Abstract
A bulk YBa2Cu3O7−δ (Y-123) superconductor synthesized by a thermal treatment method was added with different weight percentages (x = 0.0, 0.2, 1.0, 1.5, and 2.0 wt.%) of BiFeO3 (BFO) nanoparticle. X-ray diffraction (XRD), alternating current susceptibility (ACS), and field emission scanning electron microscopy (FESEM) were used to determine the properties of the samples. From the XRD results, all samples showed an orthorhombic crystal structure with a Pmmm space group. The sample x = 1.0 wt.% gave the highest value of Y-123. The high amounts of BFO degraded the crystallite size of the sample, showing that the addition did not promote the grain growth of Y-123. From ACS results, the Tc-onset value was shown to be enhanced by the addition of the BFO nanoparticle, where x = 1.5 wt.% gave the highest Tc value (91.91 K). The sample with 1.5 wt.% showed a high value of Tp (89.15 K). The FESEM analysis showed that the average grain size of the samples decreased as BFO was introduced. However, the small grain size was expected to fill in the boundary, which would help in enhancing the grain connectivity. Overall, the addition of the BFO nanoparticles in Y-123 helped to improve the superconducting properties, mainly for x = 1.5 wt.%.
1. Introduction
Superconductivity is a phenomenon where a material exhibits zero electrical resistance and does not allow any magnetic field to penetrate through it. A material that exhibits these properties is known as a superconductor. The yttrium-barium-copper-oxide (YBCO) ceramic compound is one of the greatest discoveries for high temperature superconductors (HTSs), and it was first discovered by Paul Chu and his colleagues in 1987. YBCO is a type-II superconductor with a critical temperature (Tc) of 92 K. It is the first material found to have a Tc above the boiling point of liquid nitrogen (77 K). Many applications have been explored that primarily use a superconductor, such as those in the medical and transportation fields. In the medical industry, magnetic resistance imaging (MRI) for instance, is one of its most powerful applications in diagnostic medical imaging. The superconducting magnet in MRI is a very influential tool as the magnet can achieve field values that cannot be reached by another conventional magnet. High quality, high resolution, and precise imaging can be produced as an outcome [1]. This superconductor technology has also taken place within transportation development. The Maglev Train is one of the best transportation systems in the world, where an advanced-speed train levitates above the track using superconducting magnets. This train does not depend on the friction of the track to move and stop as it travels along the guided route without any wheels. As a result, this train can reach speeds of more than 500 km/h [2].
In a type-II superconductor, the magnetic flux partially penetrates the bulk superconductor in the form of thin fibers called vortices. As the applied magnetic field is introduced, the loop of electric current, which is known as a screening current, is produced. The screening current interacts with the magnetic field and the external current, and this interaction moves the vortices. This vortex movement produces friction and increases energy dissipation. As more energy is dissipated, electric resistance arises, and superconductivity decreases. To reduce the dissipation of energy, the vortices need to be locked and pinned to reduce the movement. This motion of vortices can be stopped with crystal defects such as impurities and crystal lattice distortion [3]. Previous research found that additions and doping might help to produce the pinning defect in the system. This defective structure might enhance the flux pinning and result in an improvement of the critical current density Jc [4,5]. However, the Tc is also sensitive to doping and impurities such that the added element might suppress superconductivity [6].
In this research, a thermal treatment method is used in synthesizing the samples. This thermal treatment method, with the aid of a capping agent, has been widely used recently [7,8,9] and was chosen among other methods such as the solid-state [10], sol gel [11], and co-precipitation [12] methods because it was found that it can produce more homogeneous and finer powders, in addition to its low cost and faster and simpler step preparations [13]. The role of polyvinyl pyrrolidone (PVP) in this method, which acts as a capping agent, plays a significant role in stabilizing the particle and preventing agglomerations [8,14]. The superconductor yttrium-barium-copper-oxide (YBa2Cu3O), with the addition of a bismuth-ferrite (BFO) nanoparticle, was synthesized using the thermal treatment method. These nanoparticle materials were used in this research since they can diffuse easily during the high-temperature process. This will help to enhance the superconducting properties as they can function as strong pinning centers, unlike micro-particle materials [15,16]. The pinning centers are essential in reducing the dissipation of energy, hence improving the critical current density Jc of the superconductor [3]. Recently, the addition of magnetic impurities such as Fe3O4 and NiFe2O4 have been introduced in superconductors [17,18]. The addition of the magnetic materials is expected to increase the superconductor properties as there will be a strong interaction between the flux line network and the magnetic texture if the magnetic materials are of the same order magnitude as the flux line network. In this work, a BiFeO3 (BFO) nanoparticle is introduced into YBCO. BFO is a multiferroic material which exhibits both the ferromagnetic and ferroelectric order simultaneously at room temperature. It is interesting to study the effect of the addition of this magnetic material to YBCO as further studies on this material are rarely performed, especially where the thermal treatment method is used with a specific capping agent. Thus, the research was conducted to improve the properties of superconductors without significantly decreasing the Tc.
2. Materials and Methods
YBa2Cu3O7 bulk ceramic was synthesized by a thermal treatment method using high purity yttrium (III) nitrate hexadrate (Y(NO3)3∙6H2O), barium nitrate (Ba(NO3)2), and copper (II) nitrate hemi-pentahydrate CuN6·2.5H2O as the starting materials and polyvinyl pyrrolidone (PVP) as the capping agent. All materials were purchased from Alfa Aesar chemicals (Ward Hill, MA, US). In the thermal treatment method, the starting materials and PVP were dissolved and magnetically stirred in 300 mL deionized water for 2 h at a temperature of 80 °C. The solution was then dried at 110 °C for 24 h. The solid-like green gel was obtained and then crushed and ground into a fine powder. The sample was calcined two times at 600 °C for 4 h and at 910 °C for 24 h, with intermediate grinding. Figure 1 shows the proposed mechanism of how the PVP enwrapped the ions. In this process, the carbon-carbon double bond, C=C, breaks down and interacts with the PVP monomer. The polymerization occurs and forms a giant PVP molecule. The resonance structure of the PVP attracts the corresponding ion towards its fixed position, causing a good dispersion of the particles. The PVP then enwraps the ions that existed in the solution. These ions enwrapped by the PVP have limited space for the YBCO particles to nucleate, thus decreasing the grain size of the YBCO. This limited space promotes the growth in a specific direction and orientation [14], which helps to stabilize the particles and prevent agglomerations [8]. During the calcination process, the PVP and all the organic compounds were removed and the metallic oxide was formed [13]. After the calcination process, the powder was then ground and added with different weight percentages (x = 0.0, 0.2, 1.0, 1.5, and 2.0 wt.%) of the BiFeO3 nanoparticles. The sample was continuously ground and stirred to achieve a homogenous mixture. Later, the samples were palletized with 5-ton pressure to form a bulk superconductor. From this work, four pellets with 13 mm diameters and 5 mm thicknesses were produced. Finally, the sample was sintered in a furnace with flowing O2 at 980 °C for 24 h and was then ready for characterization. The crystal structure and phase formation of the samples were investigated by X-ray diffraction (XRD). The microstructures of the samples were studied using a scanning electron microscope (SEM-LEO 1455 VPSEM, Zeiss, Jena, Germany). The magnetic properties of the samples were measured to determine the critical temperature, Tc, using an AC susceptibility (ACS (SR830)) lock-in-amplifier at a frequency of 219 Hz with the applied field at 0.5 Oe. For this measurement, the bulk sample was cut to a bulk shape with dimensions of 5 mm × 2 mm × 1.5 mm.
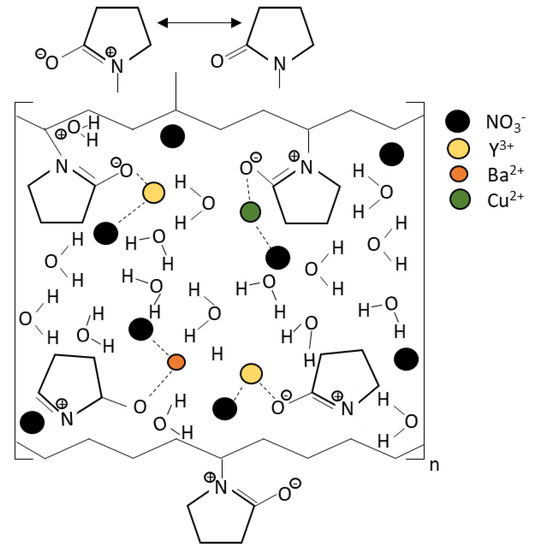
Figure 1.
Proposed mechanism of interaction between the PVP and the metal ion [13].
3. Results and Discussion
3.1. X-ray Diffraction
Figure 2 shows the XRD pattern of the Y-123 with added BFO (x = 0, 0.2, 1.0, 1.5, and 2.0 wt.%). The XRD pattern shows that most of the diffraction peaks could be indexed to Y-123, which was confirmed to be the dominant phase with an orthorhombic crystal structure and the space group Pmmm. The Y-211 phases also appeared as a minor phase. The highest intensity diffraction pattern of Y-123 was defined at 2θ ≈ 32.67°–33.04° with miller indices of [0 1 3] and [1 0 3]. As shown in Figure 3, the intensity of [0 1 3] and [1 0 3] increased until x = 1.0 wt.% and started to decrease as x = 1.5 wt.% was added. The addition of BFO into the Y-123 system did not show any unusual changes in the polycrystalline pattern of the diffractograms unless some extra peaks were assigned, which indicated the appearance of the Y-211 phase and some impurities that belonged to the BiFeO and YBaCuFeO. The Y-211 was expected to form during the solidification of the pores in the cooling process of the Y-123 phase [8]. The volume fraction of Y-123 in the x = 1.0 sample gave the highest value among the samples. The purity of the Y-123 formed by using a capping agent-aided thermal treatment was quite high compared to other methods such as co-precipitation [16]. BiFeO3 was only observed in x = 0.2 in the XRD spectrum, with a small value of 0.47%. However, as a higher amount of BFO was added, the BiFeO3 peak could not be observed in the XRD spectrum and the YBaCuFeO5 started to appear. The BiFeO3 peak could not be seen in the other addition as the wt.% of each addition was too small [16]. The weight percentages for the phases presented are stated in Table 1.
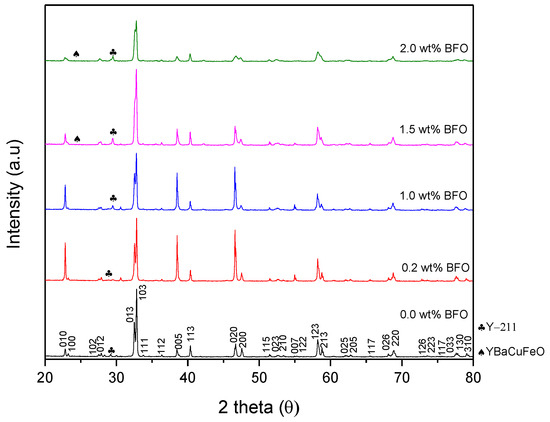
Figure 2.
X-ray diffraction pattern of the Y-123 + x wt.% of BFO.

Figure 3.
X-ray diffraction patterns corresponding to the [0 1 3] and [1 0 3] planes of the Y-123 for the Y-123 + x wt.% of BFO.

Table 1.
Weight percentages of the phases presented in the samples for the Y-123 + x wt.% of BiFeO3.
Table 2 shows the crystallite size, lattice strain, lattice parameter, unit cell volume, and orthorhombicity of all samples. The orthorhombicity was calculated using the formula [(b − a)/(a + b)], where a is the lattice constant a and b is the lattice constant b [13]. Referring to the same table, the trend of the b value of the lattice parameter is aligned with the orthorhombicity value. The change in the lattice parameter could be seen as the removal of oxygen from the copper-oxygen chains occurring along the b-axis [19]. Meanwhile, the orthorhombicity values changed with the changes on the lattice constant due to the oxygen content in the sample [20]. As the oxygen atoms were removed, the strain energy that kept the chain directed along the b-axis was diminished, thus decreasing the b value of the lattice parameter [21].

Table 2.
Crystallite size, lattice strain, lattice parameters, unit cell volume, and orthorhombicity of the samples for the Y-123 + x wt.% of BiFeO3.
Meanwhile, the average crystallite size can be calculated from the (103) peak using the Scherrer equation,
where k is the shape factor, which is usually taken as 0.9, is the wavelength of the XRD CuKα radiation source (nm), is the highest intensity of the main peak, and is the Bragg angle [22]. Referring to Figure 3, the broadening of the FWHM peak is observed as higher amounts of BFO are added. This broadening of the FWHM peak can be related to the crystallite size and lattice parameter of the samples [23]. From the figure, the broadening of the peak indicates the decrease in crystallite size, which shows that the BFO addition does not promote the crystal growth of Y-123 [24]. The lattice strain value increases as a higher amount of BFO is added, and this indicates the appearance of lattice defects such as vacancies and substitutions [23]. The addition of a high amount of magnetic material affects the symmetry of the sample [25] in which, in this sample, the appearance of the antiferromagnetic compound YBaCuFeO5 is believed to disturb the lattice of Y-123.
3.2. Alternating Current Susceptibility (ACS) Analysis
The ACS curves consist of two steps of diamagnetic transitions which are real (χ′) and imaginary (χ″) susceptibility peaks. For the real part (χ′), the onset critical temperature, Tc-onset, and phase lock-in temperature, Tcj, are observed in every sample. The Tc-onset is used to describe the transition temperature at which the sample starts to become a superconductor, where this transition occurs within the grain (intra-grain), while Tcj occurred due to the superconducting coupling between the grains (inter-grains). Meanwhile, in the imaginary part (χ″), the coupling peak temperature, Tp, is observed. The Tp indicates the maximum hysteresis loss due to the motion of the intergranular vortices [26].
Figure 4 shows the temperature dependence of the real (χ′) and imaginary (χ″) susceptibility of Y-123 with the BFO addition (x = 0, 0.2, 1.0, 1.5, and 2.0 wt.%). The addition of the BFO nanoparticle enhanced the Tc-onset. However, the increment of the Tc-onset value was only up to x = 1.5 wt.%, and then it continued to decrease as higher amounts of magnetic material were added [25]. The double-step transitions indicate the presence of the Y-211 phase [20], which happened due to the shielding of flux by the intra-grain currents as the temperature decreased [27]. The Tcj value decreased as BFO was introduced; however, the x = 1.5 sample gave the best values among the added samples. The high value of Tcj indicates strong superconductor inter-granular coupling between the grains [28]. The derivatives curve of Tcj plotted by Origin software is shown in Figure 5. A general view which illustrates the inter-grain current, intra-grain current, and inter-granular coupling is illustrated in Figure 6.
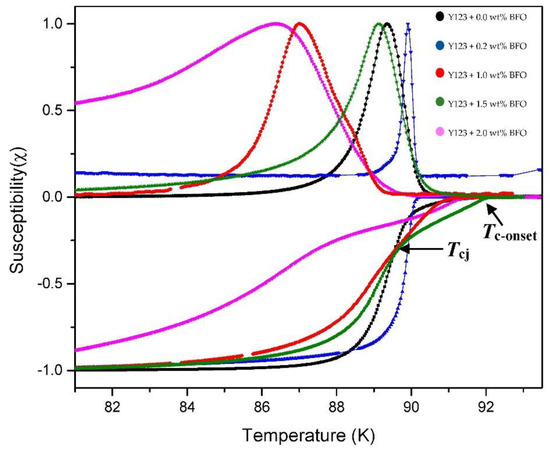
Figure 4.
Graph of the normalized susceptibility of χ′ and χ″ against the temperature of the Y-123 + x wt.% of BiFeO3.
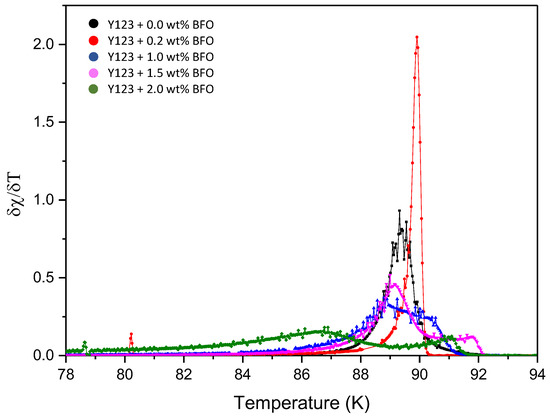
Figure 5.
Derivatives of Tcj’s susceptibility against the temperature of the Y-123 + x wt.% of BiFeO3.
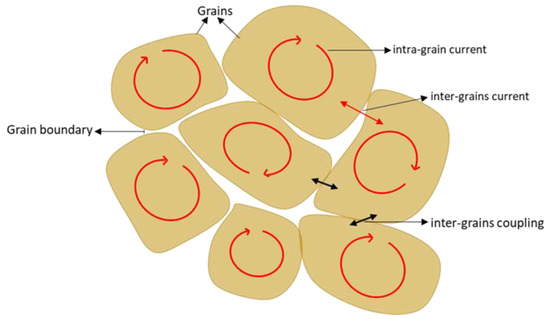
Figure 6.
A general view of a polycrystalline sample illustrates the inter-grain current, intra-grain current, and inter-grain coupling.
As shown in the imaginary part, as the weight percentage of BFO increased, the peak shifted to the left, except for x = 0.2 wt.%, where its Tp increased in value for approximately 0.56 K from the pure sample. The sample x = 1.5 wt.% also showed a high value of Tp compared to the other added samples. A high Tp value indicates strong inter-granular solid coupling between the grains [29]. The addition of BFO helped in improving the coupling between the grains that reduced the intergranular Josephson vortices’ motion, thus improving the superconductivity [26]. The ratio of the Tp/Tc-onset values in Table 3 show a declining trend where this ratio also represents the coupling between the grains. The grain coupling can be further evaluated by calculating the value of the Josephson current, Io, using the following formula:
where Tc-onset is the onset critical temperature and Tcj is the phase lock-in temperature. As shown in Table 3, the Io value increased as BFO was introduced in the Y-123, which proves that the coupling in between the grains was enhanced. The value for oxygen deficiency, δ, for all samples is also listed in the same table. These δ values are essential as the transport properties of the YBCO are strongly dependent on the δ. It is believed that the lower value of δ provided the increment of Tc on the sample [30]. Referring to Table 3, the lowest value of δ was shown by the x = 1.5 sample, which resulted in the highest Tc value. These values of oxygen deficiency were calculated using the formula
where δ is the oxygen deficiency and c is the lattice parameter’s c-axis [31]. The coupling temperature peak, Tp, onset critical temperature, Tc-onset, phase lock-in temperature, Tcj, Josephson current, Io, and oxygen deficiency, δ, can be referred to as shown in Table 3.

Table 3.
The coupling peak temperature, Tp, onset critical temperature, Tc-onset, phase lock-in temperature, Tcj, Josephson current, Io, and oxygen deficiency, δ, for the Y-123 + x wt.% of BiFeO3.
Table 4 shows the coupling peak temperature, Tp, and the intergranular critical current density, Jcm, for all samples. The value of Jcm as a function of temperature was estimated using the Bean critical state model [32] with the following formula:
where a and b are the cross-sections of the bar-shaped sample and Hac is the applied AC field. The Jcm value for the pure sample at 89.35 Tp was 3.88 A/cm2. The Jcm value decreased as 0.2 wt.% BFO was added to the sample. However, the Jcm value became higher as the BFO wt.% increased. This enhancement of the Jcm values is because of the introduction of the pinning centers in the YBCO system by the BFO [27]. The Jcm values of 1.0 wt.% and 2.0 wt.% were similar. However, the Tp value of the 1.0 wt.% was higher, which shows that the inter-grain coupling of the 1.0 wt.% samples was stronger than that of the 2.0 wt.%. The low value of Tp with a high value of Jcm may have occurred because of the flux pinning in the sample due to lattice defects such as vacancies and substitutions. The lower value of Tp shows that the flux penetration occurred in the grain boundaries at a lower temperature. The lower value of Tp also shows the weakening of the intergranular coupling between the grains [29]. In this work, x = 1.0 gave a higher value of Tp than x = 2.0, which shows that x = 1.0 could carry that amount of current at a higher Tp. This means that the flux penetration of the grain boundary at x = 1.0 happened at a higher Tp.

Table 4.
The cross-section area of a sample with the coupling peak temperature Tp and intergranular critical current density Jcm for the Y-123 + x wt.% of BiFeO3.
3.3. Field Emission Scanning Electron Microscopy (FESEM) Analysis
The morphological characterization was analyzed using the field emission scanning electron microscopy (FESEM) method. Figure 7 shows the FESEM micrographs with a 5000× magnification of the sample surface image. The average grain size of all samples was calculated from 20 randomly selected grains using ImageJ software, and they are listed in Table 5. All samples show compact microstructures with randomly distributed grains. As seen in Figure 7a, the grain size was found to be up to 20 µm; however, smaller grain sizes ranging from 0–2 µm were dominant. The average grain size for the sample was approximately 5 µm. As can be seen in Figure 7, the grain size decreased as higher wt.% amounts of BFO were added. The grain size reduction is proven with the decrease of crystallite size determined from the XRD. This reduction of grain size with the BFO addition is also observed in other methods such as the powder melt and infiltration growth (PM-IG) techniques [33] and the solid-state method [34]. However, the small grains are expected to help fill in the pores in between the grains, thus benefiting by linking the grains and enhancing the grain connectivity. These grains become tightly packed together and improve the electron transportation between the grains [13]. As could be observed in the sample x = 1.5 wt.%, the fine grains seemed to link up the voids between the grains. Referring to the ACS data, the Tp value for x = 1.5 wt.% sample was quite high, with 89.15 K, which indicates good inter-grain coupling, resulting in a high value of Tc (91.91 K) [35]. Furthermore, the fine grains also helped in increasing the vortex pinning center. Pinning may also happen with the addition of magnetic material as it may exhibit a nanophase between the YBCO grains [17]. These pinning centers can help in enhancing the Jc value of a sample. Nevertheless, as higher amounts of BFO were added, the microstructures seemed to diffuse around the sample, thus degrading the Tc value. This can be attributed to the extra addition of BFO [34]. The addition of a high magnetic moment with a lower wt.% gave a better value of Tc [25].
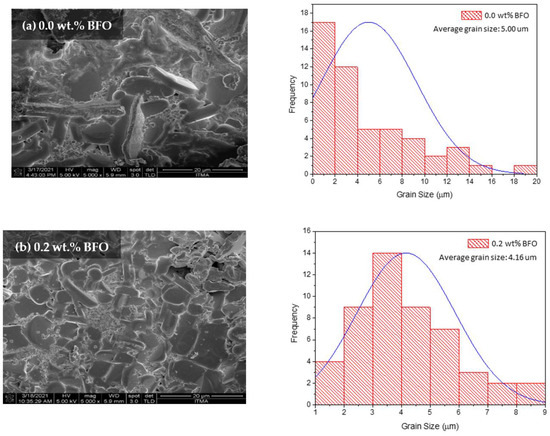
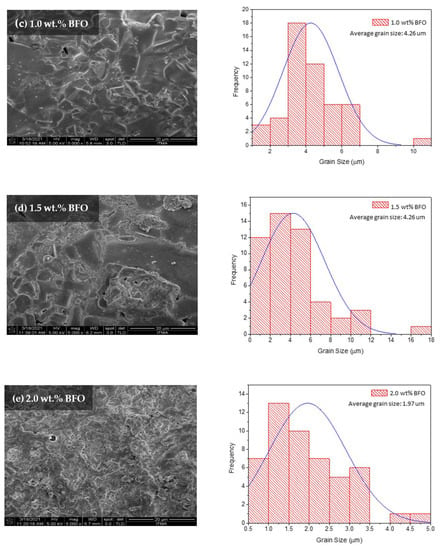
Figure 7.
The FESEM micrographs (with a 5000× magnification) and grain size distribution for the surface Y-123 with different weight percentages of BiFeO3: (a) 0.0 wt.%, (b) 0.2 wt.%, (c) 1.0 wt.%, (d) 1.5 wt.%, and (e) 2.0 wt.%.

Table 5.
The average size for the Y-123 + x weight % of BiFeO3.
3.4. Energy Dispersive X-ray (EDX) Analysis
Energy dispersive X-ray (EDX) was used to determine the elements found in the sample. The atomic percentages of every element found are tabulated in Table 6. Based on the EDX spectra in Figure 8, the existence of the yttrium (Y), barium (Ba), copper (Cu), oxygen (O), bismuth (Bi), and ferum (Fe) peaks proved that these elements were present in the samples. However, the element of carbon (C), which is an impurity, was also detected. In the pure sample, the C peaks were apparent in the EDX spectra. Previous researchers [36] have also reported the existence of this element. These impurities of BaCO3 in the XRD analysis have also been reported. The suppression of BaCO3 might lead to another stable intermediate such as Y-211 [37]. However, in this research, BaCO3 was not detected in the XRD likely because the intensity of the C element was too small, with a weight percentage of only 2.78%. However, the Y-211 was still detected in the XRD (refer to Table 1). Table 6 shows the atomic percentages and atomic ratios of every element found in the samples. However, based on the table, the atomic ratio of Y:Ba:Cu did not show the 1:2:3 ratio for the added samples. This may have happened because the elemental ratio was taken randomly from the sample.

Table 6.
The atomic percentages and atomic ratios for the Y-123 + x wt.% of BiFeO3.
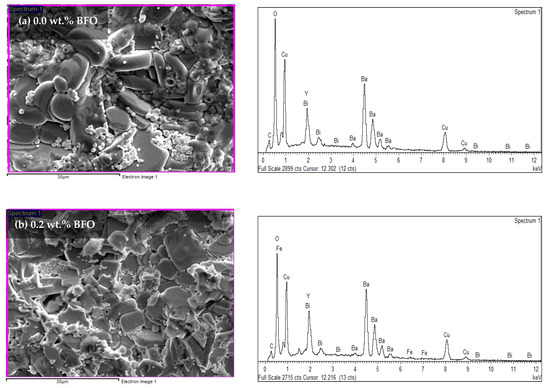
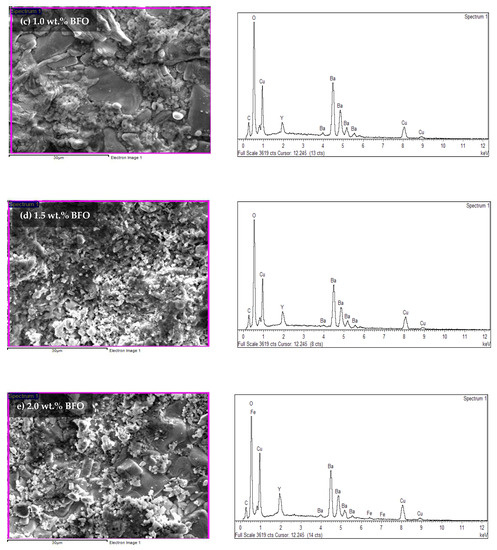
Figure 8.
The EDX spectra for the Y-123 with different weight percentages of BiFeO3: (a) 0.0 wt.%, (b) 0.2 wt.%, (c) 1.0 wt.%, (d) 1.5 wt.%, and (e) 2.0 wt.%.
4. Conclusions
The Y-123 was successfully synthesized using the thermal treatment method with PVP as the capping agent. The XRD revealed that all samples had Y-123 as the major phase and Y-211 as the secondary phase, together with some impurities such as BiFeO and YBaCuFeO. The crystallite size decreased as higher amounts of BFO were added, which shows that BFO did not promote the crystal growth of the Y-123 phase. The lattice strain came as opposition to the increment in crystallite size. The strain increased with higher percentages of BFO addition, hence showing the appearance of the lattice defects such as vacancies. From the ACS, the Tc-onset was enhanced up to 91.91 K with the BFO addition for x = 1.5 wt.%. However, the value then decreased as higher amounts of BFO were added. The oxygen deficiency, δ, was the lowest for x = 1.5 wt.%. This low value of δ gave x = 1.5 wt.% the highest Tc because if the δ was high, the charge carrier would become lower as the oxygen was removed at the Cu-O chain sites, thus degrading the Tc value. In terms of the microstructure properties, the FESEM revealed that the trend of the grain size was the same as that of the crystallite size in the XRD. The grain size degraded as BFO was introduced in the Y-123. Despite the degradation in the grain size, the small grains were expected to fill in the boundary and thus help in enhancing the grain connectivity. Furthermore, the fine grains may have helped in increasing the vortex pinning center and enhancing the Jc. The vortex pinning also can occur with the addition of magnetic material as this material exhibits a nanophase between the YBCO grains and function as pinning centers between the grains. Overall, the addition of the BFO nanoparticles in the Y-123 helped to improve the superconducting properties, mainly for x = 1.5 wt.%, as higher amounts of BFO addition may degrade the quality of the superconductor.
Author Contributions
Conceptualization, N.A.C.D.-K. and M.M.A.K.; methodology, N.A.C.D.-K. and S.I.A.S.; formal analysis, N.A.C.D.-K. and S.I.A.S.; investigation, N.A.C.D.-K. and M.M.A.K.; writing—original draft preparation, N.A.C.D.-K.; writing—review and editing, M.M.A.K., H.B., M.K.A.K., M.K.S. and K.K.M.S.; supervision, M.M.A.K., S.K.C., A.H.S. and K.P.L.; project administration, M.M.A.K.; funding acquisition, M.M. and M.M.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Ministry of Higher Education Malaysia through the Fundamental Research Grant Scheme (FRGS) under project number FRGS 1/2017/STG02/UPM/02/4, grant number FRGS 5546036. Additionally, this work was partly supported by Shibaura Institute of Technology (SIT) Inter-national Research Center for Green Electronics.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Miryala, S. Prospects of Superconducting Magnet Technology in the Medical Field: A New Paradigm on the Horizon? In Superconductivity; Springer: Cham, Germany, 2020; pp. 353–360. [Google Scholar]
- Lee, H.W.; Kim, K.C.; Lee, J. Review of Maglev Train Technologies. IEEE Trans. Magn. 2006, 42, 1917–1925. [Google Scholar]
- Ginzburg, V.L.; Andryushin, E.A. Superconductivity (Revised Edition); World Scientific: Singapore, 2004. [Google Scholar]
- Crisan, A.; Sarkar, A.; Mikheenko, P.; Dang, V.S.; Kechik, M.M.A.; Abell, J.S. Improvement of Pinning Force and Critical Current Density in Thick YBa2Cu3O7− δ Films Grown on SrTiO3 Substrates Decorated with LaNiO3 Nanodots. J. Supercond. Nov. Magn. 2009, 22, 631–636. [Google Scholar] [CrossRef]
- Lu, X.Y.; Yi, D.; Chen, H.; Nagata, A. Effect of SnO, MgO and Ag2O Mix-Doping on the Formation and Superconducting Properties of Bi-2223 Ag/tapes. Phys. Procedia 2016, 81, 129–132. [Google Scholar] [CrossRef]
- Ramli, A.; Halim, S.A.; Chen, S.K.; Kechik, M.M.A. The effect of Gd2O3 Nanoparticles Addition on Microstructural and Electrical Properties of YBCO Superconductor. J. Eng. Appl. Sci. 2016, 11, 13708–13715. [Google Scholar]
- Yusuf, N.N.M.; Kechik, M.M.A.; Baqiah, H.; Chen, S.K.; Lim, K.P.; Halim, S.A.; Abd-Shukor, R. Structural and Superconducting Properties of Thermal Treatment-Synthesised Bulk YBa2Cu3O7−δ Superconductor: Effect of Addition of SnO2 Nanoparticles. Materials 2019, 12, 92. [Google Scholar] [CrossRef]
- Naseri, M.G.; Saion, E.B.; Ahangar, H.A.; Hashim, M.; Halim, S.A. Synthesis and Characterization of Manganese Ferrite Nanoparticles by Thermal Treatment Method. J. Magn. Magn. 2011, 323, 1745–1749. [Google Scholar] [CrossRef]
- Zahari, R.M.; Shaari, A.H.; Abbas, Z.; Baqiah, H.; Chen, S.K.; Lim, K.P.; Kechik, M.M.A. Simple Preparation and Characterization of Bismuth Ferrites Nanoparticles by Thermal Treatment Method. J. Mater. Sci. Mater. Electron. 2017, 28, 17932–17938. [Google Scholar] [CrossRef]
- Mahto, C. Synthesis and Characterization of YBa2Cu3O7−x Superconductor. In Master Science; National Institute of Technology: Rourkela, India, 2015. [Google Scholar]
- Admaiai, L.F.; Grange, P.; Delmon, B.; Cassart, M.; Issi, J.P. Synthesis of Bulk and Film YBa2Cu3O7−x High-temperature Superconductor by the Sol-gel Method. J. Mater. Sci. 1994, 29, 5817–5825. [Google Scholar] [CrossRef]
- Pramanik, P.; Biswas, S.; Singh, C.; Bhattacharya, D.; Dey, T.K.; Sen, D.; Ghatak, S.K.; Chopra, K.L. Coprecipitation Method for Preparation of Superconducting YBa2Cu3Ox Compounds. Mater. Res. Bull. 1988, 23, 1693–1698. [Google Scholar] [CrossRef]
- Dihom, M.M.; Halim, S.A.; Baqiah, H.; Chen, S.K.; Azis, R.S.; Abd-Shukor, R.; Al-Hada, N.M.; Kechik, M.M.A.; Talib, Z.A. Calcium-substituted Y3Ba5Cu8O18 Ceramics Synthesized via Thermal Treatment Method: Structural and Superconducting Properties. J. Supercond. Nov. Magn. 2019, 32, 1875–1883. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in Nanoparticle Synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [PubMed]
- Mellekh, A.; Zouaoui, M.; Azzouz, F.B.; Annabi, M.; Salem, M.B. Nano-Al2O3 Particle Addition Effects on YBa2Cu3Oy Superconducting Properties. Solid State Commun. 2006, 140, 318–323. [Google Scholar] [CrossRef]
- Khalid, N.A.; Kechik, M.M.A.; Baharuddin, N.A.; Chen, S.K.; Baqiah, H.; Yusuf, N.N.M.; Halim, S.A.; Hashim, A.; Talib, Z.A. Impact of Carbon Nanotubes Addition on Transport and Superconducting Properties of YBa2Cu3O7−δ ceramics. Ceram. Int. 2018, 44, 9568–9573. [Google Scholar] [CrossRef]
- Kong, W.; Abd-Shukor, R. Enhanced Electrical Transport Properties of Nano NiFe2O4-added (Bi1.6Pb0 4)Sr2Ca2Cu3O10 Superconductor. J. Supercond. Nov. Magn. 2010, 23, 257–263. [Google Scholar] [CrossRef]
- Abd-Ghani, S.N.; Abd-Shukor, R.; Kong, W. Effects of Fe3O4 Nano Particles Addition in High Temperature Superconductor YBa2Cu3O7-δ. Adv. Mat. Res. 2012, 501, 309–313. [Google Scholar]
- Tranquada, J.M.; Heald, S.M.; Moodenbaugh, A.R.; Xu, Y. Mixed Valency, Hole Concentration and Tc in YBa2Cu3O6+x. Phys. Rev. B 1988, 38, 8893. [Google Scholar] [CrossRef] [PubMed]
- Inoue, K.; Sakai, N.; Murakami, M.; Hirabayashi, I. Effect of Potassium Addition on YBa2Cu3Ox Superconductors. Phys. C Supercond. Appl. 2006, 445, 128–132. [Google Scholar] [CrossRef]
- Howe, B.A. Crystal Structure and Superconductivity of YBa2Cu3O7–x; Minnesota State University: Mankato, MN, USA, 2014. [Google Scholar]
- Monshi, A.; Foroughi, M.R.; Monshi, M.R. Modified Scherrer Equation to Estimate more Accurately Nano-crystallite Size using XRD. World J. Nano Sci. Eng. 2012, 2, 160. [Google Scholar] [CrossRef]
- Serquis, A.; Zhu, Y.T.; Peterson, E.J.; Coulter, J.Y.; Peterson, D.E.; Mueller, F.M. Effect of Lattice Strain and Defects on the Superconductivity of MgB2. Appl. Phys. Lett. 2001, 79, 4399–4401. [Google Scholar] [CrossRef]
- Hassanzadeh-Tabrizi, S.A.; Mazaheri, M.; Aminzare, M.; Sadrnezhaad, S.K. Reverse Precipitation Synthesis and Characterization of CeO2 Nanopowder. J. Alloys Compd. 2010, 491, 499–502. [Google Scholar] [CrossRef]
- Rammah, Y.S.; Salama, A.H.; Elkhatib, M. MagneticMmoment and its Correlation with the Critical Temperature in YBCO. Interceram 2019, 68, 34–41. [Google Scholar]
- Zelati, A.; Amirabadizadeh, A.; Kompany, A.; Salamati, H.; Sonier, J. Effects of Dy2O3 Nanoparticle Addition on Structural and Superconducting Properties of BSCCO. Indian J. Sci Technol. 2014, 7, 123. [Google Scholar] [CrossRef]
- Ramli, A.; Halim, S.A.; Baqiah, H.; Chen, S.K.; Kechik, M.M.A.; Talib, Z.A. Role of Nd2O3 Nanoparticles Addition on Microstructural and Superconducting Properties of YBa2Cu3O7−δ ceramics. J. Rare Earths 2016, 34, 895–900. [Google Scholar] [CrossRef]
- Özdaş, E.; First, T. Influence of the Hole Concentration on Tc in 110K BiPbSrCaCuO Superconductors: The Intergrain and Intragrain Effects. In Physics and Materials Science of High Temperature Superconductors; Springer: Dordrecht, Germany, 1992; pp. 381–393. [Google Scholar]
- Rani, P.; Jha, R.; Awana, V.P.S. AC Susceptibility Study of Superconducting YBa2Cu3O7: Agx Bulk Composites (x = 0.0–0.20): The Role of Intra and Intergranular Coupling. J. Supercond. Nov. Magn. 2013, 26, 2347–2352. [Google Scholar] [CrossRef]
- Singh, R.K.; Varshney, D.; Khaskalam, A.K. Effect of Oxygen Deficiency (δ) on Transition Temperature of Yttrium Cuprate Superconductors. Bull. Mater. Sci. 1996, 19, 737–747. [Google Scholar] [CrossRef]
- Benzi, P.; Bottizzo, E.; Rizzi, N. Determination from Cell Dimensions in YBCO Superconductors. J. Cryst. Growth Oxyg. 2004, 269, 625–629. [Google Scholar] [CrossRef]
- Bean, C.P. Magnetization of Hard Superconductors. Phys. Rev. Lett. 1962, 8, 250. [Google Scholar] [CrossRef]
- Zhang, N.; Ye, C.; Yan, H.; Li, L.; He, H.; Wang, D.; Li, Y. Single-atom Site Catalysts for Environmental Catalysis. Nano Res. 2020, 13, 3165–3182. [Google Scholar] [CrossRef]
- Bahboh, A.; Halim, S.A.; Baqiah, H.; Chen, S.K.; Kechik, M.M.A.; Talib, Z.A.; Dihom, M.M. Effect of Sol-gel Synthesized BiFeO3 Nanoparticle Addition in YBa2Cu3O7–δ (Y123) Superconductor Synthesized by Standard Solid State Reaction Method. Solid State Phenom. 2019, 290, 245–251. [Google Scholar] [CrossRef]
- Attaf, S.; Mosbah, M.F.; Vecchione, A.; Fittipaldi, R. The influence of Doping with Ca and Mg in YBa2Cu3O7−δ Ceramic. EDP Sci. 2019, 29, 00003. [Google Scholar]
- Jongprateep, O.; Chansuriya, A.; Rugthaichareoncheep, S. Composition and Particle Size of ReBa2Cu3O7−X Superconductor Powders Synthesized by Solid State Reactions. Suranaree. J. Sci. Technol. 2013, 19, 155–160. [Google Scholar]
- Boston, R.; Awaya, K.; Nakayama, T.; Ogasawara, W.; Hall, S.R. Formation of Superconducting Yttrium Barium Copper Oxide using Sulphur-containing Templates. RSC Adv. 2014, 4, 26824–26828. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).