Pack Cementation Route to Ag2Se: Correlating Structure, Phase Formation, and Thermoelectric Performance
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
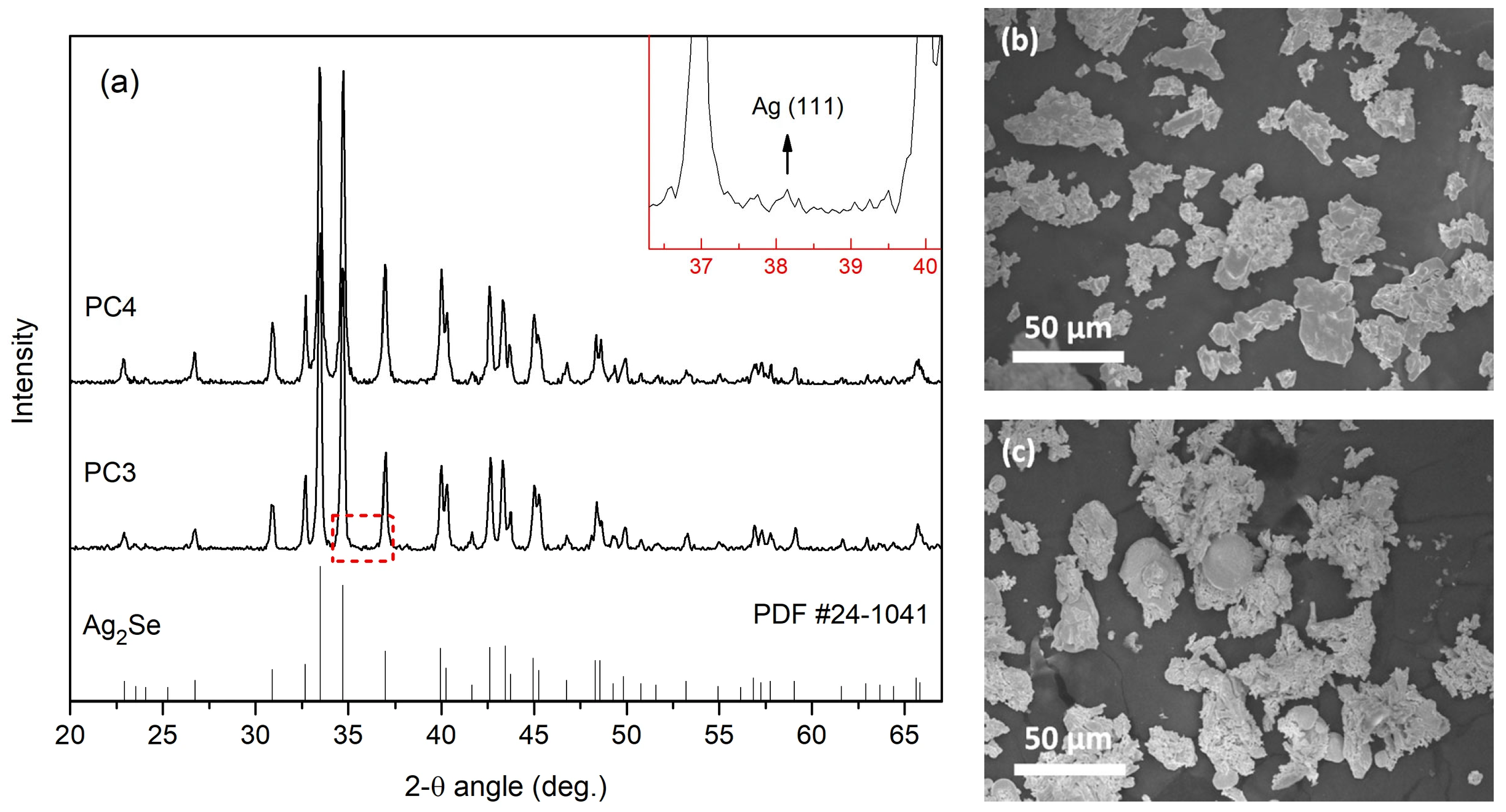
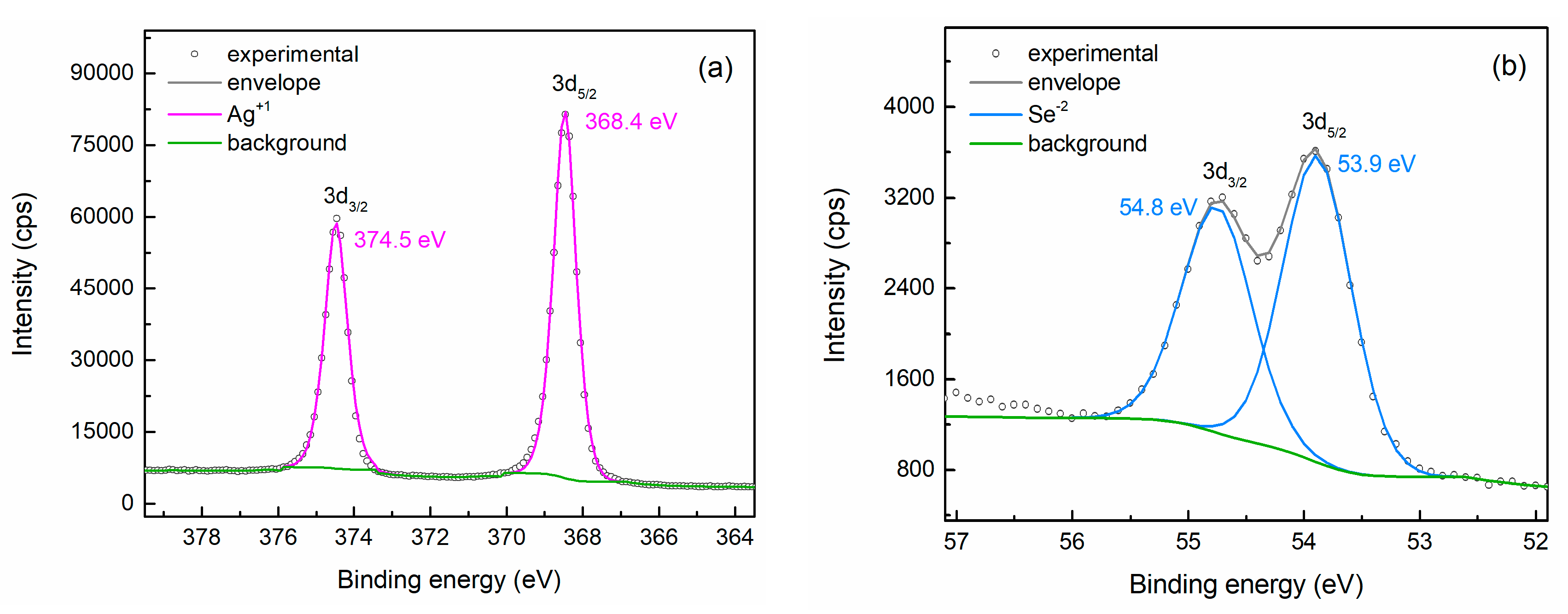
3.1. Phase Composition, Structure, and Chemical Analysis
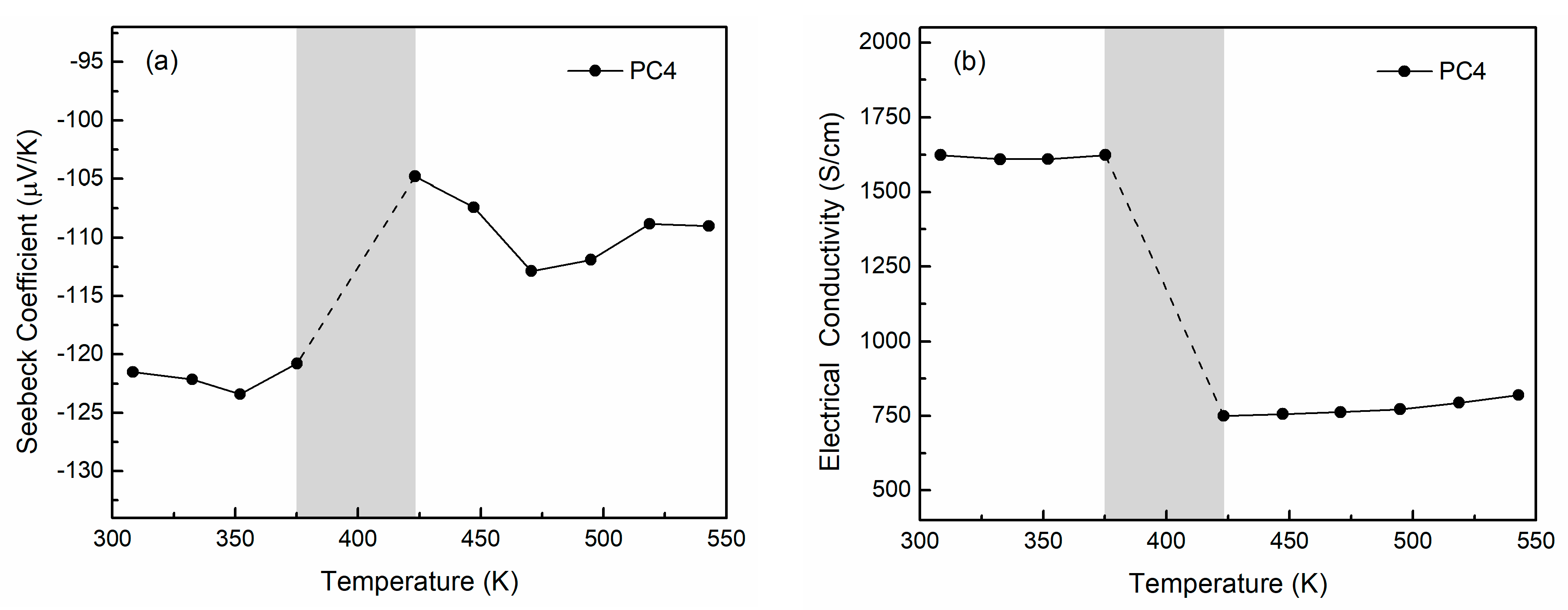
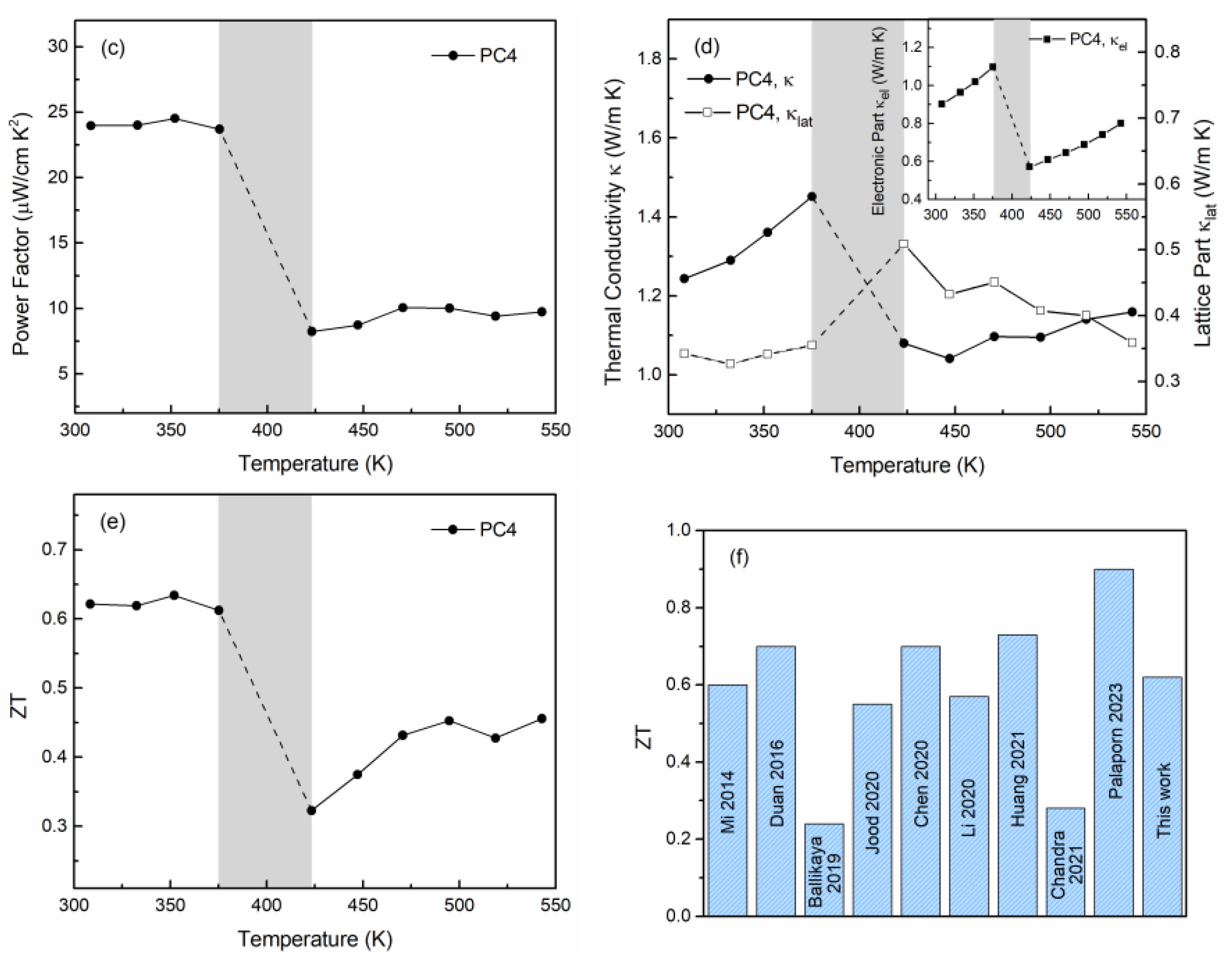
3.2. Thermoelectric Properties
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- D’Angelo, M.; Galassi, C.; Lecis, N. Thermoelectric Materials and Applications: A Review. Energies 2023, 16, 6409. [Google Scholar] [CrossRef]
- Neeli, G.; Behara, D.K.; Kumar, M.K. State of the Art Review on Thermoelectric Materials. Int. J. Sci. Res. 2016, 5, 1833–1844. [Google Scholar] [CrossRef]
- Yu, K.; Zhou, Y.; Liu, Y.; Liu, F.; Hu, L.; Ao, W.; Zhang, C.; Li, Y.; Li, J.; Xie, H. Near-room-temperature thermoelectric materials and their application prospects in geothermal power generation. Geomech. Geophys. Geo-Energy Geo-Resour. 2020, 6, 12. [Google Scholar] [CrossRef]
- Nan, B.; Li, M.; Zhang, Y.; Xiao, K.; Lim, K.H.; Chang, C.; Han, X.; Zuo, Y.; Li, J.; Arbiol, J.; et al. Engineering of Thermoelectric Composites Based on Silver Selenide in Aqueous Solution and Ambient Temperature. ACS Appl. Electron. Mater. 2023, 6, 2807–2815. [Google Scholar] [CrossRef]
- Santhosh, R.; Abinaya, R.; Archana, J.; Ponnusamy, S.; Harish, S.; Navaneethan, M.J. Controlled grain boundary interfaces of reduced graphene oxide in Ag2Se matrix for low lattice thermal conductivity and enhanced power factor for thermoelectric applications. Power Sources 2022, 525, 231045. [Google Scholar] [CrossRef]
- Yang, D.; Su, X.; Meng, F.; Wang, S.; Yan, Y.; Yang, J.; He, J.; Zhang, Q.; Uher, C.; Kanatzidis, M.G.; et al. Facile room temperature solventless synthesis of high thermoelectric performance Ag2Se: Via a dissociative adsorption reaction. J. Mater. Chem. A 2017, 5, 23243–23251. [Google Scholar] [CrossRef]
- Ferhat, M.; Nagao, J. Thermoelectric and transport properties of β-Ag2Se compounds. J. Appl. Phys. 2000, 88, 813–816. [Google Scholar] [CrossRef]
- Singh, S.; Hirata, K.; Byeon, D.; Matsunaga, T.; Muthusamy, O.; Ghodke, S.; Adachi, M.; Yamamoto, Y.; Matsunami, M.; Takeuchi, T. Investigation of Thermoelectric Properties of Ag2SxSe1−x (x = 0.0, 0.2 and 0.4). J. Electron. Mater. 2020, 49, 2846–2854. [Google Scholar] [CrossRef]
- Jin, M.; Liang, J.; Qiu, P.; Huang, H.; Yue, Z.; Zhou, L.; Li, R.; Chen, L.; Shi, X. Investigation on Low-Temperature Thermoelectric Properties of Ag2Se Polycrystal Fabricated by Using Zone-Melting Method. J. Phys. Chem. Lett. 2021, 12, 8246–8255. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Park, Y.H.; Hashimoto, H. Effect of nonstoichiometry on the thermoelectric properties of a Ag2Se alloy prepared by a mechanical alloying process. J. Appl. Phys. 2007, 101, 024920. [Google Scholar] [CrossRef]
- Chen, J.; Sun, Q.; Bao, D.; Liu, T.; Di Liu, W.; Liu, C.; Tang, J.; Zhou, D.; Yang, L.; Chen, Z.G. Hierarchical Structures Advance Thermoelectric Properties of Porous n-type β-Ag2Se. ACS Appl. Mater. Interfaces 2020, 12, 51523–51529. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.Z.; Li, Y.L.; Zhao, K.P.; Qiu, P.F.; Shi, X.; Chen, L.D. Ultra-Fast Synthesis for Ag2Se and CuAgSe Thermoelectric Materials. JOM 2016, 68, 2659–2665. [Google Scholar] [CrossRef]
- Jood, P.; Chetty, R.; Ohta, M. Structural stability enables high thermoelectric performance in room temperature Ag2Se. J. Mater. Chem. A 2020, 8, 13024–13037. [Google Scholar] [CrossRef]
- Mi, W.; Qiu, P.; Zhang, T.; Lv, Y.; Shi, X.; Chen, L. Efficient degradation of high concentration azo-dye wastewater by heterogeneous Fenton process with iron-based metal-organic framework. Appl. Phys. Lett. 2014, 104, 133903. [Google Scholar] [CrossRef]
- Aliev, F.F.; Jafarov, M.B.; Eminova, V.I. Thermoelectric figure of merit of Ag2Se with Ag and Se excess. Semiconductors 2009, 43, 977–979. [Google Scholar] [CrossRef]
- Perez-Taborda, J.A.; Caballero-Calero, O.; Vera-Londono, L.; Briones, F.; Martin-Gonzalez, M. High Thermoelectric zT in n-Type Silver Selenide films at Room Temperature. Adv. Energy Mater. 2018, 8, 1702024. [Google Scholar] [CrossRef]
- Wang, P.; Chen, J.L.; Zhou, Q.; Liao, Y.T.; Peng, Y.; Liang, J.S.; Miao, L. Enhancing the thermoelectric performance of Ag2Se by non-stoichiometric defects. Appl. Phys. Lett. 2022, 120, 193902. [Google Scholar] [CrossRef]
- Rettie, A.J.E.; Malliakas, C.D.; Botana, A.S.; Hodges, J.M.; Han, F.; Huang, R.; Chung, D.Y.; Kanatzidis, M.G. Ag2Se to KAg3Se2: Suppressing Order-Disorder Transitions via Reduced Dimensionality. J. Am. Chem. Soc. 2018, 140, 9193–9202. [Google Scholar] [CrossRef]
- Santhosh Kumar, M.C.; Pradeep, B. Electrical properties of silver selenide thin films prepared by reactive evaporation. Bull. Mater. Sci. 2002, 25, 407–411. [Google Scholar] [CrossRef]
- Day, T.; Drymiotis, F.; Zhang, T.; Rhodes, D.; Shi, X.; Chen, L.; Jeffrey Snyder, G. Evaluating the potential for high thermoelectric efficiency of silver selenide. J. Mater. Chem. C 2013, 1, 7568–7573. [Google Scholar] [CrossRef]
- Wang, H.; Chu, W.; Wang, D.; Mao, W.; Pan, W.; Guo, Y.; Xiong, Y.; Jin, H. Low-Temperature Thermoelectric Properties of β-Ag2Se Synthesized by Hydrothermal Reaction. J. Electron. Mater. 2011, 40, 624–628. [Google Scholar] [CrossRef]
- Wang, D.; Xie, T.; Peng, Q.; Li, Y. Ag, Ag2S, and Ag2Se nanocrystals: Synthesis, assembly, and construction of mesoporous structures. J. Am. Chem. Soc. 2008, 130, 4016–4022. [Google Scholar] [CrossRef]
- Sahu, A.; Qi, L.; Kang, M.S.; Deng, D.; Norris, D.J. Facile synthesis of silver chalcogenide (Ag2E; E = Se, S, Te) semiconductor nanocrystals. J. Am. Chem. Soc. 2011, 133, 6509–6512. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, J.H.; Li, J.M.; Zhang, J.; Qin, X.Y. High thermoelectric performance for an Ag2Se-based material prepared by a wet chemical method. Mater. Chem. Front. 2020, 4, 875–880. [Google Scholar] [CrossRef]
- Genovese, L.; Cocchiara, C.; Piazza, S.; Sunseri, C.; Inguanta, R. Electrochemical deposition of Ag2Se nanostructures. Mater. Res. Bull. 2017, 86, 10–18. [Google Scholar] [CrossRef]
- Chaliampalias, D.; Papazoglou, M.; Tsipas, S.; Pavlidou, E.; Skolianos, S.; Stergioudis, G.; Vourlias, G. The effect of Al and Cr additions on pack cementation zinc coatings. Appl. Surf. Sci. 2010, 256, 3618–3623. [Google Scholar] [CrossRef]
- Michel, G.; Berthod, P.; Mathieu, S.; Vilasi, M.; Steinmetz, P. Chromium Deposition on Cobalt-Based Alloys by Pack-Cementation and Behaviour of the Coated Alloys in High Temperature Oxidation. Open Corros. J. 2011, 4, 27–33. [Google Scholar] [CrossRef][Green Version]
- Stathokostopoulos, D.; Teknetzi, A.; Tarani, E.; Karfaridis, D.; Chrissafis, K.; Hatzikraniotis, E.; Vourlias, G. Synthesis and characterization of nanostructured Mg2Si by pack cementation process. Results Mater. 2022, 13, 100252. [Google Scholar] [CrossRef]
- Tarani, E.; Chaliampalias, D.; Pavlidou, E.; Chrissafis, K.; Vourlias, G. Thermal oxidation kinetics of CrSi2 powder synthesized by pack cementation process. J. Therm. Anal. Calorim. 2016, 125, 111–120. [Google Scholar] [CrossRef]
- Teknetzi, A.; Tarani, E.; Symeou, E.; Karfaridis, D.; Stathokostopoulos, D.; Pavlidou, E.; Kyratsi, T.; Hatzikraniotis, E.; Chrissafis, K.; Vourlias, G. Structure and thermoelectric properties of higher manganese silicides synthesized by pack cementation. Ceram. Int. 2021, 47, 243–251. [Google Scholar] [CrossRef]
- Teknetzi, A.; Stathokostopoulos, D.; Ioannou, I.; Tarani, E.; Hastas, N.; Kyratsi, T.; Vourlias, G. Synthesis and thermoelectric properties of Cr-substituted higher manganese silicides prepared by pack cementation. Mater. Res. Bull. 2023, 160, 112118. [Google Scholar] [CrossRef]
- International Centre for Diffraction Data (ICDD) (Formerly Joint Committee on Powder Diffraction Standards-JCPDS). Powder Diffraction File (PDF); International Centre for Diffraction Data: Newtown Square, PA, USA, 2003. [Google Scholar]
- Niu, J.; Chen, T.; Liang, G.; Ma, H.; Zhang, X.; Fan, P.; Zheng, Z. In-situ growth of high room temperature thermoelectric performance Ag2Se thin films. Mater. Lett. 2022, 312, 131662. [Google Scholar] [CrossRef]
- Park, D.; Kim, M.; Kim, J. Fabrication of pedot: Pss/Ag2Se nanowires for polymer-based thermoelectric applications. Polymers 2020, 12, 2932. [Google Scholar] [CrossRef]
- Yang, X.; Wang, C.; Zhang, X.; Wang, Y.; Gao, F.; Sun, L.; Xu, W.; Qiao, C.; Zhang, G. Photothermal and adsorption effects of silver selenide nanoparticles modified by different surfactants in nursing care of cancer patients. Sci. Technol. Adv. Mater. 2020, 21, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Vongehr, S.; Tang, S.; Dai, Y.; Huang, X.; Meng, X. Scalable Synthesis of Ag Networks with Optimized Sub-monolayer Au-Pd Nanoparticle Covering for Highly Enhanced SERS Detection and Catalysis. Sci. Rep. 2016, 6, 37092. [Google Scholar] [CrossRef]
- De Paiva, A.B.; Vargas, L.M.B.; da Silva, M.J.; Rodrigues, A.D.G.; Soares, D.A.W.; Peres, M.L.; de Godoy, M.P.F. The Negative Photoconductivity of Ag/AgO Grown by Spray-Pyrolysis. Surfaces 2022, 5, 209–217. [Google Scholar] [CrossRef]
- Sun, F.; Li, Y.; Wu, Z.; Liu, Y.; Tang, H.; Li, X.; Yue, Z.; Zhou, L. In situ reactive coating of metallic and selenophilic Ag2Se on Se/C cathode materials for high performance Li-Se batteries. RSC Adv. 2018, 8, 32808–32813. [Google Scholar] [CrossRef]
- Tee, S.Y.; Tan, X.Y.; Wang, X.; Lee, C.J.J.; Win, K.Y.; Ni, X.P.; Teo, S.L.; Seng, D.H.L.; Tanaka, Y.; Han, M.Y. Aqueous Synthesis, Doping, and Processing of n-Type Ag2Se for High Thermoelectric Performance at Near-Room-Temperature. Inorg. Chem. 2022, 61, 6451–6458. [Google Scholar] [CrossRef]
- Korte, C.; Janek, J. Ionic Conductivity, Partial Thermopowers, Heats of Transport and the Soret Effect of α-Ag2+δSe—An Experimental Study. Z. Phys. Chem. 1998, 206, 29–63. [Google Scholar] [CrossRef]
- Hou, S.; Liu, Y.; Yin, L.; Chen, C.; Wu, Z.; Wang, J.; Luo, Y.; Xue, W.; Liu, X.; Zhang, Q.; et al. High performance wearable thermoelectric generators using Ag2Se films with large carrier mobility. Nano Energy 2021, 87, 106223. [Google Scholar] [CrossRef]
- Abrikosov, A.A. Quantum linear magnetoresistance. Europhys. Lett. 2000, 49, 789. [Google Scholar] [CrossRef]
- Lin, S.; Guo, L.; Wang, X.; Liu, Y.; Wu, Y.; Li, R.; Shao, H.; Jin, M. Revealing the promising near-room-temperature thermoelectric performance in Ag2Se single crystals. J. Mater. 2023, 9, 754–761. [Google Scholar] [CrossRef]
- Tarani, E.; Stathokostopoulos, D.; Karfaridis, D.; Malletzidou, L.; Sfampa, I.K.; Stergioudi, F.; Maliaris, G.; Michailidis, N.; Chrissafis, K.; Vourlias, G. Effect of ball milling time on the formation and thermal properties of Ag2Se and Cu2Se compounds. J. Therm. Anal. Calorim. 2023, 148, 13065–13081. [Google Scholar] [CrossRef]
- Batabyal, S.K.; Basu, C.; Das, A.R.; Sanyal, G.S.; Banerjee, D.; Bandyopadhyay, N.R. Studies on nanocrystalline Ag2Se. Mater. Manuf. Process. 2006, 21, 694–697. [Google Scholar] [CrossRef]
- Ohachi, T.; Taniguchi, I. Measurement of the thermal diffusion coefficient of silver atoms in α-silver selenide. Solid State Ion. 1981, 3–4, 89–92. [Google Scholar] [CrossRef]
- Huang, S.; Wei, T.R.; Chen, H.; Xiao, J.; Zhu, M.; Zhao, K.; Shi, X. Thermoelectric Ag2Se: Imperfection, Homogeneity, and Reproducibility. ACS Appl. Mater. Interfaces 2021, 13, 60192–60199. [Google Scholar] [CrossRef]
- Bolghanabadi, N.; Sajjadi, S.A.; Babakhani, A.; Saberi, Y. Effects of Synthesis Parameters and Thickness on Thermoelectric Properties of Bi2Te3 Fabricated Using Mechanical Alloying and Spark Plasma Sintering. J. Electron. Mater. 2021, 50, 1331–1339. [Google Scholar] [CrossRef]
- Wu, C.F.; Wei, T.R.; Sun, F.H.; Li, J.F. Nanoporous PbSe–SiO2 Thermoelectric Composites. Adv. Sci. 2017, 4, 1700199. [Google Scholar] [CrossRef]
- Wu, H.; Shi, X.L.; Duan, J.; Liu, Q.; Chen, Z.G. Advances in Ag2Se-based thermoelectrics from materials to applications. Energy Environ. Sci. 2023, 16, 1870–1906. [Google Scholar] [CrossRef]
- Grønvold, F.; Stølen, S.; Semenov, Y. Heat capacity and thermodynamic properties of silver(I) selenide, oP-Ag2Se from 300 to 406 K and of cI-Ag2Se from 406 to 900 K: Transitional behavior and formation properties. Thermochim. Acta 2003, 399, 213–224. [Google Scholar] [CrossRef]
- Goldsmid, H.J.; Sharp, J.W. Estimation of the thermal band gap of a semiconductor from Seebeck measurements. J. Electron. Mater. 1999, 28, 869–872. [Google Scholar] [CrossRef]
- Palaporn, D.; Kurosaki, K.; Pinitsoontorn, S. Effect of Sintering Temperature on the Thermoelectric Properties of Ag2Se Fabricated by Spark Plasma Sintering with High Compression. Adv. Energy Sustain. Res. 2023, 4, 2300082. [Google Scholar] [CrossRef]
- Ding, Y.; Qiu, Y.; Cai, K.; Yao, Q.; Chen, S.; Chen, L.; He, J. High performance n-type Ag2Se film on nylon membrane for flexible thermoelectric power generator. Nat. Commun. 2019, 10, 841. [Google Scholar] [CrossRef]
- Sharath Chandra, L.S.; Ramjan, S.K.; Banik, S.; Sagdeo, A.; Chattopadhyay, M.K. Temperature-induced first-order electronic topological transition in β-Ag2Se. Appl. Phys. Lett. 2021, 118, 143905. [Google Scholar] [CrossRef]
- Ballikaya, S.; Oner, Y.; Temel, T.; Ozkal, B.; Bailey, T.P.; Toprak, M.S.; Uher, C. Thermoelectric and thermal stability improvements in Nano-Cu2Se included Ag2Se. J. Solid State Chem. 2019, 273, 122–127. [Google Scholar] [CrossRef]

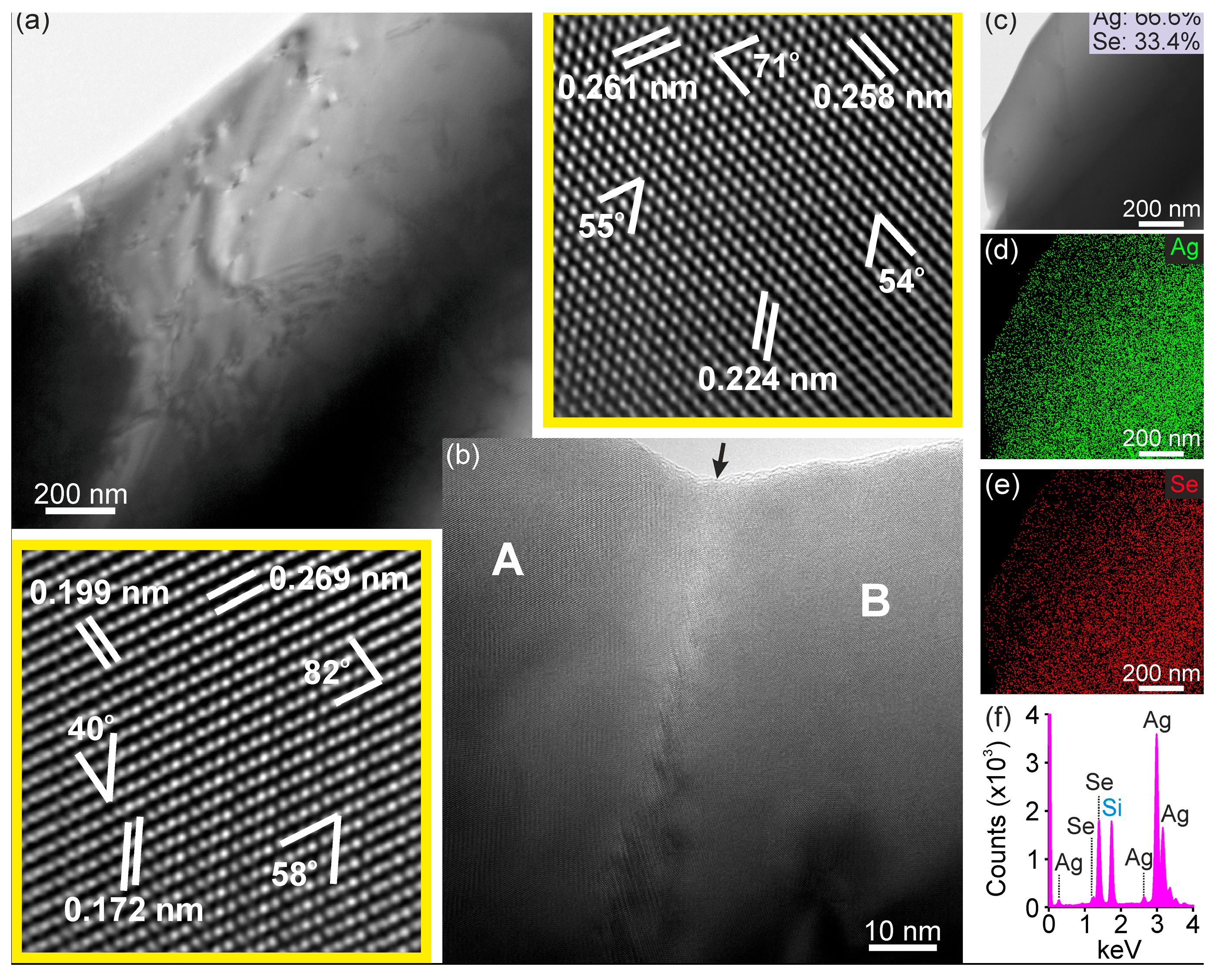


| d-Spacing Grain A (nm) | Angle (°) | d-Spacing Grain B (nm) | Angle (°) |
|---|---|---|---|
| d1 = 0.269 nm | <d1d2> = 82 | d1 = 0.261 nm | <d1d2> = 71 |
| d2 = 0.199 nm | <d2d3> = 40 | d2 = 0.258 nm | <d2d3> = 54 |
| d3 = 0.172 nm | <d3d1> = 58 | d3 = 0.224 nm | <d3d1> = 55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teknetzi, A.; Stathokostopoulos, D.; Hadjipanteli, S.; Vasileiadis, I.; Tarani, E.; Hastas, N.; Pavlidou, E.; Kehagias, T.; Kyratsi, T.; Vourlias, G. Pack Cementation Route to Ag2Se: Correlating Structure, Phase Formation, and Thermoelectric Performance. Nanomaterials 2025, 15, 1676. https://doi.org/10.3390/nano15211676
Teknetzi A, Stathokostopoulos D, Hadjipanteli S, Vasileiadis I, Tarani E, Hastas N, Pavlidou E, Kehagias T, Kyratsi T, Vourlias G. Pack Cementation Route to Ag2Se: Correlating Structure, Phase Formation, and Thermoelectric Performance. Nanomaterials. 2025; 15(21):1676. https://doi.org/10.3390/nano15211676
Chicago/Turabian StyleTeknetzi, Aikaterini, Dimitrios Stathokostopoulos, Savvas Hadjipanteli, Isaak Vasileiadis, Evangelia Tarani, Nikolaos Hastas, Eleni Pavlidou, Thomas Kehagias, Theodora Kyratsi, and George Vourlias. 2025. "Pack Cementation Route to Ag2Se: Correlating Structure, Phase Formation, and Thermoelectric Performance" Nanomaterials 15, no. 21: 1676. https://doi.org/10.3390/nano15211676
APA StyleTeknetzi, A., Stathokostopoulos, D., Hadjipanteli, S., Vasileiadis, I., Tarani, E., Hastas, N., Pavlidou, E., Kehagias, T., Kyratsi, T., & Vourlias, G. (2025). Pack Cementation Route to Ag2Se: Correlating Structure, Phase Formation, and Thermoelectric Performance. Nanomaterials, 15(21), 1676. https://doi.org/10.3390/nano15211676











