fe3O4 Magnetic Nanoparticles Under Static Magnetic Field Improve Osteogenesis via RUNX-2 and Inhibit Osteoclastogenesis by the Induction of Apoptosis. Int. J. Nanomed. 2020, 15, 10127–10148. [Google Scholar] [CrossRef] [PubMed]
- Toropova, Y.; Golovkin, A.; Malashicheva, A.; Korolev, D.; Gorshkov, A.; Gareev, K.; Afonin, M.; Galagudza, M. In Vitro Toxicity of FemOn, FemOn-SiO2 Composite, and SiO2-FemOn Core–Shell Magnetic Nanoparticles. Int. J. Nanomed. 2017, 12, 593–603. [Google Scholar] [CrossRef]
- Fiedler, R.; Sivakumaran, G.; Mallén, J.; Lindén, M. Superparamagnetic Core–Mesoporous Silica Shell Nanoparticles with Tunable Extra- and Intracellular Dissolution Rates. Chem. Mater. 2024, 36, 2790–2798. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).