Characterization of Iron Oxide Nanoparticles Inside the Myxococcus xanthus Encapsulin
Abstract
1. Introduction
2. Materials and Methods
2.1. Strains and Culturing Conditions
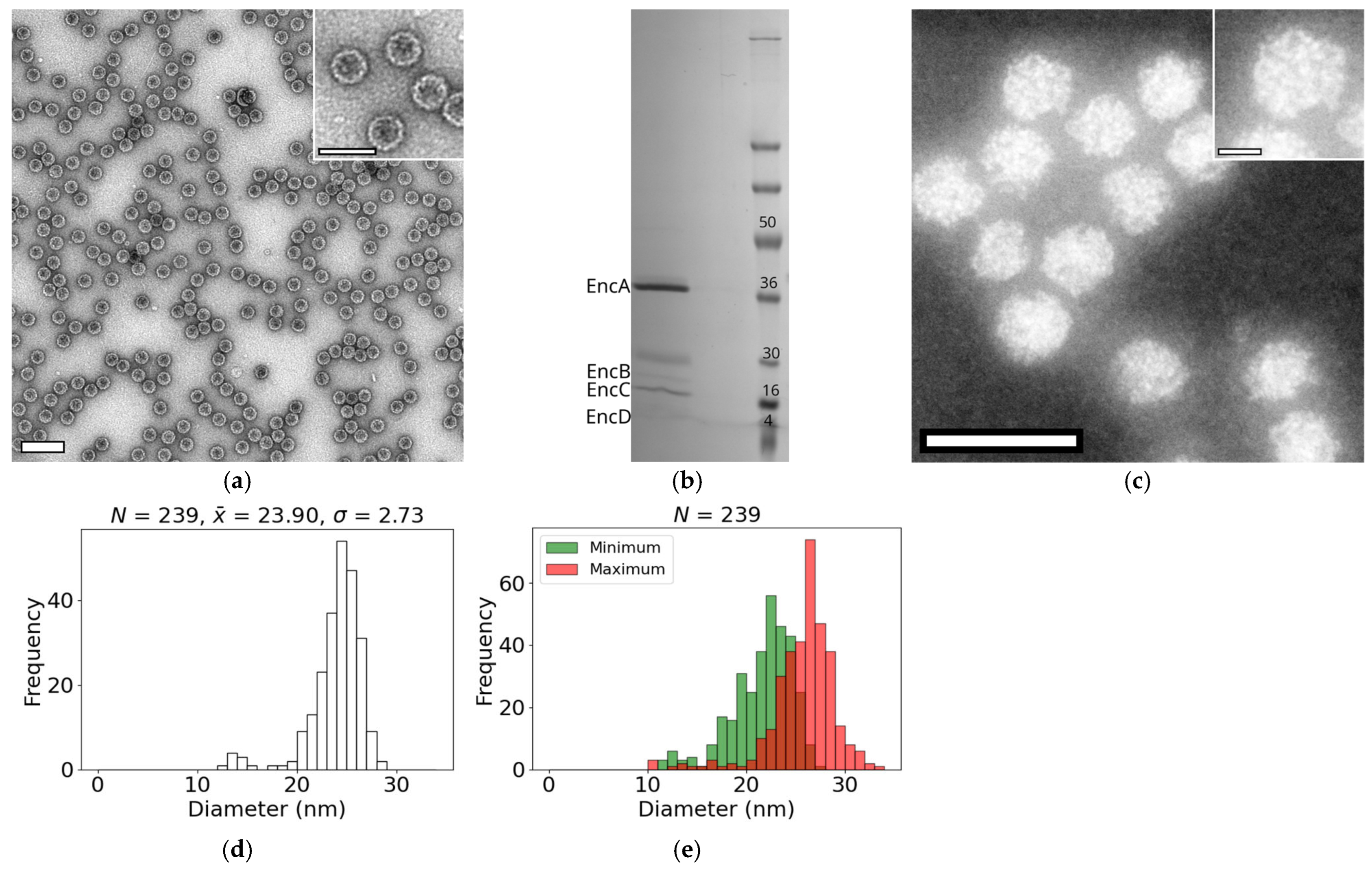
2.2. Isolation of Native Encapsulin Nanocompartments
2.3. Molecular Biology and Cloning
2.4. Protein Expression and Purification
2.5. Loading Encapsulins with Iron
2.6. Electron Microscopy
2.7. Data Analysis
2.7.1. Electron Micrograph Analysis
2.7.2. 4D-STEM Data Analysis
2.7.3. HRSTEM Data Analysis
2.7.4. EELS Spectrum Image Analysis
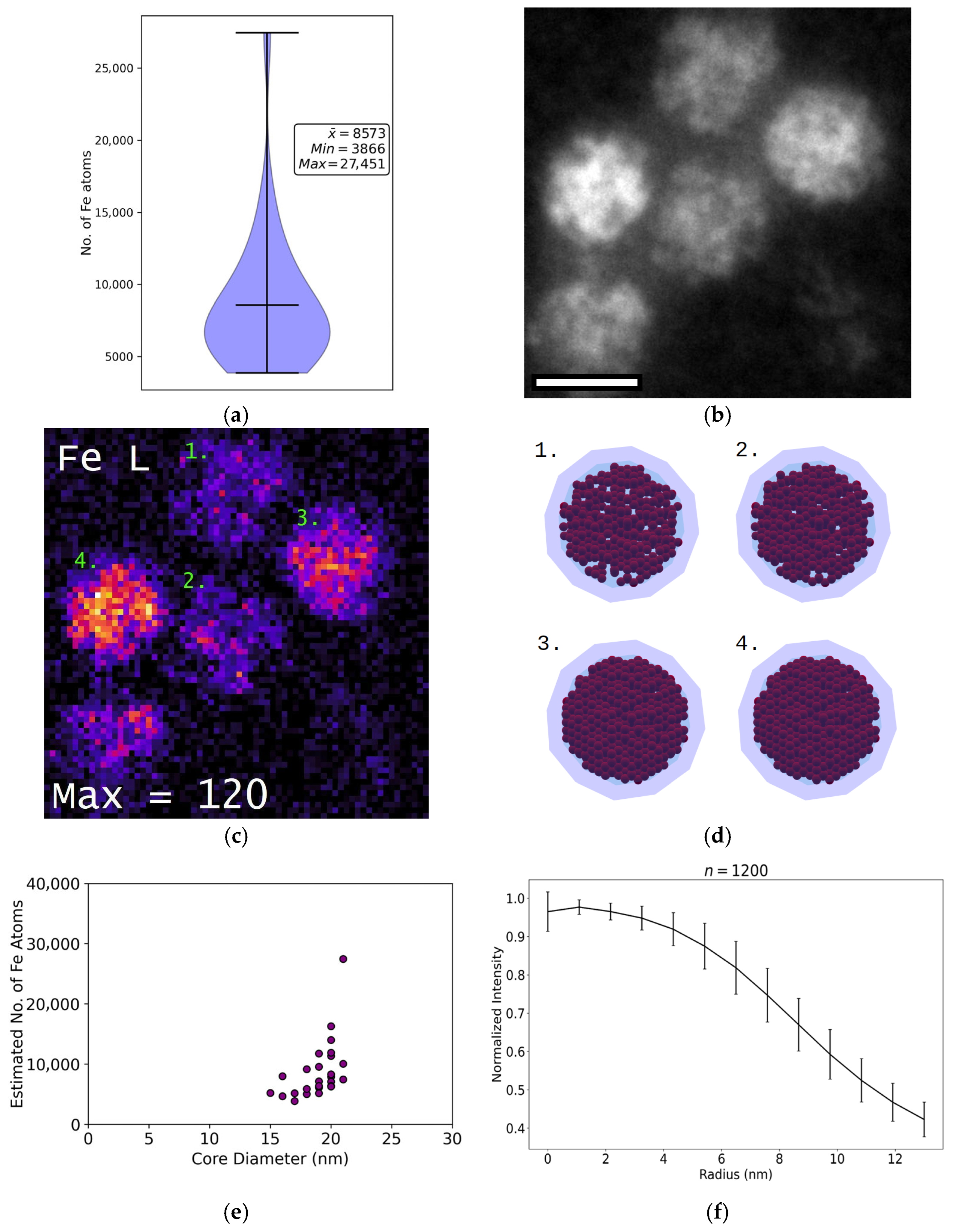
2.7.5. Estimation of the Number of Fe Atoms
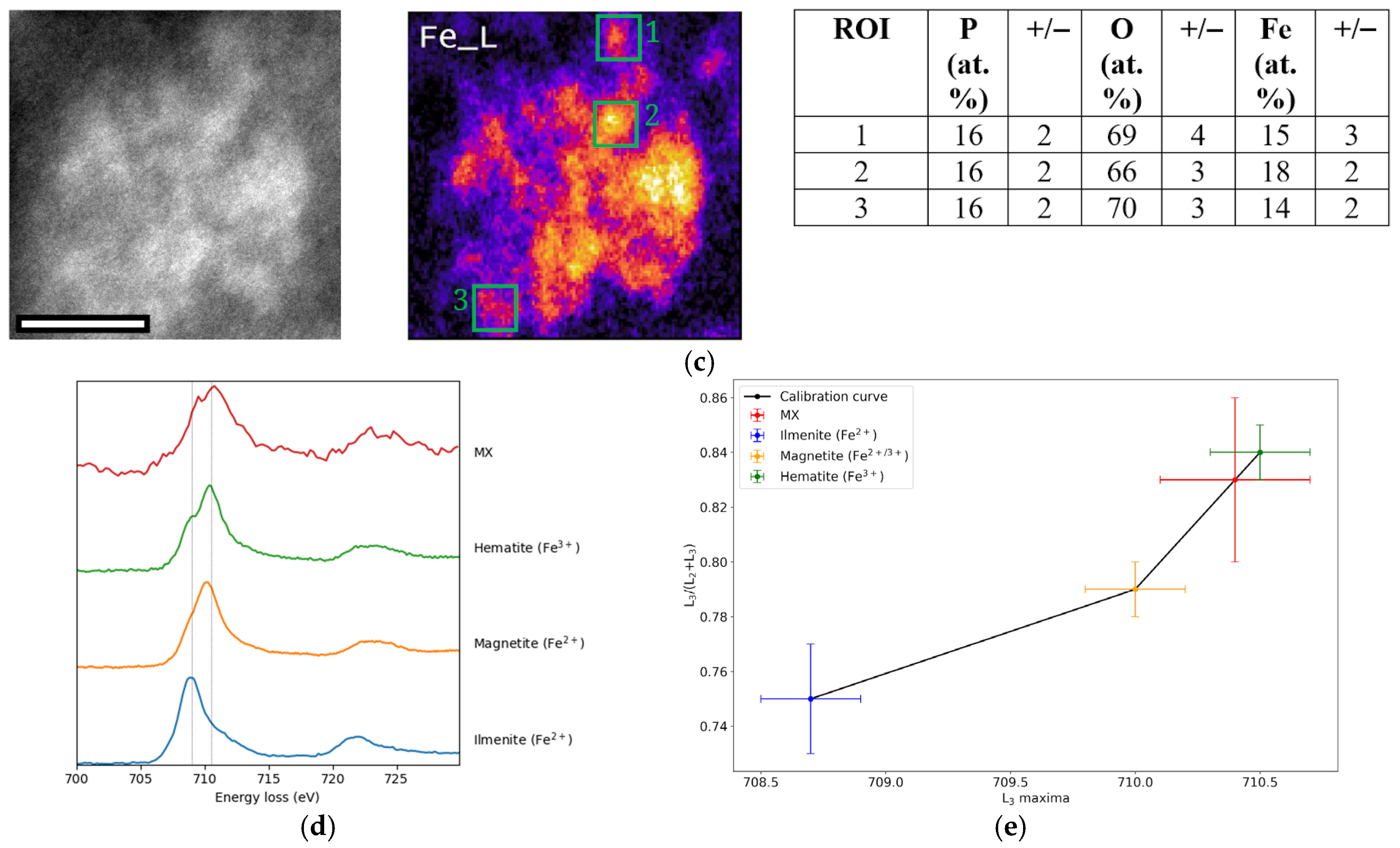
2.7.6. Fe L2,3 Edge Fingerprinting and Quantification
3. Results
3.1. Diameters of Native Encapsulins from M. xanthus
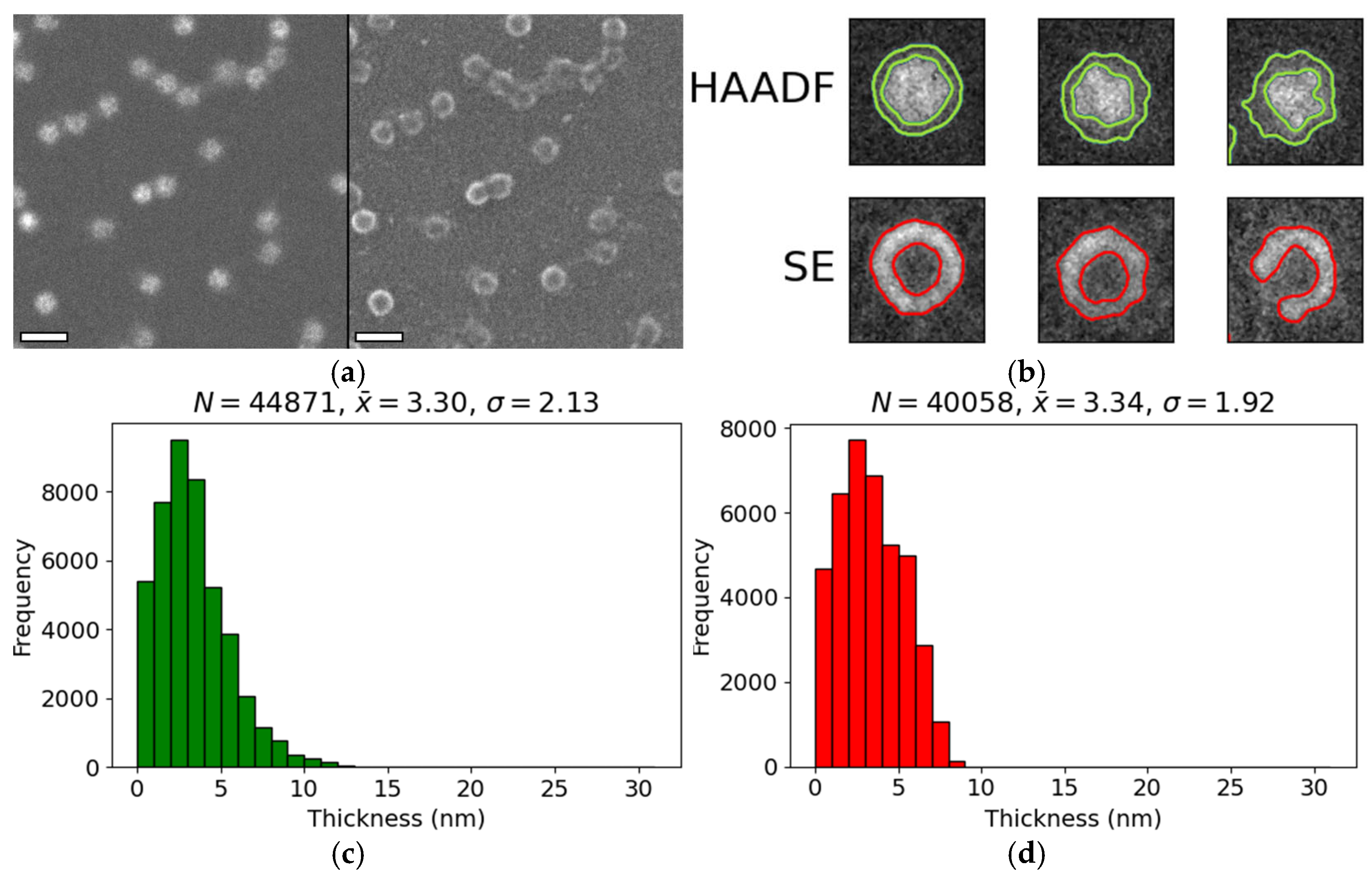
3.2. Encapsulin Shell Thickness
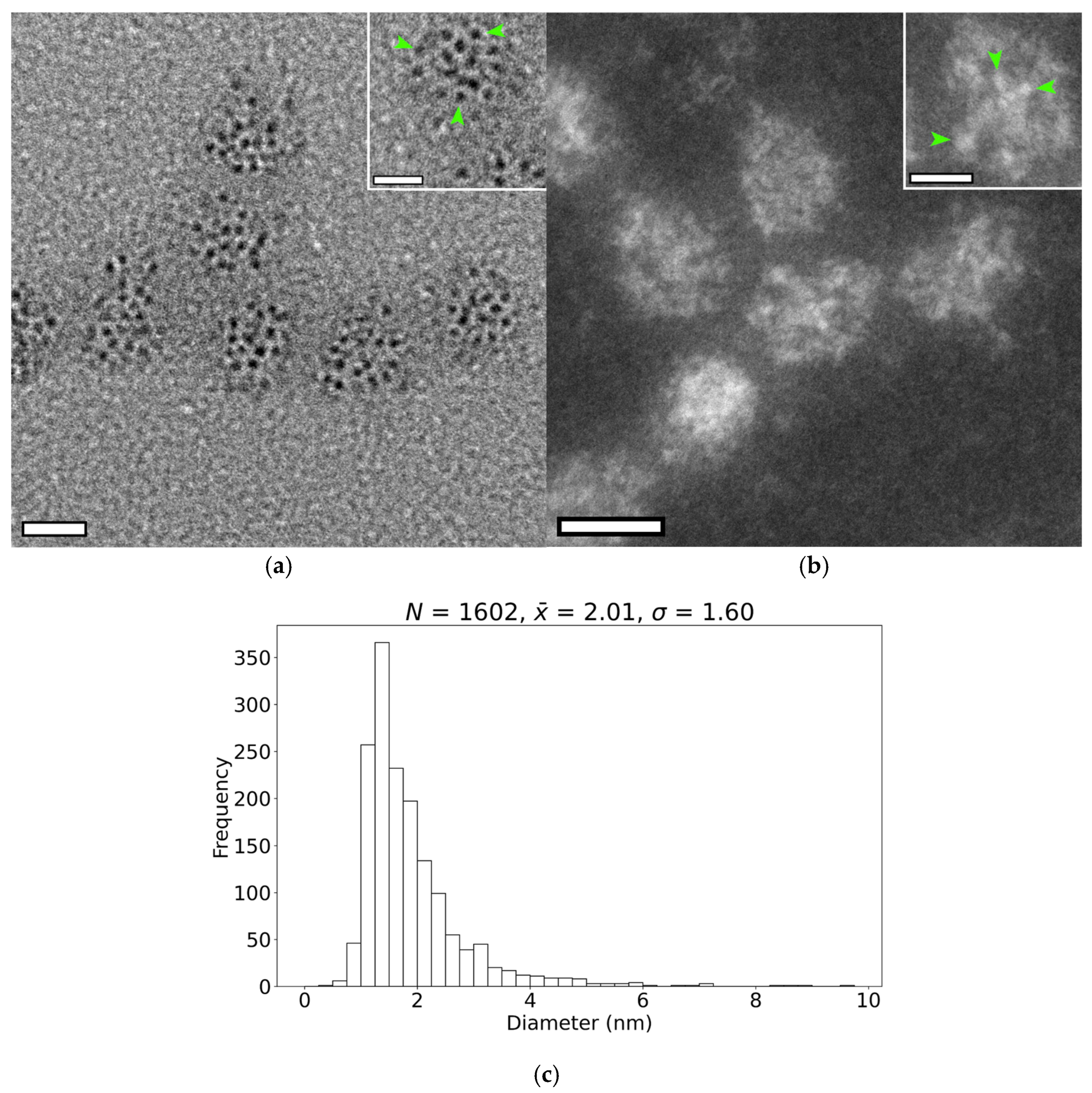
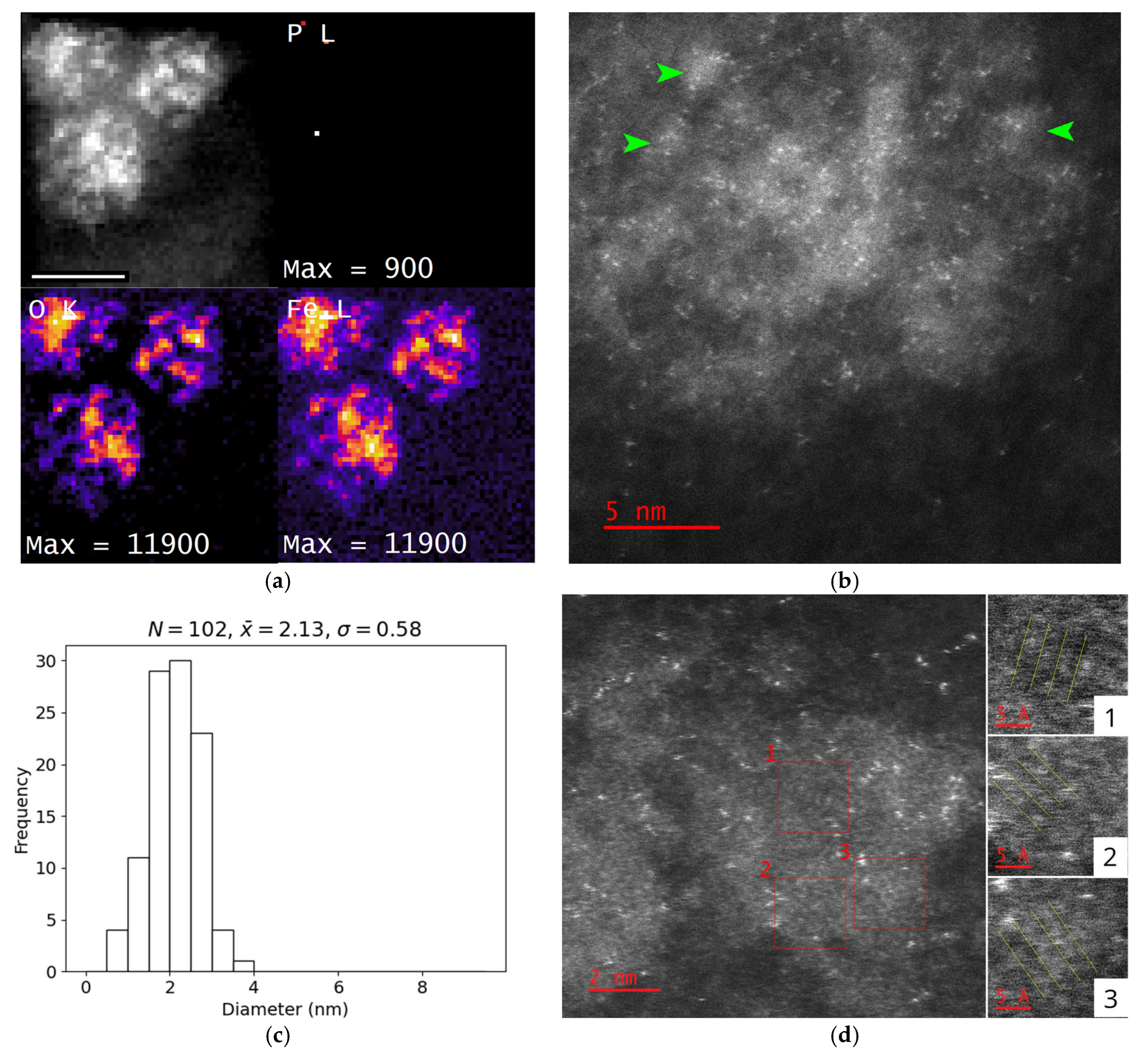
3.3. Size and Shape of the Iron-Containing Nanoparticles
3.4. Crystallinity of the Nanoparticles
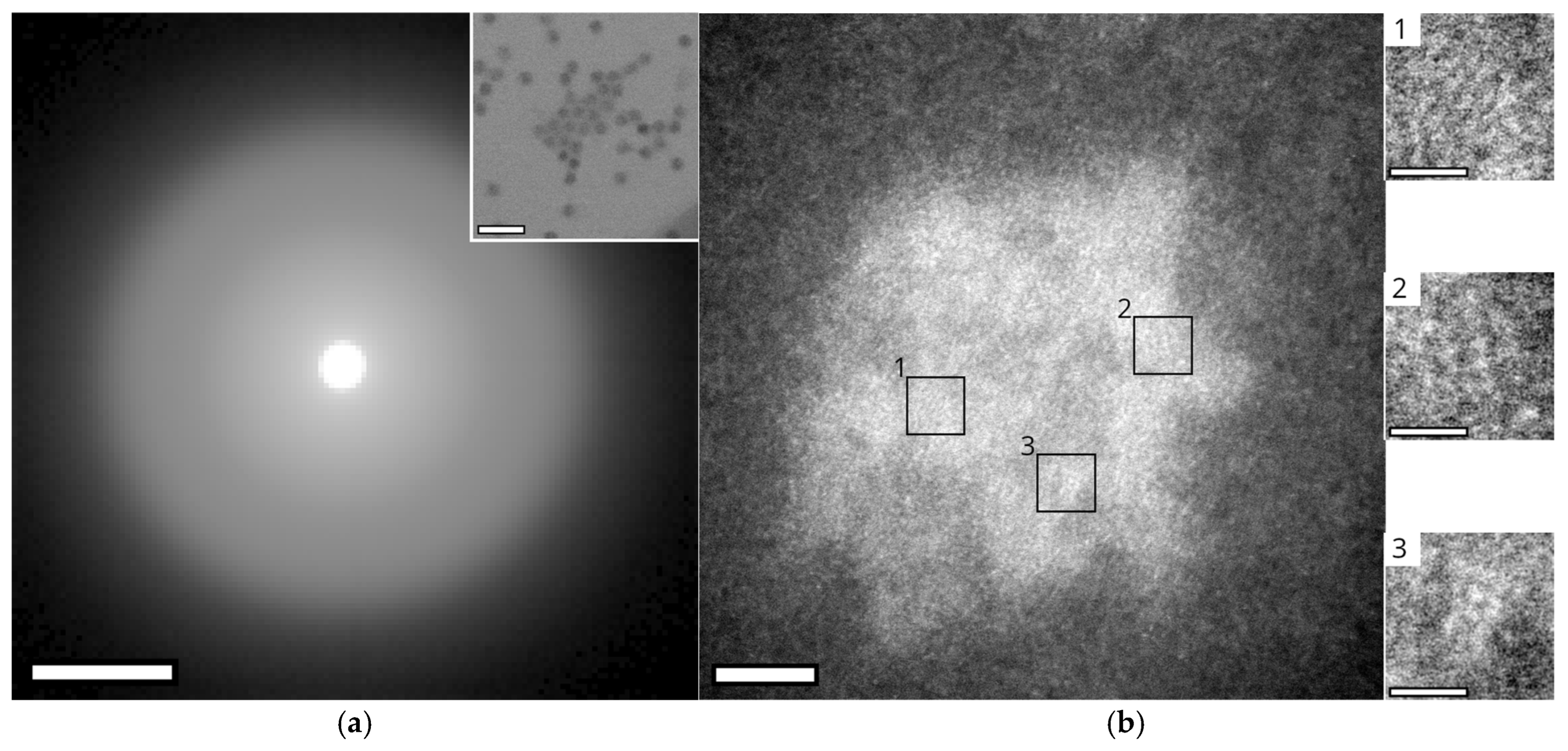
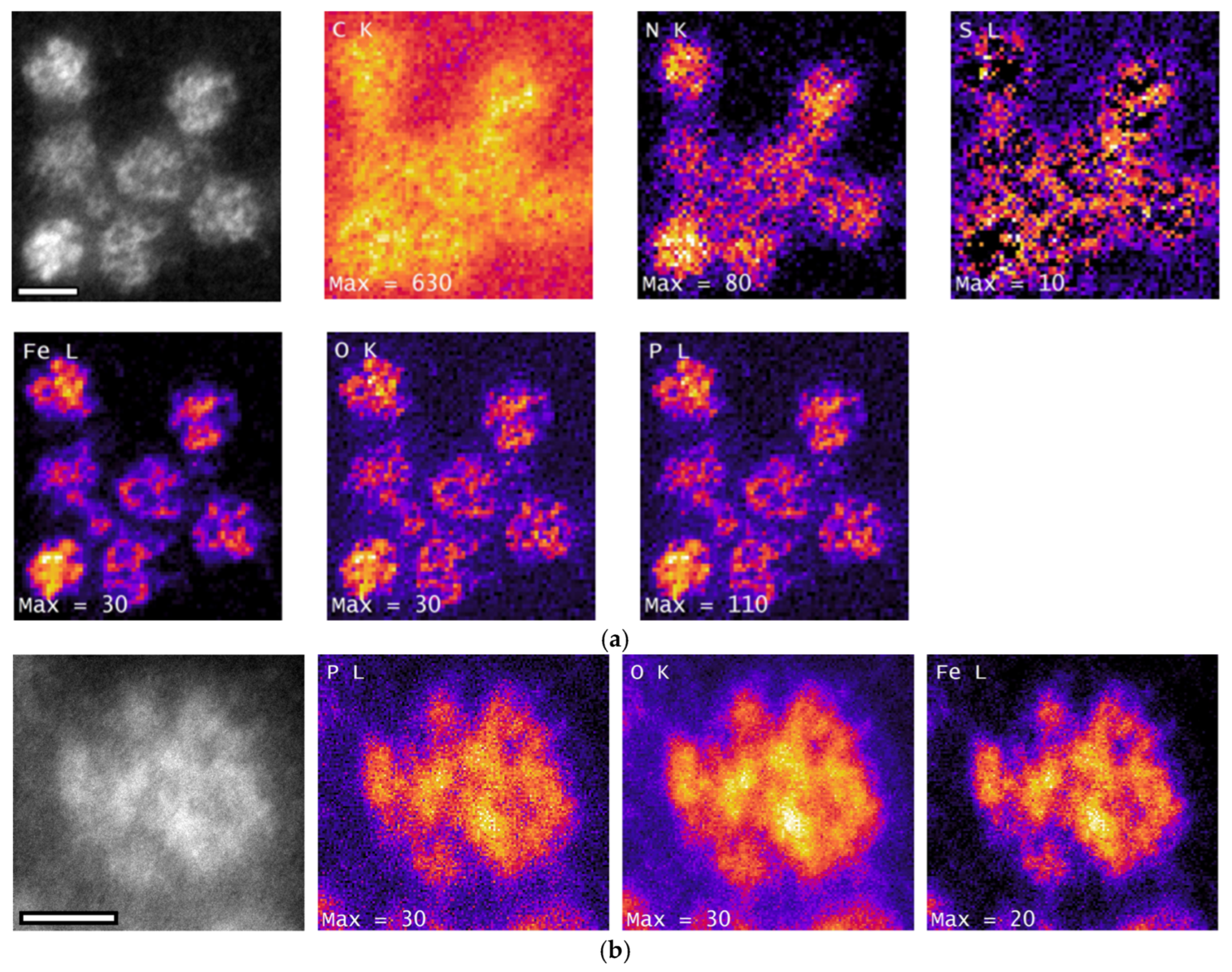
3.5. Chemical Composition of Encapsulin-Derived Materials
3.6. Iron Content
3.7. Phosphorus Content
4. Discussion
4.1. Encapsulin Content
4.2. HAADF and SE Measurements of the Protein Shell
4.3. Nanoparticle Morphology
4.4. Nanoparticle Chemistry
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BF | bright field |
| CTT | casitone tris growth medium |
| dd | double distilled |
| EDXS | energy-dispersive X-ray spectroscopy |
| EELS | electron energy-loss spectroscopy |
| HAADF | high angle annular dark field |
| HR | high resolution |
| M. | Myxococcus |
| RPM | revolutions per minute |
| SE | secondary electron |
| TEM | transmission electron microscopy |
| TPM | tris-phosphate magnesium starvation medium |
| STEM | scanning transmission electron microscopy |
Appendix A
Appendix A.1. Nanoparticle Segmentation
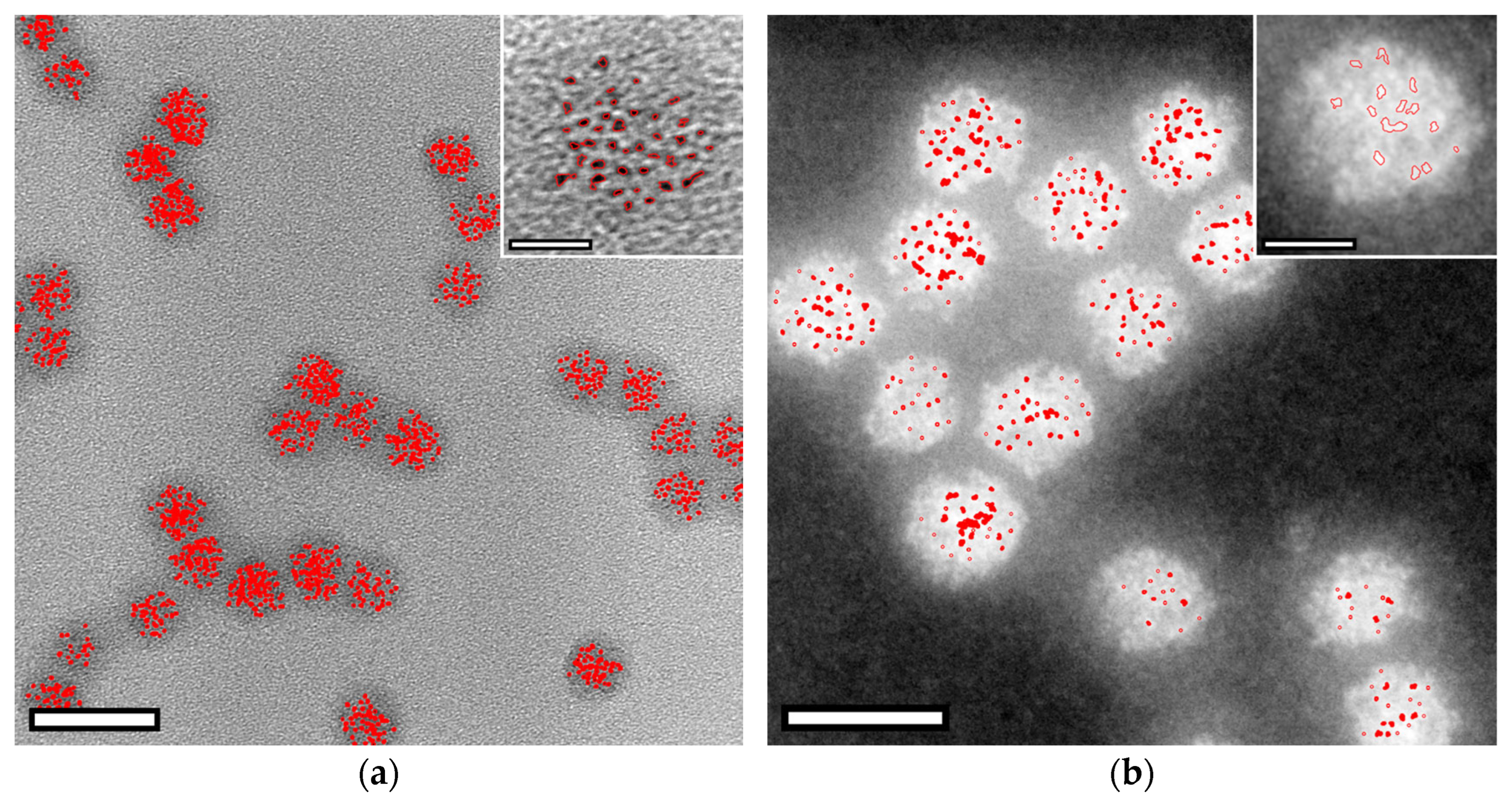
Appendix A.2. Published Iron Oxide Lattice Parameters
| Valence | Chemistry | Name | Space Group | No. | Comment | Lattice Parameters (pm) | Source |
|---|---|---|---|---|---|---|---|
| II | FeO | wuestite | Fm3m | 225 | cubic | a = 430 | [53] |
| II/III | Fe3O4 | magnetite | Fd3m | 227 | cubic, inverse spinel | a = 840 | [54] |
| III | α-Fe2O3 | hematite | R3c | 167 | hexagonal | a = 504, c = 1375 | [55] |
| γ-Fe2O3 | maghemite | Fd3m | 227 | cubic, spinel | a = 833 | [56] | |
| α-FeO(OH) | goethite | Pbnm | 62 | orthorhombic | a = 460, b = 995, c = 302 | [57] | |
| Fe2O3-0.5H2O | 6-line ferrihydrite | P63mc | 189 | hexagonal | a = 296, c = 940 | [58] | |
| α-FePO4 | iron (III) phosphate | P3121 | 152 | trigonal | a = 500, c = 1125 | [59] |
Appendix A.3. Measured Lattice Fringes and Phase Assignments
| d-Spacing (pm) | Error (pm) | Matching Phases and Miller Indices hkl (in Brackets) | Predicted d-Spacing (pm) |
|---|---|---|---|
| ferrihydrite (123) | 163 | ||
| goethite (300) | 153 | ||
| hematite (123) | 155 | ||
| hematite (210) | 165 | ||
| hematite (312) | 160 | ||
| iron (III) phosphate (311) | 160 | ||
| iron (III) phosphate (122) | 155 | ||
| magnetite (333) | 162 | ||
| wuestite (022) | 152 | ||
| 195 | 9 | ferrihydrite (013) | 198 |
| hematite (214) | 203 | ||
| hematite (223) | 197 | ||
| hematite (114) | 203 | ||
| iron (III) phosphate (022) | 199 | ||
| magnetite (024) | 188 | ||
| magnetite (133) | 193 | ||
| maghemite (024) | 186 | ||
| maghemite (133) | 191 | ||
| 215 | 11 | ferrihydrite (112) | 225 |
| goethite (220) | 209 | ||
| goethite (210) | 224 | ||
| hematite (-202) | 208 | ||
| iron (III) phosphate(112) | 225 | ||
| iron (III) phosphate (200) | 214 | ||
| iron (III) phosphate (201) | 210 | ||
| iron (III) phosphate (113) | 205 | ||
| magnetite (004) | 210 | ||
| maghemite (004) | 208 | ||
| wuestite (002) | 215 | ||
| 242 | 12 | ferrihydrite (011) | 248 |
| ferrihydrite (004) | 248 | ||
| goethite (200) | 230 | ||
| goethite (101) | 252 | ||
| goethite (111) | 245 | ||
| hematite (112) | 236 | ||
| hematite (211) | 247 | ||
| hematite (111) | 247 | ||
| hematite (122) | 236 | ||
| iron (III) phosphate (110) | 247 | ||
| iron (III) phosphate (111) | 241 | ||
| iron (III) phosphate (014) | 232 | ||
| magnetite (222) | 242 | ||
| magnetite (113) | 253 | ||
| maghemite (222) | 240 | ||
| maghemite (113) | 251 | ||
| wuestite (111) | 248 |
Appendix A.4. Characterization by Energy-Dispersive X-Ray Spectroscopy (EDXS)
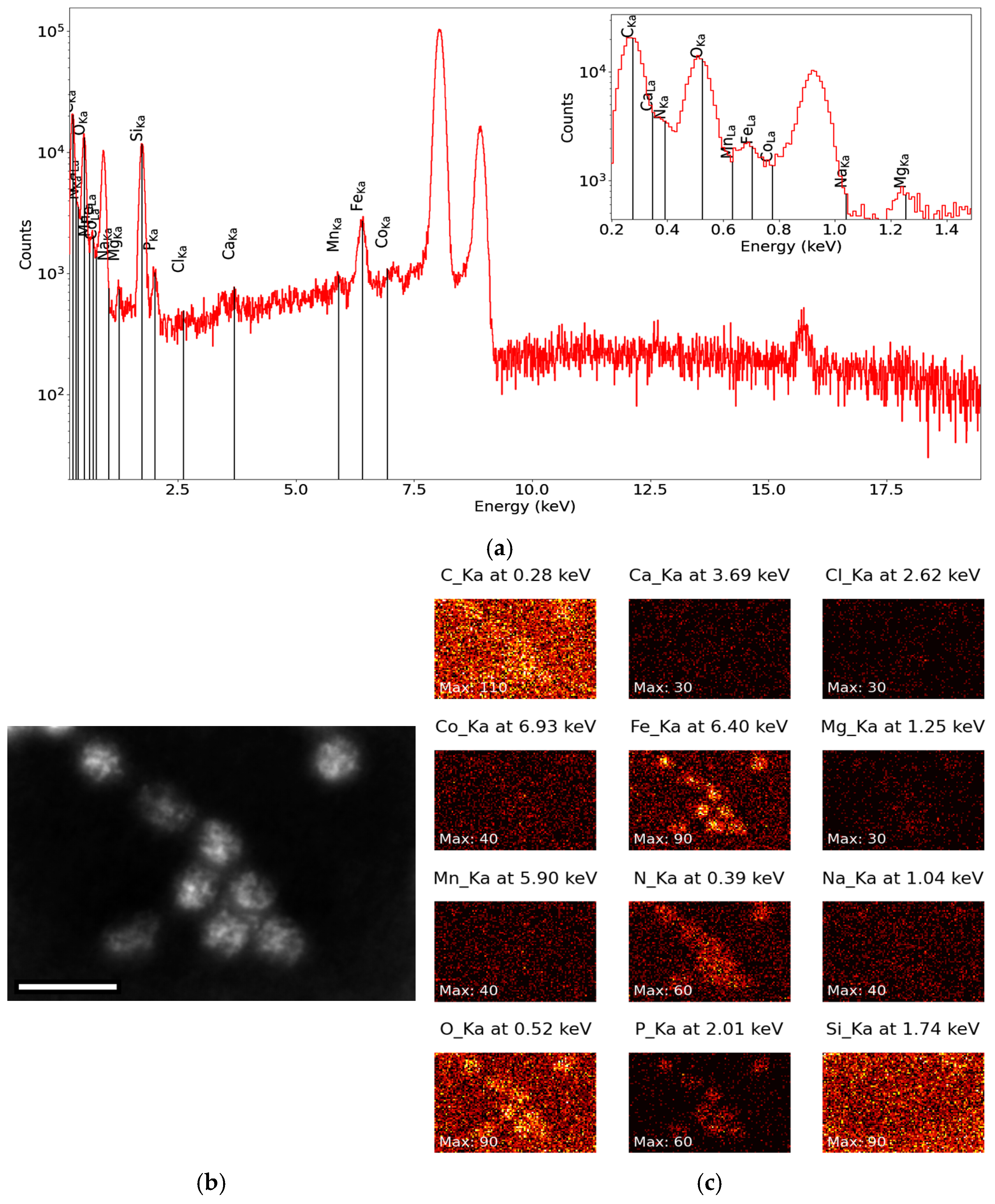
Appendix A.5. Generation and Purification of EncA + EncB + EncC
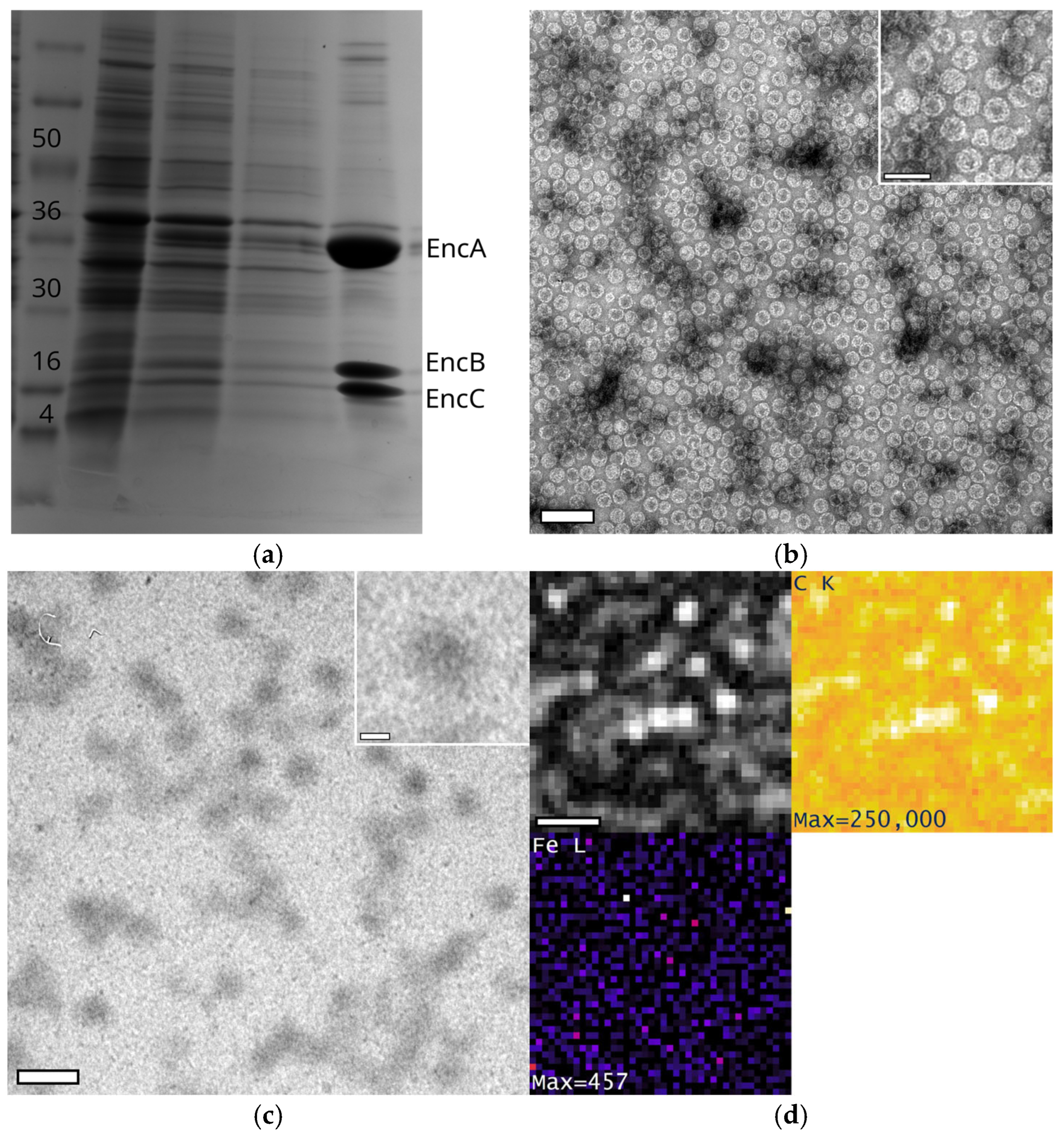
Appendix A.6. Analysis of Bright Spots in HRSTEM Images of EncA + EncB + EncC Loaded with Iron in a Phosphorous-Free Environment
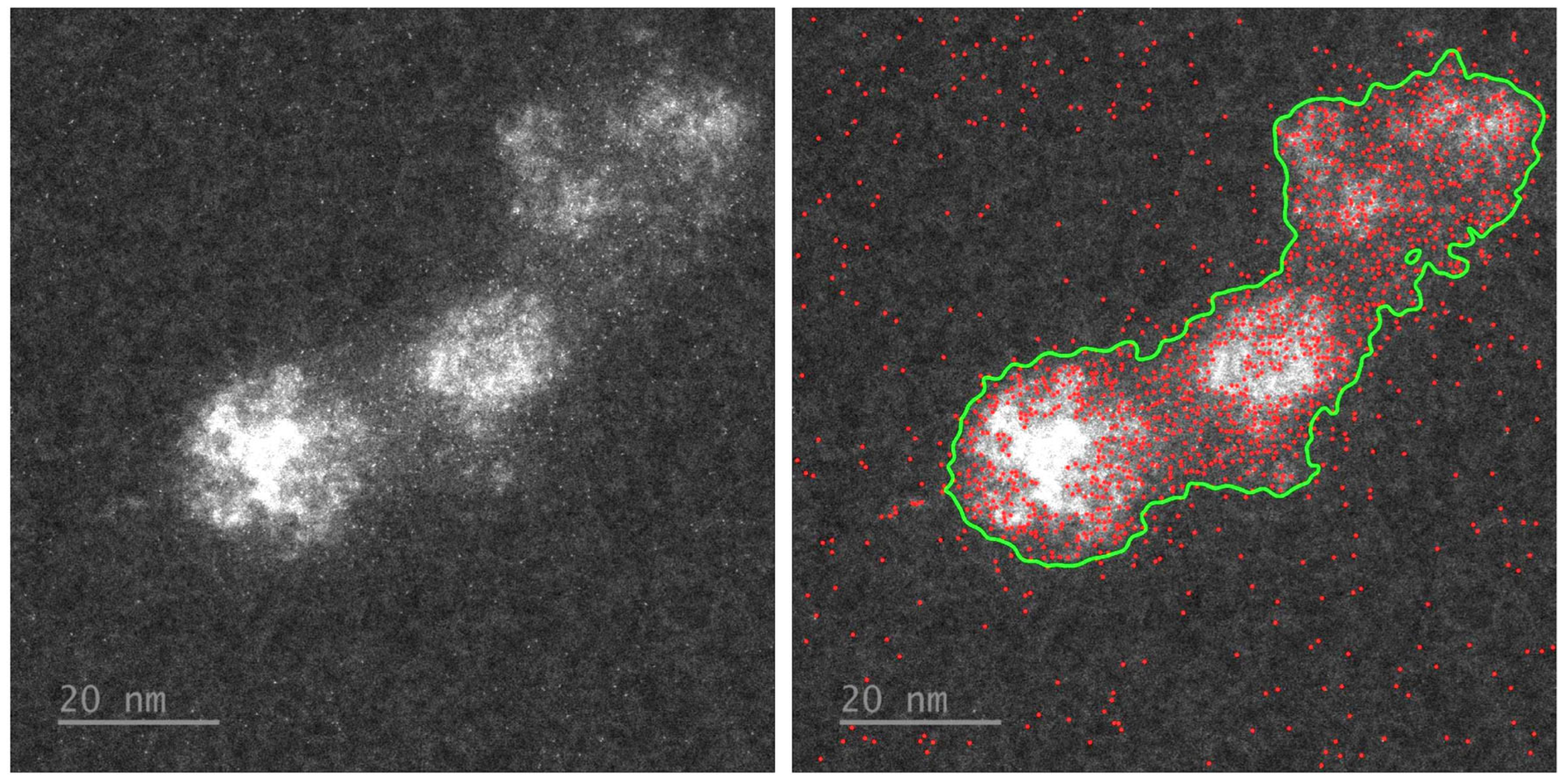
References
- Giessen, T.W.; Silver, P.A. Widespread Distribution of Encapsulin Nanocompartments Reveals Functional Diversity. Nat. Microbiol. 2017, 2, 17029. [Google Scholar] [CrossRef] [PubMed]
- Andreas, M.P.; Giessen, T.W. Large-Scale Computational Discovery and Analysis of Virus-Derived Microbial Nanocompartments. Nat. Commun. 2021, 12, 4748. [Google Scholar] [CrossRef]
- McDowell, H.B.; Hoiczyk, E. Bacterial Nanocompartments: Structures, Functions, and Applications. J. Bacteriol. 2021, 204, e0034621. [Google Scholar] [CrossRef]
- Sutter, M.; Boehringer, D.; Gutmann, S.; Günther, S.; Prangishvili, D.; Loessner, M.J.; Stetter, K.O.; Weber-Ban, E.; Ban, N. Structural Basis of Enzyme Encapsulation into a Bacterial Nanocompartment. Nat. Struct. Mol. Biol. 2008, 15, 939–947. [Google Scholar] [CrossRef] [PubMed]
- McHugh, C.A.; Fontana, J.; Nemecek, D.; Cheng, N.; Aksyuk, A.A.; Heymann, J.B.; Winkler, D.C.; Lam, A.S.; Wall, J.S.; Steven, A.C.; et al. A Virus Capsid-like Nanocompartment That Stores Iron and Protects Bacteria from Oxidative Stress. EMBO J. 2014, 33, 1896–1911. [Google Scholar] [CrossRef]
- Giessen, T.W.; Orlando, B.J.; Verdegaal, A.A.; Chambers, M.G.; Gardener, J.; Bell, D.C.; Birrane, G.; Liao, M.; Silver, P.A. Large Protein Organelles Form a New Iron Sequestration System with High Storage Capacity. eLife 2019, 8, e46070. [Google Scholar] [CrossRef]
- Cassidy-Amstutz, C.; Oltrogge, L.; Going, C.C.; Lee, A.; Teng, P.; Quintanilla, D.; East-Seletsky, A.; Williams, E.R.; Savage, D.F. Identification of a Minimal Peptide Tag for in Vivo and in Vitro Loading of Encapsulin. Biochemistry 2016, 55, 3461–3468. [Google Scholar] [CrossRef]
- Nichols, R.J.; LaFrance, B.; Phillips, N.R.; Radford, D.R.; Oltrogge, L.M.; Valentin-Alvarado, L.E.; Bischoff, A.J.; Nogales, E.; Savage, D.F. Discovery and Characterization of a Novel Family of Prokaryotic Nanocompartments Involved in Sulfur Metabolism. eLife 2021, 10, e59288. [Google Scholar] [CrossRef]
- Tracey, J.C.; Coronado, M.; Giessen, T.W.; Lau, M.C.Y.; Silver, P.A.; Ward, B.B. The Discovery of Twenty-Eight New Encapsulin Sequences, Including Three in Anammox Bacteria. Sci. Rep. 2019, 9, 20122. [Google Scholar] [CrossRef]
- Xing, C.-Y.; Fan, Y.-C.; Chen, X.; Guo, J.-S.; Shen, Y.; Yan, P.; Fang, F.; Chen, Y.-P. A Self-Assembled Nanocompartment in Anammox Bacteria for Resisting Intracelluar Hydroxylamine Stress. Sci. Total Environ. 2020, 717, 137030. [Google Scholar] [CrossRef] [PubMed]
- Rahmanpour, R.; Bugg, T.D.H. Assembly in Vitro of Rhodococcus Jostii RHA1 Encapsulin and Peroxidase DypB to Form a Nanocompartment. FEBS J. 2013, 280, 2097–2104. [Google Scholar] [CrossRef]
- Giessen, T.W. Encapsulins. Annu. Rev. Biochem. 2022, 91, 353–380. [Google Scholar] [CrossRef]
- Eren, E.; Wang, B.; Winkler, D.C.; Watts, N.R.; Steven, A.C.; Wingfield, P.T. Structural Characterization of the Myxococcus Xanthus Encapsulin and Ferritin-like Cargo System Gives Insight into Its Iron Storage Mechanism. Structure 2022, 30, 551–563.e4. [Google Scholar] [CrossRef]
- Eren, E.; Watts, N.R.; Conway, J.F.; Wingfield, P.T. Encapsulin Cargo Protein EncD Is a Flavin-Binding Protein with Ferric Reductase Activity. Proc. Natl. Acad. Sci. USA 2024, 121, e2400426121. [Google Scholar] [CrossRef]
- Zeth, K.; Hoiczyk, E.; Okuda, M. Ferroxidase-Mediated Iron Oxide Biomineralization: Novel Pathways to Multifunctional Nanoparticles. Trends Biochem. Sci. 2016, 41, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shimon, S.; Stein, D.; Zarivach, R. Current View of Iron Biomineralization in Magnetotactic Bacteria. J. Struct. Biol. X 2021, 5, 100052. [Google Scholar] [CrossRef] [PubMed]
- Keim, C.N.; Martins, J.L.; Abreu, F.; Rosado, A.S.; de Barros, H.L.; Borojevic, R.; Lins, U.; Farina, M. Multicellular Life Cycle of Magnetotactic Prokaryotes. FEMS Microbiol. Lett. 2004, 240, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.D.; Schüler, D.; Pfeiffer, D. A Compass To Boost Navigation: Cell Biology of Bacterial Magnetotaxis. J. Bacteriol. 2020, 202, 10–1128. [Google Scholar] [CrossRef]
- Efremova, M.V.; Wiedwald, U.; Sigmund, F.; Bodea, S.-V.; Ohldag, H.; Feggeler, T.; Meckenstock, R.; Panzl, L.N.; Francke, J.; Beer, I.; et al. Genetically Controlled Iron Oxide Biomineralization in Encapsulin Nanocompartments for Magnetic Manipulation of a Mammalian Cell Line. Adv. Funct. Mater. 2025, 35, 2418013. [Google Scholar] [CrossRef]
- Sigmund, F.; Massner, C.; Erdmann, P.; Stelzl, A.; Rolbieski, H.; Desai, M.; Bricault, S.; Wörner, T.P.; Snijder, J.; Geerlof, A.; et al. Bacterial Encapsulins as Orthogonal Compartments for Mammalian Cell Engineering. Nat. Commun. 2018, 9, 1990. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X.; Chu, C.; Zhou, Z.; Chen, B.; Pang, X.; Lin, G.; Lin, H.; Guo, Y.; Ren, E.; et al. Genetically Engineered Magnetic Nanocages for Cancer Magneto-Catalytic Theranostics. Nat. Commun. 2020, 11, 5421. [Google Scholar] [CrossRef]
- Kaiser, D. Social Gliding Is Correlated with the Presence of Pili in Myxococcus Xanthus. Proc. Natl. Acad. Sci. USA 1979, 76, 5952–5956. [Google Scholar] [CrossRef]
- Hodgkin, J.; Kaiser, D. Genetics of Gliding Motility in Myxococcus Xanthus (Myxobacterales): Two Gene Systems Control Movement. Mol. Gen. Genet. 1979, 171, 177–191. [Google Scholar] [CrossRef]
- van der Walt, S.; Schönberger, J.L.; Nunez-Iglesias, J.; Boulogne, F.; Warner, J.D.; Yager, N.; Gouillart, E.; Yu, T. Scikit-Image: Image Processing in Python. PeerJ 2014, 2, e453. [Google Scholar] [CrossRef]
- Bradski, G. The OpenCV Library. Dr. Dobb’s J. Softw. Tools Prof. Program. 2000, 25, 120–123. [Google Scholar]
- Pan, Y.-H.; Brown, A.; Sader, K.; Brydson, R.; Gass, M.; Bleloch, A. Quantification of Absolute Iron Content in Mineral Cores of Cytosolic Ferritin Molecules in Human Liver. Mater. Sci. Technol. 2008, 24, 689–694. [Google Scholar] [CrossRef]
- Pan, Y.-H.; Sader, K.; Powell, J.J.; Bleloch, A.; Gass, M.; Trinick, J.; Warley, A.; Li, A.; Brydson, R.; Brown, A. 3D Morphology of the Human Hepatic Ferritin Mineral Core: New Evidence for a Subunit Structure Revealed by Single Particle Analysis of HAADF-STEM Images. J. Struct. Biol. 2009, 166, 22–31. [Google Scholar] [CrossRef]
- van Aken, P.A.; Liebscher, B.; Styrsa, V.J. Quantitative Determination of Iron Oxidation States in Minerals Using Fe L 2,3-Edge Electron Energy-Loss near-Edge Structure Spectroscopy. Phys. Chem. Miner. 1998, 25, 323–327. [Google Scholar] [CrossRef]
- van Aken, P.A.; Liebscher, B. Quantification of Ferrous/ferric Ratios in Minerals: New Evaluation Schemes of Fe L 23 Electron Energy-Loss near-Edge Spectra. Phys. Chem. Miner. 2002, 29, 188–200. [Google Scholar] [CrossRef]
- Kwon, S.; Andreas, M.P.; Giessen, T.W. Pore Engineering as a General Strategy to Improve Protein-Based Enzyme Nanoreactor Performance. ACS Nano 2024, 18, 25740–25753. [Google Scholar] [CrossRef] [PubMed]
- Walther, T. The Limits of Lattice Imaging Compared to Annular Dark-Field Imaging for Determining Size Distributions of Nano-Particles. In Proceedings of the EMC2004, Antwerp, Belgium, 27 August 2004; Volume 1, pp. 119–120. [Google Scholar]
- Quintana, C.; Lancin, M.; Marhic, C.; Pérez, M.; Martin-Benito, J.; Avila, J.; Carrascosa, J.L. Initial Studies with High Resolution TEM and Electron Energy Loss Spectroscopy Studies of Ferritin Cores Extracted from Brains of Patients with Progressive Supranuclear Palsy and Alzheimer Disease. Cell. Mol. Biol. 2000, 46, 807–820. [Google Scholar]
- Quintana, C.; Cowley, J.M.; Marhic, C. Electron Nanodiffraction and High-Resolution Electron Microscopy Studies of the Structure and Composition of Physiological and Pathological Ferritin. J. Struct. Biol. 2004, 147, 166–178. [Google Scholar] [CrossRef]
- Quintana, C.; Bellefqih, S.; Laval, J.Y.; Guerquin-Kern, J.L.; Wu, T.D.; Avila, J.; Ferrer, I.; Arranz, R.; Patiño, C. Study of the Localization of Iron, Ferritin, and Hemosiderin in Alzheimer’s Disease Hippocampus by Analytical Microscopy at the Subcellular Level. J. Struct. Biol. 2006, 153, 42–54. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. 637. The Stability of Transition-Metal Complexes. J. Chem. Soc. 1953, 637, 3192–3210. [Google Scholar] [CrossRef]
- YPan, A.; Brown, R.; Brydson, A.; Warley, A.; Li, J. Powell. Electron Beam Damage Studies of Synthetic 6-Line Ferrihydrite and Ferritin Molecule Cores within a Human Liver Biopsy. Micron 2006, 37, 403–411. [Google Scholar]
- Walther, T. A New Experimental Procedure to Quantify Annular Dark Field Images in Scanning Transmission Electron Microscopy. J. Microsc. 2006, 221 Pt 2, 137–144. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides. Acta Crystallogr. Sect. A Cryst. Phys. Diffr. Theor. Gen. Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- He, D.; Hughes, S.; Vanden-Hehir, S.; Georgiev, A.; Altenbach, K.; Tarrant, E.; Mackay, C.L.; Waldron, K.J.; Clarke, D.J.; Marles-Wright, J. Structural Characterization of Encapsulated Ferritin Provides Insight into Iron Storage in Bacterial Nanocompartments. eLife 2016, 5, e18972. [Google Scholar] [CrossRef]
- Massover, W.H. Radiation Damage to Protein Specimens from Electron Beam Imaging and Diffraction: A Mini-Review of Anti-Damage Approaches, with Special Reference to Synchrotron X-Ray Crystallography. J. Synchrotron Radiat. 2007, 14 Pt 1, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Sohlberg, K.; Pennycook, T.J.; Zhou, W.; Pennycook, S.J. Insights into the Physical Chemistry of Materials from Advances in HAADF-STEM. Phys. Chem. 2015, 17, 3982–4006. [Google Scholar] [CrossRef]
- Watt, G.D.; Frankel, R.B.; Jacobs, D.; Huang, H.; Papaefthymiou, G.C. Fe2+ and Phosphate Interactions in Bacterial Ferritin from Azotobacter Vinelandii. Biochemistry 1992, 31, 5672–5679. [Google Scholar] [CrossRef]
- Watt, R.K.; Hilton, R.J.; Graff, D.M. Oxido-Reduction Is Not the Only Mechanism Allowing Ions to Traverse the Ferritin Protein Shell. Biochim. Biophys. Acta 2010, 1800, 745–759. [Google Scholar] [CrossRef]
- Gopal, N.; Jose, A.H.C.; Fermin, H.A.; Bianca, M.G.V.; Alexandre, M.; Flavio, G.; Diego, M.; Giorgio, Z.; Jose, M.V.; Surender, K.S. Tuning the Shape, Size, Phase Composition and Stoichiometry of Iron Oxide Nanoparticles: The Role of Phosphate Anions. J. Alloys Compd. 2021, 856, 156940. [Google Scholar]
- Reutovich, A.A.; Srivastava, A.K.; Smith, G.L.; Foucher, A.; Yates, D.M.; Stach, E.A.; Papaefthymiou, G.C.; Arosio, P.; Bou-Abdallah, F. Effect of Phosphate and Ferritin Subunit Composition on the Kinetics, Structure, and Reactivity of the Iron Core in Human Homo- and Heteropolymer Ferritins. Biochemistry 2022, 61, 2106–2117. [Google Scholar] [CrossRef]
- Rohrer, J.S.; Islam, Q.T.; Watt, G.D.; Sayers, D.E.; Theil, E.C. Iron Environment in Ferritin with Large Amounts of Phosphate, from Azotobacter Vinelandii and Horse Spleen, Analyzed Using Extended X-Ray Absorption Fine Structure (EXAFS). Biochemistry 1990, 29, 259–264. [Google Scholar] [CrossRef]
- Doig, P.; Austin, J.W.; Trust, T.J. The Helicobacter Pylori 19.6-Kilodalton Protein Is an Iron-Containing Protein Resembling Ferritin. J. Bacteriol. 1993, 175, 557–560. [Google Scholar] [CrossRef][Green Version]
- Aitken-Rogers, H.; Singleton, C.; Lewin, A.; Taylor-Gee, A.; Moore, G.R.; Le Brun, N.E. Effect of Phosphate on Bacterioferritin-Catalysed iron(II) Oxidation. J. Biol. Inorg. Chem. 2004, 9, 161–170. [Google Scholar] [CrossRef]
- García-Prieto, A.; Alonso, J.; Muñoz, D.; Marcano, L.; Abad Díaz de Cerio, A.; Fernández de Luis, R.; Orue, I.; Mathon, O.; Muela, A.; Fdez-Gubieda, M.L. On the Mineral Core of Ferritin-like Proteins: Structural and Magnetic Characterization. Nanoscale 2016, 8, 1088–1099. [Google Scholar] [CrossRef]
- Mann, S.; Bannister, J.V.; Williams, R.J. Structure and Composition of Ferritin Cores Isolated from Human Spleen, Limpet (Patella Vulgata) Hemolymph and Bacterial (Pseudomonas Aeruginosa) Cells. J. Mol. Biol. 1986, 188, 225–232. [Google Scholar] [CrossRef]
- Treffry, A.; Harrison, P.M.; Cleton, M.I.; de Bruijn, W.C.; Mann, S. A Note on the Composition and Properties of Ferritin Iron Cores. J. Inorg. Biochem. 1987, 31, 1–6. [Google Scholar] [CrossRef]
- Wade, V.J.; Treffry, A.; Laulhère, J.P.; Bauminger, E.R.; Cleton, M.I.; Mann, S.; Briat, J.F.; Harrison, P.M. Structure and Composition of Ferritin Cores from Pea Seed (Pisum Sativum). Biochim. Biophys. Acta 1993, 1161, 91–96. [Google Scholar] [CrossRef] [PubMed]
- WyckofF, R.W.G.; Crittenden, E.D., VI. Herstellung Und Kristallstruktur von Ferrooxyd (FeO). Z. Kristallogr. Cryst. Mater. 1926, 63, 144–147. [Google Scholar] [CrossRef]
- Claassen, A.A. The Scattering Power of Oxygen and Iron for X-Rays. Proc. Phys. Soc. Lond. 1925, 38, 482–487. [Google Scholar] [CrossRef]
- Blake, R.L.; Zoltai, T.; Hessevick, R.E.; Finger, L.W. Refinement of Hematite Crystal Structure. In Report of Investigations of the Bureau of Mines; US Department of the Interior: Washington, DC, USA, 1970; pp. 1–20. [Google Scholar]
- Pecharromán, C.; González-Carreño, T.; Iglesias, J.E. The Infrared Dielectric Properties of Maghemite, γ-Fe2O3, from Reflectance Measurement on Pressed Powders. Phys. Chem. Miner. 1995, 22, 21–29. [Google Scholar] [CrossRef]
- Hazemann, J.-L.; Bérar, J.F.; Manceau, A. Rietveld Studies of the Aluminium-Iron Substitution in Synthetic Goethite. Mater. Sci. Forum 1991, 79–82, 821–826. [Google Scholar] [CrossRef]
- Drits, V.A.; Sakharov, B.A.; Salyn, A.L.; Manceau, A. Structural Model for Ferrihydrite. Clay Miner. 1993, 28, 185–207. [Google Scholar] [CrossRef]
- Long, G.J.; Cheetham, A.K.; Battle, P.D. Study of the Iron-Phosphorus-Oxygen System by Moessbauer Effect, Neutron Diffraction, Magnetic Susceptibility, and Analytical Electron Microscopy: Some Pitfalls and Solutions in the Analysis of a Complex Mixture. Inorg. Chem. 1983, 22, 3012–3016. [Google Scholar] [CrossRef]
| Element | at. % | +/− (Std. Dev.) |
|---|---|---|
| Fe | 15 | 2 |
| O | 75 | 4 |
| P | 12 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDowell, H.B.; Hoiczyk, E.; Walther, T. Characterization of Iron Oxide Nanoparticles Inside the Myxococcus xanthus Encapsulin. Nanomaterials 2025, 15, 1793. https://doi.org/10.3390/nano15231793
McDowell HB, Hoiczyk E, Walther T. Characterization of Iron Oxide Nanoparticles Inside the Myxococcus xanthus Encapsulin. Nanomaterials. 2025; 15(23):1793. https://doi.org/10.3390/nano15231793
Chicago/Turabian StyleMcDowell, Harry B., Egbert Hoiczyk, and Thomas Walther. 2025. "Characterization of Iron Oxide Nanoparticles Inside the Myxococcus xanthus Encapsulin" Nanomaterials 15, no. 23: 1793. https://doi.org/10.3390/nano15231793
APA StyleMcDowell, H. B., Hoiczyk, E., & Walther, T. (2025). Characterization of Iron Oxide Nanoparticles Inside the Myxococcus xanthus Encapsulin. Nanomaterials, 15(23), 1793. https://doi.org/10.3390/nano15231793








