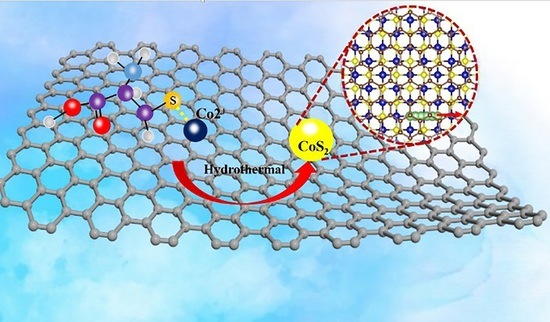
Composite Nanoarchitectonics with CoS2 Nanoparticles Embedded in Graphene Sheets for an Anode for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
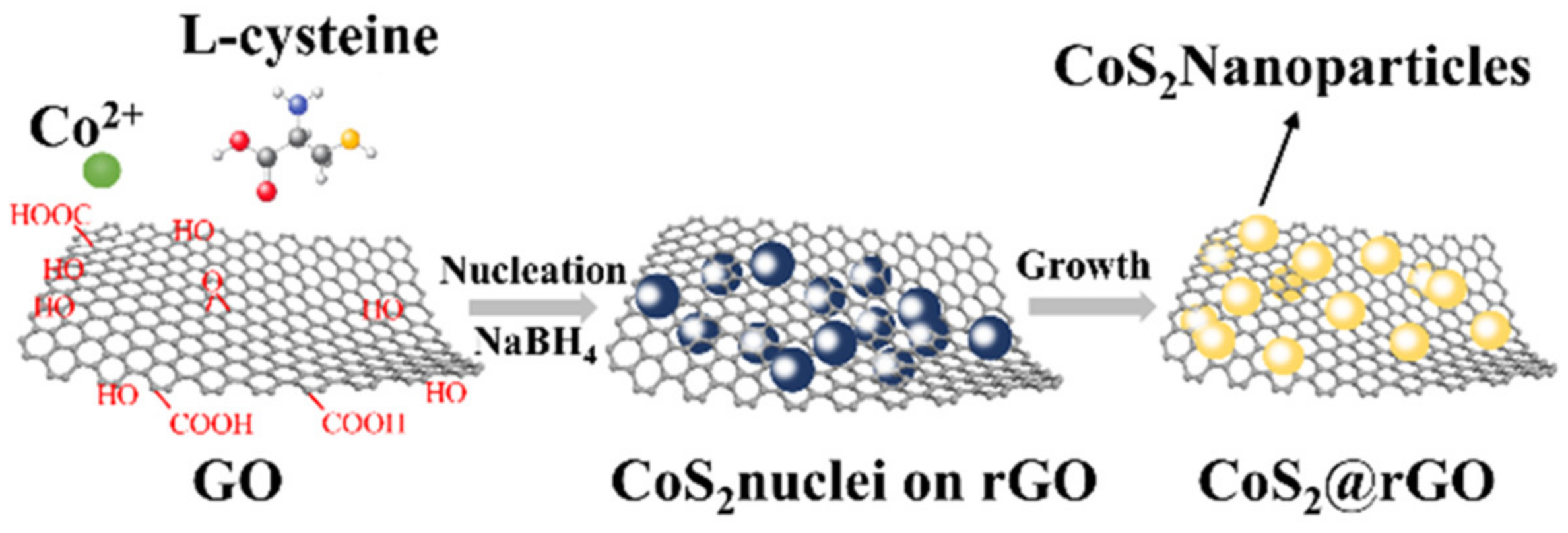
2.1. Preparation of Materials
2.2. Characterization
2.3. Fabrication of the Half Cells
3. Results and Discussion
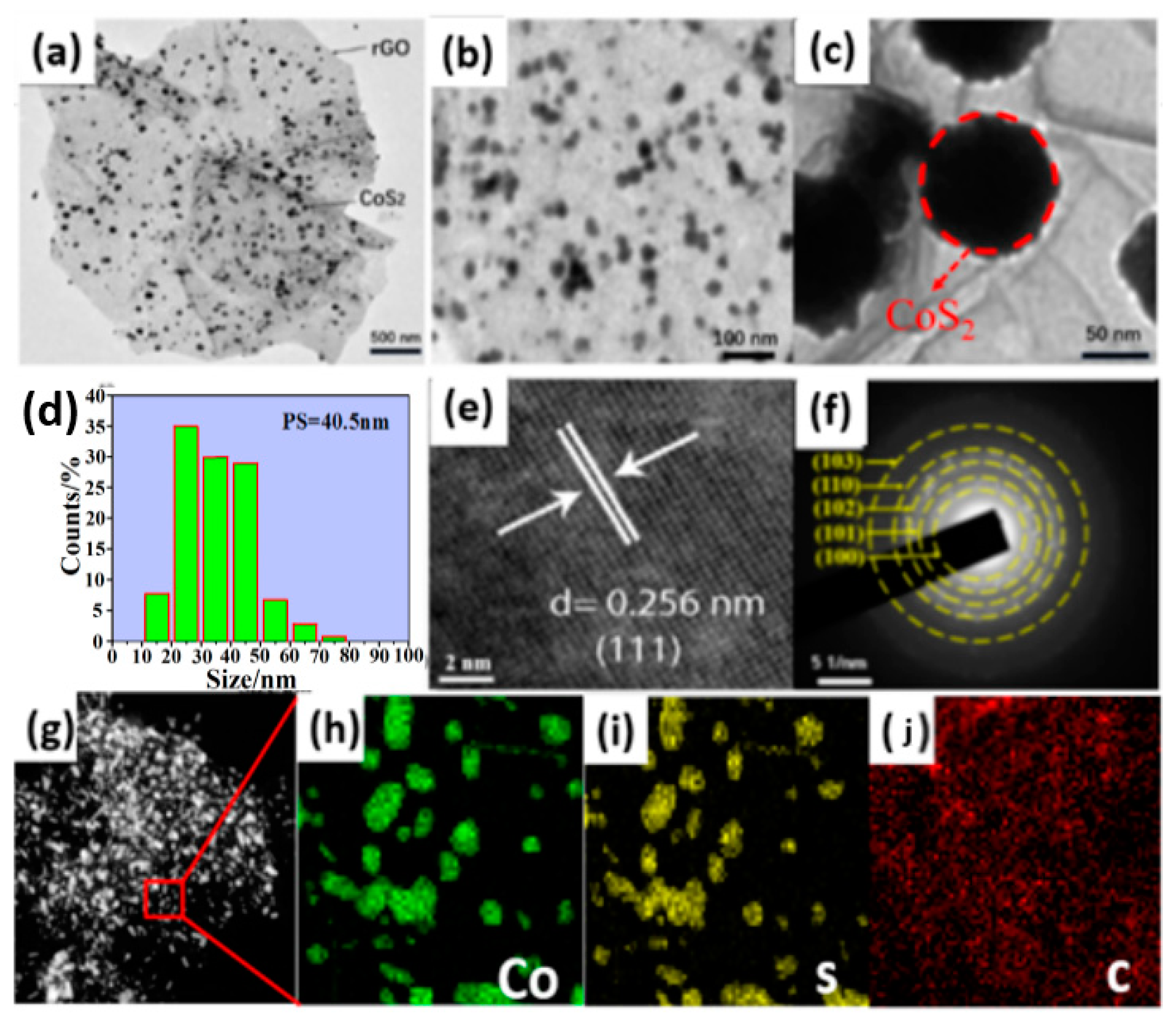
3.1. Morphology Analysis
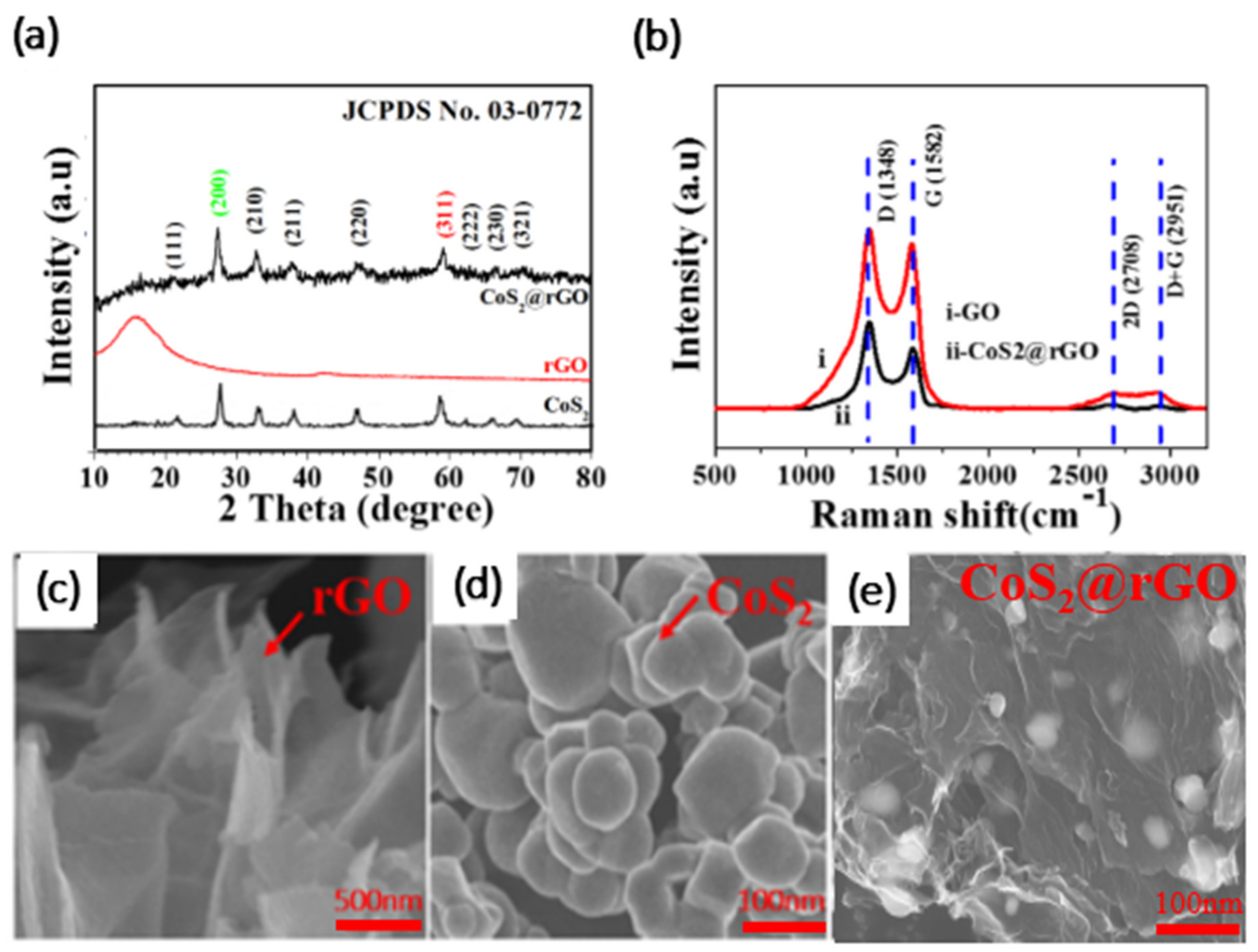
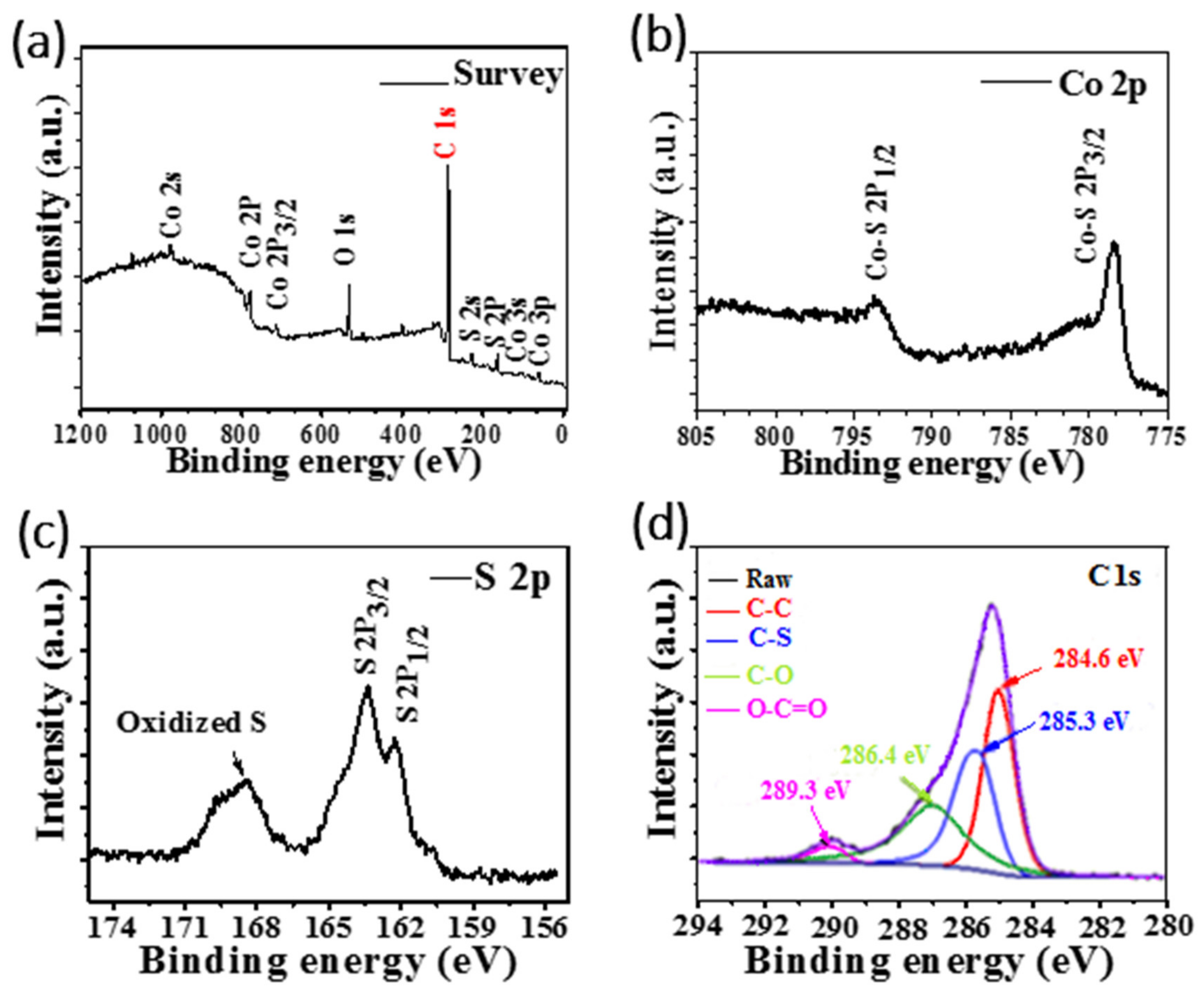
3.2. Structure Analysis
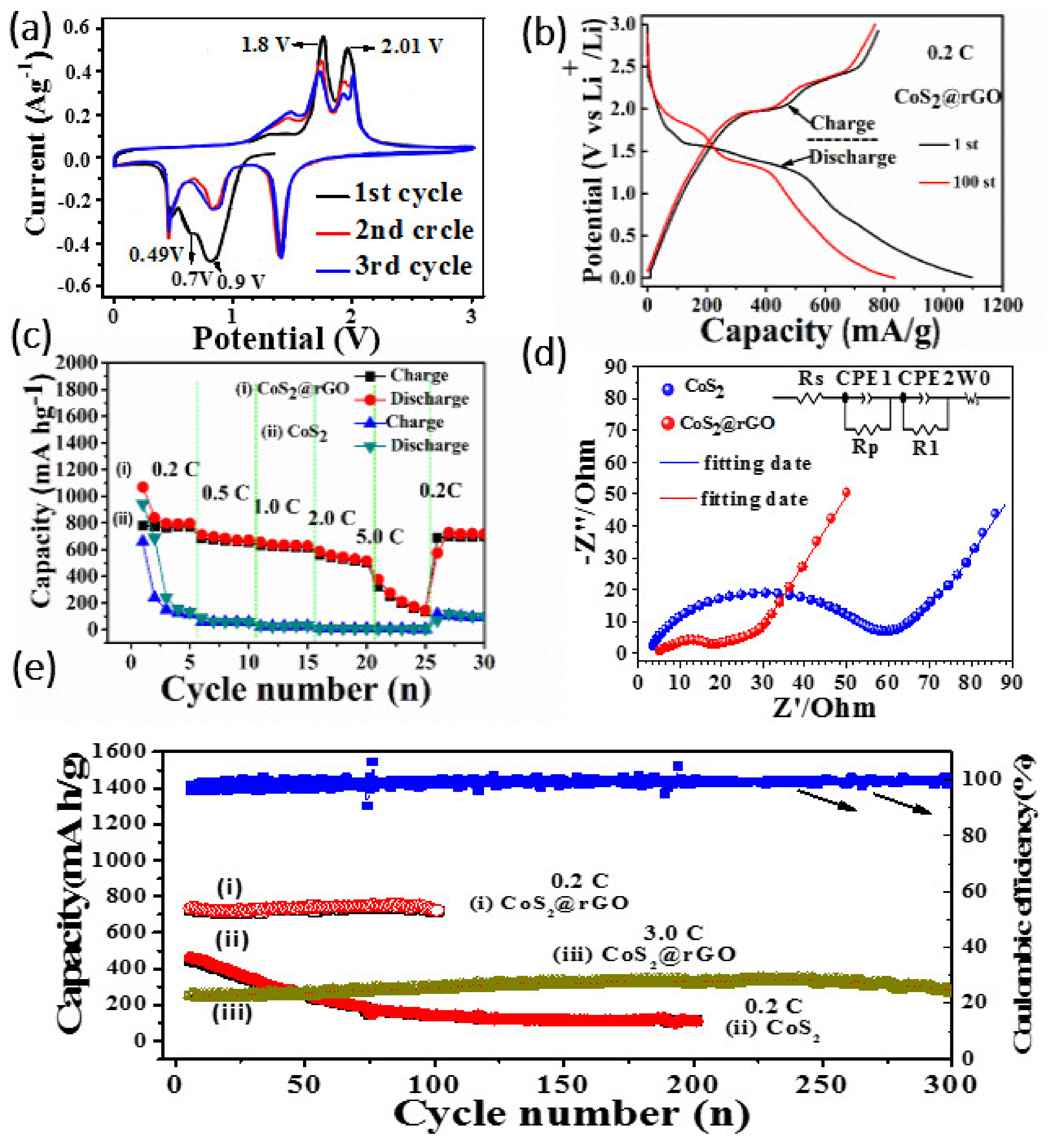
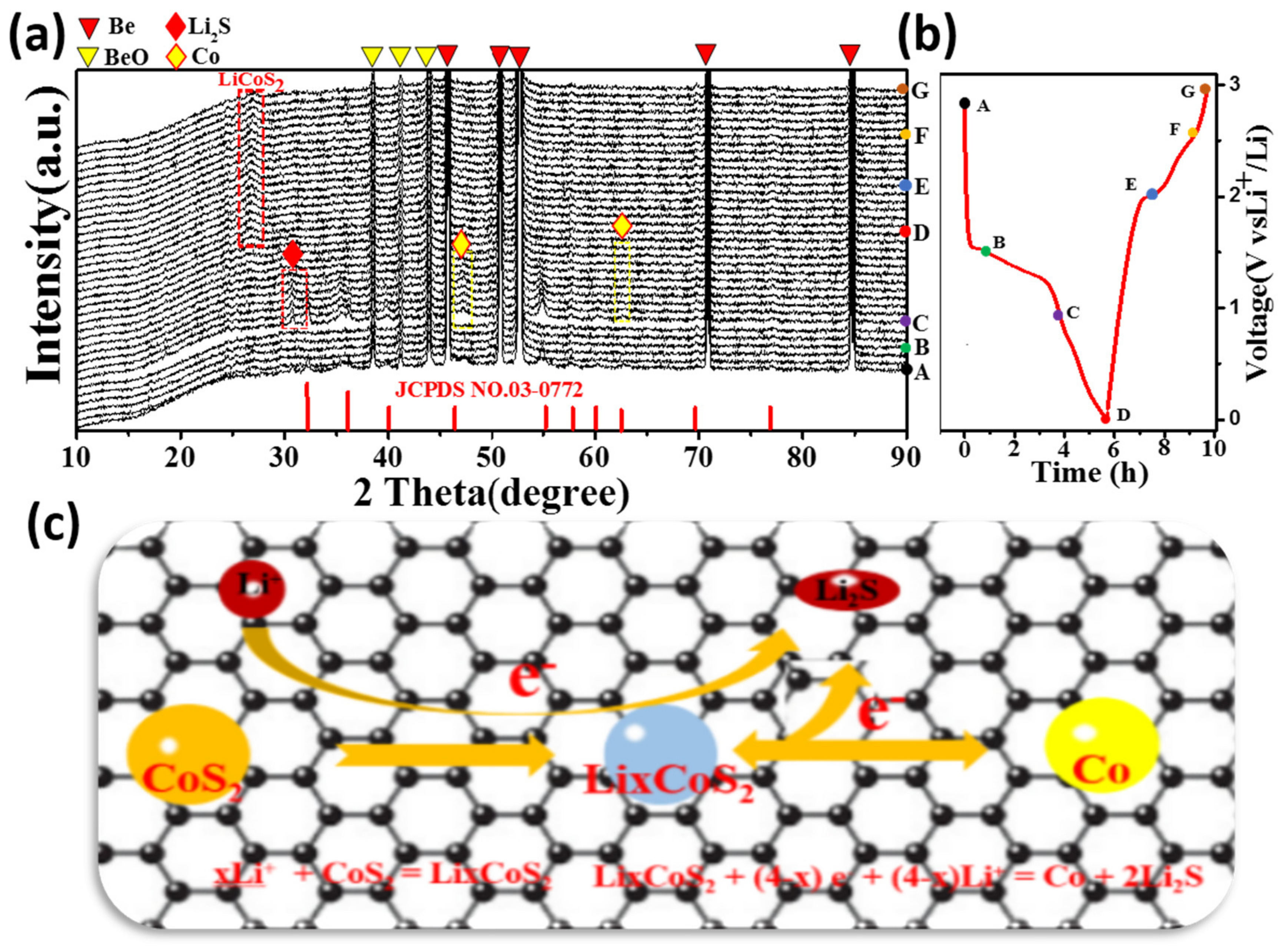
3.3. Electrochemical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, H.P.; Chen, Z.L.; Zeng, L.Y.; Wang, Z.J.; Zheng, G.F.; Zhou, R. The Effect of rGO-Doping on the Performance of SnO2/rGO Flexible Humidity SensorActa. Nanomaterials 2021, 11, 3368. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Casalongue, H.S.; Liang, Y.; Dai, H.J. Ni(OH)2 Nanoplates Grown on Graphene as Advanced Electrochemical Pseudocapacitor Materials. J. Am. Chem. Soc. 2010, 132, 7472–7477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fei, L.; Lin, Q.L.; Yuan, L.; Xie, P.; Li, Y.L.; Xu, Y.; Deng, S.G.; Luo, H.M.; Sergei, S. Reduced Graphene Oxide Wrapped FeS Nanocomposite for Lithium-Ion Battery Anode with Improved Performance. ACS Appl. Mater. Interfaces 2013, 11, 5330. [Google Scholar] [CrossRef] [PubMed]
- Schanze, K.S.; Mallett, J.J. Preface to Forum on “Current Trends in Functional Surfaces and Interfaces for Biomedical Applications”. ACS Appl. Mater. Interfaces 2016, 8, 14895. [Google Scholar] [CrossRef] [Green Version]
- Tu, C.B.; Peng, A.P.; Zhang, Z.; Qi, X.T.; Zhang, D.K.; Wang, M.J.; Huang, Y.N.; Yang, Z.Y. Surface-seeding secondary growth for CoO@Co9S8 P-N heterojunction hollow nanocube encapsulated into graphene as superior anode toward lithium ion storage. Chem. Eng. J. 2021, 425, 130648. [Google Scholar] [CrossRef]
- Xie, J.L.; Yang, Y.F.; Li, G.; Xia, H.C.; Wang, P.J.; Sun, P.H.; Li, X.L.; Cai, H.R.; Xiong, J. One-step sulfuration synthesis of hierarchical Ni-Co2S4@NiCo2S4 nanotube/nanosheet arrays on carbon cloth as advanced electrodes for high-performance flexible solid-state hybrid supercapacitors. RSC Adv. 2019, 9, 3041. [Google Scholar] [CrossRef] [Green Version]
- Li, X.N.; Zhang, H.S.; Cao, Z.X. Study on N-doped carbon phosphorus composite anode mate-rials for lithium ion batteries and their properties. J. Power Sources 2016, 140, 1163–1166. [Google Scholar]
- Zhao, Y.J.; Liu, J.L.; Ding, C.H. Rare-Earth Transition-Metal Chalcogenides. Cryst. Eng. Comm. 2018, 20, 2175–2182. [Google Scholar] [CrossRef]
- Reddy, M.G.; Rao, B.; Chowdari, B.V.R. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef]
- Shu, Q.M.; Xie, J.; Zhang, J.; Zhong, Y.J.; Du, G.H.; Xu, B.S. Facile In Situ Transmission Electron Microscopy Observation of Electrochemical Behavior of CoS2 in Lithium-Ion Battery. ACS Appl. Mater. Interfaces 2014, 6, 3016–3022. [Google Scholar]
- Galushkin, N.E.; Yazvinskaya, N.N.; Galushkin, D.N. A Critical Study of Using the Peukert Equation and Its Gener-alizations for Determining the Remaining Capacity of Lithi-um-Ion Batteries. Appl. Sci. 2020, 10, 5518. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Wang, N.N.; Sun, C.H.; Lu, Z.X.; Xue, P.; Tang, B.; Bai, Z.C.; Dou, S.X. 3D spongy CoS2 nanoparticles/carbon composite as high-performance anode material for lithium/sodium ion batteries. Chem. Eng. J. 2018, 332, 370–376. [Google Scholar] [CrossRef]
- Huang, H.H.; De Silva, K.; Kanishka, H.; Kumara, G.R.A.; Yoshimura, M. Structural Evolution of Hydrothermally Derived Reduced Graphene Oxide. Sci. Rep. 2018, 8, 6849–6853. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Li, L.Y.; Li, D.; Li, X.G.; Zhong, H.; Li, S.M.; Lei, L.M.; Li, J.B.; Zhang, Y. Microstructural evolution and mechanical properties of laser repaired 12Cr12Mo stainless steel. Mater. Sci. Eng. A 2022, 830, 142292. [Google Scholar] [CrossRef]
- Shu, H.T.; Mei, L.; Liang, J.F.; Zhao, Z.P.; Li, M.F.; Han, G.L.; Huang, Y.; Duan, X.F. Three-dimensional holey-graphene/niobia composite architectures for ultrahigh-rate energy storage. Science 2017, 356, 599–604. [Google Scholar]
- Bresser, D.; Mueler, F.; Franziska, M.; Martin, F.; Steffen, K.; Richard, K.; Dietmar, B.; Winter, M.; Elie, P.; Stefano, P. Transition-Metal-Doped Zinc Oxide Nanoparticles as a New Lithium-Ion Anode Material. Chem. Mater. 2013, 25, 4977–4985. [Google Scholar] [CrossRef]
- Liu, X.; Xu, H.; Ma, H.; Yue, D.; Wang, Q.Z.; Li, M.F.; Li, Y.L.; Chen, Y.L.; Wang, D. One pot synthesis and capacitive sodium storage properties of rGO confined CoS2 anode materials. J. Alloys Compd. 2020, 813, 151598. [Google Scholar] [CrossRef]
- Liu, T.C.; Lin, L.P.; Bi, X.X.; Tian, L.L.; Yang, K.; Liu, J.J.; Li, M.F.; Chen, Z.H.; Lu, J.; Amine, K.; et al. In situ quantification of interphasial chemistry in Li-ion battery. Nat. Nanotechnol. 2019, 14, 50. [Google Scholar] [CrossRef]
- Huang, X.; Zeng, Z.Y.; Fan, Z.X.; Liu, J.Q.; Zhang, H. Graphene-Based Electrodes. Adv. Mater. 2012, 24, 5979–6004. [Google Scholar] [CrossRef]
- Wen, Z.; Zhu, Z.; Jin, B.; Li, H.; Yao, W.M.; Jiang, Q. In-situ synthesis of Co1-xS-rGO composite for high-rate lithium-ion storage. J. Electoroanal. Chem. 2018, 833, 380–386. [Google Scholar] [CrossRef]
- Chen, L.; Wei, B.; Zhang, X.T.; Li, C. Bifunctional Graphene/γ-Fe2O3 Hybrid Aerogels with Double Nanocrystalline Networks for Enzyme Immobilization. Small 2013, 13, 2331–2340. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.T.; Yu, C.; Liu, S.H.; Yang, J.; Fan, X.M.; Qiu, J.S. Facile Fabrication of Bicomponent CoO/CoFe2O4-N-Doped Graphene Hybrids with Ultrahigh Lithium Storage. Paticle 2015, 32, 91–97. [Google Scholar] [CrossRef]
- Choi, S.H.; Lee, J.K.; Kang, Y.C. Three-dimensional porous graphene-metal oxide composite microspheres: Preparation and application in Li-ion batteries. Adv. Mater. 2015, 8, 1584–1594. [Google Scholar] [CrossRef]
- Li, X.N.; Yu, M.M.; Fan, Y.; Wang, Q.X.; Zhang, H.S.; Yang, S.T. Study on Electrochemical Performances of N-doped P/C Composite as Anode Material of Lithium Ion Batteries. Chem. J. Chin. Univ. 2019, 40, 2360–2366. [Google Scholar]
- Pan, Y.L.; Chen, X.D.; Gong, L.L.; Shi, L.; Zhou, T.; Deng, Y.R.; Zhang, H.P. Double-morphology CoS2 anchored on N-doped multichannel carbon nanofibers as high-performance anode materials for Na-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 31441–31451. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, J.; Wu, H.T.; Kong, F.J.; Yin, W.Y.; Cheng, H.J.; Tang, X.Y.; Qin, B.; Tao, S.; Yi, J.; et al. MOFs derived Co1-xS nanoparticles embedded in N-doped carbon nanosheets with improved electrochemical performance for lithium ion batteries. Appl. Surf. Sci. 2019, 479, 693–699. [Google Scholar] [CrossRef]
- Wang, Q.F.; Zou, R.Q.; Xia, W.; Ma, J.; Qiu, B.; Yang, Y.C.; Xia, T.G.; Xu, Q. Facile Synthesis of Ultrasmall CoS2 Nanoparticles within Thin N-Doped Porous Carbon Shell for High Performance Lithium-Ion Batteries. Small 2015, 11, 2511–2517. [Google Scholar] [CrossRef]
- Pan, Y.L.; Cheng, X.D.; Gong, L.; Zhang, H.P. Highly reversible Na ion storage in N-doped polyhedral car-bon-coated transition-metal chalcogenides by optimizing the nanostructure and surface engineerin. J. Mater. Chem. A 2018, 6, 18967–18978. [Google Scholar] [CrossRef]
- Chen, X.R.; Yao, Y.X.; Zhang, R.; Cheng, X.B.; Zhang, Q. A Diffusion-Reaction Competition Mechanism to Tailor Lithium Deposition for Lithium-Metal Batteries. Angew. Chem. 2020, 132, 7817–7821. [Google Scholar] [CrossRef]
- Ma, Y.; Bresser, D.; Ji, Y.; Geiger, D.; Kaiser, U.; Streb, C.; Varzi, A.; Passerini, S. Dominic Bresser Cobalt Disulfide Nanoparticles Embedded in Porous Carbonaceous Micro-Polyhedrons Interlinked by Car-bon Nanotubes for Superior Lithium and Sodium Storage. ACS Nano. 2018, 12, 7220–7231. [Google Scholar] [CrossRef]
- Xiong, Q.Q. Controllable growth of MoS2/C flower-like microspheres with enhanced electrochemical performance for lithium ion batteries. J. Alloys Compd. 2016, 673, 215–219. [Google Scholar] [CrossRef]
- Hu, Y.G.; Liu, Z.; Ran, F. New comprehensions on structure superiority of asymmetric carbon membrane and controlled construction of advanced hierarchical inner-structure for high performance supercapacitors. Microporous Mater. 2019, 275, 14–25. [Google Scholar]
- Wang, H.X.; Cui, Y. Nano diamonds for energy. Carbon Energy. 2019, 1, 13–18. [Google Scholar] [CrossRef] [Green Version]
- Kong, L.J.; Liu, Y.Y.; Huang, H.; Liu, M.; Xu, W.; Li, B.Y.; Bu, X.H. High-Performance Anode for Li-ion Batteries: Cross-Linked CoS2/NC-CNTs Networks. Sci. China Mater. 2021, 64, 820–829. [Google Scholar] [CrossRef]
- Pan, Y.L.; Cheng, X.D.; Huang, Y.; Zhang, H.P. CoS2 Nanoparticles Wrapping on Flexible Freestanding Multichannel Carbon Nanofibers with High Performance for Na-Ion Batteries. ACS Appl. Mater. Interfaces 2017, 9, 35820–35828. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.S.; Won, J.M.; Kang, Y.Z.; Lee, J.K. Promises and challenges of nanomaterials for lithium-based rechargeable batteries. Nano Energy 2016, 26, 466–478. [Google Scholar] [CrossRef]
- Lu, S.J.; Wang, Z.T.; Zheng, J.C.; He, Z.J.; Tong, H.; Zang, J.C.I. In situ-formed hollow cobalt sulfide wrapped by reduced graphene oxide as an anode for high-performance lithium-ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 2671–2678. [Google Scholar] [CrossRef]
- Nasrullah, S.; Touseef, R.; Li, X.M.; Yang, G.; Zhao, K.; Dong, L. Fabrication of an anode composed of a N, S co-doped carbon nanotube hollow architecture with CoS2 confined within: Toward Li and Na storage. Nanoscale 2019, 11, 20996–21007. [Google Scholar]
- Ying, J.; Xie, M.; Hu, F.; Ye, Z.Q.; Zhang, Y.X.; Wang, Z.H.; Zhou, Y.Z.; Li, L.; Chen, R.J. Cobalt Selenide Hollow Polyhedron Encapsulated in Graphene for High-Performance Lithium/Sodium Storage. Small 2021, 17, 2102893. [Google Scholar]
- Lin, J.; Peng, H.; Kim, J.-H.; Wygant, B.R.; Meyerson, M.L.; Rodriguez, R.; Liu, Y.; Kawashima, K.; Gu, D.; Peng, D.-L.; et al. Lithium fluoride coated silicon nanocolumns as anodes for lithium ion batteries. ACS Appl. Mater. Interfaces 2020, 12, 18465–18472. [Google Scholar] [CrossRef]
- Qiu, B.; Zhao, X.Y.; Xia, D.G. In situ synthesis of CoS2/RGO nanocomposites with enhanced electrode performance for lithium-ion batterie. J. Alloys Compd. 2013, 579, 372–376. [Google Scholar] [CrossRef]
- Xie, S.; Deng, Y.F.; Mei, J.; Yang, Z.T.; Liu, H. Facile synthesis of CoS2/CNTs composite and its exploitation in thermal battery fabrication. Compos. B Eng. 2016, 93, 203–209. [Google Scholar] [CrossRef]
- Liao, S.-Y.; Ciu, T.-T.; Zhang, S.-Y.; Cai, J.-J.; Zheng, F.; Liu, Y.-D.; Min, Y.-G. Cross-nanoflower CoS2 in-situ self-assembled on rGO sheet as advanced anode for lithium/sodium ion battery. Electrochim. Acta 2019, 326, 134992. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, T.; Dong, H.; Shi, Z.; Yue, H.; Yin, Y.; Li, X.; Zhang, H.; Wu, X.; Li, B.; Yang, S. Composite Nanoarchitectonics with CoS2 Nanoparticles Embedded in Graphene Sheets for an Anode for Lithium-Ion Batteries. Nanomaterials 2022, 12, 724. https://doi.org/10.3390/nano12040724
Li T, Dong H, Shi Z, Yue H, Yin Y, Li X, Zhang H, Wu X, Li B, Yang S. Composite Nanoarchitectonics with CoS2 Nanoparticles Embedded in Graphene Sheets for an Anode for Lithium-Ion Batteries. Nanomaterials. 2022; 12(4):724. https://doi.org/10.3390/nano12040724
Chicago/Turabian StyleLi, Tongjun, Hongyu Dong, Zhenpu Shi, Hongyun Yue, Yanhong Yin, Xiangnan Li, Huishuang Zhang, Xianli Wu, Baojun Li, and Shuting Yang. 2022. "Composite Nanoarchitectonics with CoS2 Nanoparticles Embedded in Graphene Sheets for an Anode for Lithium-Ion Batteries" Nanomaterials 12, no. 4: 724. https://doi.org/10.3390/nano12040724
APA StyleLi, T., Dong, H., Shi, Z., Yue, H., Yin, Y., Li, X., Zhang, H., Wu, X., Li, B., & Yang, S. (2022). Composite Nanoarchitectonics with CoS2 Nanoparticles Embedded in Graphene Sheets for an Anode for Lithium-Ion Batteries. Nanomaterials, 12(4), 724. https://doi.org/10.3390/nano12040724