Engineering Mesoporous Silica Hosts for Ultrasmall ZnO Nanoparticles: A Dendritic Polymer-Assisted Strategy Towards Sustainable, Safe, and Effective Antibacterial Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Mesoporous Silica Hosts
2.3. Synthesis of ZnO/SiO2 Nanocomposites
2.4. Materials Characterization
2.5. Evaluation of Antibacterial Activity
2.6. Evaluation of In Vitro Cytotoxicity
2.7. LCA of the ZnO/SiO2 Nanocomposites
3. Results and Discussion
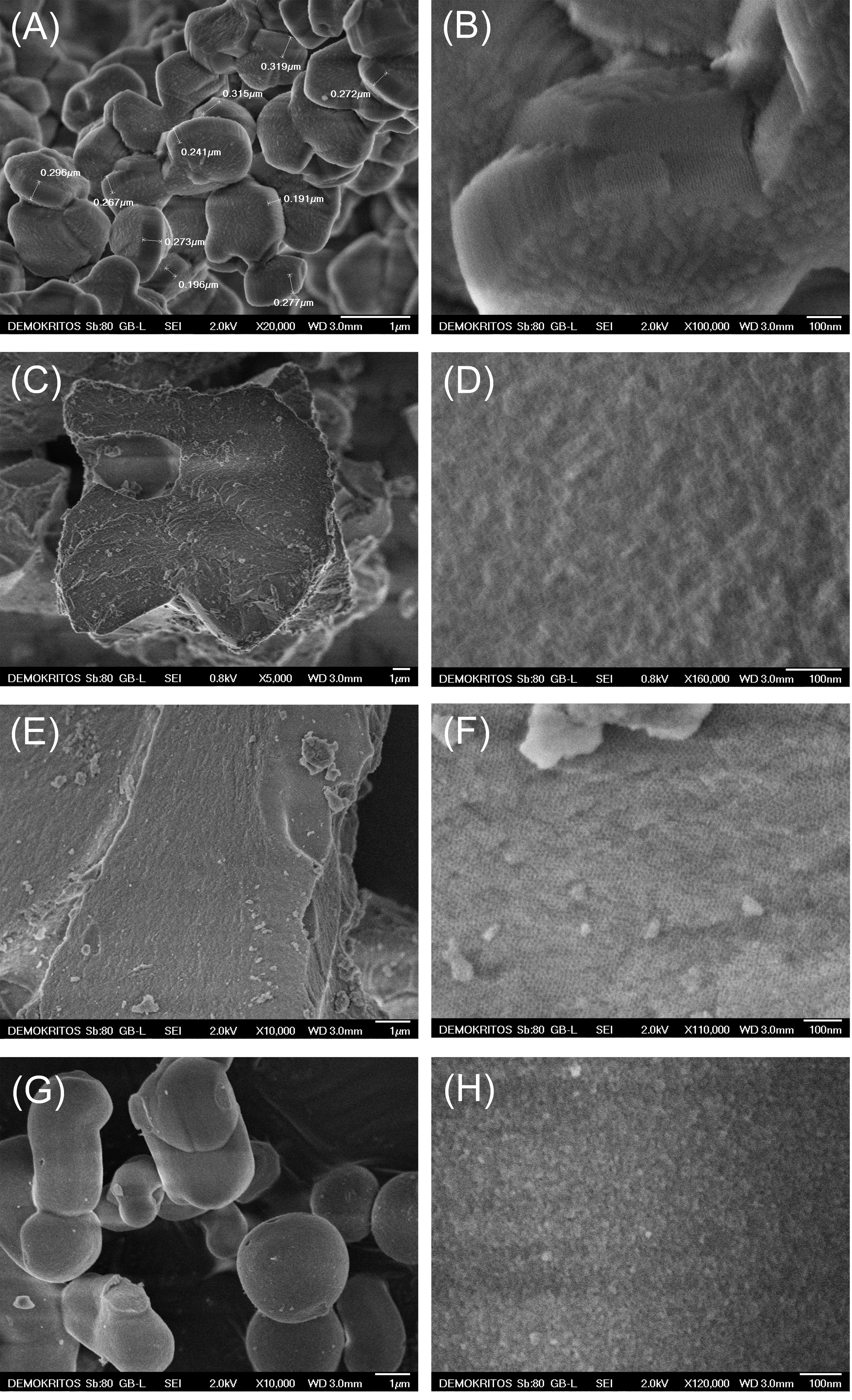
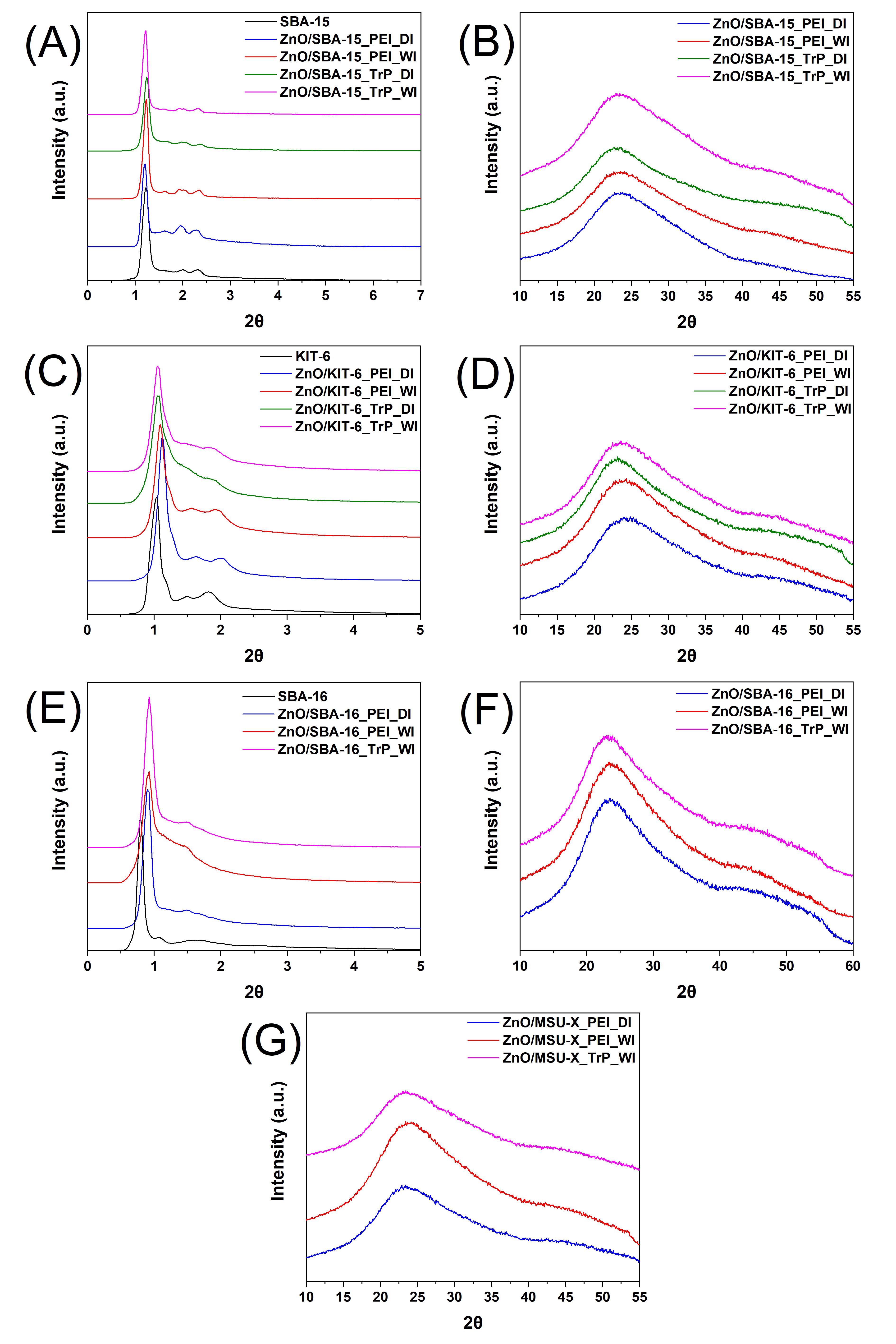
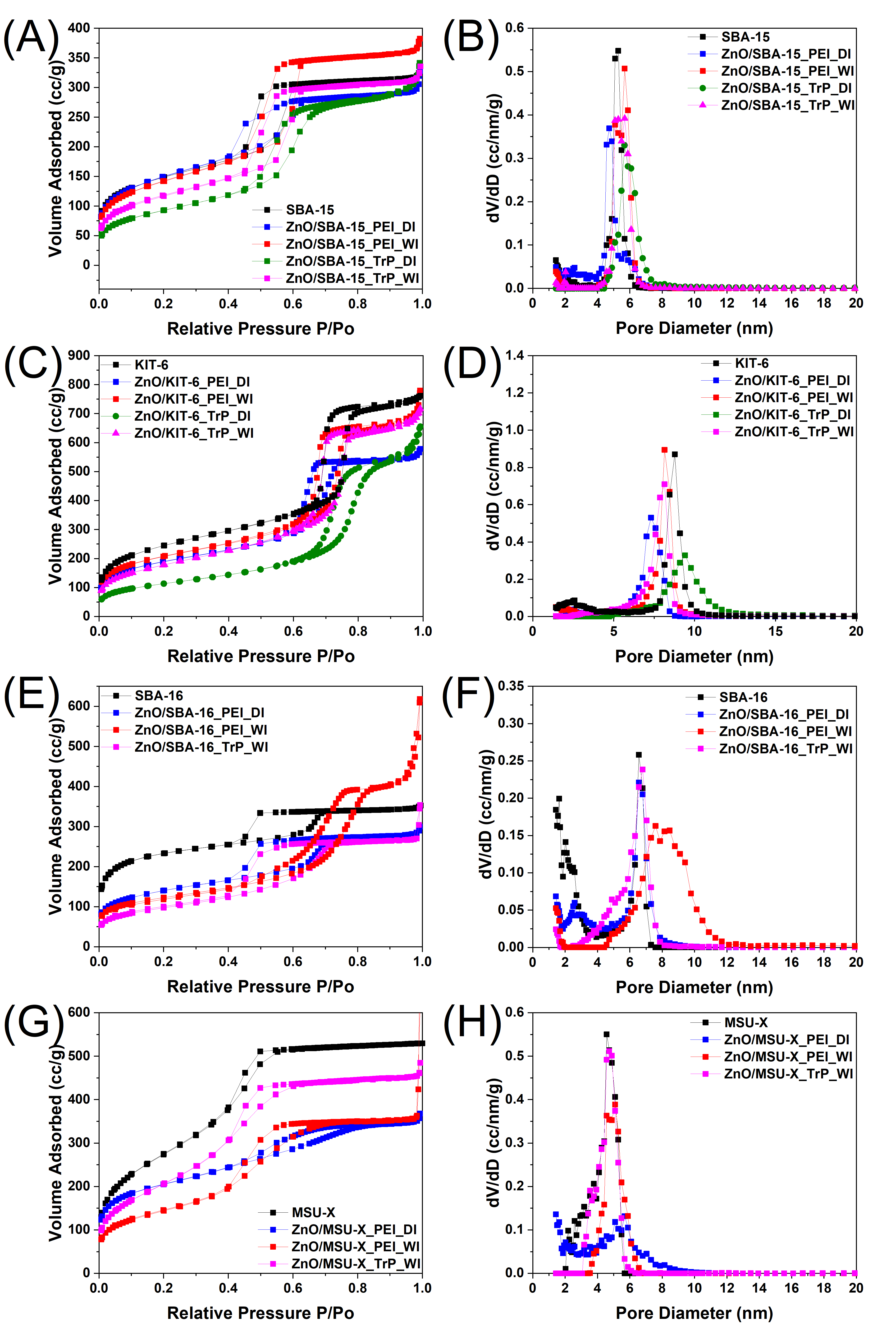
3.1. Characterization of the Pristine Mesoporous Silicas
3.2. Characterization of Organically Modified Mesoporous Silicas
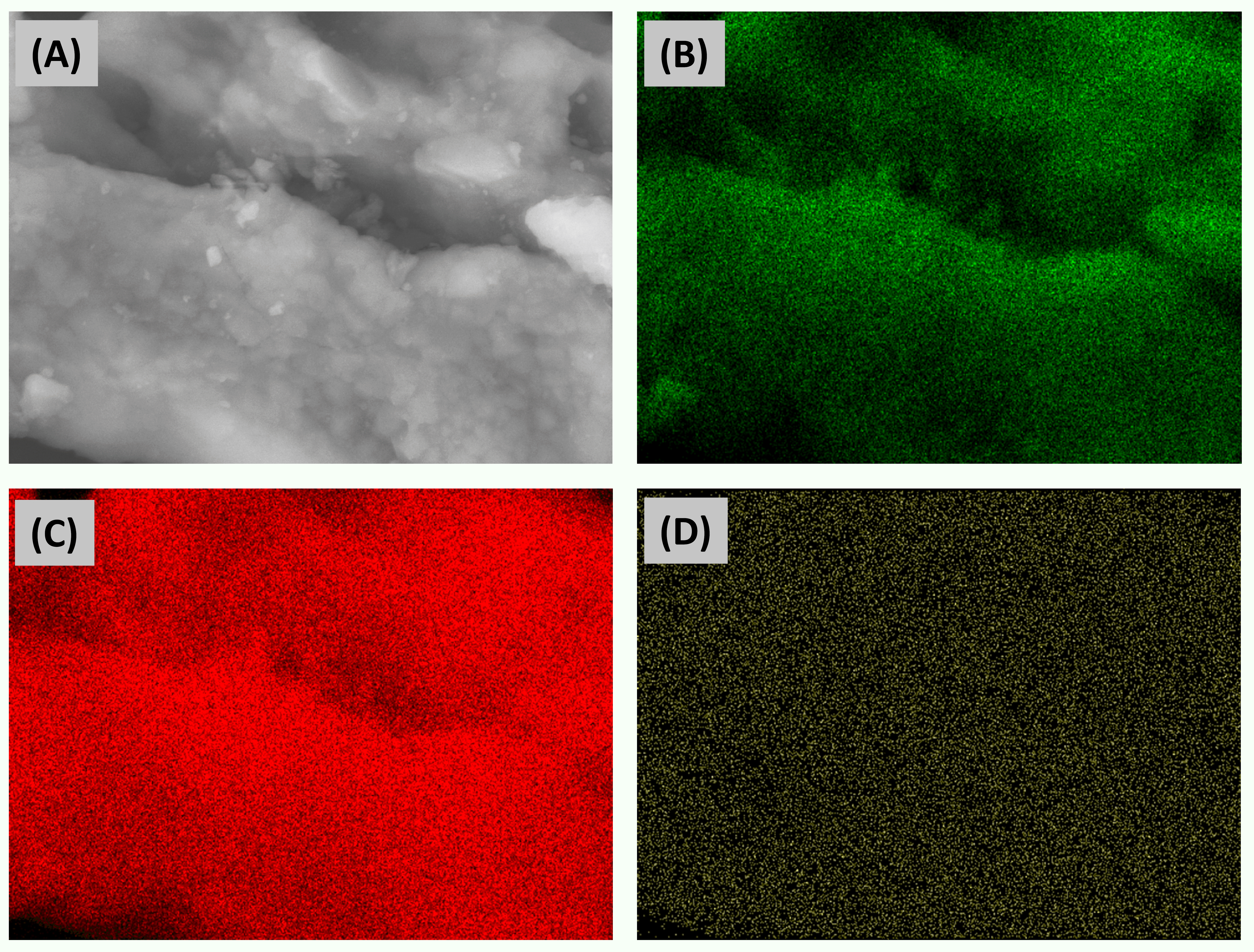
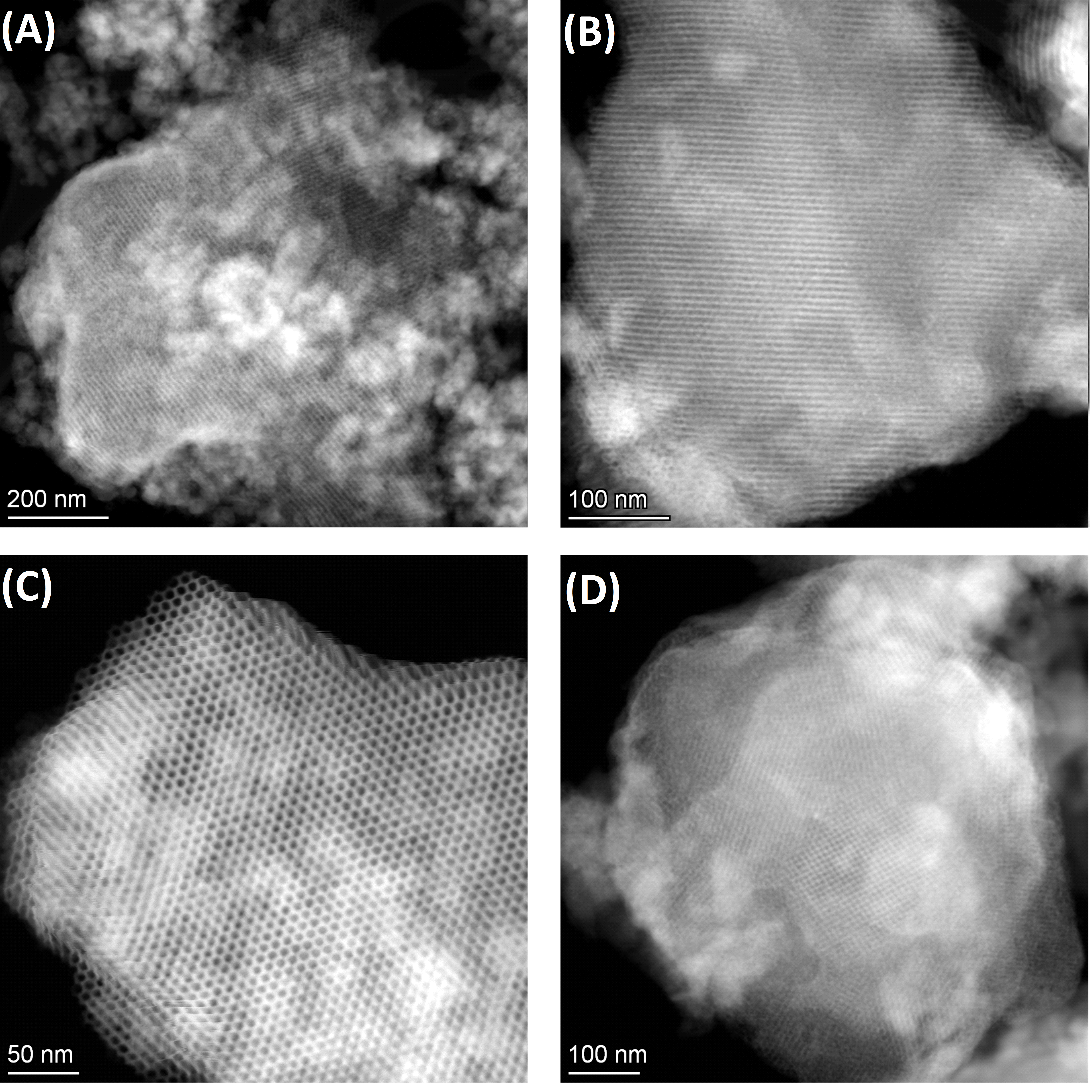
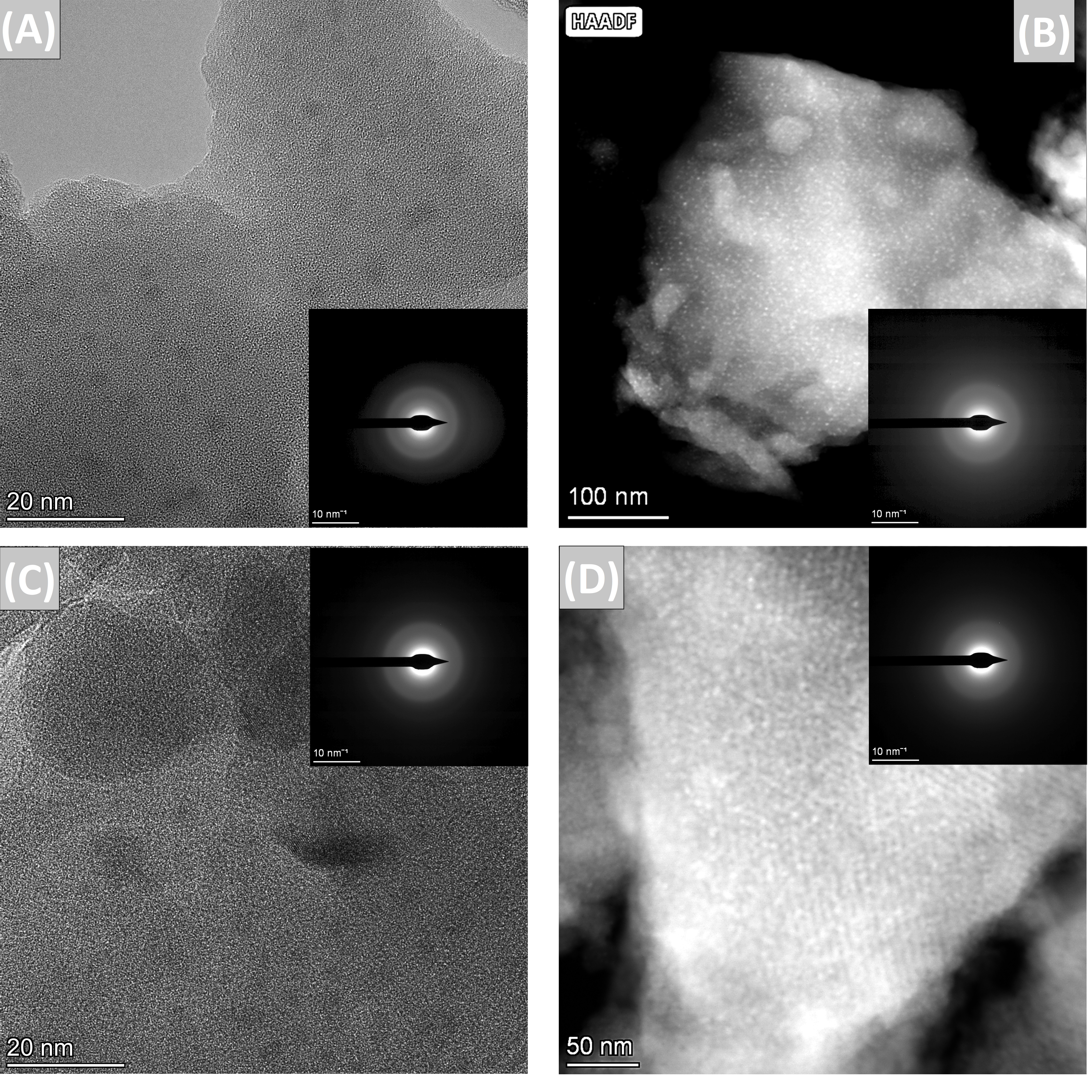
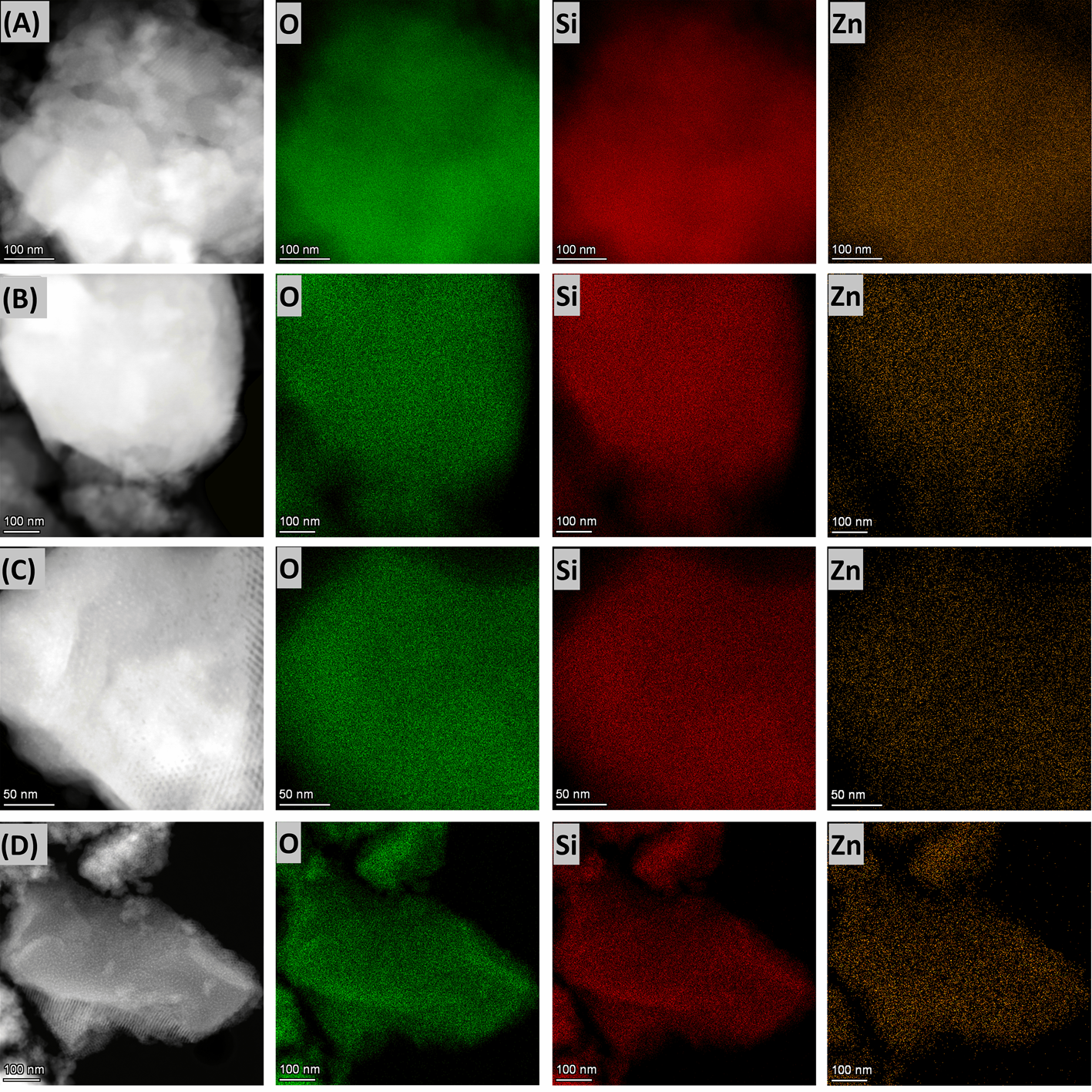
3.3. Characterization of ZnO/SiO2 Nanocomposites
3.4. Antibacterial Activity of ZnO/SiO2 Nanocomposites
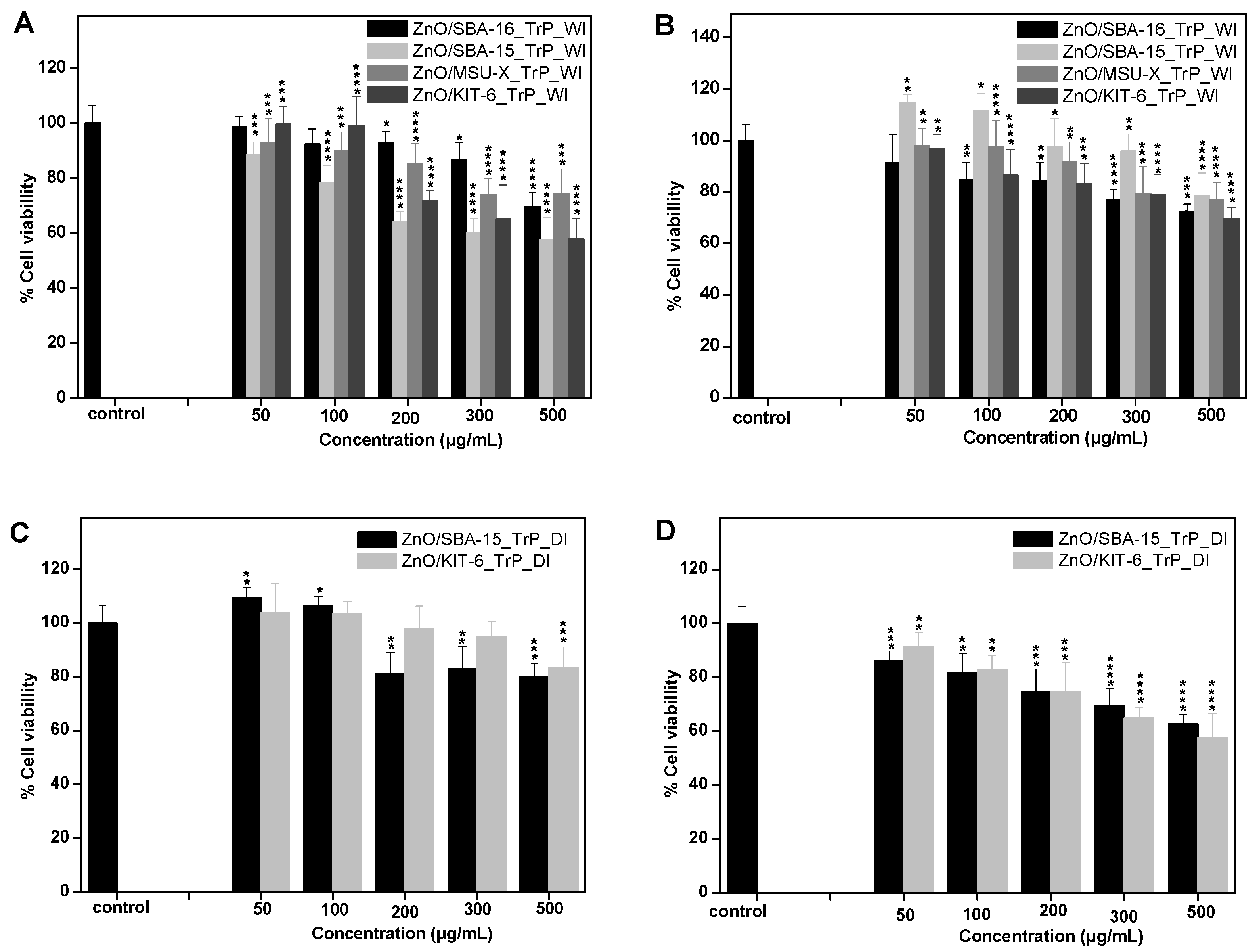
3.5. In Vitro Cytotoxicity Evaluation of ZnO/SiO2 Nanocomposites
3.6. Life Cycle Assessment of Selected ZnO/SiO2 Nanocomposites
3.6.1. Life Cycle Assessment Results
3.6.2. Life Cycle Assessment Limitations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ATR | Attenuated Total Reflection |
| BET | Brunauer–Emmett–Teller (surface area analysis method) |
| CFU | Colony Forming Units |
| CLSI | Clinical and Laboratory Standards Institute |
| CO2e | Carbon Dioxide Equivalent |
| DI | Dry Impregnation |
| EDS | Energy-Dispersive X-ray Spectroscopy |
| FBS | Fetal Bovine Serum |
| FTIR | Fourier Transform Infrared Spectroscopy |
| HAADF-STEM | High-Angle Annular Dark Field Scanning Transmission Electron Microscopy |
| HCl | Hydrochloride |
| HEK293 | Human Embryonic Kidney 293 Cells |
| HRTEM | High-Resolution Transmission Electron Microscopy |
| KIT-6 | Korea Institute of Science and Technology-6 |
| LCA | Life Cycle Assessment |
| LCI | Life Cycle Inventory |
| MBC | Minimum Bactericidal Concentration |
| MIC | Minimum Inhibitory Concentration |
| MSU-X | Michigan State University-X |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (cell viability reagent) |
| NLDFT | Nonlocal Density Functional Theory (pore size distribution model) |
| NPs | Nanoparticles |
| PBS | Phosphate-Buffered Saline |
| PC3 | Human Prostate Cancer Cells |
| PEI | Hyperbranched Polyethyleneimine |
| PSD | Pore Size Distribution |
| ROS | Reactive Oxygen Species |
| RT | Room Temperature |
| S. aureus | Staphylococcus aureus |
| SBA-15 | Santa Barbara Amorphous-15 |
| SBA-16 | Santa Barbara Amorphous-16 |
| SEM | Scanning Electron Microscopy |
| SiO2 | Silicon Dioxide |
| SSA | Specific Surface Area |
| STEM | Scanning Transmission Electron Microscopy |
| TGA | Thermogravimetric Analysis |
| TEM | Transmission Electron Microscopy |
| TEOS | Tetraethyl Orthosilicate |
| TPV | Total Pore Volume |
| TSB | Tryptic soy broth |
| TrP | Carboxy-Methylated Hyperbranched Polyethyleneimine (Trilon-P) |
| WI | Wet Impregnation |
| UV–vis | Ultraviolet-visible spectroscopy |
| XRD | X-ray Diffraction |
| ZnO | Zinc Oxide |
References
- Okeke, I.N.; De Kraker, M.E.A.; Van Boeckel, T.P.; Kumar, C.K.; Schmitt, H.; Gales, A.C.; Bertagnolio, S.; Sharland, M.; Laxminarayan, R. The scope of the antimicrobial resistance challenge. Lancet 2024, 403, 2426–2438. [Google Scholar] [CrossRef] [PubMed]
- de Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 million people die a year due to antimicrobial resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef] [PubMed]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Tsikourkitoudi, V.; Henriques-Normark, B.; Sotiriou, G.A. Inorganic nanoparticle engineering against bacterial infections. Curr. Opin. Chem. Eng. 2022, 38, 100872. [Google Scholar] [CrossRef]
- Lushniak, B.D. Antibiotic resistance: A public health crisis. Public Health Rep. 2014, 129, 314–316. [Google Scholar] [CrossRef]
- Paladini, F.; D’Urso, F.; Broccolo, F.; Pollini, M. Combating Healthcare-Associated Infections in Modern Hospitals: Nanotechnology-Based Approaches in the Era of Antimicrobial Resistance. Nanomaterials 2025, 15, 1405. [Google Scholar] [CrossRef]
- Mubeen, B.; Ansar, A.N.; Rasool, R.; Ullah, I.; Imam, S.S.; Alshehri, S.; Ghoneim, M.M.; Alzarea, S.I.; Nadeem, M.S.; Kazmi, I. Nanotechnology as a Novel Approach in Combating Microbes Providing an Alternative to Antibiotics. Antibiotics 2021, 10, 1473. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef]
- Jiang, S.; Lin, K.; Cai, M. ZnO nanomaterials: Current advancements in antibacterial mechanisms and applications. Front. Chem. 2020, 8, 580. [Google Scholar] [CrossRef]
- Kumar, R.; Umar, A.; Kumar, G.; Nalwa, H.S. Antimicrobial properties of ZnO nanomaterials: A review. Ceram. Int. 2016, 43, 3940–3961. [Google Scholar] [CrossRef]
- Laurenti, M.; Grochowicz, M.; Dragoni, E.; Carofiglio, M.; Limongi, T.; Cauda, V. Biodegradable and drug-eluting inorganic composites based on mesoporous zinc oxide for urinary stent applications. Materials 2020, 13, 3821. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-based nanoparticles as antimicrobial agents: An overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef] [PubMed]
- Raha, S.; Ahmaruzzaman, M. ZnO nanostructured materials and their potential applications: Progress, challenges and perspectives. Nanoscale Adv. 2022, 4, 1868–1925. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Pareek, A. Zinc oxide nanoparticles: A comprehensive review on its synthesis, characterization, and role in biomedical applications as well as health risks. Inorg. Chem. Commun. 2025, 181, 115314. [Google Scholar] [CrossRef]
- Franklin, N.M.; Rogers, N.J.; Apte, S.C.; Batley, G.E.; Gadd, G.E.; Casey, P.S. Comparative Toxicity of Nanoparticulate ZnO, Bulk ZnO, and ZnCl2 to a freshwater Microalga (Pseudokirchneriella subcapitata): The importance of particle solubility. Environ. Sci. Technol. 2007, 41, 8484–8490. [Google Scholar] [CrossRef]
- Bathi, J.R.; Wright, L.; Khan, E. Critical Review of Engineered Nanoparticles: Environmental Concentrations and Toxicity. Curr. Pollut. Rep. 2022, 8, 498–518. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on Zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Raghupathi, K.R.; Koodali, R.T.; Manna, A.C. Size-Dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir 2011, 27, 4020–4028. [Google Scholar] [CrossRef]
- Babayevska, N.; Przysiecka, Ł.; Iatsunskyi, I.; Nowaczyk, G.; Jarek, M.; Janiszewska, E.; Jurga, S. ZnO size and shape effect on antibacterial activity and cytotoxicity profile. Sci. Rep. 2022, 12, 8148. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, J.; Cai, L.; Wu, Y.; Ji, M.; Jiang, H.; Chen, J. Dissection of the antibacterial mechanism of zinc oxide nanoparticles with manipulable nanoscale morphologies. J. Hazard. Mater. 2022, 430, 128436. [Google Scholar] [CrossRef]
- Applerot, G.; Lipovsky, A.; Dror, R.; Perkas, N.; Nitzan, Y.; Lubart, R.; Gedanken, A. Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS--mediated cell injury. Adv. Funct. Mater. 2009, 19, 842–852. [Google Scholar] [CrossRef]
- Donnadio, A.; Cardinali, G.; Latterini, L.; Roscini, L.; Ambrogi, V. Nanostructured zinc oxide on silica surface: Preparation, physicochemical characterization and antimicrobial activity. Mater. Sci. Eng. C 2019, 104, 109977. [Google Scholar] [CrossRef]
- Niño-Martínez, N.; Orozco, M.F.S.; Martínez-Castañón, G.-A.; Méndez, F.T.; Ruiz, F. Molecular mechanisms of bacterial resistance to metal and metal oxide nanoparticles. Int. J. Mol. Sci. 2019, 20, 2808. [Google Scholar] [CrossRef]
- Tang, S.; Wang, J.; Zhu, X.; Shen, D. Ecological risks of zinc oxide nanoparticles for early life stages of obscure puffer (Takifugu obscurus). Toxics 2024, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.; Lin, C.; Wang, Y.; Ma, J.; Wang, X.; Yao, X.; Tang, B. Preparation of Zn doped mesoporous silica nanoparticles (Zn-MSNs) for the improvement of mechanical and antibacterial properties of dental resin composites. Dent. Mater. 2020, 36, 794–807. [Google Scholar] [CrossRef]
- Hou, Y.-X.; Abdullah, H.; Kuo, D.-H.; Leu, S.-J.; Gultom, N.S.; Su, C.-H. A comparison study of SiO2/nano metal oxide composite sphere for antibacterial application. Compos. B Eng. 2018, 133, 166–176. [Google Scholar] [CrossRef]
- Krakor, E.; Saniternik, S.; Gessner, I.; Frohnhoven, R.; Wilhelm, M.; Drexelius, M.; Tosun, N.; Neundorf, I.; Mathur, S. Hollow mesoporous silica capsules loaded with copper, silver, and zinc oxide nanoclusters for sustained antibacterial efficacy. J. Am. Ceram. Soc. 2022, 105, 1685–1696. [Google Scholar] [CrossRef]
- Colilla, M.; Vallet-Regí, M. Organically modified mesoporous silica nanoparticles against bacterial resistance. Chem. Mater. 2023, 35, 8788–8805. [Google Scholar] [CrossRef]
- González, B.; Colilla, M.; Díez, J.; Pedraza, D.; Guembe, M.; Izquierdo-Barba, I.; Vallet-Regí, M. Mesoporous silica nanoparticles decorated with polycationic dendrimers for infection treatment. Acta Biomater. 2018, 68, 261–271. [Google Scholar] [CrossRef]
- Zhuang, J.; Yu, Y.; Lu, R. Mesoporous silica nanoparticles as carrier to overcome bacterial drug resistant barriers. Int. J. Pharm. 2023, 631, 122529. [Google Scholar] [CrossRef]
- Colilla, M.; Vallet-Regí, M. Targeted stimuli-responsive mesoporous silica nanoparticles for bacterial infection treatment. Int. J. Mol. Sci. 2020, 21, 8605. [Google Scholar] [CrossRef]
- Kankala, R.K.; Han, Y.-H.; Na, J.; Lee, C.-H.; Sun, Z.; Wang, S.; Kimura, T.; Ok, Y.S.; Yamauchi, Y.; Chen, A.-Z.; et al. Nanoarchitectured structure and surface biofunctionality of mesoporous silica nanoparticles. Adv. Mater. 2020, 32, 1907035. [Google Scholar] [CrossRef]
- Deze, E.G.; Papavasiliou, A.; Papageorgiou, S.K.; Katsaros, F.K.; Kouvelos, E.P.; Romanos, G.E.; Boukos, N.; Xin, Q.; Nyalosaso, J.L.; Cool, P. Metal loaded nanoporous silicas with tailor-made properties through hyperbranched polymer assisted templating approaches. Micropor. Mesopor. Mat. 2016, 235, 107–119. [Google Scholar] [CrossRef]
- Álvarez, E.; Estévez, M.; Jiménez-Jiménez, C.; Colilla, M.; Izquierdo-Barba, I.; González, B.; Vallet-Regí, M. A versatile multicomponent mesoporous silica nanosystem with dual antimicrobial and osteogenic effects. Acta Biomater. 2021, 136, 570–581. [Google Scholar] [CrossRef]
- Kankala, R.K.; Lin, W.-Z.; Lee, C.-H. Combating antibiotic resistance through the synergistic effects of mesoporous silica-based hierarchical nanocomposites. Nanomaterials 2020, 10, 597. [Google Scholar] [CrossRef]
- Qiu, S.; Zhou, H.; Shen, Z.; Hao, L.; Chen, H.; Zhou, X. Synthesis, characterization, and comparison of antibacterial effects and elucidating the mechanism of ZnO, CuO and CuZnO nanoparticles supported on mesoporous silica SBA-3. RSC Adv. 2020, 10, 2767–2785. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Zhou, X.; Shen, Z.; Peng, Z.; Chen, H.; Hao, L.; Zhou, H. Synthesis of ZnO nanoparticles supported on mesoporous SBA-15 with coordination effect -assist for anti-bacterial assessment. Colloids Surf. B Biointerfaces 2019, 181, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Papavasiliou, A.; Tsiourvas, D.; Deze, E.G.; Papageorgiou, S.K.; Katsaros, F.K.; Poulakis, E.; Philippopoulos, C.J.; Boukos, N.; Xin, Q.; Cool, P. Hyperbranched polyethyleneimine towards the development of homogeneous and highly porous CuO–CeO2–SiO2 catalytic materials. Chem. Eng. J. 2016, 300, 343–357. [Google Scholar] [CrossRef]
- Tsiourvas, D.; Papavasiliou, A.; Deze, E.; Papageorgiou, S.; Katsaros, F.; Romanos, G.; Poulakis, E.; Philippopoulos, C.; Xin, Q.; Cool, P. A green route to copper loaded silica nanoparticles using hyperbranched Poly(Ethylene imine) as a biomimetic template: Application in heterogeneous catalysis. Catalysts 2017, 7, 390. [Google Scholar] [CrossRef]
- Papavasiliou, A.; Deze, E.G.; Papageorgiou, S.K.; Sideratou, Z.; Boukos, N.; Poulakis, E.; Philippopoulos, C.J.; Glisenti, A.; Van Everbroeck, T.; Cool, P.; et al. A hyperbranched polymer synthetic strategy for the efficient fixation of metal species within nanoporous structures: Application in automotive catalysis. Chem. Eng. J. 2021, 421, 129496. [Google Scholar] [CrossRef]
- Kosuge, K.; Sato, T.; Kikukawa, N.; Takemori, M. Morphological control of rod- and fiberlike SBA-15 type mesoporous silica using water-soluble sodium silicate. Chem. Mater. 2004, 16, 899–905. [Google Scholar] [CrossRef]
- Grudzien, R.M.; Grabicka, B.E.; Jaroniec, M. Adsorption studies of thermal stability of SBA-16 mesoporous silicas. Appl. Surf. Sci. 2007, 253, 5660–5665. [Google Scholar] [CrossRef]
- Boissière, C.; Larbot, A.; Van Der Lee, A.; Kooyman, P.J.; Prouzet, E. A new synthesis of mesoporous MSU-X silica controlled by a two-step pathway. Chem. Mater. 2000, 12, 2902–2913. [Google Scholar] [CrossRef]
- Kleitz, F.; Choi, S.H.; Ryoo, R. Cubic Ia3d large mesoporous silica: Synthesis and replication to platinum nanowires, carbon nanorods and carbon nanotubes. Chem. Commun. 2003, 17, 2136–2137. [Google Scholar] [CrossRef]
- CLSI M26; Methods for Determining Bactericidal Activity of Antimicrobial Agents. Clinical and Laboratory Standards Institute (CLSI): Malvern, PA, USA, 1999.
- CLSI M07; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Clinical and Laboratory Standards Institute (CLSI): Malvern, PA, USA, 2012.
- Panagiotaki, K.N.; Lyra, K.-M.; Papavasiliou, A.; Stamatakis, K.; Sideratou, Z. Synthesis of N--sulfopropylated hyperbranched polyethyleneimine with enhanced biocompatibility and antimicrobial activity. ChemPlusChem 2025, 90, e202400454. [Google Scholar] [CrossRef]
- Piffet, C.; Thomassin, J.-M.; Stierlin, E.; Tchoumtchoua, J.; Fernández, C.; Mateo, M.; Hernández, L.; Lyra, K.M.; Papavasiliou, A.; Sakellis, E.; et al. Sustainable antibacterial chitin nanofiber/ZnO nanohybrid materials: Ex situ and in situ synthesis, characterization and evaluation. Nanomaterials 2025, 15, 809. [Google Scholar] [CrossRef]
- EC-European Commission. Commission Recommendation 2013/179/EU of 9 April 2013 on the use of common methods to measure and communicate the life cycle environmental performance of products and organisations. ANNEX II: Product Environmental Footprint (PEF) Guide. Off. J. Eur. Union 2013, L124, 1–210. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Kaplun, M.; Sandström, M.; Boström, D.; Shchukarev, A.; Persson, P. Crystal structures and spectroscopic properties of palladium complexes isolated from Pd–EDTA solutions. Inorg. Chim. Acta 2005, 358, 527–534. [Google Scholar] [CrossRef]
- Croissant, J.G.; Fatieiev, Y.; Almalik, A.; Khashab, N.M. Mesoporous silica and organosilica nanoparticles: Physical chemistry, biosafety, delivery strategies, and biomedical applications. Adv. Healthcare Mater. 2018, 7, 1700831. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Shi, J. Mesoporous silica nanoparticle based nano drug delivery systems: Synthesis, controlled drug release and delivery, pharmacokinetics and biocompatibility. J. Mater. Chem. 2011, 21, 5845–5855. [Google Scholar] [CrossRef]
- Trinh, H.T.; Tran, T.K.A.; Arora, S.; George, S.M.; Sheri, J.; Li, Z.; Yang, J.H.; Naruphontjirakul, P.; Balani, K.; Karakoti, A.; et al. Zn-Loaded SBA-1 and SBA-15 Molecular Sieves for Combined Antimicrobial and Osteogenic Activity. Adv. Mater. Technol. 2023, 8, 2201169. [Google Scholar] [CrossRef]
- Kodeh, F.S.; El-Nahhal, I.M. Exploring of Potential Antibacterial Activity of Hypochlorite and Chloroamine Ad-sorbed Ammonium Functionalized Mesoporous SBA-15 Silica. Chem. Afr. 2021, 4, 599–605. [Google Scholar] [CrossRef]
- Shahriarinour, M.; Divsar, F.; Eskandari, Z. Synthesis, characterization, and antibacterial activity of thymol loaded SBA-15 mesoporous silica nanoparticles. Inorg. Nano-Metal Chem. 2019, 49, 182–189. [Google Scholar] [CrossRef]
- Xu, Z.; Xu, D.; Li, S.; Liu, Z.; Gao, X.; Cheng, L.; Liu, Y.; Sun, J. Preparation, sustained-release and antibacterial activity of SBA-15/CG antibacterial agent. Mater. Lett. 2023, 344, 134432. [Google Scholar] [CrossRef]
- Banafti, S.; Jahanshahi, M.; Peyravi, M.; Khalili, S. Controllable release activity of antibacterial Ag/SBA-16 cage-like synthesized by one-pot method. Micropor. Mesopor. Mat. 2020, 299, 110107. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A mini review of antibacterial properties of ZnO nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Abebe, B.; Zereffa, E.A.; Tadesse, A.; Murthy, H.C.A. A review on enhancing the antibacterial activity of ZnO: Mechanisms and microscopic investigation. Nanoscale Res. Lett. 2020, 15, 190. [Google Scholar] [CrossRef]
- Siddiqi, K.S.; Rahman, A.U.; Tajuddin, N.; Husen, A. Properties of zinc oxide nanoparticles and their activity against microbes. Nanoscale Res. Lett. 2018, 13, 141. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, L.; Wen, D.; Ding, Y. Role of physical and chemical interactions in the antibacterial behavior of ZnO nanoparticles against E. coli. Mater. Sci. Eng. C 2016, 69, 1361–1366. [Google Scholar] [CrossRef]
- Shehata, S.; Elkholy, Y.N.; Hussien, M.S.; Yahia, I.S.; Aboshanab, K.M. Antibacterial, antibiofilm and cyto-toxic activity of synthesized metal-incorporated mesoporous silica nanoparticles. AMB Express 2025, 15, 130. [Google Scholar] [CrossRef]
- Agarwal, N.H.; Menon, N.S.; Kumar, S.V.; Rajeshkumar, S. Mechanistic study on antibacterial action of zinc oxide nanoparticles synthesized using green route. Chem. Biol. Interact. 2018, 286, 60–70. [Google Scholar] [CrossRef]
- Mendes, C.R.; Dilarri, G.; Forsan, C.F.; De Moraes Ruy Sapata, V.; Lopes, P.R.M.; De Moraes, P.B.; Montagnolli, R.N.; Ferreira, H.; Bidoia, E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022, 12, 2658. [Google Scholar] [CrossRef]
- da Silva, B.L.; Abuçafy, M.P.; Manaia, E.B.; Oshiro-Junior, J.A.; Chiari-Andréo, B.G.; Pietro, R.C.R.; Chiavacci, L.A. Relationship between structure and antimicrobial activity of zinc oxide nanoparticles: An overview. Int. J. Nanomed. 2019, 14, 9395–9410. [Google Scholar] [CrossRef]
- Patra, J.K.; Baek, K.-H. Antibacterial Activity and Synergistic Antibacterial Potential of Biosynthesized Silver Na-noparticles against Foodborne Pathogenic Bacteria along with Its Anticandidal and Antioxidant Effects. Front. Microbiol. 2017, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Canta, M.; Cauda, V. The investigation of the parameters affecting the ZnO nanoparticle cytotoxicity behaviour: A tutorial review. Biomater. Sci. 2020, 8, 6157–6174. [Google Scholar] [CrossRef] [PubMed]
- Vagena, I.-A.; Gatou, M.-A.; Theocharous, G.; Pantelis, P.; Gazouli, M.; Pippa, N.; Gorgoulis, V.G.; Pavlatou, E.A.; Lagopati, N. Functionalized ZnO-Based Nanocomposites for Diverse Biological Applications: Current Trends and Future Perspectives. Nanomaterials 2024, 14, 397. [Google Scholar] [CrossRef]
- Liao, C.; Jin, Y.; Li, Y.; Tjong, S.C. Interactions of Zinc Oxide Nanostructures with Mammalian Cells: Cytotoxicity and Photocatalytic Toxicity. Int. J. Mol. Sci. 2020, 21, 6305. [Google Scholar] [CrossRef]
- Song, W.; Zhang, J.; Guo, J.; Zhang, J.; Ding, F.; Li, L.; Sun, Z. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar] [CrossRef]
- Camaioni, A.; Massimiani, M.; Lacconi, V.; Magrini, A.; Salustri, A.; Sotiriou, G.A.; Singh, D.; Bitounis, D.; Bocca, B.; Pino, A.; et al. Silica encapsulation of ZnO nanoparticles reduces their toxicity for cumulus cell-oocyte-complex expansion. Part. Fibre Toxicol. 2021, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.O.; Grice, J.E.; Roberts, M.S.; Haridass, I.N.; Duque, M.D.; Lopes, P.S.; Leite-Silva, V.R.; Martins, T.S. ZnO: SBA-15 nanocomposites for potential use in sunscreen: Preparation, properties, human skin penetration and toxicity. Skin Pharmacol. Physiol. 2018, 32, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Chia, S.L.; Leong, D.T. Reducing ZnO nanoparticles toxicity through silica coating. Heliyon 2016, 2, e00177. [Google Scholar] [CrossRef] [PubMed]
- ISO 14040:2006; Environmental Management—Life-Cycle Assessment—Principles and Framework. International Organization for Standardization: Geneva, Switzerland, 2006.
- ISO 14044:2006; Environmental Management—Life Cycle Assessment—Requirements and Guidelines. International Organization for Standardization: Geneva, Switzerland, 2006.
- Ros-Lis, J.V.; Vetter, S.; Smith, P. A comparative life cycle assessment of the synthesis of mesoporous silica materials on a small and a large scale. Green Chem. 2024, 26, 10107–10114. [Google Scholar] [CrossRef]


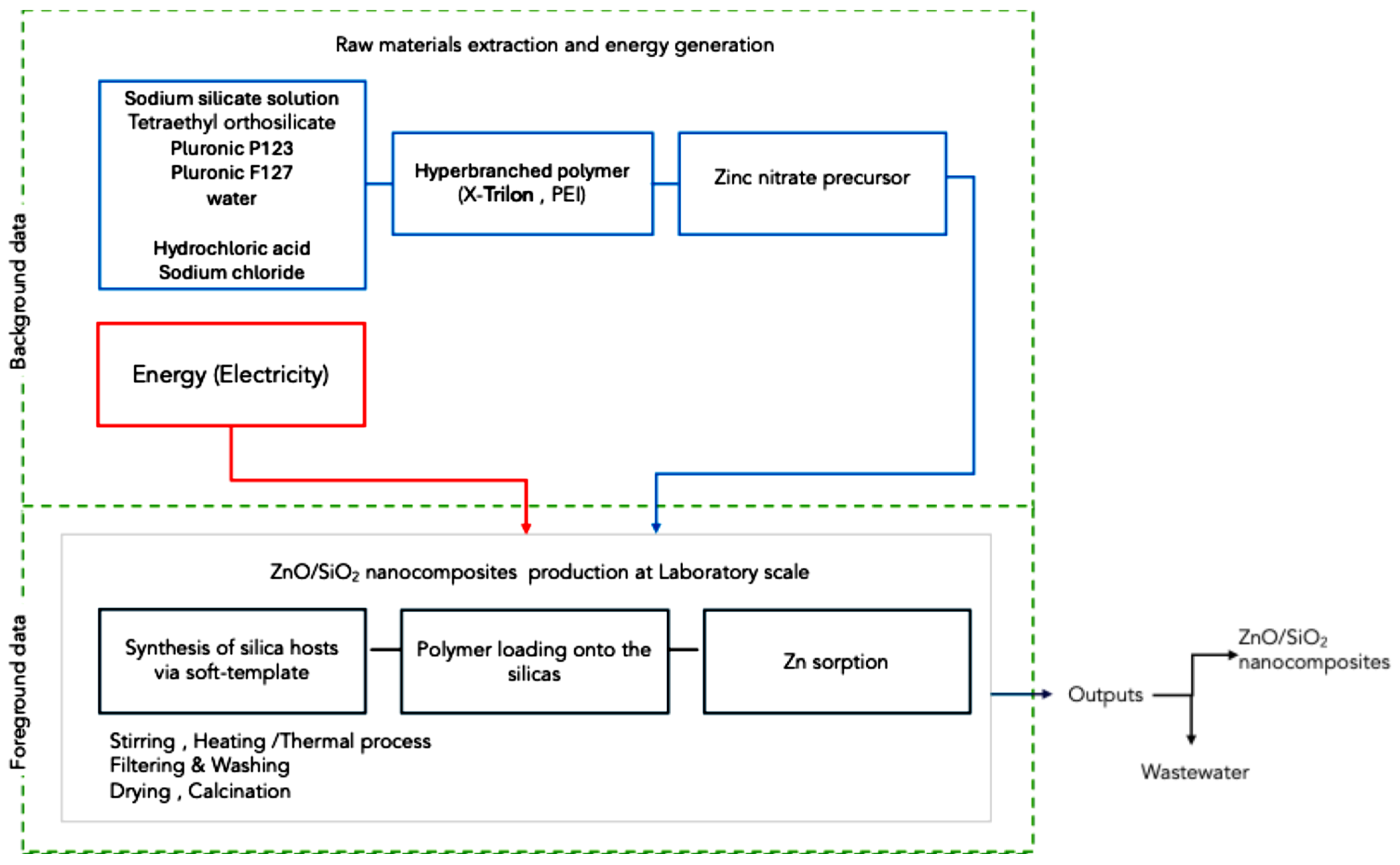
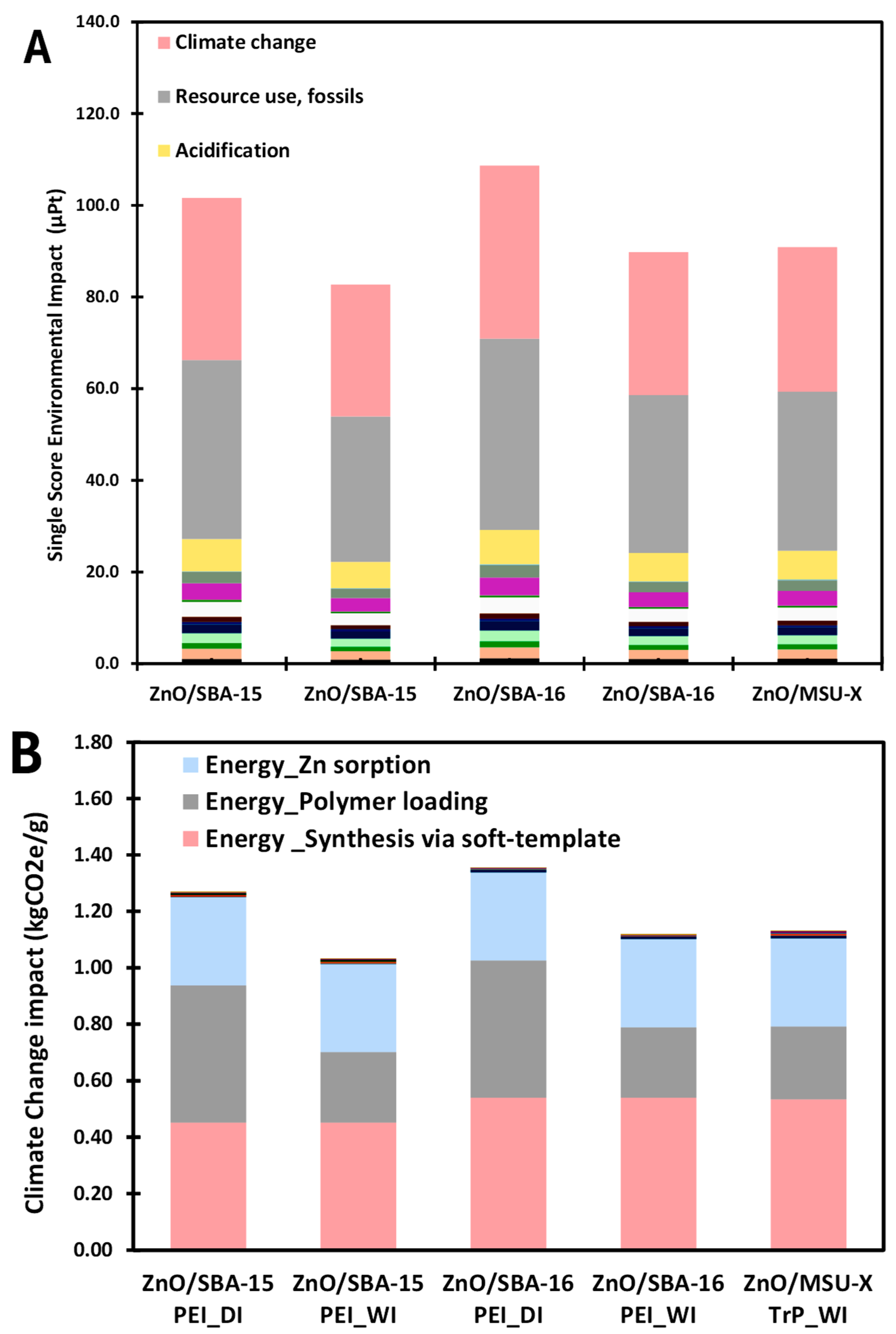
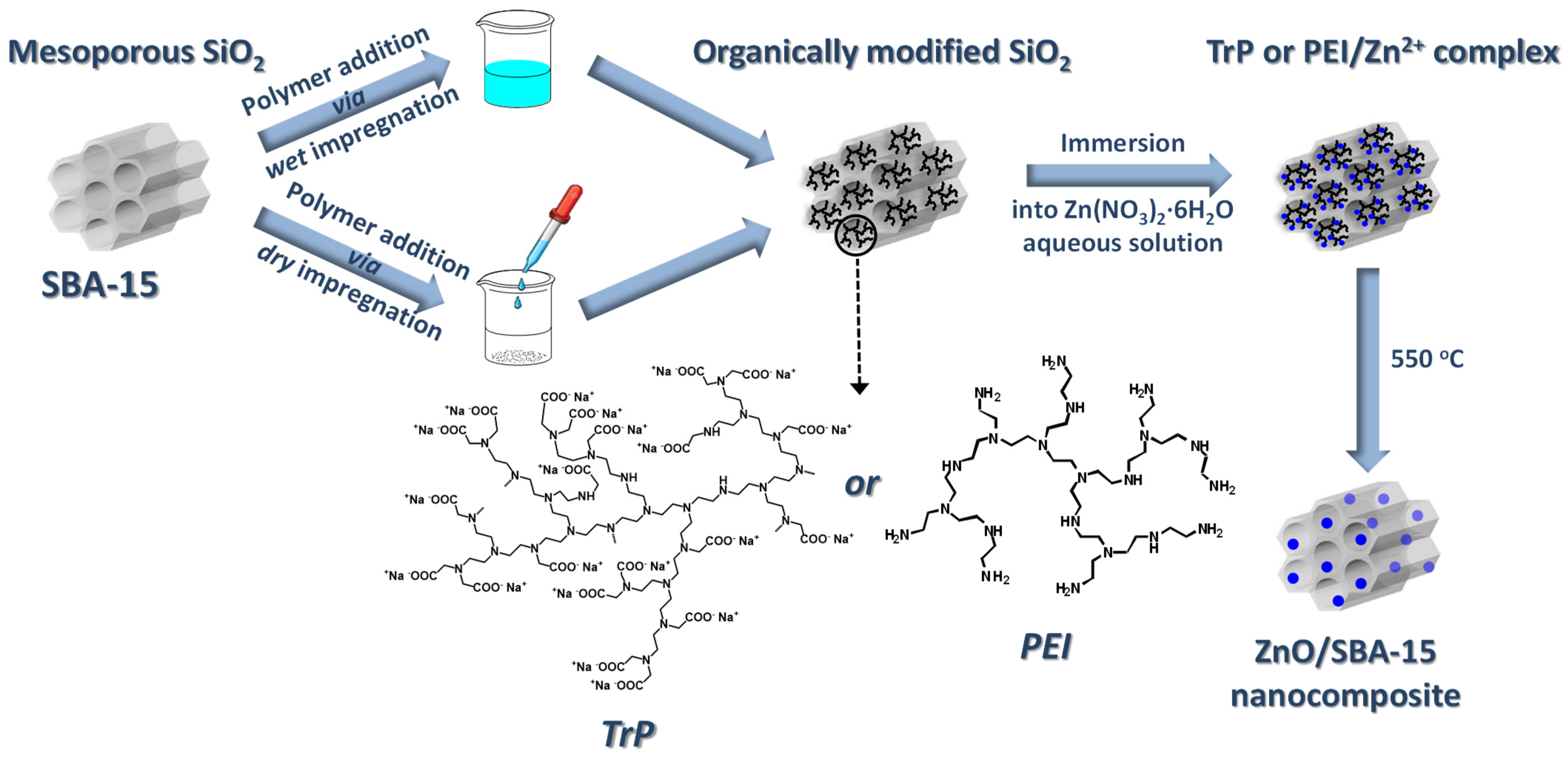
| Functional Unit | 1 g of Synthesized ZnO/SiO2 |
|---|---|
| Method | EF3.1 (adapted) |
| Geography | Europe |
| Dataset | Ecoinvent v3.11 |
| Data type | Primary data (experimental cases at laboratory scale under the scope of this work) |
| Samples | SSA (m2 g−1) | TPV (cc g−1) | Average Pore Diameter (nm) |
|---|---|---|---|
| SBA-15 | 546 | 0.49 | 3.6 |
| KIT-6 | 876 | 1.16 | 5.3 |
| SBA-16 | 871 | 0.53 | 2.4 |
| MSU-X | 1001 | 0.82 | 3.3 |
| Samples | SSA (m2 g−1) | TPV (cc g−1) | Average Pore Diameter (nm) | Polymer Content (wt.%) |
|---|---|---|---|---|
| SBA-15_PEI_WI | 223 | 0.28 | 5 | 17 |
| KIT-6_PEI_WI | 297 | 0.57 | 7.7 | 27 |
| SBA-16_PEI_WI | 191 | 0.39 | 8.3 | 21 |
| MSU-X_PEI_WI | 432 | 0.38 | 3.5 | 22 |
| SBA-15_PEI_DI | 33.2 | 0.046 | 5.6 | 24 |
| KIT-6_PEI_DI | 277 | 0.46 | 6.7 | 30 |
| SBA-16_PEI_DI | 359 | 0.34 | 3.8 | 9 |
| MSU-X_PEI_DI | 94 | 0.12 | 5.3 | 25 |
| SBA-15_TrP_WI | 436 | 0.47 | 4.3 | 5.5 |
| KIT-6_TrP_WI | 600 | 0.96 | 6.4 | 11 |
| SBA-16_TrP_WI | 534 | 0.45 | 3.3 | 6 |
| MSU-X_TrP_WI | 705 | 0.65 | 3.7 | 7.5 |
| SBA-15_TrP_DI | 333 | 0.45 | 5.4 | 5 |
| KIT-6_TrP_DI | 391 | 0.83 | 8.4 | 9 |
| Samples | Zn Loading (wt.%) | SSA (m2 g−1) | TPV (cc g−1) | Average Pore Diameter (nm) |
|---|---|---|---|---|
| ZnO/SBA-15_PEI_WI | 2.6 | 511 | 0.56 | 4.4 |
| ZnO/KIT-6_PEI_WI | 5.9 | 742 | 1.05 | 5.7 |
| ZnO/SBA-16_PEI_WI | 1.8 | 442 | 0.67 | 6.0 |
| ZnO/MSU-X_PEI_WI | 4.3 | 519 | 0.55 | 4.2 |
| ZnO/SBA-15_PEI_DI | 2.7 | 535 | 0.45 | 3.4 |
| ZnO/KIT-6_PEI_DI | 8.1 | 683 | 0.85 | 5.0 |
| ZnO/SBA-16_PEI_DI | 1.3 | 507 | 0.43 | 3.4 |
| ZnO/MSU-X_PEI_DI | 4.2 | 742 | 0.54 | 2.9 |
| ZnO/SBA-15_TrP_WI | 0.9 | 420 | 0.48 | 4.6 |
| ZnO/KIT-6_TrP_WI | 1.8 | 644 | 1.04 | 6.4 |
| ZnO/SBA-16_TrP_WI | 0.75 | 350 | 0.41 | 4.7 |
| ZnO/MSU-X_TrP_WI | 1.0 | 769 | 0.70 | 3.6 |
| ZnO/SBA-15_TrP_DI | 1.0 | 335 | 0.47 | 5.6 |
| ZnO/KIT-6_TrP_DI | 0.9 | 411 | 0.88 | 8.6 |
| Samples | MIC (μg/mL) | MBC (μg/mL) | |
|---|---|---|---|
| ZnO/SBA-16_PEI_WI | Total | 50 | 1200 |
| Zn content | 0.9 | 21.6 | |
| ZnO/SBA-15_PEI_WI | Total | 100 | 1000 |
| Zn content | 2.6 | 26.0 | |
| ZnO/MSU-X_PEI_WI | Total | 100 | 600 |
| Zn content | 4.3 | 25.8 | |
| ZnO/KIT-6_PEI_WI | Total | 100 | 500 |
| Zn content | 5.9 | 29.5 | |
| ZnO/SBA-16_PEI_DI | Total | 50 | 1200 |
| Zn content | 0.65 | 15.6 | |
| ZnO/SBA-15_PEI_DI | Total | 50 | 900 |
| Zn content | 1.35 | 24.3 | |
| ZnO/MSU-X_PEI_DI | Total | 100 | 600 |
| Zn content | 4.2 | 25.3 | |
| ZnO/KIT-6_PEI_DI | Total | 200 | 500 |
| Zn content | 16.2 | 40.5 |
| Samples | MIC (μg/mL) | MBC (μg/mL) | |
|---|---|---|---|
| ZnO/SBA-16_TrP_WI | Total | 400 | 1500 |
| Zn content | 3.0 | 11.25 | |
| ZnO/SBA-15_TrP_WI | Total | 400 | 1200 |
| Zn content | 3.6 | 10.8 | |
| ZnO/MSU-X_TrP_WI | Total | 200 | 500 |
| Zn content | 2.0 | 5.0 | |
| ZnO/KIT-6_TrP_WI | Total | 200 | 600 |
| Zn content | 3.6 | 10.8 | |
| ZnO/SBA-15_TrP_DI | Total | 300 | 500 |
| Zn content | 3.0 | 5.0 | |
| ZnO/KIT-6_TrP_DI | Total | 400 | 900 |
| Zn content | 3.6 | 8.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papavasiliou, A.; Lyra, K.M.; Sakellis, E.; Lozano Násner, A.M.; Gallego, J.; Katsaros, F.K.; Sideratou, Z. Engineering Mesoporous Silica Hosts for Ultrasmall ZnO Nanoparticles: A Dendritic Polymer-Assisted Strategy Towards Sustainable, Safe, and Effective Antibacterial Systems. Nanomaterials 2025, 15, 1697. https://doi.org/10.3390/nano15221697
Papavasiliou A, Lyra KM, Sakellis E, Lozano Násner AM, Gallego J, Katsaros FK, Sideratou Z. Engineering Mesoporous Silica Hosts for Ultrasmall ZnO Nanoparticles: A Dendritic Polymer-Assisted Strategy Towards Sustainable, Safe, and Effective Antibacterial Systems. Nanomaterials. 2025; 15(22):1697. https://doi.org/10.3390/nano15221697
Chicago/Turabian StylePapavasiliou, Aggeliki, Kyriaki Marina Lyra, Elias Sakellis, Albany Milena Lozano Násner, Jose Gallego, Fotios K. Katsaros, and Zili Sideratou. 2025. "Engineering Mesoporous Silica Hosts for Ultrasmall ZnO Nanoparticles: A Dendritic Polymer-Assisted Strategy Towards Sustainable, Safe, and Effective Antibacterial Systems" Nanomaterials 15, no. 22: 1697. https://doi.org/10.3390/nano15221697
APA StylePapavasiliou, A., Lyra, K. M., Sakellis, E., Lozano Násner, A. M., Gallego, J., Katsaros, F. K., & Sideratou, Z. (2025). Engineering Mesoporous Silica Hosts for Ultrasmall ZnO Nanoparticles: A Dendritic Polymer-Assisted Strategy Towards Sustainable, Safe, and Effective Antibacterial Systems. Nanomaterials, 15(22), 1697. https://doi.org/10.3390/nano15221697








