A Brief Review of Recent Results in Arsenic Adsorption Process from Aquatic Environments by Metal-Organic Frameworks: Classification Based on Kinetics, Isotherms and Thermodynamics Behaviors
Abstract
1. Introduction
1.1. Prospects for Removing Arsenic Ions from Water
1.2. Sorbents in Use for Arsenic Adsorptive Removal from the Aquatic Environment
2. Result and Discussion
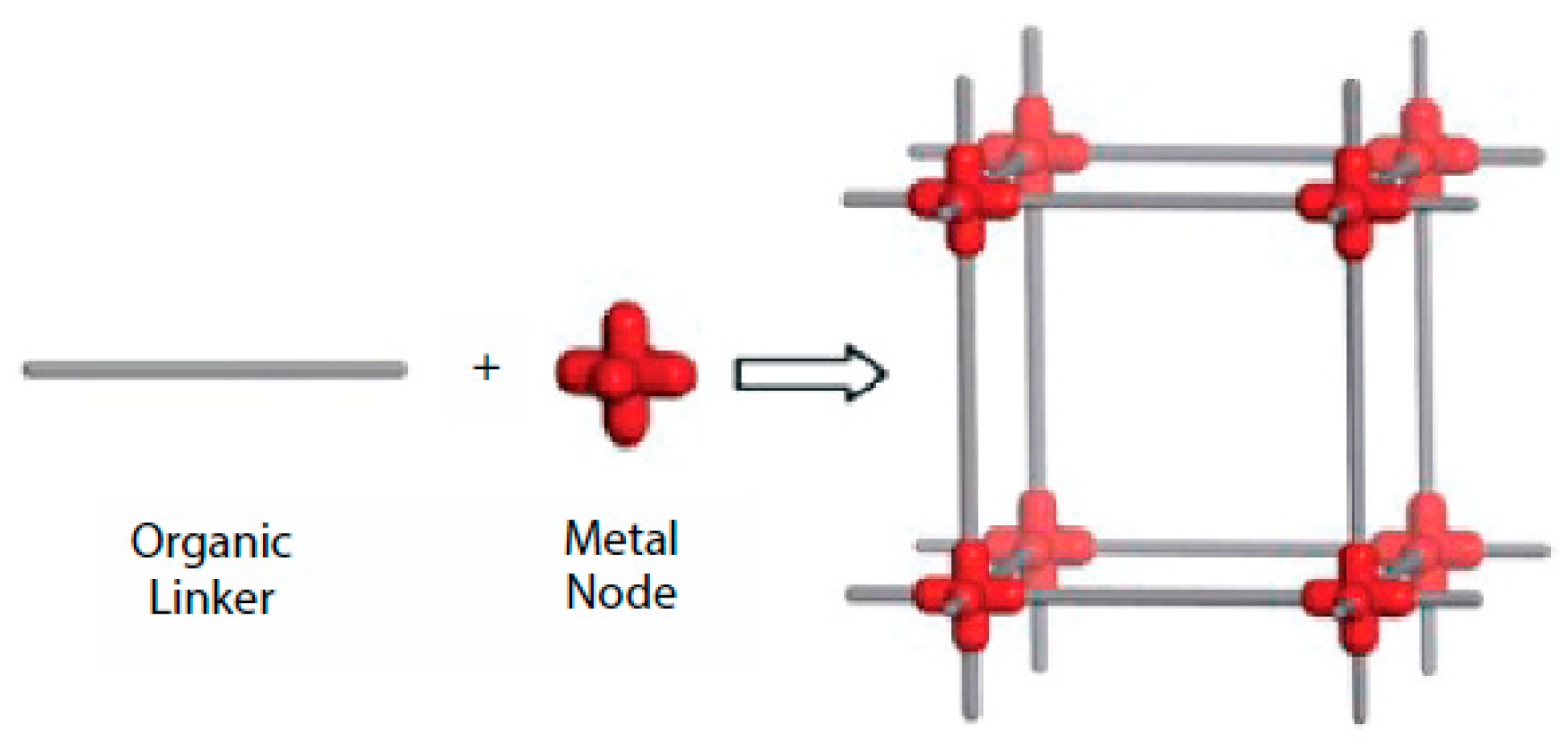
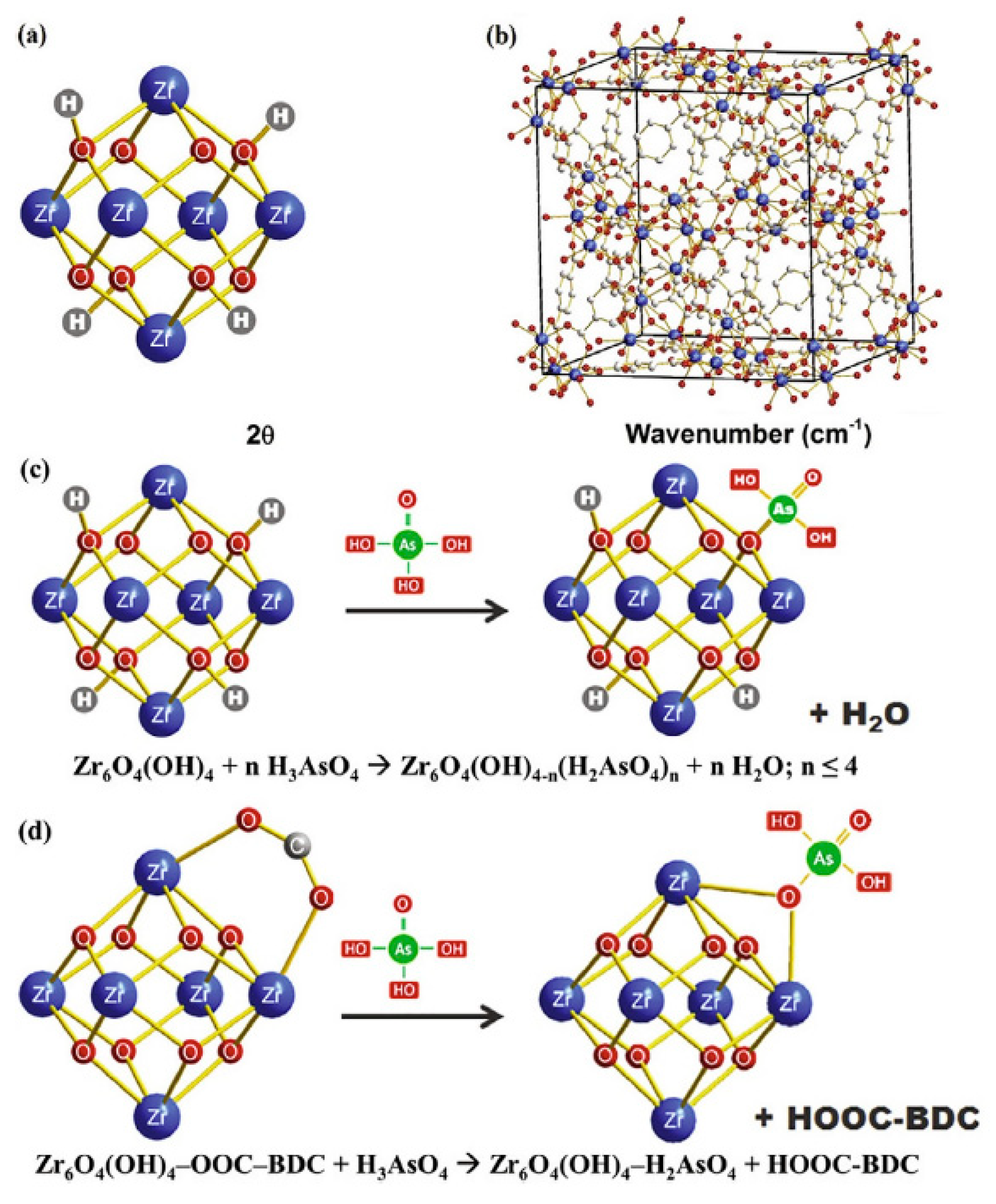
2.1. MOFs Used for Arsenic Removal in the Last Decade
2.2. Operational Factors Affecting Adsorption for Arsenic Removal by MOFs
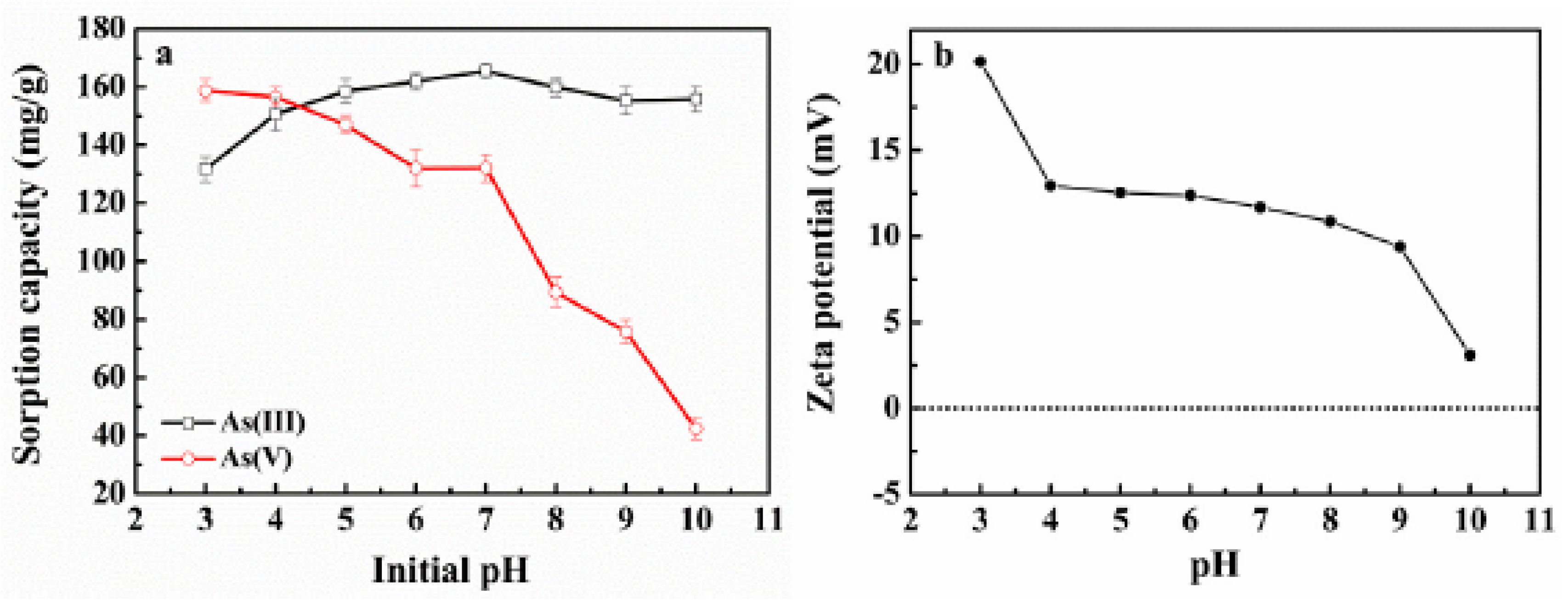
2.2.1. The Effect of Solution pH
2.2.2. The Effect of Initial Arsenic Concentration
2.3. Adsorption Kinetic Studies in Arsenic Removal Using MOFs
2.4. Adsorption Isotherm Studies in Arsenic Removal Using MOFs
2.5. Adsorption Thermodynamic Studies in Arsenic Removal Using MOFs
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mladin, G.; Ciopec, M.; Negrea, A.; Duteanu, N.; Negrea, P.; Ianasi, P.; Ianași, C. Silica-Iron Oxide Nanocomposite Enhanced with Porogen Agent Used for Arsenic Removal. Materials 2022, 15, 5366. [Google Scholar] [CrossRef]
- Ciopec, M.; Biliuta, G.; Negrea, A.; Duțeanu, N.; Coseri, S.; Negrea, P.; Ghangrekar, M. Testing of Chemically Activated Cellulose Fibers as Adsorbents for Treatment of Arsenic Contaminated Water. Materials 2021, 14, 3731. [Google Scholar] [CrossRef]
- Samimi, M.; Shahriari-Moghadam, M. Isolation and identification of Delftia lacustris Strain-MS3 as a novel and efficient adsorbent for lead biosorption: Kinetics and thermodynamic studies, optimization of operating variables. Biochem. Eng. J. 2021, 173, 108091. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, J.; Zhang, Y.; Ma, J.; Huang, L.; Yu, S.; Chen, L.; Song, G.; Qiu, M.; Wang, X. Applications of water-stable metal-organic frameworks in the removal of water pollutants: A review. Environ. Pollut. 2021, 291, 118076. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, J.; Bu, X.-H. Recent advances about metal–organic frameworks in the removal of pollutants from wastewater. Coord. Chem. Rev. 2018, 378, 17–31. [Google Scholar] [CrossRef]
- Aghel, B.; Mohadesi, M.; Gouran, A.; Razmegir, M. Use of modified Iranian clinoptilolite zeolite for cadmium and lead removal from oil refinery wastewater. Int. J. Environ. Sci. Technol. 2020, 17, 1239–1250. [Google Scholar] [CrossRef]
- Samimi, M.; Moeini, S. Optimization of the Ba+ 2 uptake in the formation process of hydrogels using central composite design: Kinetics and thermodynamic studies of malachite green removal by Ba-alginate particles. J. Part. Sci. Technol. 2020, 6, 95–102. [Google Scholar]
- Safari, M.; Shahriari Moghadam, M.; Samimi, M.; Azizi, Z. Study of the Optimal Conditions for [Zn]^(+2) Removal Using the Biomass of Isolated Bacteria from Ravang Mine. Iran. J. Soil Water Res. 2019, 50, 149–160. [Google Scholar]
- Asghar, A.; Bello, M.M.; Raman, A.A.A. Metal–Organic Frameworks for Heavy Metal Removal. Appl. Water Sci. Remediat. Technol. 2021, 2, 321–356. [Google Scholar]
- Rao, Z.; Feng, K.; Tang, B.; Wu, P. Surface decoration of amino-functionalized metal-organic framework/graphene oxide composite onto polydopamine coated membrane substrate for highly efficient heavy metal removal. Appl. Mater. Interfaces 2016, 9, 2594–2605. [Google Scholar] [CrossRef]
- Moayedi, H.; Aghel, B.; Nguyen, H.; Rashid, A.S.A. Applications of rice husk ash as green and sustainable biomass. J. Clean. Prod. 2019, 237, 117851. [Google Scholar] [CrossRef]
- Li, X.; Wang, B.; Cao, Y.; Zhao, S.; Wang, H.; Feng, X.; Zhou, J.; Ma, X. Water contaminants elimination based on Metal- Organic Frameworks and perspective on their industrial applications. ACS Sustain. Chem. Eng. 2019, 7, 4548–4563. [Google Scholar] [CrossRef]
- Uddin, M.K. A review on the adsorption of heavy metals by clay minerals, with special focus on the past decade. Chem. Eng. J. 2017, 308, 438–462. [Google Scholar] [CrossRef]
- Azimi, N.; Azimi, P.; Samimi, M.; Jalilian, T.M. Ultrasonic-assisted adsorption of Ni (II) ions from aqueous solution onto Fe3O4 nanoparticles. Adv. Nanochem. 2019, 1, 66–72. [Google Scholar]
- Xu, J.; Cao, Z.; Zhang, Y.; Yuan, Z.; Lou, Z.; Xu, X.; Wang, X. A review of functionalized carbon nanotubes and graphene for heavy metal adsorption from water: Preparation, application, and mechanism. Chemosphere 2018, 195, 351–364. [Google Scholar] [CrossRef]
- Abbas, A.; Al-Amer, A.M.; Laoui, T.; Al-Marri, M.J.; Nasser, M.S.; Khraisheh, M.; Atieh, M.A. Heavy metal removal from aqueous solution by advanced carbon nanotubes: Critical review of adsorption applications. Sep. Purif. Technol. 2016, 157, 141–161. [Google Scholar]
- Sharma, M.; Singh, J.; Hazra, S.; Basu, S. Adsorption of heavy metal ions by mesoporous ZnO and TiO2@ZnO monoliths: Adsorption and kinetic studies. Microchem. J. 2019, 145, 105–112. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Zhang, L. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef]
- Ciopec, M.; Davidescu, C.M.; Negrea, A.; Duţeanu, N.; Rusu, G.; Grad, O.; Negrea, P. Amberlite XAD7 resin functionalized with crown ether and Fe (III) used for arsenic removal from water. Pure Appl. Chem. 2019, 91, 375–388. [Google Scholar] [CrossRef]
- Li, Z.Q.; Yang, J.C.; Sui, K.W.; Yin, N. Facile synthesis of metal-organic framework MOF-808 for arsenic removal. Mater. Lett. 2015, 160, 412–414. [Google Scholar] [CrossRef]
- Rebelo, F.M.; Caldas, E.D. Arsenic, lead, mercury and cadmium: Toxicity, levels in breast milk and the risks for breastfed infants. Environ. Res. 2016, 151, 671–688. [Google Scholar] [CrossRef]
- Garelick, H.; Jones, H.; Dybowska, A.; Valsami-Jones, E. Arsenic pollution sources. Rev. Environ. Contam. 2009, 197, 17–60. [Google Scholar]
- Jian, M.; Liu, B.; Zhang, G.; Liu, R.; Zhang, X. Adsorptive removal of arsenic from aqueous solution by zeolitic imidazolate framework-8 (ZIF-8) nanoparticle. Colloids Surf. A Physicochem. Eng. Asp. 2015, 465, 67–76. [Google Scholar] [CrossRef]
- Atallah, H.; Mahmoud, M.E.; Jelle, A.; Loughcand, A.; Hmadeh, M. A highly stable indium based metal organicframework for efficient arsenic removal from water. Dalton Trans. 2017, 47, 799–806. [Google Scholar] [CrossRef]
- Gabor, A.; Davidescu, C.M.; Negrea, A.; Ciopec, M.; Lupa, L. Behaviour of silica and florisil as solid supports in the removal process of As (V) from aqueous solutions. J. Anal. Methods Chem. 2015, 2015, 562780. [Google Scholar] [CrossRef]
- Yin, C.Y.; Aroua, M.K.; WanDaud, W.M.A. Review of modifications of activated carbon for enhancing contaminant uptakes from aqueous solutions. Sep. Purif. Technol. 2007, 52, 403–415. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, F.-S.; Sasai, R. Arsenate removal from water using Fe3O4-loaded activated carbon prepared from waste biomass. Chem. Eng. J. 2010, 160, 57–62. [Google Scholar] [CrossRef]
- Vitela-Rodriguez, A.V.; Rangel-Mendez, J.R. Arsenic removal by modified activated carbons with iron hydro(oxide) nanoparticles. J. Environ. Manag. 2013, 114, 225–231. [Google Scholar] [CrossRef]
- Yao, S.; Liu, Z.; Shi, Z. Arsenic removal from aqueous solutions by adsorption onto iron oxide/activated carbon magnetic composite. Environ. Health Sci. Eng. 2014, 58, 58. [Google Scholar] [CrossRef]
- Jais, F.M.; Ibrahim, S.; Yoon, Y.; Jang, M. Enhanced arsenate removal by lanthanum and nano–magnetite composite incorporated palm shell waste–based activated carbon. Sep. Purif. Technol. 2016, 169, 93–102. [Google Scholar] [CrossRef]
- Velazquez-Peña, G.C.; Solache-Ríos, M.; Olguin, M.T.; Fall, C. As(V) sorption by different natural zeolite frameworks modified with Fe, Zr and FeZr. Microporous Mesoporous Mater. 2019, 273, 133–141. [Google Scholar] [CrossRef]
- Pillewan, P.; Mukherjee, S.; Meher, A.K.; Rayalu, S.; Bansiwal, A. Removal of Arsenic (III) and Arsenic (V) Using Copper Exchange Zeolite-A. Environ. Prog. Sustain. Energy 2014, 33, 1274–1282. [Google Scholar] [CrossRef]
- Jaafarzadeh, N.; Ahmadi, M.; Amiri, H.; Yassin, M.H.; SilvaMartinez, S. Predicting Fenton modification of solid waste vegetable oil industry for arsenic removal using artificial neural networks. J. Taiwan Inst. Chem. Eng. 2012, 43, 873–878. [Google Scholar] [CrossRef]
- Bibi, S.; Farooqi, A.; Hussain, K.; Haider, N. Evaluation of industrial based adsorbents for simultaneous removal of arsenic and fluoride from drinking water. J. Clean. Prod. 2015, 87, 882–896. [Google Scholar] [CrossRef]
- Daer, S.; Kharraz, J.; Giwa, A.; Hasan, S.W. Recent applications of nanomaterials in water desalination: A critical review and future opportunities. Desalination 2015, 367, 37–48. [Google Scholar] [CrossRef]
- Liu, D.; Deng, S.; Maimaiti, A.; Wang, B.; Huang, J.; Wang, Y.; Yu, G. As(III) and As(V) adsorption on nanocomposite of hydrated zirconium oxide coated carbon nanotubes. J. Colloid Interface Sci. 2018, 511, 277–284. [Google Scholar] [CrossRef]
- Fu, D.; He, Z.; Su, S.; Xu, B.; Liu, Y.; Zhao, Y. Fabrication of α-FeOOH decorated graphene oxide-carbon nanotubes aerogel and its application in adsorption of arsenic species. J. Colloid Interface Sci. 2017, 505, 105–114. [Google Scholar] [CrossRef]
- Purohit, S.; Chini, M.K.; Chakraborty, T.; Yadav, K.L.; Satapathi, S. Rapid removal of arsenic from water using metal oxide doped recyclable cross-linked chitosan cryogel. SN Appl. Sci. 2020, 2, 768. [Google Scholar] [CrossRef]
- Siddiqui, S.I.; Chaudhry, S.A. Iron oxide and its modified forms as an adsorbent for arsenic removal: A comprehensive recent advancement. Process Saf. Environ. Prot. 2017, 111, 592–626. [Google Scholar] [CrossRef]
- Paul, B.; Parashar, V.; Mishra, A. Graphene in the Fe3O4 nano-composite switching the negative influence of humic acid coating into an enhancing effect in the removal of arsenic from water. Environ. Sci. Water Res. Technol. 2015, 1, 77–83. [Google Scholar] [CrossRef]
- Zhu, J.; Lou, Z.; Liu, Y.; Fu, R.; Baig, S.A.; Xu, X. Adsorption behavior and removal mechanism of arsenic on graphene modified by iron–manganese binary oxide (FeMnOx/RGO) from aqueous solutions. R. Soc. Chem. Adv. 2015, 5, 67951–67961. [Google Scholar] [CrossRef]
- Soni, R.; PraiseShukla, D. Synthesis of fly ash based zeolite-reduced graphene oxide composite and its evaluation as an adsorbent for arsenic removal. Chemosphere 2019, 219, 504–509. [Google Scholar] [CrossRef]
- Samimi, M.; Safari, M. TMU-24 (Zn-based MOF) as an advance and recyclable adsorbent for the efficient removal of eosin B: Characterization, equilibrium, and thermodynamic studies. Environ. Prog. Sustain. Energy 2022, 41, e13859. [Google Scholar] [CrossRef]
- Ehzari, H.; Safari, M.; Samimi, M. Signal amplification of novel sandwich-type genosensor via catalytic redox-recycling on platform MWCNTs/Fe3O4@ TMU-21 for BRCA1 gene detection. Talanta 2021, 234, 122698. [Google Scholar] [CrossRef]
- Jian, M.; Liu, B.; Liu, R.; Qu, J.; Wang, H.; Zhang, X. Water-based synthesis of zeolitic imidazolate framework-8 with high morphology level at room temperature. R. Soc. Chem. 2015, 5, 48433–48441. [Google Scholar] [CrossRef]
- Falcaro, P.; Ricco, R.; Doherty, C.M.; Liang, K.; Hill, A.J.; Styles, M.J. MOF positioning technology and device fabrication. Chem. Soc. Rev. 2014, 43, 5403–6176. [Google Scholar] [CrossRef]
- Shahsavari, M.; Jahani, P.M.; Sheikhshoaie, I.; Tajik, S.; Afshar, A.A.; Askari, M.B.; Salarizadeh, P.; Bartolomeo, A.D.; Beitollahi, H. Green Synthesis of Zeolitic Imidazolate Frameworks: A Review of Their Characterization and Industrial and Medical Applications. Materials 2022, 15, 447. [Google Scholar] [CrossRef]
- Shen, B.; Wang, B.; Zhu, L.; Jiang, L. Properties of Cobalt- and Nickel-Doped Zif-8 Framework Materials and Their Application in Heavy-Metal Removal from Wastewater. Nanomaterials 2020, 10, 1636. [Google Scholar] [CrossRef]
- Ehzari, H.; Amiri, M.; Safari, M.; Samimi, M. Zn-based metal-organic frameworks and p-aminobenzoic acid for electrochemical sensing of copper ions in milk and milk powder samples. Int. J. Environ. Anal. Chem. 2020, 110, 1–14. [Google Scholar] [CrossRef]
- Zhu, B.-J.; Yu, X.-Y.; Jia, Y.; Peng, F.-M.; Sun, B.; Zhang, M.-Y.; Luo, T.; Liu, J.-H.; Huang, X.-J. Iron and 1, 3, 5-benzenetricarboxylic metal–organic coordination polymers prepared by solvothermal method and their application in efficient As (V) removal from aqueous solutions. J. Phys. Chem. C 2012, 116, 8601–8607. [Google Scholar] [CrossRef]
- Li, J.; Wu, Y.-n.; Li, Z.; Zhu, M.; Li, F. Characteristics of arsenate removal from water by metal-organic frameworks (MOFs). Water Sci. Technol. 2014, 70, 1391–1397. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jian, M.; Liu, R.; Yao, J.; Zhang, X. Highly efficient removal of arsenic (III) from aqueous solution by zeolitic imidazolate frameworks with different morphology. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 358–366. [Google Scholar] [CrossRef]
- Vu, T.A.; Le, G.H.; Dao, C.D.; Dang, L.Q.; Nguyen, K.T.; Nguyen, Q.K.; Dang, P.T.; Tran, H.T.; Duong, Q.T.; Nguyen, T.V. Arsenic removal from aqueous solutions by adsorption using novel MIL-53 (Fe) as a highly efficient adsorbent. RSC Adv. 2015, 5, 5261–5268. [Google Scholar] [CrossRef]
- Wang, C.; Liu, X.; Chen, J.P.; Li, K. Superior removal of arsenic from water with zirconium metal-organic framework UiO-66. Sci. Rep. 2015, 5, 16613. [Google Scholar] [CrossRef]
- Kobielska, P.A.; Howarth, A.J.; Farha, O.K.; Nayak, S. Metal–organic frameworks for heavy metal removal from water. Coord. Chem. Rev. 2018, 358, 92–107. [Google Scholar] [CrossRef]
- Yang, J.-C.; Yin, X.-B. CoFe2O4@ MIL-100 (Fe) hybrid magnetic nanoparticles exhibit fast and selective adsorption of arsenic with high adsorption capacity. Sci. Rep. 2017, 7, 40955. [Google Scholar] [CrossRef]
- Huo, J.-B.; Xu, L.; Yang, J.-C.E.; Cui, H.-J.; Yuan, B.; Fu, M.-L. Magnetic responsive Fe3O4-ZIF-8 core-shell composites for efficient removal of As (III) from water. Colloids Surf. A Physicochem. Eng. Asp. 2018, 539, 59–68. [Google Scholar] [CrossRef]
- Nasir, A.M.; Nordin, N.M.; Goh, P.; Ismail, A. Application of two-dimensional leaf-shaped zeolitic imidazolate framework (2D ZIF-L) as arsenite adsorbent: Kinetic, isotherm and mechanism. J. Mol. Liq. 2018, 250, 269–277. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Zhang, A.; Liao, C. Preparation of Fe–Co based MOF-74 and its effective adsorption of arsenic from aqueous solution. J. Environ. Sci. 2019, 80, 197–207. [Google Scholar] [CrossRef]
- Yang, B.; Zhou, X.; Chen, Y.; Fang, Y.; Luo, H. Preparation of a spindle δ-MnO2@ Fe/Co-MOF-74 for effective adsorption of arsenic from water. Colloids Surf. A Physicochem. Eng. Asp. 2021, 629, 127378. [Google Scholar] [CrossRef]
- Song, T.; Feng, X.; Bao, C.; Lai, Q.; Li, Z.; Tang, W.; Shao, Z.-W.; Zhang, Z.; Dai, Z.; Liu, C. Aquatic arsenic removal with a Zr-MOF constructed via in situ nitroso coupling. Sep. Purif. Technol. 2022, 288, 120700. [Google Scholar] [CrossRef]
- Guo, Q.; Li, Y.; Zheng, L.-W.; Wei, X.-Y.; Xu, Y.; Shen, Y.-W.; Zhang, K.-G.; Yuan, C.-G. Facile fabrication of Fe/Zr binary MOFs for arsenic removal in water: High capacity, fast kinetics and good reusability. J. Environ. Sci. 2022, 128, 213–223. [Google Scholar] [CrossRef]
- Su, S.; Zhang, R.; Rao, J.; Yu, J.; Jiang, X.; Wang, S.; Yang, X. Fabrication of lanthanum-modified MOF-808 for phosphate and arsenic(V) removal from wastewater. J. Environ. Chem. Eng. 2022, 10, 108527. [Google Scholar] [CrossRef]
- Lagergren, S. Zur theorie der sogenannten adsorption geloster stoffe. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.-S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Daifullah, A.; Yakout, S.; Elreefy, S. Adsorption of fluoride in aqueous solutions using KMnO4-modified activated carbon derived from steam pyrolysis of rice straw. J. Hazard. Mater. 2007, 147, 633–643. [Google Scholar] [CrossRef]
| Adsorbent | Analyte | Optimal Conditions | Adsorption Capacity (mg/g) | Proposed Kinetic Model | Proposed Isotherm Model | Thermodynamic Behavior | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|
| pH | Equilibrium Time (min) | Initial Arsenic Concentration (mg/L) | |||||||
| Fe−BTC MOF | As(V) | 4 | 10 | 5 | 12.287 | P-S-O | L-I-M | Endothermic process Spontaneous | [50] |
| MIL-53(Al) | As(V) | 8 | 660 | 2.428 | 105.6 | P-S-O | L-I-M | - | [51] |
| Leaf-shaped ZIFs | As(III) | 8.5 | 600 | 80 | 108.5 | - | L-I-M | - | [52] |
| Dodecahedral ZIFs | 117.05 | ||||||||
| Cubic ZIFs | 122.6 | ||||||||
| ZIF-8 nanoparticles | As(III) | 7 | 780 | 100 | 49.49 | P-S-O | L-I-M | - | [23] |
| As(V) | 420 | 60.3 | |||||||
| MIL-53(Fe) | As(V) | 5 | 90 | 5 | 21.27 | P-S-O | L-I-M | - | [53] |
| MOF-808 | As(V) | 4 | 30 | 5 | 24.83 | P-S-O | - | - | [20] |
| UiO-66 | As(V) | 2 | 1440 | 50 | 303.4 | - | L-I-M | - | [54] |
| CoFe2O4@MIL-100(Fe) | As(III) | 2–8 | 720 | 1 | 143.6 | P-S-O | F-I-M | Endothermic process Spontaneous | [56] |
| As(V) | 114.8 | L-I-M | |||||||
| AUBM-1 | As(V) | 7 | 180 | 40 | 103.1 | P-S-O | L-I-M | Endothermic process non-spontaneous | [24] |
| Fe3O4-ZIF-8 | As(III) | 8 | 240 | 3.5–40 | 100 | P-S-O | L-I-M | Endothermic process Spontaneous | [57] |
| 2D ZIF-L | As(III) | 10 | 600 | 20–100 | 43.74 | P-S-O | L-I-M | - | [58] |
| Fe2Co1 MOF-74 | As(III) | 4.3 | 720 | 1–250 | 266.52 | P-S-O | L-I-M | - | [59] |
| As(V) | 292.29 | ||||||||
| δ-MnO2@Fe/Co-MOF-74 | As(III) | 10 | 1440 | 5 | 300.5 | P-S-O | L-I-M | - | [60] |
| SUM-8 | As(V) | 2 | 720 | 20 | 152.52 | P-S-O | L-I-M | - | [61] |
| UiO-66(Fe/Zr) | As(III) | 7.1 | 120 | 30 | 101.73 | P-S-O | L-I-M | - | [62] |
| As(V) | 50 | 204.1 | |||||||
| La-MOF-808 | As(V) | 8.32 | 720 | 100 | 217.54 | P-S-O | L-I-M | Endothermic process Spontaneous | [63] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samimi, M.; Zakeri, M.; Alobaid, F.; Aghel, B. A Brief Review of Recent Results in Arsenic Adsorption Process from Aquatic Environments by Metal-Organic Frameworks: Classification Based on Kinetics, Isotherms and Thermodynamics Behaviors. Nanomaterials 2023, 13, 60. https://doi.org/10.3390/nano13010060
Samimi M, Zakeri M, Alobaid F, Aghel B. A Brief Review of Recent Results in Arsenic Adsorption Process from Aquatic Environments by Metal-Organic Frameworks: Classification Based on Kinetics, Isotherms and Thermodynamics Behaviors. Nanomaterials. 2023; 13(1):60. https://doi.org/10.3390/nano13010060
Chicago/Turabian StyleSamimi, Mohsen, Mozhgan Zakeri, Falah Alobaid, and Babak Aghel. 2023. "A Brief Review of Recent Results in Arsenic Adsorption Process from Aquatic Environments by Metal-Organic Frameworks: Classification Based on Kinetics, Isotherms and Thermodynamics Behaviors" Nanomaterials 13, no. 1: 60. https://doi.org/10.3390/nano13010060
APA StyleSamimi, M., Zakeri, M., Alobaid, F., & Aghel, B. (2023). A Brief Review of Recent Results in Arsenic Adsorption Process from Aquatic Environments by Metal-Organic Frameworks: Classification Based on Kinetics, Isotherms and Thermodynamics Behaviors. Nanomaterials, 13(1), 60. https://doi.org/10.3390/nano13010060








