Effective Pore Distribution and Mechanism of CO2/CH4 Dynamic Separation by Carbon Molecular Sieves
Abstract
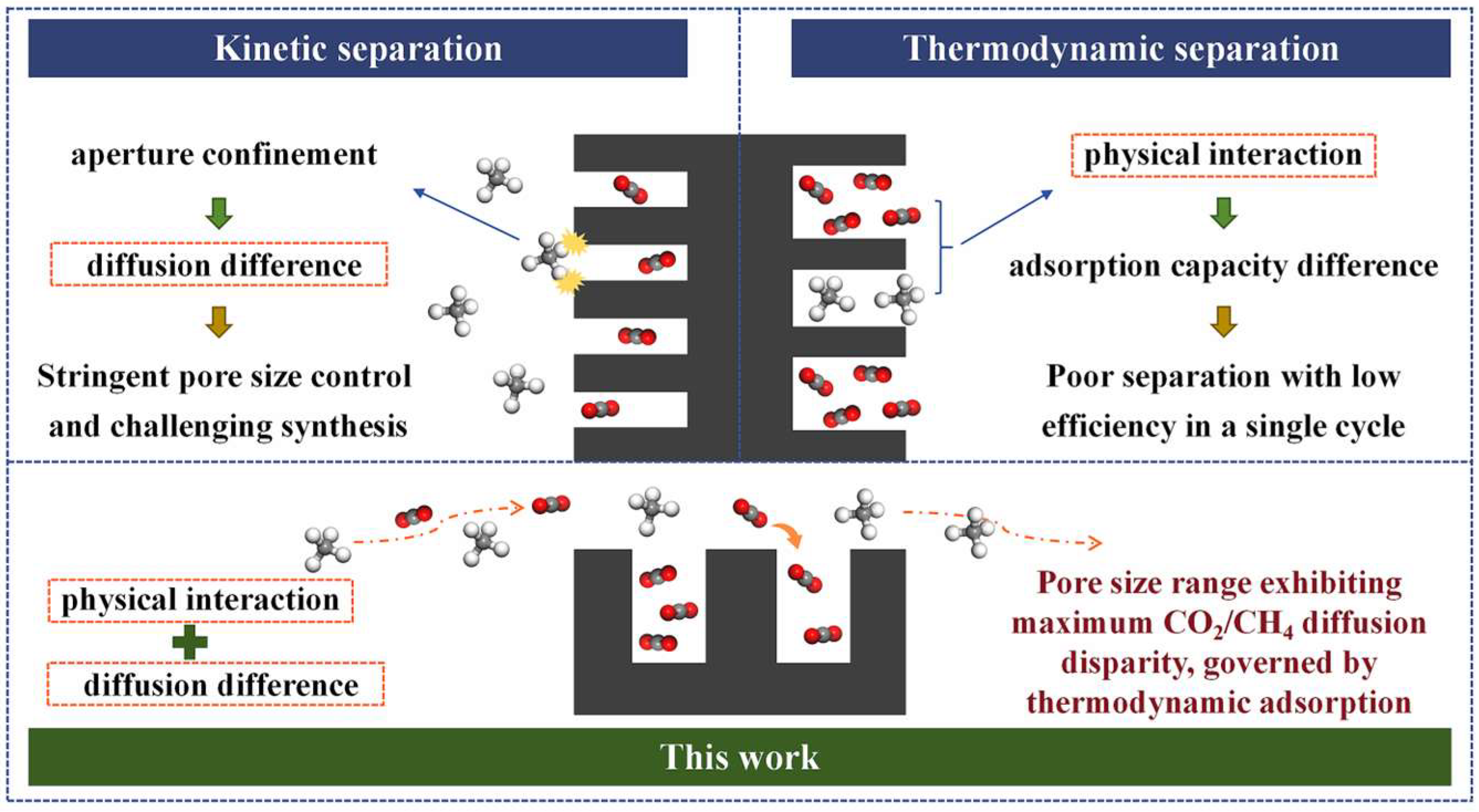
1. Introduction
2. Experimental and Methods
2.1. Sample Preparation
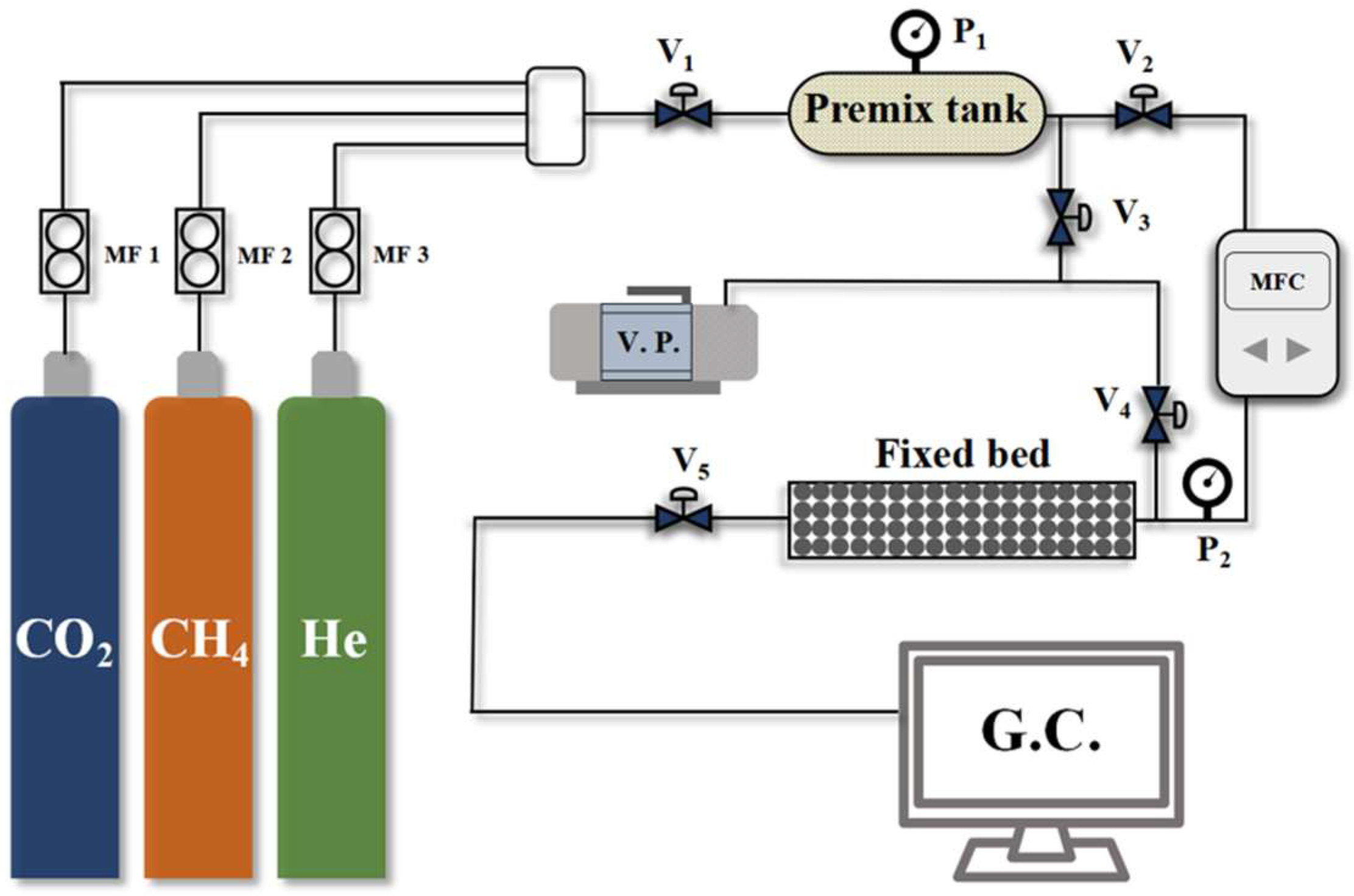
2.2. Testing and Analysis Procedures
- Samples were initially dried at 353 K for 6 h in a drying oven to prepare for adsorption measurements. Subsequently, 20 g of the dried sample was loaded into the fixed-bed adsorption column;
- Prior to testing, the system was evacuated under vacuum for 1 h to remove residual impurities. Following evacuation, all valves were closed;
- Feed gas valve V1 was opened to introduce pure component gases into the mixing tank, generating a pre-mixed gas with a CO2/CH4 ratio of 19:81;
- Valve V2 was then opened to feed the mixed gas into the fixed bed at a flow rate of 40 mL min−1 for the breakthrough test. Valves V5 was opened to direct the outlet gas from the adsorption column to a gas chromatograph for continuous analysis of the component concentrations.
2.3. Pore Structure Characterization
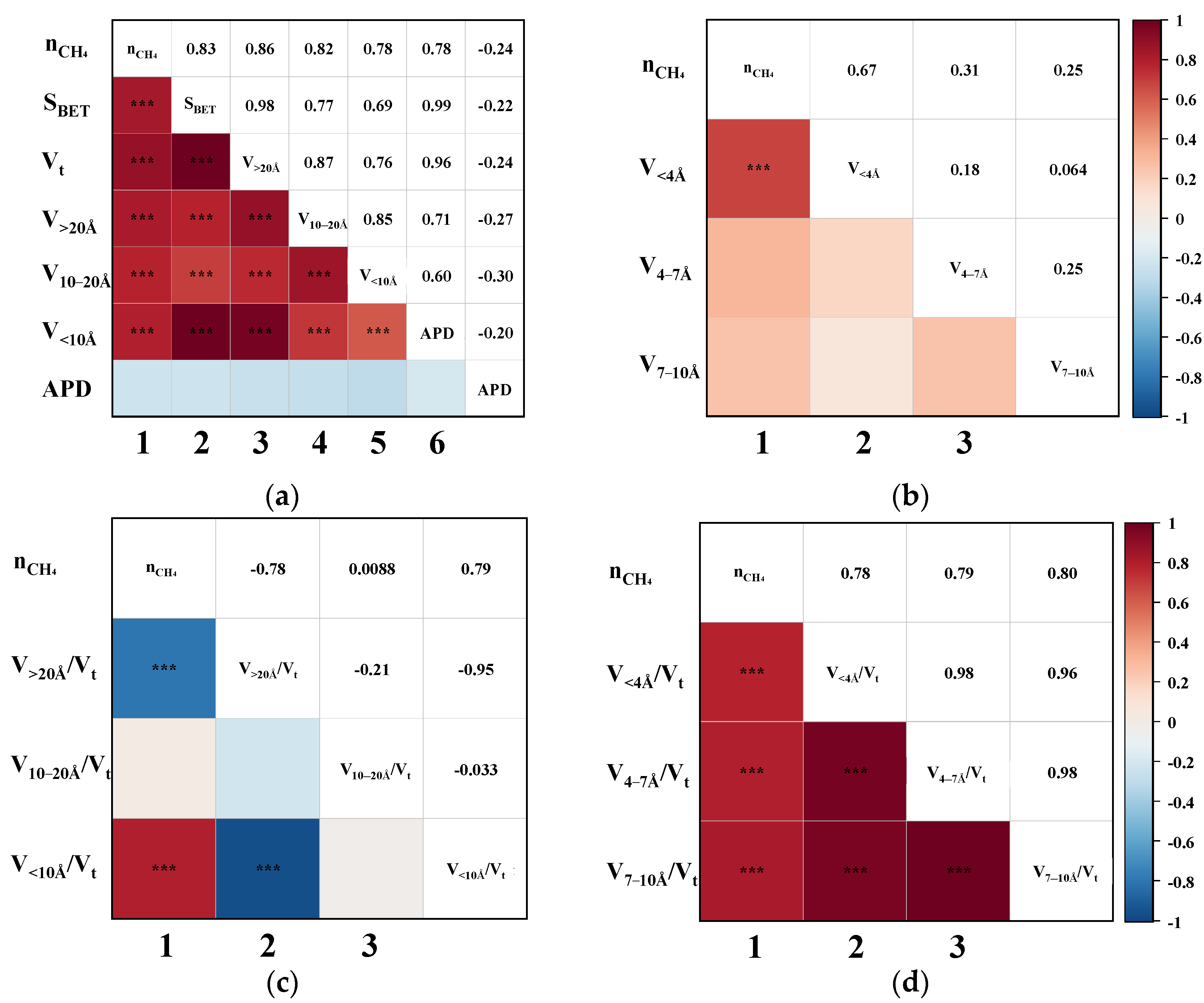
2.4. Pearson Correlation Analysis
3. Results and Discussion
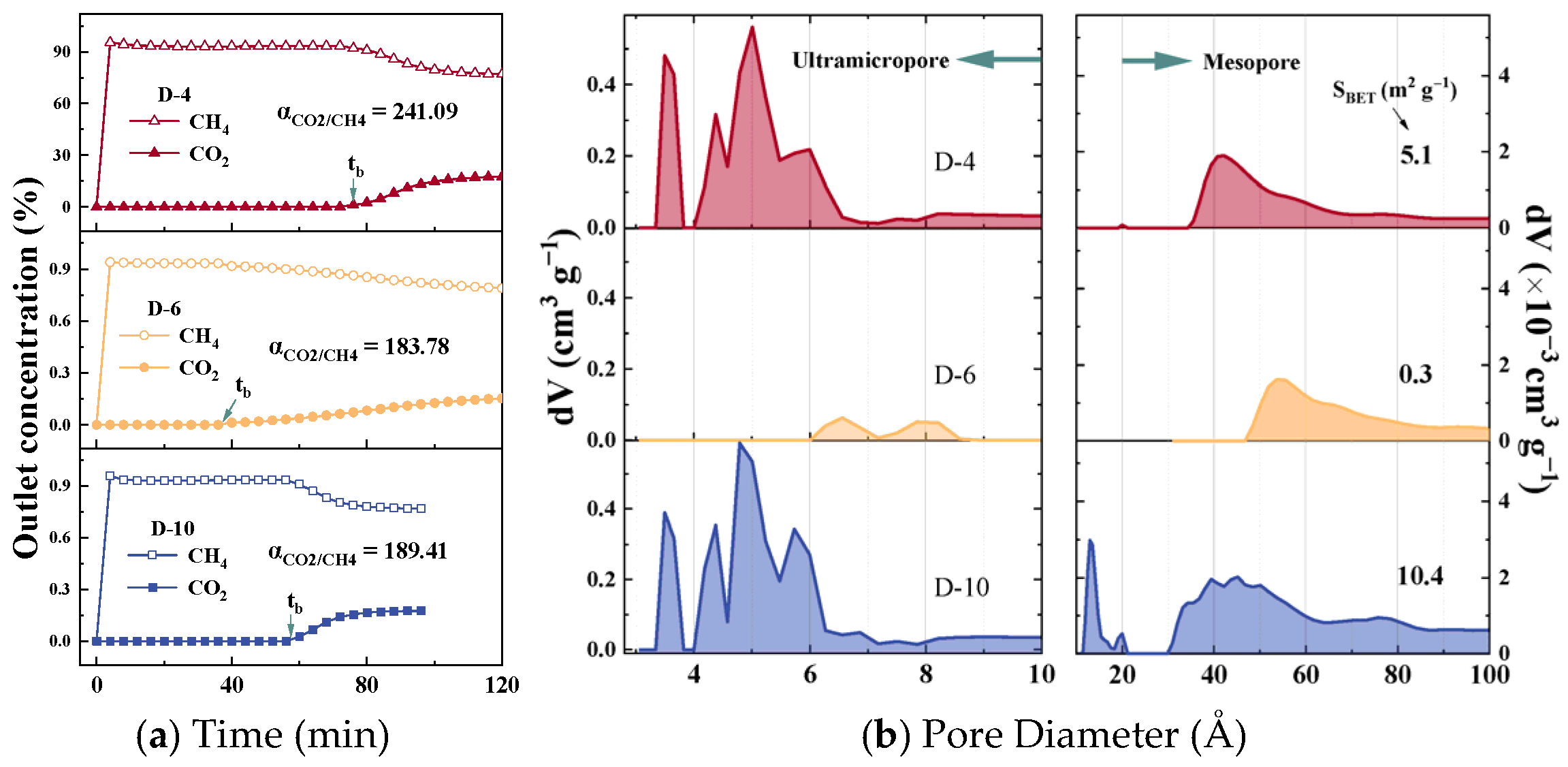
3.1. Sample Analysis
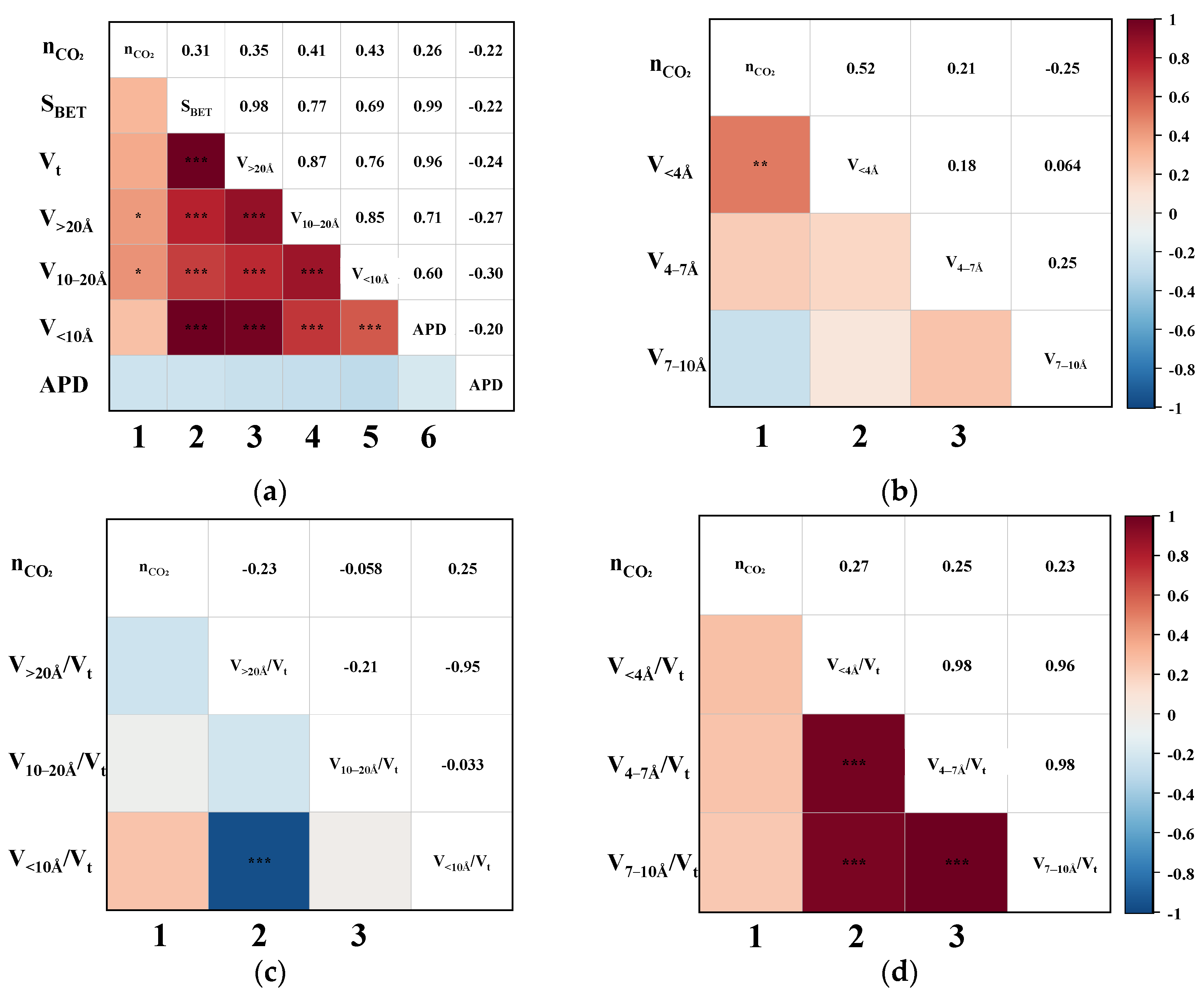
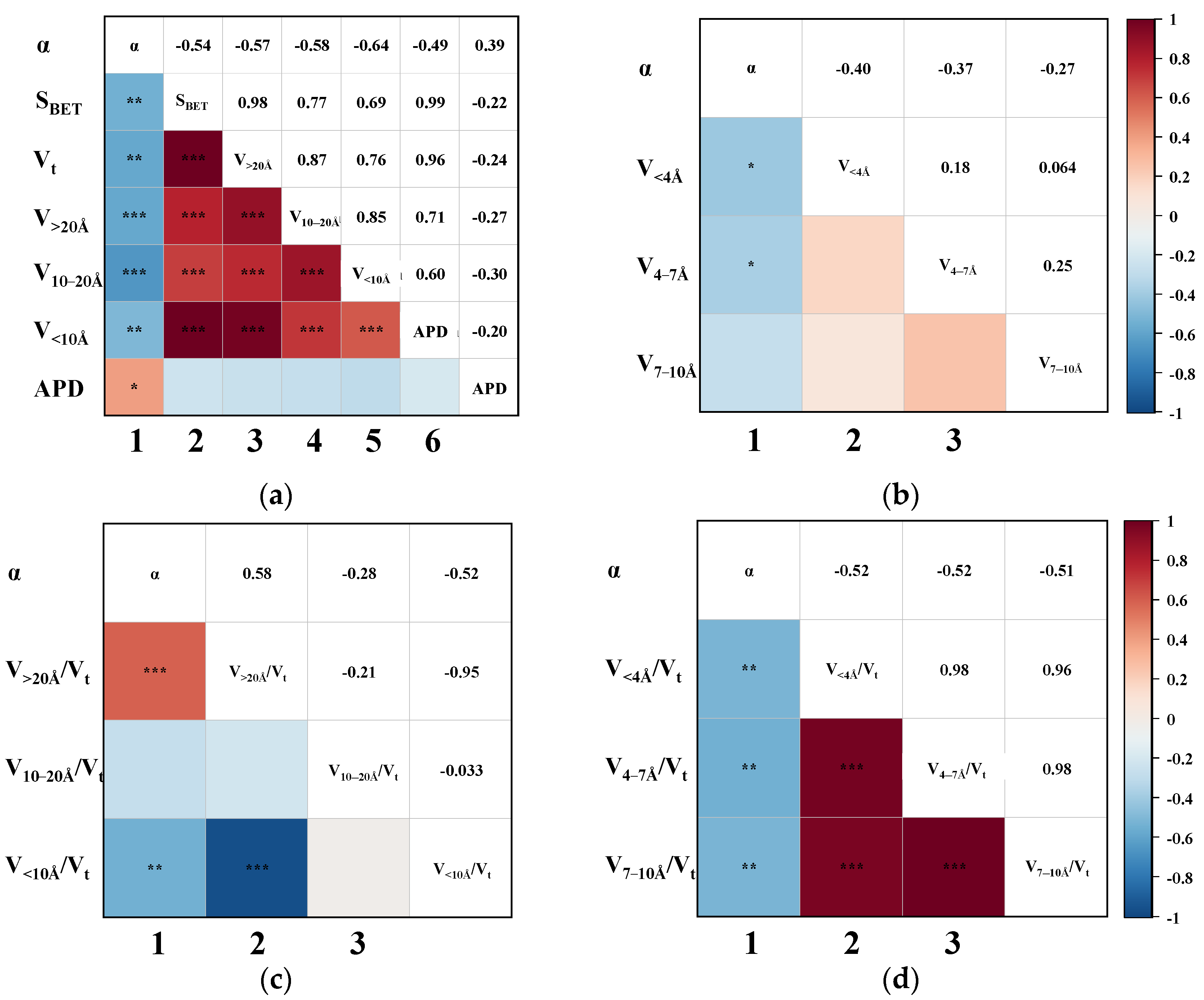
3.2. Correlation of CH4 Adsorption Capacity
3.3. Correlation of CO2 Adsorption Capacity
3.4. Correlation of CO2/CH4 Separation Performance
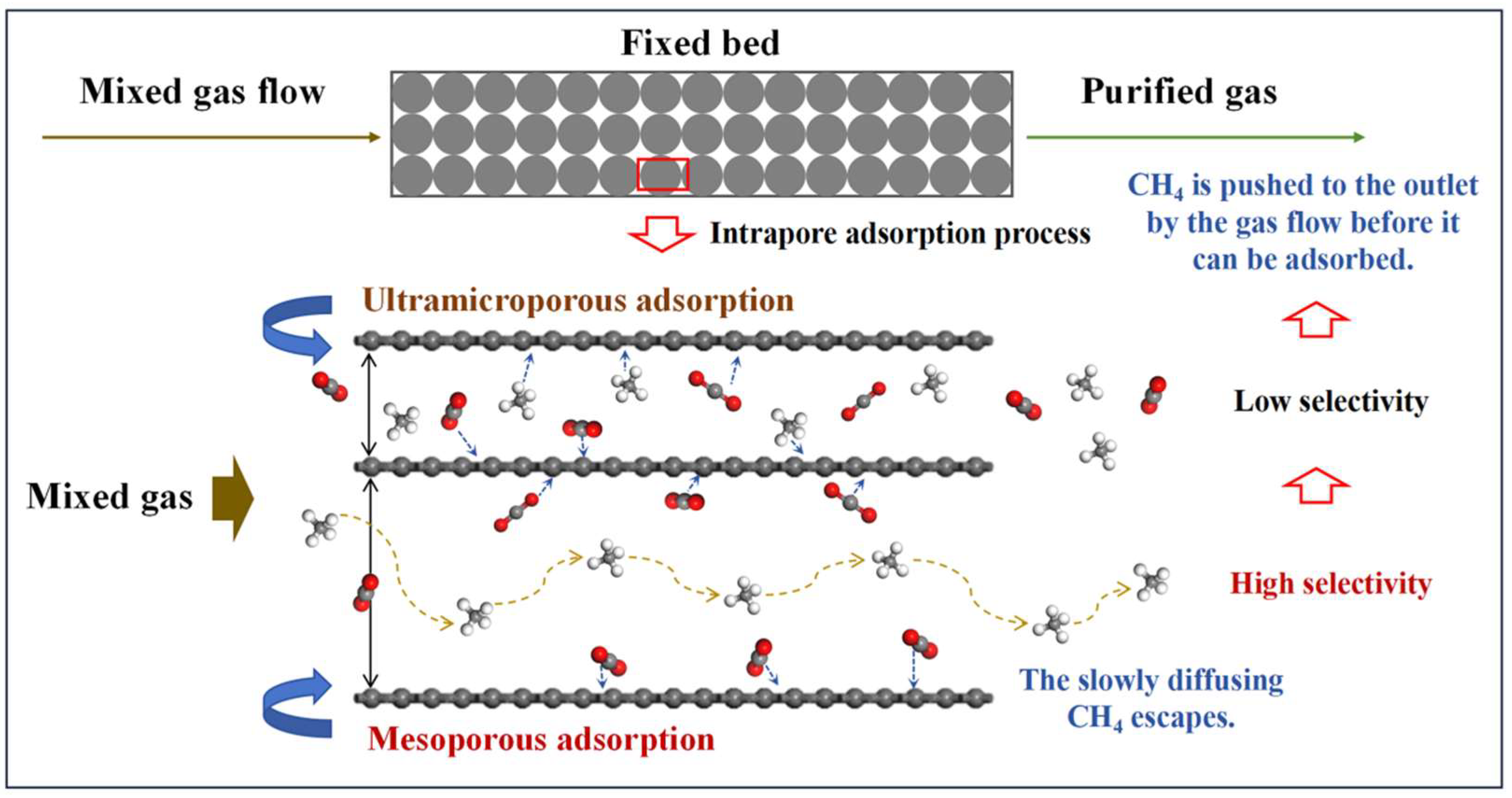
3.5. Mechanism of Effective Pores on Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, X.; Wang, L.; Gong, J.; Zhan, X.; Zeng, Y. Exploring a new method to study the effects of surface functional groups on adsorption of CO2 and CH4 on activated carbons. Langmuir 2020, 36, 3862–3870. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, R.; Wang, B.; Wei, J.; Wang, L.; Shen, M.; Yang, J. Enhanced N-doped porous carbon derived from KOH-activated waste wool: A promising material for selective adsorption of CO2/CH4 and CH4/N2. Nanomaterials 2019, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Carrott, P.J.M.; Cansado, I.P.P.; Carrott, M.M.L.R. Carbon molecular sieves from PET for separations involving CH4, CO2, O2 and N2. Appl. Surf. Sci. 2006, 252, 5948–5952. [Google Scholar] [CrossRef]
- Lozano-Castello, D.; Alcaniz-Monge, J.; Cazorla-Amorós, D.; Linares-Solano, A.; Zhu, W.; Kapteijn, F.; Moulijn, J. Adsorption properties of carbon molecular sieves prepared from an activated carbon by pitch pyrolysis. Carbon 2005, 43, 1643–1651. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, X.; He, N.; Song, F.; Wang, X. Investigation on novel deep eutectic solvents with high carbon dioxide adsorption performance. J. Environ. Chem. Eng. 2025, 13, 117870. [Google Scholar] [CrossRef]
- Fang, Q.; Sun, Q.; Ge, J.; Wang, H.; Qi, J. Multidimensional Engineering of Nanoconfined Catalysis: Frontiers in Carbon-Based Energy Conversion and Utilization. Catalysts 2025, 15, 477. [Google Scholar] [CrossRef]
- Li, B.; Wang, X.; Khurshid, A.; Saleem, S.F. Environmental governance, green finance, and mitigation technologies: Pathways to carbon neutrality in European industrial economies. Int. J. Environ. Sci. Technol. 2025, 22, 14899–14912. [Google Scholar] [CrossRef]
- Mohammadi, M.; Najafpour, G.; Mohamed, A. Production of carbon molecular sieves from palm shell through carbon deposition from methane. Chem. Ind. Chem. Eng. Q. 2011, 17, 525–533. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Wan Daud, W.M.A.; Aroua, M.K. Adsorption kinetics of various gases in carbon molecular sieves (CMS) produced from palm shell. Colloids Surf. A. 2008, 312, 131–135. [Google Scholar] [CrossRef]
- Li, Y.; Xu, R.; Wang, X.; Wang, B.; Cao, J.; Yang, J.; Wei, J. Waste wool derived nitrogen-doped hierarchical porous carbon for selective CO2 capture. RSC Adv. 2018, 8, 19818–19826. [Google Scholar] [CrossRef]
- Cui, X.; Bustin, R.M.; Dipple, G. Selective transport of CO2, CH4, and N2 in coals: Insights from modeling of experimental gas adsorption data. Fuel 2004, 83, 293–303. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Wang, B.; Wang, Y.; Wei, J. Sustainable biomass glucose-derived porous carbon spheres with high nitrogen doping: As a promising adsorbent for CO2/CH4/N2 adsorptive separation. Nanomaterials 2020, 10, 174. [Google Scholar] [CrossRef]
- Peredo-Mancilla, D.; Ghimbeu, C.M.; Réty, B.; Ho, B.-N.; Pino, D.; Vaulot, C.; Hort, C.; Bessieres, D. Surface-Modified Activated Carbon with a Superior CH4/CO2 Adsorption Selectivity for the Biogas Upgrading Process. Ind. Eng. Chem. Res. 2022, 61, 12710–12727. [Google Scholar] [CrossRef]
- Bai, R.; Yang, M.; Hu, G.; Xu, L.; Hu, X.; Li, Z.; Wang, S.; Dai, W.; Fan, M. A new nanoporous nitrogen-doped highly-efficient carbonaceous CO2 sorbent synthesized with inexpensive urea and petroleum coke. Carbon 2015, 81, 465–473. [Google Scholar] [CrossRef]
- Javadi Shokroo, E.; Farniaei, M.; Baghbani, M. Simulation study of pressure swing adsorption to purify helium using zeolite 13X. Appl. Chem. Eng. 2020, 3, 1–7. [Google Scholar] [CrossRef]
- Durán, I.; Rubiera, F.; Pevida, C. Modeling a biogas upgrading PSA unit with a sustainable activated carbon derived from pine sawdust. Sensitivity analysis on the adsorption of CO2 and CH4 mixtures. Chem. Eng. J. 2022, 428, 1–14. [Google Scholar] [CrossRef]
- Balsamo, M.; Silvestre-Albero, A.; Silvestre-Albero, J.; Erto, A.; Rodríguez-Reinoso, F.; Lancia, A. Assessment of CO2 Adsorption Capacity on Activated Carbons by a Combination of Batch and Dynamic Tests. Langmuir 2014, 30, 5840–5848. [Google Scholar] [CrossRef]
- Li, L.; Liu, D.; Zhen, D.; Ge, Z.; Zhang, X.; Xiong, B.; Li, Z.; Zhang, K.; Xing, T.; Xu, W.; et al. Thermodynamic-Kinetic Synergistic Separation for O2/N2 and CO2/CH4 on Nanoporous Carbon Molecular Sieves. ACS Appl. Nano Mater. 2022, 5, 11414–11422. [Google Scholar] [CrossRef]
- Xu, R.; Xian, X.; Song, Z.; Gu, M. The impact of effective pore percentage on CH4/N2 separation in coal-based activated carbon. J. Mater. Sci. 2023, 58, 13635–13648. [Google Scholar] [CrossRef]
- Mestre, A.S.; Freire, C.; Pires, J.; Carvalho, A.P.; Pinto, M.L. High performance microspherical activated carbons for methane storage and landfill gas or biogas upgrade. J. Mater. Chem. A. 2014, 2, 15337–15344. [Google Scholar] [CrossRef]
- Cruz, O.F.; Gómez, I.C.; Escandell, M.M.; Rambo, C.R.; Silvestre-Albero, J. Activated carbon from polyurethane residues as molecular sieves for kinetic adsorption/separation of CO2/CH4. Colloids Surf. A 2022, 652, 1–7. [Google Scholar] [CrossRef]
- Rocha, L.A.M.; Andreassen, K.A.; Grande, C.A. Separation of CO2/CH4 using carbon molecular sieve (CMS) at low and high pressure. Chem. Eng. Sci. 2017, 164, 148–157. [Google Scholar] [CrossRef]
- Neimark, A.V.; Lin, Y.; Ravikovitch, P.I.; Thommes, M. Quenched solid density functional theory and pore size analysis of micro-mesoporous carbons. Carbon 2009, 47, 1617–1628. [Google Scholar] [CrossRef]
- Zhou, H.; Guo, J.; Zhu, G.; Xie, F.; Tang, X.; Luo, X. Highly efficient preparation of crystalline yttrium carbonate in sodium carbonate system: Formation and growth mechanism. J. Rare. Earth. 2025, 43, 1492–1501. [Google Scholar] [CrossRef]
- Yang, Q.; Kang, Q.; Huang, Q.; Cui, Z.; Bai, Y.; Wei, H. Linear correlation analysis of ammunition storage environment based on Pearson correlation analysis. J. Phys. Conf. Ser. 2021, 1948, 012064. [Google Scholar] [CrossRef]
- Cai, Z.; Zheng, Z.; Jiang, X. Composition analysis and identification of glass products based on Pearson correlation analysis. Highlights Sci. Eng. Technol. 2022, 22, 174–186. [Google Scholar] [CrossRef]
- Aghel, B.; Behaein, S.; Alobaid, F. CO2 capture from biogas by biomass-based adsorbents: A review. Fuel 2022, 328, 1–18. [Google Scholar] [CrossRef]
- Foeth, F.; Andersson, M.; Bosch, H.; Aly, G.; Reith, T. Separation of Dilute CO2-CH4 Mixtures by Adsorption on Activated Carbon. Sep. Sci. Technol. 1994, 29, 93–118. [Google Scholar] [CrossRef]
- Golmakani, A.; Fatemi, S.; Tamnanloo, J. CO2 Capture from the Tail Gas of Hydrogen Purification Unit by Vacuum Swing Adsorption Process, Using SAPO-34. Ind. Eng. Chem. Res. 2015, 55, 334–350. [Google Scholar] [CrossRef]
- Hamid, U.; Vyawahare, P.; Tun, H.; Chen, C. Generalization of thermodynamic Langmuir isotherm for mixed-gas adsorption equilibria. AIChE J. 2022, 68, e17663. [Google Scholar] [CrossRef]
- Valleroy, Z.; dos Santos, G.; Lombardi, T.; Wexler, C. Adsorption of Natural Gas Mixtures of Methane, Ethane, and Propane in Nanoporous Carbon: Fully Atomistic Numerical Studies. Langmuir 2020, 36, 3690–3702. [Google Scholar] [CrossRef]
- Simonin, J.-P. On the comparison of pseudo-first order and pseudo-second order rate laws in the modeling of adsorption kinetics. Chem. Eng. J. 2016, 300, 254–263. [Google Scholar] [CrossRef]
- Gu, M.; Zhang, B.; Qi, Z.; Liu, Z.; Duan, S.; Du, X.; Xian, X. Effects of pore structure of granular activated carbons on CH4 enrichment from CH4/N2 by vacuum pressure swing adsorption. Sep. Purif. Technol. 2015, 146, 213–218. [Google Scholar] [CrossRef]
- Zhang, B.; Huang, Z.; Liu, P.; Liu, J.; Gu, M. Influence of pore structure of granular activated carbon prepared from anthracite on the adsorption of CO2, CH4 and N2. Korean J. Chem. Eng. 2022, 39, 724–735. [Google Scholar] [CrossRef]

| Fixed Bed Specifications | Value |
|---|---|
| Adsorbent packing mass | 20 g |
| Fixed bed length | 0.27 m |
| Fixed bed inner diameter | 0.014 m |
| Sample | Equilibrium Adsorption Capacity (mmol g−1) | SCO2/CH4 | Notes | |
|---|---|---|---|---|
| CO2 | CH4 | |||
| NX-1 | 0.80 | 0.36 | 2.24 | More than half of the samples have an SCO2/CH4 above 1.32. |
| ZZ-1 | 0.74 | 0.56 | 1.32 | |
| CX-1 | 0.92 | 0.60 | 1.75 | |
| D-4 | 0.78 | 0.30 | 3.21 | This study |
| D-6 | 0.83 | 0.12 | 10.98 | This study |
| D-10 | 0.74 | 0.33 | 2.93 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, J.; Xu, R.; Song, Z.; Xiao, Z.; Guo, S.; Geng, W.; Xian, X. Effective Pore Distribution and Mechanism of CO2/CH4 Dynamic Separation by Carbon Molecular Sieves. Nanomaterials 2025, 15, 1685. https://doi.org/10.3390/nano15211685
Gu J, Xu R, Song Z, Xiao Z, Guo S, Geng W, Xian X. Effective Pore Distribution and Mechanism of CO2/CH4 Dynamic Separation by Carbon Molecular Sieves. Nanomaterials. 2025; 15(21):1685. https://doi.org/10.3390/nano15211685
Chicago/Turabian StyleGu, Jianhong, Ran Xu, Zhenlong Song, Zejun Xiao, Shengli Guo, Weile Geng, and Xuefu Xian. 2025. "Effective Pore Distribution and Mechanism of CO2/CH4 Dynamic Separation by Carbon Molecular Sieves" Nanomaterials 15, no. 21: 1685. https://doi.org/10.3390/nano15211685
APA StyleGu, J., Xu, R., Song, Z., Xiao, Z., Guo, S., Geng, W., & Xian, X. (2025). Effective Pore Distribution and Mechanism of CO2/CH4 Dynamic Separation by Carbon Molecular Sieves. Nanomaterials, 15(21), 1685. https://doi.org/10.3390/nano15211685




