Directed Self-Assembly of an Acid-Responsive Block Copolymer for Hole-Shrink Process and Pattern Transfer
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Bromide-Terminated Poly (Methyl Methacrylate) Homopolymer (PMMA20k-Br)
2.3. Synthesis of Conventional Polystyrene-Block-Poly (Methyl Methacrylate) BCP (PS40k-b-PMMA20k)
2.4. Synthesis of Hydroxyterminated Poly (Methyl Methacrylate) Homopolymers (PMMA-OH)
2.5. Synthesis of Aromatic Aldehyde-Terminated Poly (Methyl Methacrylate) Homopolymers (PMMA-CHO)
2.6. Synthesis of Schiff Base-Linked PS-PMMA BCP (PS-N=CH-PMMA)
2.7. Synthesis of Random Copolymer Mats with Different PS Molar Fractions (FSt)
2.8. Self-Assembly of BCPs
2.9. Directed Self-Assembly of BCPs
2.10. Wet Etching Process
2.11. BCP Pattern Transfer
2.12. Ohta-Kawasaki Model Simulation Method
2.13. Characterization
3. Results and Discussion
3.1. Synthesis and Characterization of BCPs
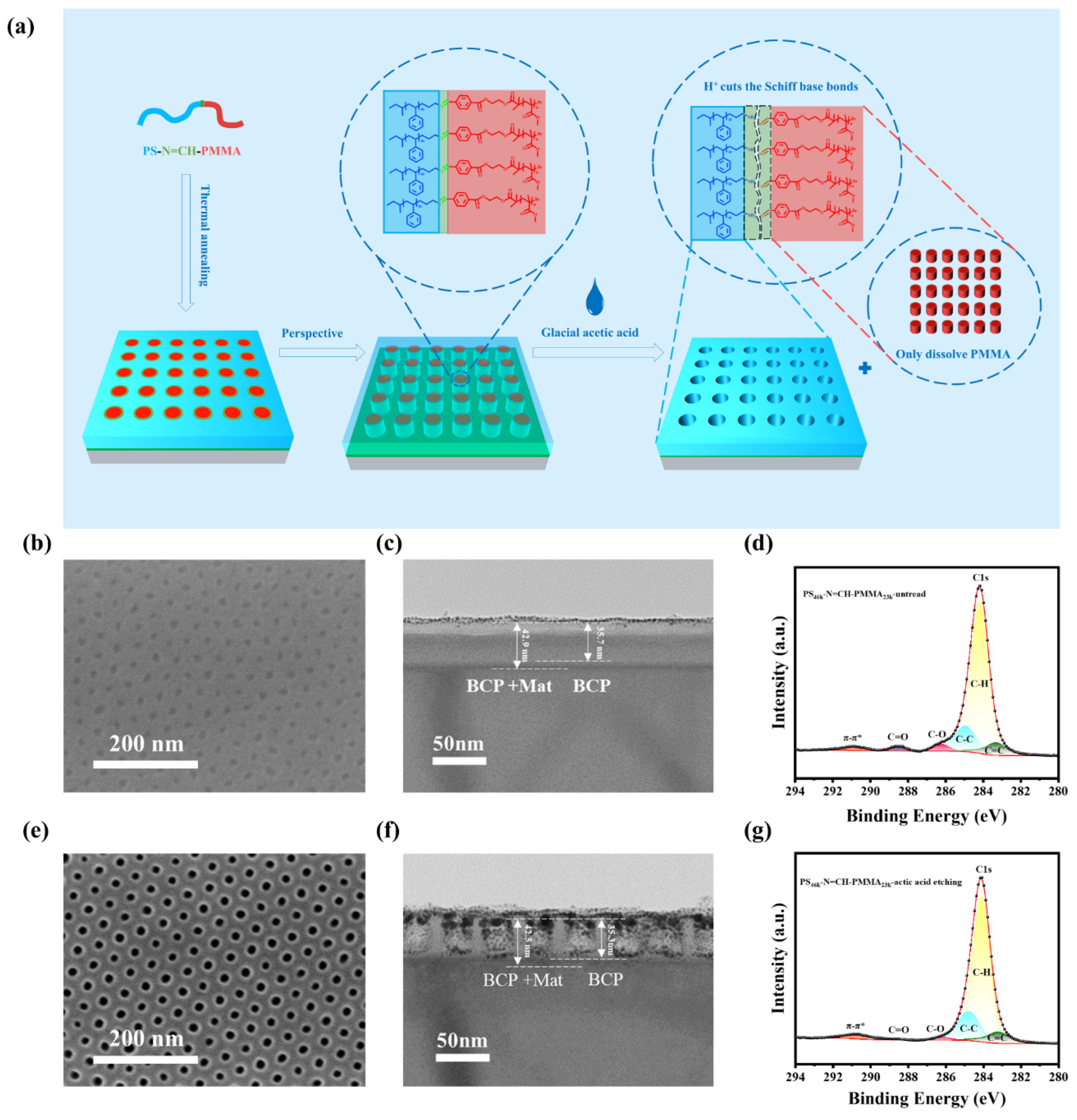
3.2. Morphological Analysis of BCP Films
3.3. Wet Etching Mechanism of the PS-N=CH-PMMA Film
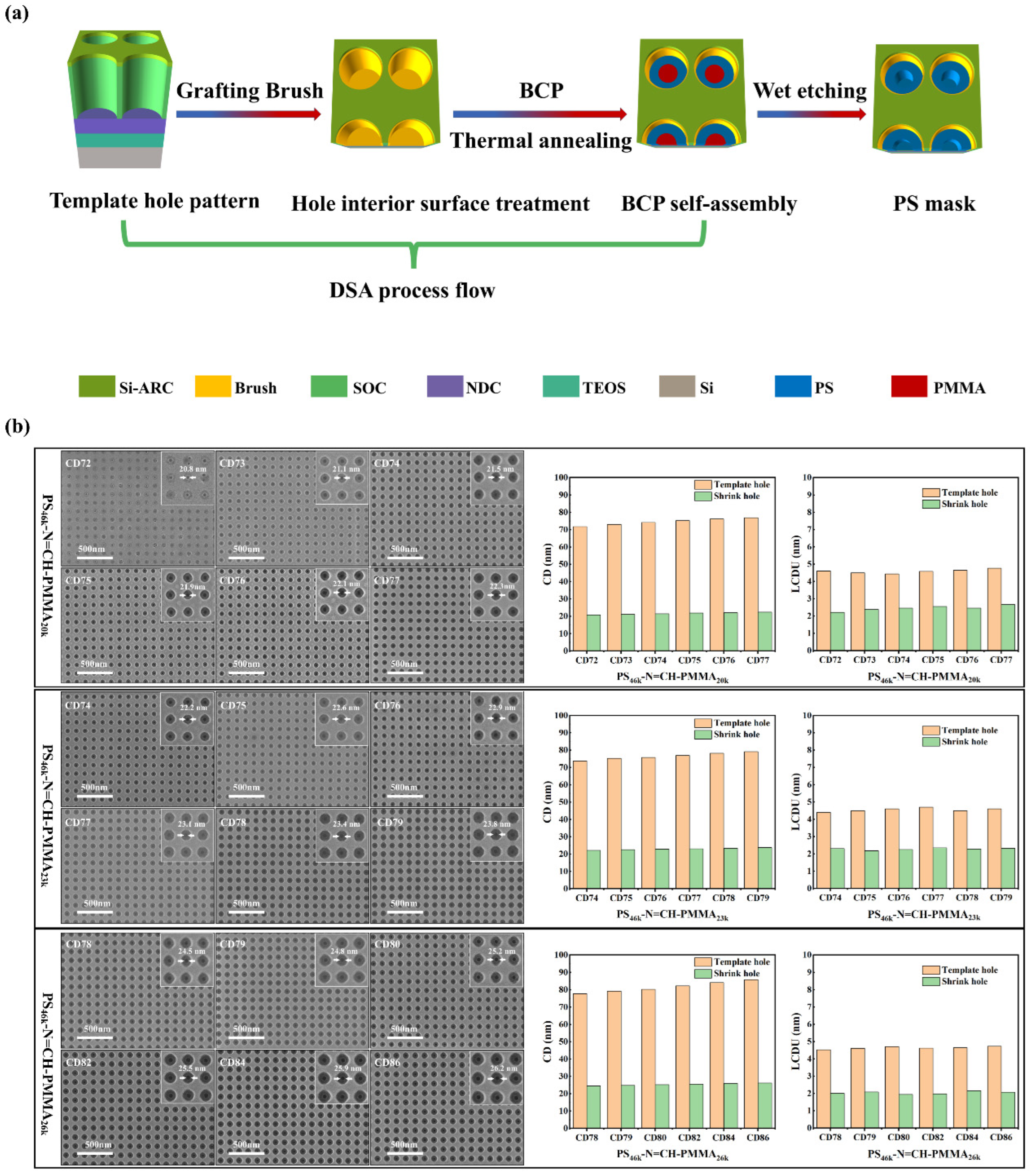
3.4. Graphoepitaxial DSA of PS-N=CH-PMMA BCPs
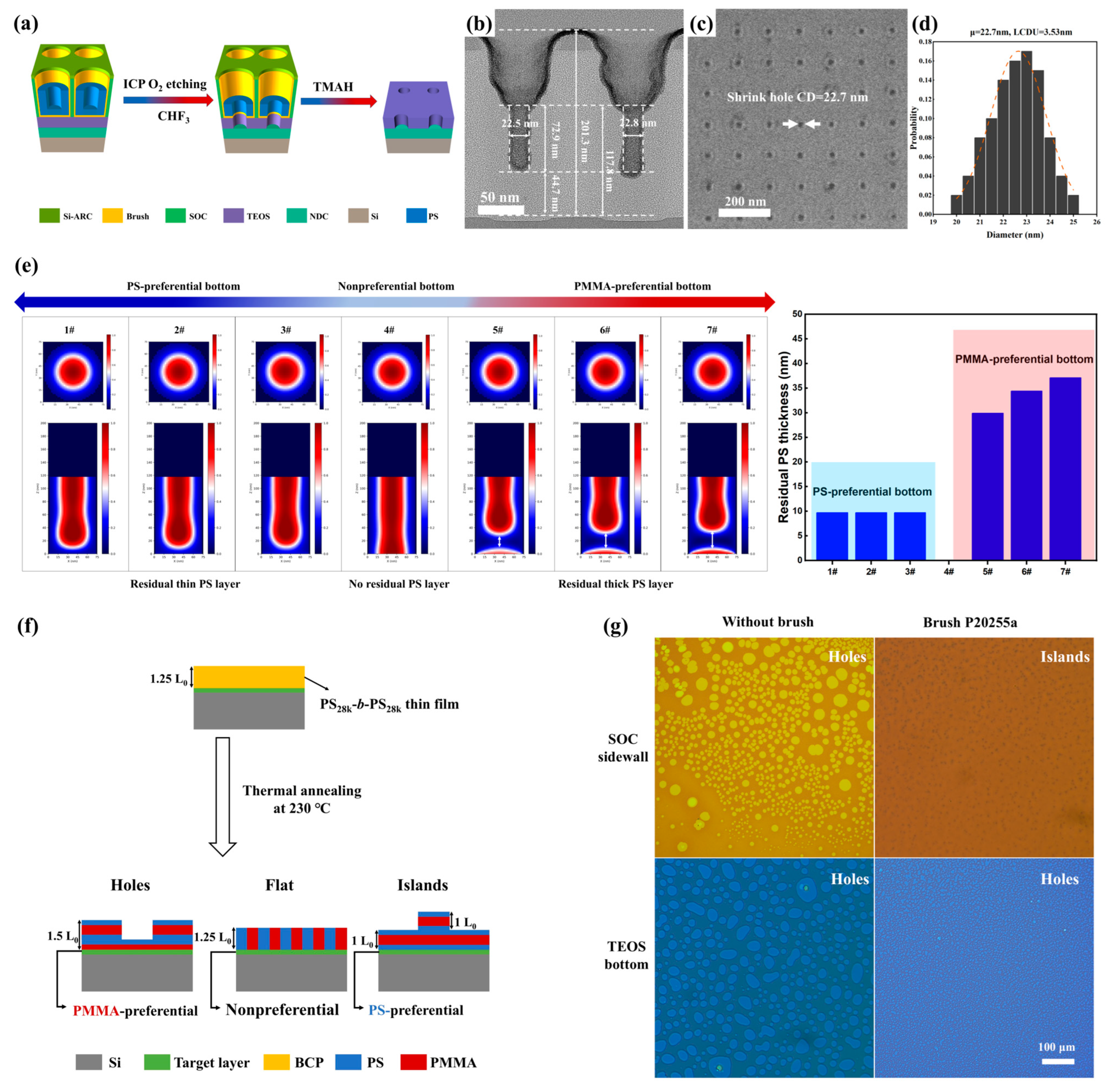
3.5. Pattern Transfer of DSA Shrink-Hole Structures into TEOS Layer
4. Conclusions
5. Patents
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Iyengar, S.A.; Bhattacharyya, S.; Roy, S.; Glavin, N.R.; Roy, A.K.; Ajayan, P.M. A researcher’s perspective on unconventional lab-to-fab for 2D semiconductor devices. ACS Nano 2023, 17, 12955–12970. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Kwon, J.; Ryu, H.; Kim, C.; Kim, H.; Lee, E.-K.; Lee, D.; Seo, S.; Han, N.M.; Suh, J.M. The future of two-dimensional semiconductors beyond Moore’s law. Nat. Nanotechnol. 2024, 19, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Stokes, K.; Clark, K.; Odetade, D.; Hardy, M.; Goldberg Oppenheimer, P. Advances in lithographic techniques for precision nanostructure fabrication in biomedical applications. Discov. Nano 2023, 18, 153. [Google Scholar] [CrossRef]
- Ryu, H.; Kwon, D.; Song, J.; Park, W.; Gwak, J.; Ko, H.; Lee, J.; Kim, J.; Ryu, K.; Hwang, S. Chemical approach to make the most of EUVL: Stochastic effect mitigation with developer and rinse. In Optical and EUV Nanolithography XXXVI, San Jose, CA, USA, 26 February–2 March 2023; SPIE: San Jose, CA, USA, 2023; Volume 12494, pp. 9–16. [Google Scholar]
- Kundu, A.; Gupta, M.; De Simone, D.; Vanelderen, P.; Suh, H.S.; De Roest, D.; Christy, D.; Davodi, F.; Patel, K.; Wallace, S. Substantial dose reduction using dry deposited underlayer for EUV lithography while maintaining roughness and minimizing defects. In Proceedings of the International Conference on Extreme Ultraviolet Lithography 2024, Monterey, CA, USA, 29 September–3 October 2024; SPIE: San Jose, CA, USA, 2024; Volume 13215, pp. 142–147. [Google Scholar]
- Zhou, K.; Zhu, X.; Li, Y.; Liu, J. Fabrication of PDMS micro through-holes using micromolding in open capillaries. RSC Adv. 2014, 4, 31988–31993. [Google Scholar] [CrossRef]
- Gentili, D.; Sonar, P.; Liscio, F.; Cramer, T.; Ferlauto, L.; Leonardi, F.; Milita, S.; Dodabalapur, A.; Cavallini, M. Logic-gate devices based on printed polymer semiconducting nanostripes. Nano Lett. 2013, 13, 3643–3647. [Google Scholar] [CrossRef]
- Wan, L.; Ruiz, R.; Gao, H.; Albrecht, T.R. Self-registered self-assembly of block copolymers. ACS Nano 2017, 11, 7666–7673. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, S. Directed self-assembly of block copolymers for sub-10 nm fabrication. Int. J. Extrem. Manuf. 2020, 2, 032006. [Google Scholar] [CrossRef]
- Huang, H.; Liu, R.; Ross, C.A.; Alexander-Katz, A. Self-directed self-assembly of 3D tailored block copolymer nanostructures. ACS Nano 2020, 14, 15182–15192. [Google Scholar] [CrossRef]
- Lazzari, M.; López-Quintela, M.A. Block copolymers as a tool for nanomaterial fabrication. Adv. Mater. 2003, 15, 1583–1594. [Google Scholar] [CrossRef]
- Kim, H.-C.; Park, S.-M.; Hinsberg, W.D. Block copolymer based nanostructures: Materials, processes, and applications to electronics. Chem. Rev. 2010, 110, 146–177. [Google Scholar] [CrossRef]
- Hu, H.; Gopinadhan, M.; Osuji, C.O. Directed self-assembly of block copolymers: A tutorial review of strategies for enabling nanotechnology with soft matter. Soft Matter 2014, 10, 3867–3889. [Google Scholar] [CrossRef]
- Ji, S.; Wan, L.; Liu, C.-C.; Nealey, P.F. Directed self-assembly of block copolymers on chemical patterns: A platform for nanofabrication. Prog. Polym. Sci. 2016, 54, 76–127. [Google Scholar] [CrossRef]
- Li, W.; Müller, M. Directed self-assembly of block copolymers by chemical or topographical guiding patterns: Optimizing molecular architecture, thin-film properties, and kinetics. Prog. Polym. Sci. 2016, 54, 47–75. [Google Scholar] [CrossRef]
- Li, J.; Rincon-Delgadillo, P.A.; Suh, H.S.; Mannaert, G.; Nealey, P.F. Understanding kinetics of defect annihilation in chemoepitaxy-directed self-assembly. ACS Appl. Mater. Interfaces 2021, 13, 25357–25364. [Google Scholar] [CrossRef]
- Radamson, H.H.; Zhu, H.; Wu, Z.; He, X.; Lin, H.; Liu, J.; Xiang, J.; Kong, Z.; Xiong, W.; Li, J. State of the art and future perspectives in advanced CMOS technology. Nanomaterials 2020, 10, 1555. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.S.; Yong, S.K.; Kim, W.; Kang, S.; Park, H.W.; Yoon, K.J.; Sheen, D.S.; Lee, S.; Hwang, C.S. Review of semiconductor flash memory devices for material and process issues. Adv. Mater. 2023, 35, 2200659. [Google Scholar] [CrossRef]
- Laachi, N.; Shykind, D.; Fredrickson, G.H. A Landau–Peierls Analysis of Contact Hole Placement in Directed Self-Assembly of Linear Arrays of Block Copolymer Cylinders. Macromolecules 2014, 47, 8819–8823. [Google Scholar] [CrossRef]
- Kato, H.; Seino, Y.; Yonemitsu, H.; Sato, H.; Kanno, M.; Kobayashi, K.; Kawanishi, A.; Imamura, T.; Omura, M.; Nakamura, N. Electrical via chain yield for DSA contact hole shrink process. J. Photopolym. Sci. Technol. 2013, 26, 21–26. [Google Scholar] [CrossRef]
- Ferrarese Lupi, F.; Giammaria, T.J.; Volpe, F.; Lotto, F.; Seguini, G.; Pivac, B.; Laus, M.; Perego, M. High aspect ratio PS-b-PMMA block copolymer masks for lithographic applications. ACS Appl. Mater. Interfaces 2014, 6, 21389–21396. [Google Scholar] [CrossRef]
- Barros, P.P.; Barnola, S.; Gharbi, A.; Argoud, M.; Servin, I.; Tiron, R.; Chevalier, X.; Navarro, C.; Nicolet, C.; Lapeyre, C. Etch challenges for DSA implementation in CMOS via patterning. In Proceedings of the Advanced Etch Technology for Nanopatterning III, San Jose, CA, USA, 24–25 February 2014; SPIE: San Jose, CA, USA, 2014; Volume 9054, pp. 87–96. [Google Scholar]
- Ting, Y.-H.; Liu, C.-C.; Park, S.-M.; Jiang, H.; Nealey, P.F.; Wendt, A.E. Surface roughening of polystyrene and poly (methyl methacrylate) in Ar/O2 plasma etching. Polymers 2010, 2, 649–663. [Google Scholar] [CrossRef]
- Rastogi, V.; Ventzek, P.L.; Ranjan, A. Etch considerations for directed self-assembly patterning using capacitively coupled plasma. J. Vac. Sci. Technol. A 2018, 36, 031301. [Google Scholar] [CrossRef]
- Sarrazin, A.; Posseme, N.; Pimenta-Barros, P.; Barnola, S.; Tiron, R.; Cardinaud, C. New CH4-N2 dry etch chemistry for poly (methyl methacrylate) removal without consuming polystyrene for lamellar copolymers application. J. Vac. Sci. Technol. B 2019, 37, 030601. [Google Scholar] [CrossRef]
- Bézard, P.; Chevalier, X.; Legrain, A.; Navarro, C.; Nicolet, C.; Fleury, G.; Cayrefourcq, I.; Tiron, R.; Zelsmann, M. Graphoepitaxy integration and pattern transfer of lamellar silicon-containing high-chi block copolymers. In Proceedings of the Advanced Etch Technology for Nanopatterning VII, San Jose, CA, USA, 26–28 January 2018; SPIE: San Jose, CA, USA, 2018; Volume 10589, pp. 39–48. [Google Scholar]
- Shimomukai, K.; Kawata, H.; Yasuda, M.; Hirai, Y. High selective plasma etching of PMMA to PS. J. Photopolym. Sci. Technol. 2015, 28, 569–572. [Google Scholar] [CrossRef]
- Yabagi, J.A.; Kimpa, M.I.; Muhammad, M.N.; Nayan, N.; Embong, Z.; Agam, M.A. Nanofabrication process by reactive ion etching of polystyrene nanosphere on silicon surface. J. Sci. Technol. 2017, 9, 145–153. [Google Scholar]
- Omura, M.; Imamura, T.; Yamamoto, H.; Sakai, I.; Hayashi, H. Dry development for a directed self-assembly lithography hole-shrink process using CO/H2 plasma. J. Micro Nanolithography MEMS MOEMS 2015, 14, 044505. [Google Scholar] [CrossRef]
- Farrell, R.A.; Petkov, N.; Shaw, M.T.; Djara, V.; Holmes, J.D.; Morris, M.A. Monitoring PMMA elimination by reactive ion etching from a lamellar PS-b-PMMA thin film by ex situ TEM methods. Macromolecules 2010, 43, 8651–8655. [Google Scholar] [CrossRef]
- Yamada, K.; Kazama, Y.; Kimura, Y. Development of Water-Resistant Autohesive Strength of Polyethylene Plates with Photografting of Alkyl (Meth) Acrylates. Macromol 2023, 3, 554–568. [Google Scholar] [CrossRef]
- Lei, P.-H.; Yang, P.-C.; Huang, P.-C. Investigation of photonic-crystal-structured p-GaN nanorods fabricated by polystyrene nanosphere lithography method to improve the light extraction efficiency of InGaN/GaN green light-emitting diodes. Materials 2021, 14, 2200. [Google Scholar] [CrossRef] [PubMed]
- Bürger, J.; Venugopal, H.; Kool, D.; de los Arcos, T.; Gonzalez Orive, A.; Grundmeier, G.; Brassat, K.; Lindner, J.K. High-Resolution Study of Changes in Morphology and Chemistry of Cylindrical PS-b-PMMA Block Copolymer Nanomasks during Mask Development. Adv. Mater. Interfaces 2022, 9, 2200962. [Google Scholar] [CrossRef]
- Hao, H.; Chen, S.; Ren, J.; Chen, X.; Nealey, P. Enhanced etching resolution of self-assembled PS-b-PMMA block copolymer films by ionic liquid additives. Nanotechnology 2023, 34, 205303. [Google Scholar] [CrossRef]
- Gharbi, A.; Tiron, R.; Pimenta Barros, P.; Argoud, M.; Servin, I.; Chevalier, X.; Nicolet, C.; Navarro, C. PMMA removal options by wet development in PS-b-PMMA block copolymer for nanolithographic mask fabrication. J. Vac. Sci. Technol. B 2015, 33, 051602. [Google Scholar] [CrossRef]
- Sarrazin, A.; Posseme, N.; Pimenta-Barros, P.; Barnola, S.; Gharbi, A.; Argoud, M.; Tiron, R.; Cardinaud, C. PMMA removal selectivity to polystyrene using dry etch approach. J. Vac. Sci. Technol. B 2016, 34, 061802. [Google Scholar] [CrossRef]
- Wu, H.-C.; Liao, M.-C.; Hirahara, E.; Iwaki, T. Wet etch process for high-resolution DSA patterning for advanced node DRAM. In Proceedings of the Advances in Patterning Materials and Processes XLI, San Jose, CA, USA, 26–29 February 2024; SPIE: San Jose, CA, USA, 2024; Volume 12957, pp. 374–383. [Google Scholar]
- Thurn-Albrecht, T.; Steiner, R.; DeRouchey, J.; Stafford, C.M.; Huang, E.; Bal, M.; Tuominen, M.; Hawker, C.J.; Russell, T.P. Nanoscopic templates from oriented block copolymer films. Adv. Mater. 2000, 12, 787–791. [Google Scholar] [CrossRef]
- Cummins, C.; Ghoshal, T.; Holmes, J.D.; Morris, M.A. Strategies for inorganic incorporation using neat block copolymer thin films for etch mask function and nanotechnological application. Adv. Mater. 2016, 28, 5586–5618. [Google Scholar] [CrossRef]
- Pola, R.; Vícha, M.; Trousil, J.; Grosmanová, E.; Pechar, M.; Rumlerová, A.; Studenovský, M.; Kučerová, E.; Ulbrich, P.; Vokatá, B. Polymer-antimicrobial peptide constructs with tailored drug-release behavior. Pharmaceutics 2023, 15, 406. [Google Scholar] [CrossRef]
- Jia, Y.; Li, J. Molecular assembly of Schiff base interactions: Construction and application. Chem. Rev. 2015, 115, 1597–1621. [Google Scholar] [CrossRef]
- Zhan, J.; Wu, Y.; Wang, H.; Liu, J.; Ma, Q.; Xiao, K.; Li, Z.; Li, J.; Luo, F.; Tan, H. An injectable hydrogel with pH-sensitive and self-healing properties based on 4armPEGDA and N-carboxyethyl chitosan for local treatment of hepatocellular carcinoma. Int. J. Biol. Macromol. 2020, 163, 1208–1222. [Google Scholar] [CrossRef]
- Xin, Y.; Yuan, J. Schiff’s base as a stimuli-responsive linker in polymer chemistry. Polym. Chem. 2012, 3, 3045–3055. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, Y.; Hu, D.; Tian, M.; Wei, Y.; Yuan, J. Construction and modulation of aggregation-induced emission materials based on dynamic covalent bonds. Aggregate 2024, 5, e624. [Google Scholar] [CrossRef]
- Rao, J.; De, S.; Khan, A. Synthesis and self-assembly of dynamic covalent block copolymers: Towards a general route to pore-functionalized membranes. Chem. Commun. 2012, 48, 3427–3429. [Google Scholar] [CrossRef]
- He, L.; Jiang, Y.; Tu, C.; Li, G.; Zhu, B.; Jin, C.; Zhu, Q.; Yan, D.; Zhu, X. Self-assembled encapsulation systems with pH tunable release property based on reversible covalent bond. Chem. Commun. 2010, 46, 7569–7571. [Google Scholar] [CrossRef]
- Zhan, J.; Shang, C.; Niu, M.; Luo, J.; Gao, S.; Wu, Z.; Niu, S.; Xu, Y.; Zhang, X.; Li, Z.; et al. An Acid-Cleavable Lamellar Block Copolymer for Sub-30-nm Line Spacing Patterning via Graphoepitaxial Directed Self-Assembly and Direct Wet Etching. Polymers 2025, 17, 2435. [Google Scholar] [CrossRef]
- Chatterjee, D.P.; Chatterjee, U.; Mandal, B.M. Atom transfer radical polymerization of methyl methacrylate at ambient temperature using soluble Cu (I) complex catalysts formed with mixed ligands of multidentate amines and halide ions. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 4132–4142. [Google Scholar] [CrossRef]
- Huang, G.-C.; Ji, S.-X. Effect of Halogen Chain End Fidelity on the Synthesis of Poly (methyl methacrylate-b-styrene) by ATRP. Chin. J. Polym. Sci. 2018, 36, 1217–1224. [Google Scholar] [CrossRef]
- Tamura, M.; Kurokawa, N.; Hotta, A. Compensation for orientation birefringence of PMMA by blending bottlebrush polymers composed of well-controlled graft chains. ACS Macro Lett. 2022, 11, 799–804. [Google Scholar] [CrossRef]
- Yang, B.; Zhao, Y.; Fu, C.; Zhu, C.; Zhang, Y.; Wang, S.; Wei, Y.; Tao, L. Introducing the Ugi reaction into polymer chemistry as a green click reaction to prepare middle-functional block copolymers. Polym. Chem. 2014, 5, 2704–2708. [Google Scholar] [CrossRef]
- Liu, C.-C.; Han, E.; Onses, M.S.; Thode, C.J.; Ji, S.; Gopalan, P.; Nealey, P.F. Fabrication of lithographically defined chemically patterned polymer brushes and mats. Macromolecules 2011, 44, 1876–1885. [Google Scholar] [CrossRef]
- Yoshimoto, K.; Fukawatase, K.; Ohshima, M.; Naka, Y.; Maeda, S.; Tanaka, S.; Morita, S.; Aoyama, H.; Mimotogi, S. Optimization of directed self-assembly hole shrink process with simplified model. J. Micro Nanolithography MEMS MOEMS 2014, 13, 031305. [Google Scholar] [CrossRef]
- Munawar, S.; Zahoor, A.F.; Hussain, S.M.; Ahmad, S.; Mansha, A.; Parveen, B.; Ali, K.G.; Irfan, A. Steglich esterification: A versatile synthetic approach toward the synthesis of natural products, their analogues/derivatives. Heliyon 2024, 10, e23416. [Google Scholar] [CrossRef]
- Kobayashi, M.; Okuyama, S.; Ishizone, T.; Nakahama, S. Stereospecific anionic polymerization of N, N-dialkylacrylamides. Macromolecules 1999, 32, 6466–6477. [Google Scholar] [CrossRef]
- Ferrarese Lupi, F.; Giammaria, T.J.; Seguini, G.; Vita, F.; Francescangeli, O.; Sparnacci, K.; Antonioli, D.; Gianotti, V.; Laus, M.; Perego, M. Fine tuning of lithographic masks through thin films of PS-b-PMMA with different molar mass by rapid thermal processing. ACS Appl. Mater. Interfaces 2014, 6, 7180–7188. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Wang, C.; Liu, Y.; Deng, H. Fast self-assembly of polystyrene-b-poly (fluoro methacrylate) into sub-5 nm microdomains for nanopatterning applications. J. Mater. Chem. C 2019, 7, 2535–2540. [Google Scholar] [CrossRef]
- Pang, Y.; Wan, L.; Huang, G.; Zhang, X.; Jin, X.; Xu, P.; Liu, Y.; Han, M.; Wu, G.-P.; Ji, S. Controlling block copolymer–substrate interactions by homopolymer brushes/mats. Macromolecules 2017, 50, 6733–6741. [Google Scholar] [CrossRef]
- Guo, R.; Kim, E.; Gong, J.; Choi, S.; Ham, S.; Ryu, D.Y. Perpendicular orientation of microdomains in PS-b-PMMA thin films on the PS brushed substrates. Soft Matter 2011, 7, 6920–6925. [Google Scholar] [CrossRef]
- Mansky, P.; Liu, Y.; Huang, E.; Russell, T.P.; Hawker, C. Controlling Polymer-Surface Interactions with Random Copolymer Brushes. Science 1997, 275, 1458–1460. [Google Scholar] [CrossRef]
- Huang, E.; Pruzinsky, S.; Russell, T.P.; Mays, J.; Hawker, C.J. Neutrality Conditions for Block Copolymer Systems on Random Copolymer Brush Surfaces. Macromolecules 1999, 32, 5299–5303. [Google Scholar] [CrossRef]
- Guedes, G.; Wang, S.; Fontana, F.; Figueiredo, P.; Lindén, J.; Correia, A.; Pinto, R.J.; Hietala, S.; Sousa, F.L.; Santos, H.A. Dual-crosslinked dynamic hydrogel incorporating {Mo154} with pH and NIR responsiveness for chemo-photothermal therapy. Adv. Mater. 2021, 33, 2007761. [Google Scholar] [CrossRef]
- Feng, W.; Li, G.; Kang, X.; Wang, R.; Liu, F.; Zhao, D.; Li, H.; Bu, F.; Yu, Y.; Moriarty, T.F. Cascade-targeting poly (amino acid) nanoparticles eliminate intracellular bacteria via on-site antibiotic delivery. Adv. Mater. 2022, 34, 2109789. [Google Scholar] [CrossRef]
- Jeong, U.; Ryu, D.Y.; Kim, J.K.; Kim, D.H.; Wu, X.; Russell, T.P. Precise control of nanopore size in thin film using mixtures of asymmetric block copolymer and homopolymer. Macromolecules 2003, 36, 10126–10129. [Google Scholar] [CrossRef]
- Xuan, Y.; Peng, J.; Wang, H.; Li, B.; Han, Y. The formation of ordered nanoholes in binary, chemically similar, symmetric diblock copolymer blend films. Macromol. Rapid Commun. 2004, 25, 1181–1185. [Google Scholar] [CrossRef]
- Huang, Y.C.; Chen, W.C.; Kuo, S. Mesoporous Phenolic/POSS Hybrids Induced by Microphase Separation Arising from Competitive Hydrogen Bonding Interactions. Macromolecules 2022, 55, 8918–8930. [Google Scholar] [CrossRef]
- Doise, J.; Chan, B.T.; Hori, M.; Gronheid, R. Dual brush process for selective surface modification in graphoepitaxy directed self-assembly. J. Micro Nanolithography MEMS MOEMS 2017, 16, 033503. [Google Scholar] [CrossRef]
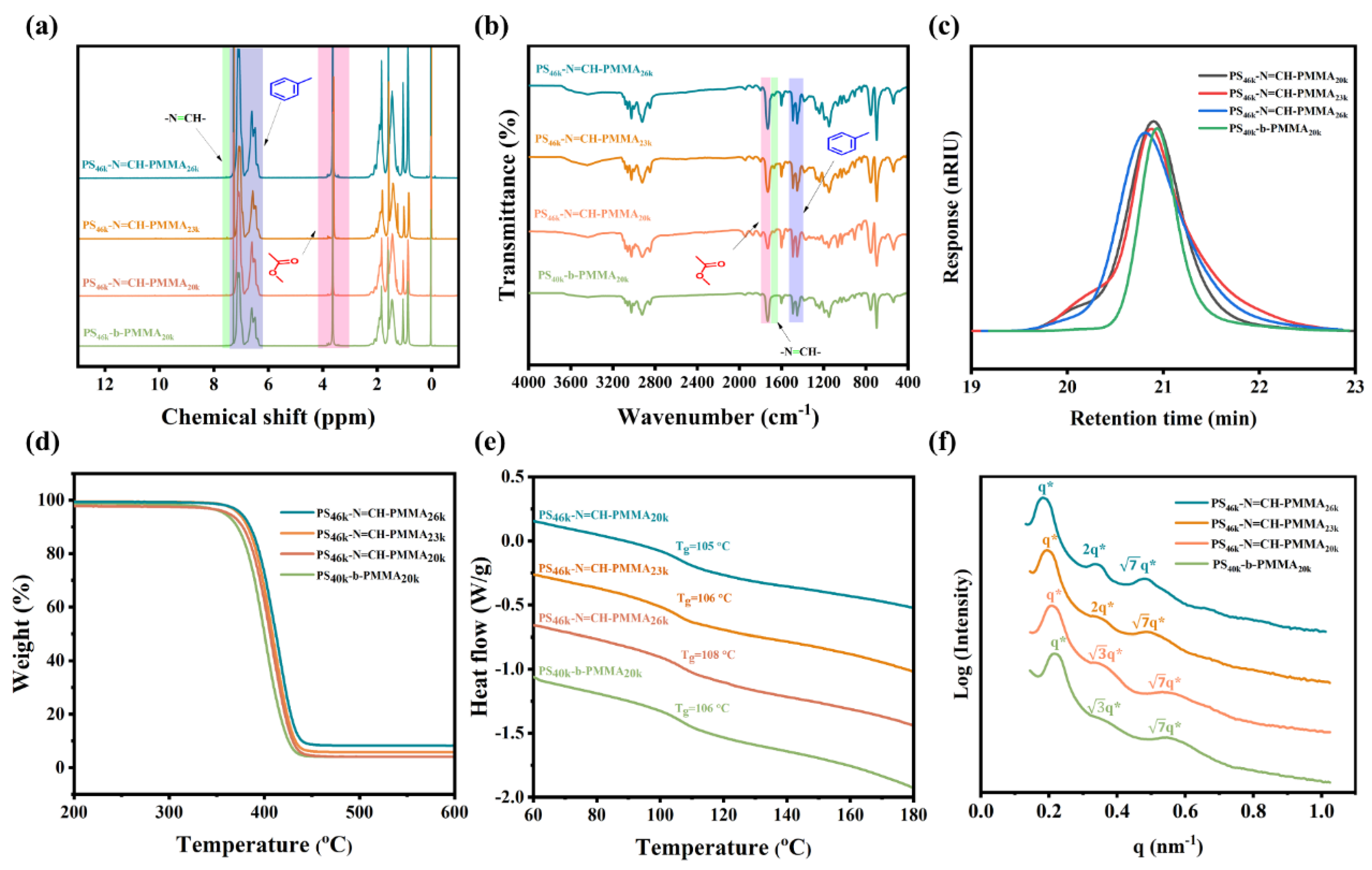


| Sample a | Mn BCP b (kg/mol) | Mn PMMA b (kg/mol) | PDI b | ƒSt c | L0 SEM d (nm) | L0 SAXS e (nm) | Film Thickness f (nm) |
|---|---|---|---|---|---|---|---|
| PS46k-N=CH-PMMA20k | 65.5k | 20.1k | 1.12 | 0.725 | 35.9 | 31.1 | 32.8 |
| PS46k-N=CH-PMMA23k | 67.7k | 23.3k | 1.12 | 0.703 | 37.9 | 32.8 | 35.3 |
| PS46k-N=CH-PMMA26k | 70.4k | 26.1k | 1.12 | 0.674 | 39.6 | 34.3 | 37.5 |
| PS40k-b-PMMA20k | 60.0k | 20.5k | 1.09 | 0.702 | 32.2 | 27.9 | 30.3 |
| Sample a | Mn b (kg/mol) | PDI b | FSt c (%) | Film Thickness d (nm) |
|---|---|---|---|---|
| Mat63 | 24.4 | 1.55 | 63.2 | 6.4 |
| Mat65 | 25.2 | 1.57 | 65.4 | 6.5 |
| Mat67 | 28.5 | 1.55 | 67.7 | 7.3 |
| Mat72 | 28.1 | 1.56 | 72.3 | 6.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, J.; Luo, J.; Zhuo, Z.; Shang, C.; Li, Z.; Xiong, S. Directed Self-Assembly of an Acid-Responsive Block Copolymer for Hole-Shrink Process and Pattern Transfer. Nanomaterials 2025, 15, 1571. https://doi.org/10.3390/nano15201571
Zhan J, Luo J, Zhuo Z, Shang C, Li Z, Xiong S. Directed Self-Assembly of an Acid-Responsive Block Copolymer for Hole-Shrink Process and Pattern Transfer. Nanomaterials. 2025; 15(20):1571. https://doi.org/10.3390/nano15201571
Chicago/Turabian StyleZhan, Jianghao, Jiacheng Luo, Zixin Zhuo, Caiwei Shang, Zili Li, and Shisheng Xiong. 2025. "Directed Self-Assembly of an Acid-Responsive Block Copolymer for Hole-Shrink Process and Pattern Transfer" Nanomaterials 15, no. 20: 1571. https://doi.org/10.3390/nano15201571
APA StyleZhan, J., Luo, J., Zhuo, Z., Shang, C., Li, Z., & Xiong, S. (2025). Directed Self-Assembly of an Acid-Responsive Block Copolymer for Hole-Shrink Process and Pattern Transfer. Nanomaterials, 15(20), 1571. https://doi.org/10.3390/nano15201571









