Abstract
Chemical energy conversion strategies by photocatalysis and electrocatalysis are promising approaches to alleviating our energy shortages and environmental issues. Due to the 2D layer structure, adjustable composition, unique thermal decomposition and memory properties, abundant surface hydroxyl, and low cost, layered double hydroxides (LDHs) have attracted extensive attention in electrocatalysis, photocatalysis, and photoelectrocatalysis. This review summarizes the main structural characteristics of LDHs, including tunable composition, thermal decomposition and memory properties, delaminated layer, and surface hydroxyl. Next, the influences of the structural characteristics on the photo(electro)catalytic process are briefly introduced to understand the structure–performance correlations of LDHs materials. Recent progress and advances of LDHs in photocatalysis and photoelectrocatalysis applications are summarized. Finally, the challenges and future development of LDHs are prospected from the aspect of structural design and exploring structure-activity relationships in the photo(electro)catalysis applications.
1. Introduce
Globalization and industrialization development have accelerated population increase and economic development, which greatly increase demand of fossil fuels [1]. As of 2018, fossil energy still accounted for 80% of the world’s primary energy [2]. The huge consumption of fossil energy brings numerous ecological and social problems, such as the greenhouse effect [3,4], environmental pollution [5,6], and reduction of fossil energy [7,8,9]. Solar and hydrogen energy attract attention as promising green and clean energy sources to address our energy shortages and environmental problems. However, it is necessary to convert solar energy and excess electrical energy into chemical energy stored in chemical molecules, due to limits of time and space [10]. Among various chemical energy conversion strategies, photocatalysis and electrocatalysis are attractive approaches for converting solar energy and produce hydrogen or hydrocarbon fuels, such as water splitting and CO2 reduction by photo(electro)catalysis [11,12,13,14,15,16]. A variety of materials have been exploited in photo(electro)catalytic energy conversion. Among these materials, two-dimensional (2D) materials have attracted tremendous interest due to its high charge mobility and large specific surface area [17,18,19].
Layered double hydroxide (LDH) is a classical 2D layered material. Natural LDH (hydrotalcite) was discovered in 1842 [20]. In 1942, Feitknecht and Gerber first synthesized LDHs and proposed the concept of a double sheet structure [20,21]. Until 1969, the lamellar structure of LDHs was determined by Allmann and Taylor using single crystal X-ray diffraction [22]. Due to the unique structures and properties, the interest in LDHs is gradually increasing and the application is also widely explored [23,24,25,26,27,28]. For example, the interlayer space, exchangeable guest anions, and high specific surface areas are very beneficial for the removal of pollutants (organic contaminant, heavy metal ions, etc.) and the loading of drugs, resulting in potential applications in the fields of environmental protection and drug delivery [24,26]. Due to the 2D layer structure, adjustable composition, high specific surface area and abundant active sites, and mass producibility, LDHs have attracted extensive attention in electrocatalysis [29,30,31,32], photocatalysis [33,34,35], and photoelectrocatalysis [36,37,38]. Among the many structural features of LDHs, tunable structure, such as controllable composition (tunable metal cations and guest anions) and size (delamination of LDH), are huge advantages for function-oriented design of LDHs in photo(electro)catalysis. Thus, it is crucial to understand the relationship between structure and photo(electro)catalytic performance of LDHs.
In this review, we briefly highlight the influences of the intrinsic structural characteristics of LDHs on the photo(electro)catalytic process to better understand the structure–performance correlations. Recent progress and advances of LDHs in photocatalysis and photoelectrocatalysis applications (water splitting, CO2 reduction, and contaminant degradation) are summarized in Section 2. Finally, the challenges and future development of LDHs are also examined from the aspect of structural design and exploration of structure–activity relationships in photo(electro)catalysis applications.
2. Structures and Properties of LDH
LDHs, also called hydrotalcite-like compounds, are two-dimensional layered clays. LDHs consist of host layers with metal cations and interlayer anions to keep charge balance with H2O molecules. Thus, the general formula for LDHs is written as [M2+1−xM3+x(OH)2]x+[Ap−x/p]x−·mH2O, where M2+ is divalent metal cation (e.g., Fe2+, Mg2+, Ni2+, Co2+, Cu2+, Mn2+, Zn2+, Cd2+, Pd2+, and Ca2+), M3+ is trivalent metal cation (e.g., Co3+, Al3+, Mn3+, Fe3+, Cr3+, Ga3+, V3+, and Tb3+), X = M3+/(M2++M3+), (0.2 ≤ x ≤ 0.33), Ap- is an interlayer anion, and m represents the number of H2O molecules [2,39,40], as shown in Figure 1. On occasion, there are metal cations of M+ and M4+ in LDHs, but only with the exception of M+ being Li+ and M4+ being Ti4 + [18]. The main structural properties of LDH are tunable composition, thermal decomposition, memory properties, delaminated layer, and abundant surface hydroxyls [17]. Next, we will elaborate on the effect of these structural properties on photo(electro)catalysis performance.
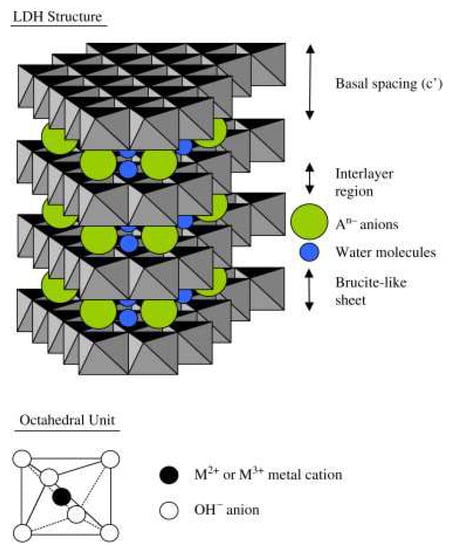
Figure 1.
Structural model of LDH [40]. Copyright 2007 Elsevier.
2.1. Adjustable Composition
The most significant structural property of LDH is the compositional flexibility, including tunable metal cations in the host layer and guest anions in the interlayer. The tunability of composition significantly affects the physicochemical properties of LDH. We will discuss the influences of tunable composition on the physicochemical properties and photoelectrocatalytic performance of LDH.
2.1.1. Regulating Energy Band Structure
The varied metal cation species and ratios modulate the composition of LDHs, and their physicochemical properties change significantly. The band structure of the LDH is usually regulated by the changed types and ratios of metal cations in the host layer, which change the range of light absorption and oxidation–reduction potential of LDH. Xu et al. [41] found band gaps of Mg and Zn-based LDH were greater than 3.1 eV, whereas the Co and Ni-based LDH samples absorbed visible light with a band gap lower than 3.1 eV (Figure 2). Guo et al. [42] loaded TiO2 to three different cobalt-based LDHs (CoAl-LDH, CoCr-LDH, and CoFe-LDH). The Ti-TiO2@CoCr-LDH had the optimal photoelectrocatalytic (PEC) performance with a 43% increase in photocurrent in those samples. This is because the band structure of CoCr-LDH has the best matching with reduced TiO2, resulting in the best water oxidation performance.
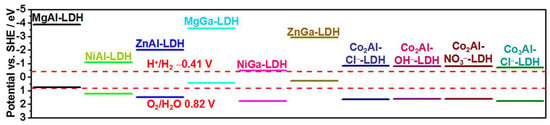
Figure 2.
The position of MIIMIII−LDHs band edge [41]. Copyright 2015 American Chemical Society.
The changed ratio of metal cations can also adjust the band gap and light absorption of LDHs. Han and Yang et al. [43] reported that BiVO4/NiFe-LDH core/shell heterostructure films had four times higher photocurrent intensity than that of pure BiVO4 at 1.23V vs. reversible hydrogen electrode (RHE). The higher content of Fe3+ in NiFe-LDH resulted in a smaller band gap and stronger light absorbance and conductivity. Parida et al. [44] fabricated the ternary series of Mg/Al + Fe-CO3 LDHs by adjusting the rate of Al/Fe. The Fe3+ doping increased the visible-light absorption of MgAl-LDHs, resulting in the better H2 evolution performance.
2.1.2. Promoting Electron-Hole Pairs Separation
The variable valence state of metal cations of LDHs directly promotes the transfer and separation of charge carriers. Low-valence metal cations are oxidized to high-valence metal cations by the photogenerated holes, which improve the transfer and separation of photogenerated charge carriers. For example, Bai et al. [45] synthesized the NiFe-LDH/Mo-BiVO4 heterostructure by an electrodeposition method. The photogenerated holes transferred from BiVO4 nanoparticles to NiFe-LDH due to a type II staggered band structure of the heterostructures. At the same time, the photogenerated holes oxidized Ni2+ from NiFe-LDH to Ni3+and Ni4+. The Ni3+and Ni4+ take part in oxygen evolution reaction (OER) and improve the performance of PEC for decomposing water (Figure 3a). In the work of Shao et al. [14], a ZnO@CoNi–LDH core−shell nanoarray was prepared by an electrosynthesis method. The Co2+ was oxidized to Co3+/Co4+ by the photogenerated holes, which enhanced the efficiency of photogenerated charge carrier separation. Moreover, the Co3+/Co4+ served as co-catalysts to improve water splitting ability.
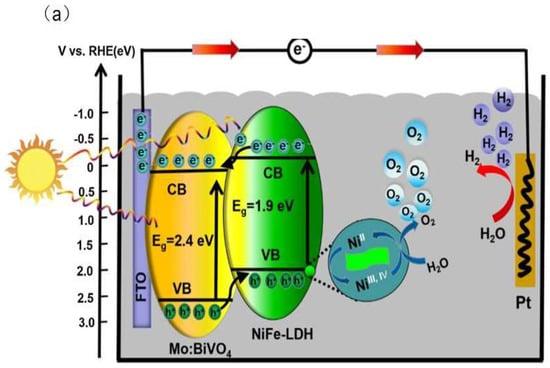
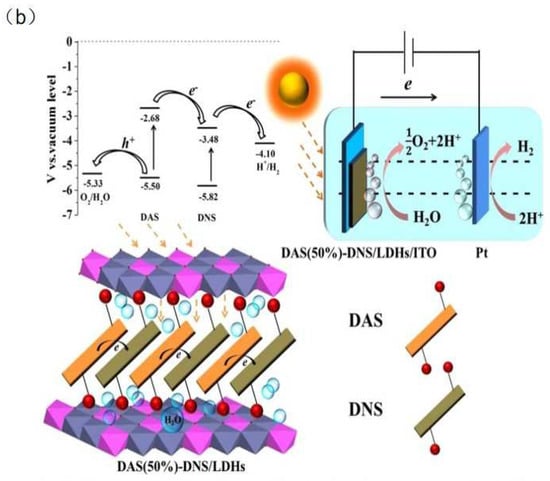
Figure 3.
(a) PEC water splitting mechanism using the NiFe−LDH/Mo−BiVO4 heterostructure photoanode [45]. Copyright 2018 Elsevier. (b) The possible photo−generated charges transfer mechanism of DAS (50%)−DNS/LDHs photoanode [47]. Copyright 2015 Springer Nature.
Suitable interlayer anions facilitate the transport and separation of charge carriers. Hunter et al. [46] synthesized different interlayer anions inserted into NiFe-LDH samples. The experimental results indicated that all interlayer anions were replaced by CO2 in the air to CO32−, which had the highest catalytic activity. In non-CO32− interlayer anions, the catalytic activity is a function of the alkalinity of the interlayer anion. The interlayer anions with more negative charges act as stronger proton acceptors and electron donors than interlayer anions with one negative charge. Zheng et al. [47] obtained 4,4-diaminostilbene-2,2-disulfonate (DAS) and 4,4-dinitro-stilbene-2,2-disulfonate (DNS) co-intercalated Zn2Al-LDH nanosheets. Due to the matched HOMO/LOMO energy levels of DAS and DNS, the photogenerated electrons of DAS efficiently migrate to DNS under UV-visible-light illumination (Figure 3b). When the percentage of DAS is 50%, the DAS (50%)-DNS/LDHs exhibit excellent photogenerated charge separation ability and stability. Photogenerated electron transfer within the interlayer anion was achieved with water splitting.
2.1.3. Adjusting Selectivity of Reactions
The different types of metal cations of LDHs lead to different active sites of the reaction and thus different products. The different positions of the conduction bands of the photocatalysts determine the different reduction capabilities, leading to the different selectivity in photo(electro)catalytic reactions [48]. Xiong et al. [49] prepared a series of Zn-based layered ZnM-LDH (M = Ti4+, Fe3+, Co3+, Ga3+, Al3+) by a co-precipitation method. The varied M3+ or M4+ in the ZnM-LDH could precisely adjust the product selectivity of the CO2 reduction. The experimental and computational results revealed that d-band center positions of the metal cations dominated the adsorption strength of CO2 and, ultimately, product selectivity. The d-band centers of intralayer metal ions of ZnTi-LDH, ZnGa-LDH, and ZnAl-LDH were relatively adjacent to the Fermi level, which facilitated the reduction of CO2 to CH4 (ZnTi-LDH) and CO (ZnGa-LDH and ZnAl-LDH). ZnFe-LDH and ZnCo-LDH cannot reduce CO2 but induce water desorption and hydrogenation due to the d-band centers of Fe3+ and Co3+ further away from the Fermi level. Zhao et al. [50] investigated the electronic properties, reaction path, and reaction kinetics of CO2PR in 10 MII2MIII/IV-NO3-LDHs (MII = Mg2+, Co2+, Ni2+, Zn2+; MIII = Al3+, In3+, Cr3+, Fe3+; MIV = Ti4+) by Hubbard-corrected density functional theory. The calculation showed that all LDHs might exhibit CO2PR except Ni2Al-LDH and Ni2FeNO3-LDH. Among the remaining eight LDHs, the favorable products of the others were CH4, except for the product of Co2Fe-NO3LDH, which was HCOOH. According to the relationship between the effective driving force (ΔΔGb) of CO2 reduction to CH4 or CO and the adsorption energy of CO2, which resembled the relationship between ΔΔGb and valence band maximum (VBM) of LDH, Mg2In-LDH was most likely to photocatalytically reduce CO2 to CH4, whereas Mg2Al-NO3-LDH was most likely to reduce CO2 to CO.
2.1.4. Improving Absorption Capacity
LDHs with exchangeable interlayer anions are widely used to adsorb harmful anions or contaminants of wastewater and polluting soil. The type of interlayer anion affects the adsorption capacity of LDH for the anions in solution. HONGO et al. [51] prepared MgAl-LDH with Cl−, , or as the interlayer anion to adsorb harmful anions (F−, , , and ) using a co-precipitation method. The LDHs with different interlayer anions showed excellent attraction for harmful anions and display adsorption capacity in the order > Cl− > . They concluded that the two adsorption mechanisms of LDH for anions were fast adsorption on the surface and slow interlayer anion exchange. The exchange rate of interlayer anions depends on the strength of the interaction between interlayer anions and LDH. The stronger the interaction of interlayer anion and LDH, the weaker the ion exchange capacity of the LDH, resulting in a poorer adsorption capacity of anion. In the first minute, the fast adsorption process is caused by the synergy of two adsorption mechanisms. However, the surface anion adsorption by anion exchange is usually relatively slow. Based on the experimental results and analysis, they believe that the nanocrystallization and highly Al substituted phase of NO3-formed Mg-Al LDH obviously improve the anion adsorption ability, resulting in fast surface adsorption. The fast surface adsorption dominates the adsorption ability for NO3-formed Mg-Al LDH.
The interaction of interlayer anion and metal ions dominates the adsorption capacity of LDH and selectivity for different metal ions. Jawad et al. [52] synthesized intercalated FeMgAl-LDH as an absorber to removal heavy metals. The results showed the following order of selection for adsorption: Hg2+ ∼ Ag+ > Pb2+ > Cu2+ > Cr6+ > As3+ > Ni2+ ∼ Zn2+ ∼ Co2+. The adsorbed metal cations can form coordination complexes in the interlayer channels. At the same time, the layered structure of LDH provide a protective space for Fe-MoS4 to prevent its oxidation. The adsorption capacity of samples for metal ions was determined by the strength of soft-soft acid base bonding interactions between Fe-MoS and metal ions.
2.2. Thermal Decomposition and Memory Property
The calcination process causes significant change of the structure and properties of LDHs. The thermal decomposition process of LDHs generally includes three stages [17]: First, the calcination temperature is below 300 °C, adsorbed water of the interlayer and surface is removed, and the layer structure of LDHs is well maintained. Second, during the calcination process at 300–450 °C, the intralayer hydroxyl groups and water are gradually removed. Third, when the calcination temperature is above 450 °C, the layer structure of LDHs gradually collapses, and a composite oxide (M2+M3+O) is formed.
Calcined LDHs decompose into complex metal oxides and thus form in-situ heterojunctions between the metal oxides, resulting in improved photo/electrocatalytic performance. Suárez-Quezada et al. [53] synthesized a series of ZnAl-LDH samples calcined at different temperatures. They found that Zn was present as hexagonal ZnO in all samples, and Al was present as ZnAl2O4 and Zn6Al2O9 depending on calcination temperature. Both ZnAl2O4 and Zn6Al2O9 can form heterojunctions with ZnO. As the temperature increased, the higher crystallinity led to higher hydrogen production efficiency, reaching a peak at 600 ℃. Mostafa et al. [54] prepared novel 1D CoBiTi-LDH with a bandgap of 2.4 eV and 2D CoBiTi layered double oxides (LDO) with high infrared (IR)-responsivity. After drying at 150 °C for 1D CoBiTi-LDH, 1D CoBiTi-LDH and in-situ formed 2D CoBiTi-LDO formed a novel 3D-heterojunction. The hydrogen evolution reaction (HER) of CoBiTi-LDH/CoBiTiO heterojunction increased nearly four times (∼1255 μmolg−1h−1) compared with the 1D CoBiTi-LDH. The increased HER of the heterojunction was attributed to the enhancement of light absorbance in the IR-region (53% of sunlight) and the trapping of photoexcited species by the functional groups of CBT-LDH.
The calcined LDHs have a stronger adsorption capacity for anionic dyes than the pristine LDHs due to higher specific surface areas and better reconstruction ability [17]. Li et al. [55] prepared hierarchical ZnAl-LDH by ZnAl-LDOs reaction with carbonate solution. The adsorption capacity of ZnAl-LDHfor methyl orange (MO) is far less than that of LDOs due to the decreased specific surface area, adsorption sites, or positive surface charge. Kim et al. [56] reported that calcination process of MgAl-LDHs induced the crystal deformation and formation of an interlayer structure of layered double oxides, leading to the development of mesopores and increased specific surface area. When LDHs were calcined at 500 °C for 10 h and transformed to LDOs, the specific surface area of LDHs obtained by hydrothermal reaction for 1 day (H1-LDH) and 3 days (H3-LDH) increased from 18.4 and 11.3 m2/g to 206 and 187 m2/g, respectively. The enhanced specific surface area originated from the developed mesopores of the LDO and larger pore volume.
Interestingly, at a certain temperature, the disordered lamellar structure of calcinated LDHs are restored to its original layer structure by immersing it in water or an aqueous solution containing anions [57]. This unique property of LDH is known as the “memory effect”. Peng et al. [58] obtained MgAl-LDH by intercalating 5-Fluorouracil anions using the memory effect of the LDH. The as-prepared samples not only showed improved corrosion resistance, but also inhibited human bile duct cancer cells. Thus, the intercalation of anions in interlayer by the memory effect of LDH is an efficient approach for designing functionalized LDHs [18]. However, some LDHs, such as Ni–Cr, Ca–Al, and Co–Al, have irreversible thermal decomposition behavior, and their lamellar structure cannot be recovered [59].
2.3. Delamination of LDH
The LDHs possess a typical layered structure whose layers are connected by strong interlayer electrostatic interactions and interlamellar hydrogen bonding [18]. Although delamination of LDHs remains a big challenge (especially for monolayer LDH) [60], delamination is still an attractive way to improve photo(electro)chemical activity and expand the applications for LDH nanomaterials. This is because the delaminated LDHs have a larger specific surface area, more active sites, and higher electron transport efficiency. Hu et al. [61] delaminated CoCo-LDH, NiCo-LDH, and NiFe-LDH using a liquid phase exfoliation method. The delaminated nanosheets have lower overpotential. When η = 300 mV, the current densities of the CoCo-LDH, NiCo-LDH, and NiFe-LDH nanosheets were 2.6, 3.4, and 4.5 times that of their bulk LDHs, respectively.
Delamination of LDHs introduces more vacancy defects and thus increases number of reactive sites [19]. For example, Wang et al. [62] prepared ultrathin CoFe LDH nanosheets by exfoliation of bulk CoFe LDHs with nitrogen plasma. The exfoliation process induces formation of defects of ultrathin CoFe LDHs nanosheets. The defects increase the dangling bonds near reactive sites and decrease the coordination number of reactive sites, resulting in the improved electrocatalytic activity.
2.4. Hydroxyl Groups on the LDH Surface
The LDH surface has abundant surface hydroxyl groups that are nearly perpendicular to the host layer [18]. The hydroxyl groups not only effectively adsorb reactants [63], but also form interfacial chemical bonds with other semiconductor surfaces, thereby facilitating the transport of interfacial charge carriers [15]. For example, Liu et al. [13] deposited NiFe-LDH onto Co-intercalated TiO2 by electrodeposition. The hydroxyl groups of NiFe-LDH form hydrogen bonds with TiO2 (Figure 4). Therefore, under illumination, the holes in Co-TiO2 VB can be transferred to the VB of NiFe-LDH through hydrogen bonding in time to participate in water decomposition, which improves transfer and separation of interfacial photogenerated charge.
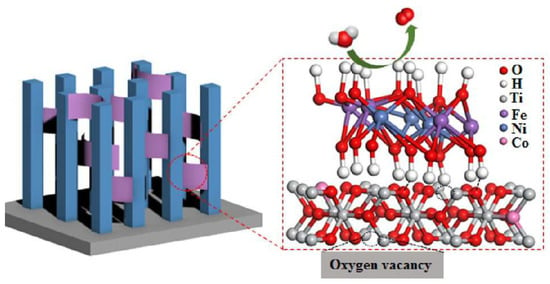
Figure 4.
The hydrogen bonds formed between NiFe-LDH and Co-TiO2 [13]. Copyright 2020 American Chemical Society.
3. The Photo(Electro)Chemical Applications of LDHs
3.1. Water Splitting
When the energy of light harvesting is more than the bandgap energy of LDHs, the electrons in the valence band of LDHs would inject into the conduction band and leave photogenerated holes in the valence band. The photogenerated electrons and holes will migrate to LDH surfaces to participate in a hydrogen evolution reaction and an oxygen evolution reaction. However, in photoelectrocatalysis, the photogenerated electrons will drift to the cathode to participate in a hydrogen evolution reaction, whereas the photogenerated holes will drift to the anode to participate in an oxygen evolution reaction.
Water splitting by photochemistry can be divided into three steps [17]: (i) water adsorption. LDHs and LDH compounds will directly contact water without a concentration gradient. The ability of water adsorption is determined by the specific surface area of LDHs. (ii) Separation and migration of charge carriers: High separation and migration rate of carriers greatly improve the performances of LDH water splitting by photochemistry [64]. (iii) Surface redox reaction: The valence band maximum should be greater than the potential of O2/H2O (1.23 V vs. normal hydrogen electrode (NHE)), and the conduction minimum should be less than the potential of H+/H2 (0 V vs. NHE) [65].
Hydrogen is an excellent clean energy and has many potential applications [66], such as hydrogen electric vehicles [67], reduction iron in industry [68], treatment in clinical applications [69], and so on. Water splitting by photochemistry is one effective method to evolve hydrogen. However, the high charge carrier recombination and the low efficiency of hydrogen evolution limit commercial scale production. Among many photo/ electrocatalysts, LDHs have attracted wide attention in photo(electro)catalytic water splitting, due to high specific surface areas, highly dispersed metal active sites, adjustable composition, and low cost [17]. However, many drawbacks of LDHs, such as low conductivity, low carrier mobility, and high electron-hole recombination rate, greatly hinder the photo(electro)catalytic applications [17,70]. Thus, many modification methods of LDHs have been used to improve the photo(electro)catalytic performance.
Constructing LDH-based heterostructure is an effective strategy to enhance the photochemical hydrogen evolution performance for LDHs. Chen et al. [71] successfully prepared hierarchical CoNi-LDH modified TiO2 nanotube arrays (NTAs) by a quick electrochemical deposition method. The photocurrent density of TiO2@CoNi-LDH NTAs was 4.4 mA·cm−2 (vs. RHE), which was 3.3 times higher than that of pure TiO2. The band gap of TiO2@CoNi-LDH NTAs was smaller than that of pristine TiO2. When light radiation is introduced during the synthesis of the heterostructure, the interface of heterostructure become more compact, leading to better separation ability of charge carriers. Zhang et al. [72] obtained two types of ZnFe-LDH/TiO2 nanoarrys (NAs) by photo-assisted electrodeposition (TiO2/ZnFe-LDH-PE) method and electrochemical deposition method (TiO2/ZnFe-LDH-E), respectively. The photocurrent density of TiO2/ZnFe-LDH-PE was 2.29 and 1.31 times than that of pure TiO2 and TiO2/ZnFe-LDH-E, respectively. For pristine TiO2, the interface formed between TiO2 and ZnFe-LDH reduced the recombination of photogenerated electrons and holes (Figure 5a). At the same time, Fe species captured photogenerated holes and served as active sites for oxygen evolution reaction. For TiO2/ZnFe-LDH-E, the light radiation resulted in the stronger interaction between Zn 2p3/2 and Ti 2p3/2, resulting in the enhanced separation and transfer efficiency of photogenerated charges (Figure 5b,c). Carbon nanodots (CDs) have superior rapid charge separation due to their unique structure [73]. Lv et al. [74] reported the introduction of CDs further improved carrier mobility and reduced overpotential for oxygen evolution of CDs/NiFe-LDH/BiVO4 photoanode, leading to enhanced water splitting ability. Yang et al. [75] constructed a novel CoFe-LDH/NiFe-LDH core-shell architecture supported on nickel foam by a hydrothermal and electrodeposition strategy. The heterostructure showed the lowest Tafel slope of 88.88 mV dec−1, indicating excellent HER kinetics. The outstanding kinetics of the HER reaction was attributed to the strong synergistic effect as well as the typical 3D interconnected architectures. The HER activity of the core-shell architecture electrode is similar to or better than many state-of-the-art HER electrocatalysts. Zhang et al. [76] fabricated hierarchical NiFe-LDH@NiCoP nanowires on nickel foam as electrodes by a hydrothermal–phosphorization–hydrothermal strategy. The 3D heterostructure NiFe-LDH@NiCoP/NF electrodes require a low overpotential of 120 and 220 mV to deliver 10 mA cm−2 for the HER and OER, respectively. The overall water splitting of the heterostructure electrodes showed a cell voltage of 1.57 V at 10 mA cm−2 and excellent stability. Due to the strong electronic interaction between the NiFe-LDH and NiCoP, the synthetic strategy and interface engineering of the heterostructure facilitated charge transfer and improved reaction kinetics.
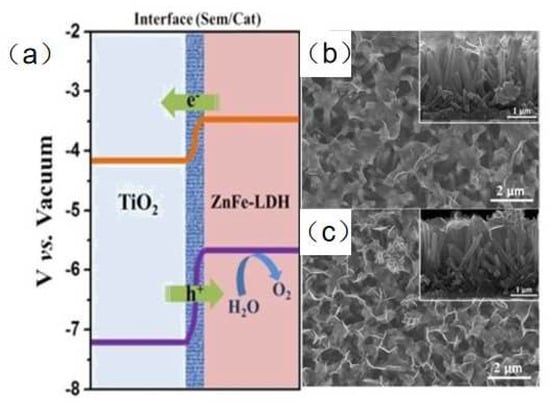
Figure 5.
(a) The band structure of TiO2/ZnFe−LDH heterostructure. SEM images of (b) TiO2/ZnFe−LDH-E and (c) TiO2/ZnFe−LDH-PE NAs [72]. Copyright 2017 Elsevier.
The formation of positive–negative (PN) junctions is a common and effective method to improve photochemical water splitting performance. Yang et al. [77] used NiV-LDH and CdS to form P-N heterojunctions by physically mixing them together in a mass ratio of 1:10 (Figure 6a). The formed NiV-LDH/CdS heterostructure had excellent electron-hole separation ability, and the hydrogen evolution efficiency is significantly greater than that of pure NiV-LDH and CdS (Figure 6b). Sahoo et al. [78] constructed a heterojunction between Co(OH)2 and ZnCr-LDH by an ultrasonication method. The H2 and O2 evolution apparent conversion of optimized Co(OH)2-modified ZnCr LDH sample reached 13.12% and 6.25% in 2 h, respectively.
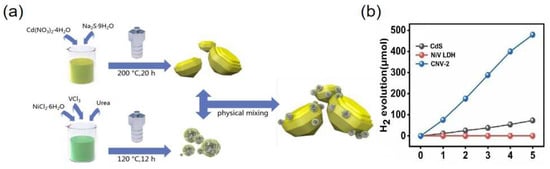
Figure 6.
(a) The synthesis process of NiV-LDH/CdS. (b) The amount of hydrogen produced by CdS, NiV-LDH, and CNV-2. Copyright 2021 Elsevier [77].
3.2. CO2 Reduction
Currently, the greatest threat to ecosystems is climate change. In order to achieve the plan specified in Conference of the Parties 21, energy and industrial processes need to reduce carbon emissions by 60% to limit global temperature rise to 2 °C [79]. There are also a number of ways to reduce the environmental impact of CO2: carbon capture and storage (ccs) chemical cycle capture, thermal decarbonization, photo(-electro)chemical reduction, and so on [80]. While reducing CO2, it is highly anticipated that CO2 can be used to generate electricity and convert it into more valuable compounds [81,82]. However, the traditional CO2 absorption method requires high temperature and pressure. A fresh LDH is not able to capture CO2, but LDHs forming a metal oxide mixture will have the ability to capture CO2 [83].
At present, the photochemical CO2 reduction attracts much attention due to the mild reaction condition. For photochemical CO2 reduction, when the energy of the absorbed light is greater than the band gap energy of LDHs, electron–hole pairs are produced. The photochemical CO2 reduction is roughly divided into three steps: (1) CO2 adsorption; hydroxyl groups adsorption on the surface [84], and interlayer anions adsorption [85]; (2) separation and migration of photogenerated charges [86]; (3) CO2 reduction reaction; CO2 will be reduced to hydrocarbons or CO by electrons [87]. The difference between PEC and photocatalytic (PC) CO2 reduction is that photoelectrocatalysis uses light and bias voltage to reduce CO2. Light performs as the drive, and the bias voltage improves the catalysis efficiency. Photosemiconductor structure, intrinsic properties, and active centers on the surface affect the efficiency of CO2 reduction in PEC [88].
In the photocatalytic CO2 reduction by LDH, the amount of CO2 absorbed depends on the type of divalent metal cation. Wang et al. [89] reported the bond strength between CO2 and MAl-LDH was relevant to the position of the d-band center. The higher the position of the d-band center, the higher the photocatalytic activity for CO2 reduction (Figure 7). The reduction capacity of CO2 was as follows: NiAl-LDHs > CuAl-LDHs > ZnAl-LDHs > MgAl-LDHs.
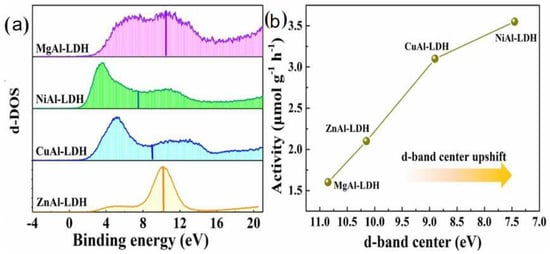
Figure 7.
(a) Position of d−band center of the different LDHs. (b) Relationship of activity and the d−band center position for LDHs [89]. Copyright 2021 Elsevier.
In photochemical reduction of CO2, the reduction of H2O tends to compete with the reduction of CO2 for electrons [90]. Therefore, much effort has been made to improve the selective reduction of CO2 by LDHs. Tan et al. [91] successfully obtained a composited photocatalyst with ruthenium and NiAl-LDH. The experimental results confirmed that a monolayer NiAl-LDH (m-NiAl-LDH) could completely suppress the hydrogen evolution reaction under a longer wavelength irradiation (λ > 600 nm). This phenomenon was attributed to the metal-induced defect states in the forbidden zone of m-NiAl-LDH. Photogenerated electrons only localized at the defect state, and the driving force of the defect state (0.313 eV) could reduce CO2 to CH4 instead of H2O reduction. Wang et al. [92] successfully prepared NiO samples with different vacancy amounts by calcinating NiAl-LDH. The vacancy concentrations of Ni and O determine the selectivity of CO2 reduction under visible light irradiation. The NiAl-275 sample with the highest defect concentration has the highest selectivity for CH4 (22.8%).
Constructing heterostructure still effectively improves the photo(electro)chemical performance of CO2 reduction. Lin et al. [93] prepared a FeWO4/NiAl-LDH(FWLDH) heterostructure using NiAl-LDH flower-like spheres and FeWO4 nanoflakes. The NiAl-LDH and FeWO4 formed a direct Z-scheme heterostructure. Tight binding of the heterostructure interface resulted in a larger specific surface area and thus formed more active sites. The internal electric field enhanced the separation and transport of photogenerated electrons, leading to the prominent improved photoelectron reduction ability of the NiAl-LDH (Figure 8). The photocatalytic CO yield of 10%FWLDH was 2.4 times than that of the original NiAl-LDH. Song et al. [94] fabricated a MgAl-LDO/carbon nitride with nitrogen defect (MgAl LDO/Nv-CN) 2D heterostructure. The photocatalytic activity of 10% MgAl LDO/Nv-CN for CO2 reduction was seven times than that of pure g-C3N4 under visible light illumination. Liu et al. [95] synthesized ultrathin Cu2O/CuCoCr-LDH p-n type heterojunction nanosheets (U-Cu2O/CuCoCr-LDH) as the cathodes of PEC. The photogenerated electrons at the photocathode reduced CO2 to CO and CH4. The maximum CO product yield of photoelectrocatalysis was 1167.6 mmol g−1h−1, which was approximately four times higher than that of electrocatalysis. The obvious improvement of photoelectrocatalytic performance was attributed to the internal electric field constructed by Cu2O and CuCr-LDH, which accelerates the separation of carriers.
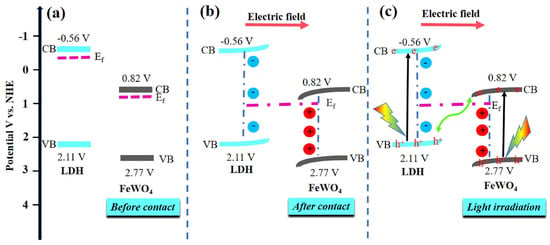
Figure 8.
Schematic energy band diagrams of FeWO4 and NiAl−LDH (a) before and (b) after heterojunction formation. (c) Photogenerated charges transfer pathway in FeWO4/NiAl−LDH heterostructure under light irradiation [93]. Copyright 2021 Elsevier.
LDHs have also been used in the electrocatalytic reduction of CO2. Fu et al. [96] prepared a monolayer NiFe-LDH catalyst using a solid-phase exfoliation method as an electrode for CO2 electroreduction. The optimized NiFe-CN-1 catalyst (NiFe-LDH was 1 wt%) exhibited a faradaic efficiency of CO generation of 93.5 % at 0.8v (vs. RHE). The excellent electrocatalytic performance originates from effective exposure of Ni and Fe active sites doped on the char material and efficient proton transfer channels of NiFe-LDH. Iwase et al. [97] prepared 2D CuAl-LDH as an electrocatalyst for electrochemical CO2 reduction (CO2RR). The optimized CuAl-LDH exhibited a faradaic efficiency of 42% for CO2 reduction to CO and 22% formate generation. It was found that the size of the LDH sheet was a key of CO2RR activity.
3.3. Contaminant Degradation
In 2015, about 9 million people died from environmental pollution, of which 1.8 million people died from diseases caused by water pollution [81]. Organic contaminants, as an important source of water pollution, are difficult to biodegrade because of their stability [98]. Photo(electro)catalysis has attracted extensive attention for solving environmental pollution problems, due to low cost, no pollution, and mild conditions [99,100].
Organic contaminant degradation by photo(electro)catalysis can be broadly divided into three steps: (1) The adsorption of the organic contaminant [101]. (2) The separation and transfer of photogenerated charges: This step is the key to improving photochemical activity [102]. (3) Redox reactions: Organic contaminant are converted to carbon dioxide, water, and inorganic acids by participating in redox reactions [103].
Recently, LDHs, particularly, transition metal-based LDHs (TLDHs), have emerged as promising candidates for contaminant degradation by photo(electro)catalysis [104]. Baliarsingh et al. [105] investigated the effect of M2+ (Co, Ni, Cu, and Zn) in MII/Cr-LDH to photodegrade methyl orange (MO). Among them, CoCr-LDH showed the highest photoactivity for MO (90% MO removal in 3 h). The improved photocatalytic activity of CoCr-LDH is mainly attributed to the excitation of M2+–O–Cr3+ bridge bonds under visible light irradiation and the effective transfer of photogenerated charge through the bridge bonds, which leads to the production of hydroxyl radicals and superoxide radicals. Zhao et al. [106] synthesized a series of MCr-LDH (M = Cu, Ni, Zn) samples with visible light response. The MCr-LDH samples have excellent photocatalytic activity for degradation of Sulforhodamine-B, Congo red, chlorinated phenol, and salicylic acid sodium. Experimental and computational results indicate that the obvious excellent visible light photocatalytic activity of MCr-NO3-LDHs is attributed to the low band gap and the abundant surface OH groups. The visible light response was induced by a d–d transition of CrO6 octahedra.
Construction of heterostructures is a common and effective approach to address the low photogenerated charge transport efficiency of LDHs. Megala et al. [107] obtained NiAl-LDH/CuWO4 heterostructures by a one-pot hydrothermal method. The photodegradation rate of LDH with 5% CuWO4 for methylene blue (MB) dye reached 87.5% in 5 h. The enhanced photocatalytic ability of NiAl-LDH/CuWO4 nanocomposite mainly originates from the heterojunction, which effectively promotes the separation of photogenerated charges. Ma et al. [108] synthesized BiOCl-NiFe-LDH composites using NiFe-Cl-LDH and Bi(NO3)3 as precursors. Photocatalytic activity of BiOCl-NiFe-LDH composites for Rhodamine B (RhB) degradation was 4.11 times higher than that of BiOCl. The heterostructure formed by BiOCl and NiFe-LDH can transfer photogenerated electron-holes in time (Figure 9a). At the same time, the highly dispersed BiOCl on the NiFe-LDH surface facilitates the formation of ·OH. Walaa R. Abd-Ellatif et al. [109] prepared ZnCo-LDH by a co-precipitation method and then obtained LDO (ZnO/CoO composite) by calcination. The ZnO/CoO composite formed S-scheme heterojunctions, as shown in Figure 9b. The removal rates of LDH calcinated at 300 °C for ponceau 4R (E124) and tartrazine (E102) were 90% and 80%, respectively. Pirkarami et al. [110] prepared CdS/NiCo-LDH heterojunctions by a hydrothermal method to construct photoelectrodes (Figure 9c). The degradation efficiency of Allura Red under an alkaline environment was over 90%. During the degradation process, the N = N of Allura Red would first break and it would eventually become H2O, NO3, NO2, CO2, SO3, Na+. Lu et al. [111] prepared Ni foam@ZnO@ZnFe-LDH photoelectrodes through electrodeposition of ZnO and hydrothermally grown ZnFe-LDH. Ni foam@ZnO@ZnFe-LDH acted as photoelectrodes in the PEC process and effectively removed Cr (VI) and Acid Red 1 by the synergistic effect of photoelectrocatalysis. Experimental results indicated that the 2D/2D core-shell heterojunction formed by ZnO and ZnFe-LDH not only narrowed the bandgap of ZnO and increased visible light absorption, but also promoted electron-hole separation. Argote-Fuentes et al. [112] synthesized activated MgAl-LDH through the co-precipitation method as heterogeneous catalysts for degradation of Congo red dye. In the photoelectrocatalysis process under 0.5v bias, the photoelectrocatalytic degradation rate of the MgAl-LDH/Cu electrode reached 95% and was the highest compared with other degradation processes. The synergistic effect of the Cu2+ ions induced by electric current and the photogenerated electrons suppressed the recombination of the electron–hole in the catalyst, resulting in excellent catalytic activity of Congo red degradation.
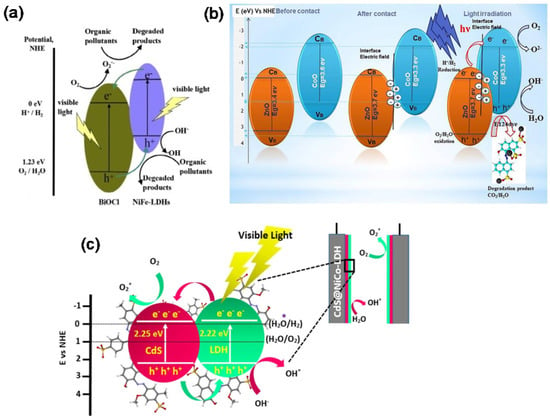
Figure 9.
(a) The photocatalytic mechanism of BiOCl/NiFe−LDH heterostructure [108]. Copyright 2015 Elsevier. (b) The possible photodegradation mechanism of ZnO/CoO [109]. Copyright 2022 Elsevier. (c) Proposed photoelectrocatalytic degradation mechanism of CdS/NiCo−LDH heterojunctions [110]. Copyright 2022 Elsevier.
4. Conclusion and Outlook
LDHs are promising 2D photo(electro)catalysts with the advantages of low cost, tunable composition, unique thermal decomposition and memory properties, delaminated layer, and abundant surface hydroxyls. The compositional flexibility of LDHs can tune band structure, improve the absorption capacity and separation of charge carriers, and change the selectivity of the reaction. Calcined LDHs form in situ heterojunctions between the metal oxides, resulting in improved photo/electrocatalytic performance. Delamination and calcination of LDHs introduces more vacancy defects and specific surface areas, leading to an increased number of reactive sites. These insights into structure–activity relationships of LDHs provide a theoretical basis for function-oriented design of LDH-based photo(electro)catalytic materials.
Although a great deal of exciting research has appeared for improving the photo(electro)catalytic performance and fulfilling the practical applications, the two major drawbacks that need to be addressed for LDHs are the structural instability in a low pH environment and a low quantum efficiency induced by its low conductivity. The structure regulation of LDHs should be an ideal strategy to overcome the drawbacks. LDH is calcined at a certain temperature and then recovered by the memory effect, which improves its structural stability in an acidic environment and its photo(electro)catalytic performance. However, the corrosion resistance mechanism of calcined LDH has not yet been given a certain explanation. Taking advantage of the tunable composition characteristics of LDHs, the doping and defect introduction can effectively improve the conductivity of LDHs, resulting in enhanced quantum efficiency. How to precisely control the composition and structures of LDHs is still a huge challenge, such as precisely controlling the thickness of LDH and adjusting the ratio between metal cations by the electrodeposition method.
The structure–performance correlations of LDH-based photo(electro)catalytic materials needs to be more deeply understood to provide theoretical guidance for the design of efficient LDH photo(electro)catalysts. In order to better explore the structure–activity relationship of LDHs, advanced and effective characterization methods should be vigorously developed and applied. In situ characterization techniques would more precisely investigate structural changes of LDHs under reaction conditions. Transient spectroscopic techniques facilitate the research of photogenerated electron-hole separation and transfer dynamics and should be wildly utilized. In addition, theoretical simulations, especially density functional theory calculations, are powerful tools to study the relationship between the structure and properties of LDHs. The combination of advanced and valid characterization technologies and theoretical simulations is necessary to reveal complex charge dynamics, which supply a more detailed understanding of photo(electro)catalytic mechanisms. Further creative investigations will overcome the challenges in photochemistry of LDHs and will continue to advance the photo(electro)catalytic applications of LDHs.
Author Contributions
Writing—original draft preparation, C.L.; writing—review and editing, D.J.; visualization, C.L. and H.J.; supervision, D.J.; project administration, D.J.; funding acquisition, Z.W. and D.J. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Hunan Province, grant number 2022JJ30596, 2022JJ90004, and 2020JJ4094, the National Natural Science Foundation of China, grant number 52171078, Natural Science Foundation of Tianjin, grant number 21JCZDJC00440, the Scientific Research Fund of Hunan Provincial Education Department, grant numbers 19C0079 and 21C0169, the Open Research Fund of Hunan Provincial Key Laboratory of Flexible Electronic Materials Genome Engineering, grant number 202011, and Changsha University of Science and Technology Postgraduate Research Innovation Project, grant number CXCLY2022143.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| LDH | Layered double hydroxide |
| RHE | Reversible hydrogen electrode |
| NHE | Normal hydrogen electrode |
| OER | Oxygen evolution reaction |
| HER | Hydrogen evolution reaction |
| PEC | Photoelectrocatalytic |
| VBM | Valence band maximum |
| LDO | Layered double oxide |
| PN | Positive–negative |
| NTAs | Nanotube arrays |
| NAs | Nanoarrays |
| CD | Carbon nanodot |
| Nv | Nitrogen defect |
| PE | Photoassisted electrodeposition method |
| MO | Methyl orange |
References
- Akizu-Gardoki, O.; Bueno, G.; Wiedmann, T.; Lopez-Guede, J.M.; Arto, I.; Hernandez, P.; Moran, D. Decoupling between human development and energy consumption within footprint accounts. J. Clean. Prod. 2018, 202, 1145–1157. [Google Scholar] [CrossRef]
- Johnsson, F.; Kjärstad, J.; Rootzén, J. The threat to climate change mitigation posed by the abundance of fossil fuels. Clim. Policy 2018, 19, 258–274. [Google Scholar] [CrossRef]
- Soltani, S.M.; Lahiri, A.; Bahzad, H.; Clough, P.; Gorbounov, M.; Yan, Y. Sorption-enhanced Steam Methane Reforming for Combined CO2 Capture and Hydrogen Production: A State-of-the-Art Review. Carbon Capture Sci. Technol. 2021, 1, 100003. [Google Scholar] [CrossRef]
- Wang, Q.; Geng, B.; Wang, S. ZnO/Au Hybrid Nanoarchitectures: Wet-Chemical Synthesis and Structurally Enhanced Photocatalytic Performance. Environ. Sci. Technol. 2009, 43, 8968–8973. [Google Scholar] [CrossRef]
- Zheng, Y.; Cheng, B.; You, W.; Yu, J.; Ho, W. 3D hierarchical graphene oxide-NiFe LDH composite with enhanced adsorption affinity to Congo red, methyl orange and Cr (VI) ions. J. Hazard. Mater. 2019, 369, 214–225. [Google Scholar] [CrossRef]
- Davis, L.J.; Milligan, R.; Stauber, C.E.; Jelks, N.O.; Casanova, L.; Ledford, S.H. Environmental injustice and Escherichia coli in urban streams: Potential for community-led response. WIREs Water 2022, 9, e1583. [Google Scholar] [CrossRef]
- Torimoto, T.; Horibe, H.; Kameyama, T.; Okazaki, K.-I.; Ikeda, S.; Matsumura, M.; Ishikawa, A.; Ishihara, H. Plasmon-Enhanced Photocatalytic Activity of Cadmium Sulfide Nanoparticle Immobilized on Silica-Coated Gold Particles. J. Phys. Chem. Lett. 2011, 2, 2057–2062. [Google Scholar] [CrossRef]
- He, Y.; Yuan, R.; Yan, J.; Li, J. A highly efficient NiFe-layer double hydroxide/TiO2 heterojunction photoanode-based high-performance bifunctional photocatalytic fuel cell. Mater. Mater. Today Commun. 2021, 26, 102177. [Google Scholar] [CrossRef]
- Ibrahim, H.; Ilinca, A.; Perron, J. Energy storage systems—Characteristics and comparisons. Renew. Sustain. Energy Rev. 2008, 12, 1221–1250. [Google Scholar] [CrossRef]
- Seh, Z.W.; Kibsgaard, J.; Dickens, C.F.; Chorkendorff, I.; Nørskov, J.K.; Jaramillo, T.F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998. [Google Scholar] [CrossRef]
- Mahala, C.; Sharma, M.D.; Basu, M. Type-II Heterostructure of ZnO and Carbon Dots Demonstrates Enhanced Photoanodic Performance in Photoelectrochemical Water Splitting. Inorg. Chem. 2020, 59, 6988–6999. [Google Scholar] [CrossRef]
- Cao, X.; Xu, C.; Ma, J.; Dong, Y.; Dong, C.; Yue, M.; Ding, Y. Enhanced Photoelectrochemical Performance of WO 3 -Based Composite Photoanode Coupled with Carbon Quantum Dots and NiFe Layered Double Hydroxide. ChemSusChem 2019, 12, 4685–4692. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, C.; Ji, D.; Yin, G.; Wang, W.; Chen, Z. Cobalt-Doped TiO2 Nanowire Arrays Coated with NiFe Layered-Double-Hydroxide Nanoplatelets as Photoanodes for Photoelectrochemical Water Oxidation. ACS Appl. Nano Mater. 2020, 3, 6598–6608. [Google Scholar] [CrossRef]
- Shao, M.; Ning, F.; Wei, M.; Evans, D.G.; Duan, X. Hierarchical Nanowire Arrays Based on ZnO Core−Layered Double Hydroxide Shell for Largely Enhanced Photoelectrochemical Water Splitting. Adv. Funct. Mater. 2013, 24, 580–586. [Google Scholar] [CrossRef]
- Jiang, D.; Liu, Z.; Fu, L.; Yang, H. Interfacial Chemical-Bond-Modulated Charge Transfer of Heterostructures for Improving Photocatalytic Performance. ACS Appl. Mater. Interfaces 2020, 12, 9872–9880. [Google Scholar] [CrossRef]
- Jiang, D.; Xue, J.; Wu, L.; Zhou, W.; Zhang, Y.; Li, X. Photocatalytic performance enhancement of CuO/Cu2O heterostructures for photodegradation of organic dyes: Effects of CuO morphology. Appl. Catal. B Environ. 2017, 211, 199–204. [Google Scholar] [CrossRef]
- Wang, K.; Wang, T.; Islam, Q.A.; Wu, Y. Layered double hydroxide photocatalysts for solar fuel production. Chin. J. Catal. 2021, 42, 1944–1975. [Google Scholar] [CrossRef]
- Wang, Q.; O’Hare, D. Recent Advances in the Synthesis and Application of Layered Double Hydroxide (LDH) Nanosheets. Chem. Rev. 2012, 112, 4124–4155. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, X.; Waterhouse, G.I.N.; Wu, L.-Z.; Tung, C.-H.; O’Hare, D.; Zhang, T. Layered double hydroxide nanostructured photocatalysts for renewable energy production. Adv. Mater. 2016, 6, 1501974. [Google Scholar] [CrossRef]
- Khan, A.I.; O’Hare, D. Intercalation chemistry of layered double hydroxides: Recent developments and applications. J. Mater. Chem. 2002, 12, 3191–3198. [Google Scholar] [CrossRef]
- Feitknecht, W.; Gerber, M. Zur Kenntnis der Doppelhydroxyde und basischen Doppelsalze III. Über Magnesium-Aluminiumdoppelhydroxyd. Helv. Chim. Acta 1942, 25, 131–137. [Google Scholar] [CrossRef]
- Tcherniak, A.; Ha, J.W.; Dominguez-Medina, S.; Slaughter, L.S.; Link, S. Probing a Century Old Prediction One Plasmonic Particle at a Time. Nano Lett. 2010, 10, 1398–1404. [Google Scholar] [CrossRef]
- Yu, J.; Wang, Q.; O'Hare, D.; Sun, L. Preparation of two dimensional layered double hydroxide nanosheets and their applications. Chem. Soc. Rev. 2017, 46, 5950–5974. [Google Scholar] [CrossRef]
- Dong, Y.; Kong, X.; Luo, X.; Wang, H. Adsorptive removal of heavy metal anions from water by layered double hydroxide: A review. Chemosphere 2022, 303, 134685. [Google Scholar] [CrossRef]
- Chatterjee, A.; Bharadiya, P.; Hansora, D. Layered double hydroxide based bionanocomposites. Appl. Clay Sci. 2019, 177, 19–36. [Google Scholar] [CrossRef]
- Shirin, V.A.; Sankar, R.; Johnson, A.P.; Gangadharappa, H.; Pramod, K. Advanced drug delivery applications of layered double hydroxide. J. Control. Release 2020, 330, 398–426. [Google Scholar] [CrossRef]
- Bian, X.; Zhang, S.; Zhao, Y.; Shi, R.; Zhang, T. Layered double hydroxide-based photocatalytic materials toward renewable solar fuels production. InfoMat 2021, 3, 719–738. [Google Scholar] [CrossRef]
- Xu, M.; Wei, M. Layered Double Hydroxide-Based Catalysts: Recent Advances in Preparation, Structure, and Applications. Adv. Funct. Mater. 2018, 28, 1802943. [Google Scholar] [CrossRef]
- Jiao, S.; Yao, Z.; Li, M.; Mu, C.; Liang, H.; Zeng, Y.-J.; Huang, H. Accelerating oxygen evolution electrocatalysis of two-dimensional NiFe layered double hydroxide nanosheets via space-confined amorphization. Nanoscale 2019, 11, 18894–18899. [Google Scholar] [CrossRef]
- Guo, W.; Dun, C.; Yu, C.; Song, X.; Yang, F.; Kuang, W.; Xie, Y.; Li, S.; Wang, Z.; Yu, J.; et al. Mismatching integration-enabled strains and defects engineering in LDH microstructure for high-rate and long-life charge storage. Nat. Commun. 2022, 13, 1409. [Google Scholar] [CrossRef]
- Fan, K.; Chen, H.; Ji, Y.; Huang, H.; Claesson, H.H.P.M.; Daniel, Q.; Philippe, B.; Rensmo, B.P.H.; Li, F.; Luo, Y.; et al. Nickel–vanadium monolayer double hydroxide for efficient electrochemical water oxidation. Nat. Commun. 2016, 7, 11981. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lee, S.C.; Patil, U.; Shackery, I.; Kang, S.; Zhang, K.; Park, J.H.; Chung, K.Y.; Chan Jun, S. Hierarchical MnCo-layered double hydroxides@Ni(OH)2 core–shell heterostructures as advanced electrodes for supercapacitors. J. Mater. Chem. A 2017, 5, 1043–1049. [Google Scholar] [CrossRef]
- Zhou, H.; Song, Y.; Liu, Y.; Li, H.; Li, W.; Chang, Z. Fabrication of CdS/Ni Fe LDH heterostructure for improved photocatalytic hydrogen evolution from aqueous methanol solution. Int. J. Hydrogen Energy 2018, 43, 14328–14336. [Google Scholar] [CrossRef]
- Nayak, S.; Swain, G.; Parida, K. Enhanced Photocatalytic Activities of RhB Degradation and H2 Evolution from in Situ Formation of the Electrostatic Heterostructure MoS2/NiFe LDH Nanocomposite through the Z-Scheme Mechanism via p-n Heterojunctions. ACS Appl. Mater. Interfaces 2019, 11, 20923–20942. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Li, J.; Ma, X.; Xu, S.-M.; Gao, R.; Zhao, Y.; Song, Y.-F. Highly selective photo-hydroxylation of phenol using ultrathin NiFe-layered double hydroxide nanosheets under visible-light up to 550 nm. Green Chem. 2020, 22, 8604–8613. [Google Scholar] [CrossRef]
- Huang, J.; Hu, G.; Ding, Y.; Pang, M.; Ma, B. Mn-doping and NiFe layered double hydroxide coating: Effective approaches to enhancing the performance of α-Fe2O3 in photoelectrochemical water oxidation. J. Catal. 2016, 340, 261–269. [Google Scholar] [CrossRef]
- Liu, X.; Liang, J.; Song, X.; Yang, H.; Li, X.; Dai, H.; Song, Y.; Liu, Y.; Hu, J.; Pan, X.; et al. Enhanced water dissociation performance of graphitic-C3N4 assembled with ZnCr-layered double hydroxide. Chem. Eng. J. 2018, 337, 560–566. [Google Scholar] [CrossRef]
- Aboubakr, A.E.A.; El Rouby, W.M.A.; Khan, M.D.; Farghali, A.A.; Revaprasadu, N. ZnCr-CO3 LDH/ruptured tubular g-C3N4 composite with increased specific surface area for enhanced photoelectrochemical water splitting. Appl. Surf. Sci. 2020, 508, 145100. [Google Scholar] [CrossRef]
- Nayak, S.; Parida, K. Superactive NiFe-LDH/graphene nanocomposites as competent catalysts for water splitting reactions. Inorg. Chem. Front. 2020, 7, 3805–3836. [Google Scholar] [CrossRef]
- Goh, K.-H.; Lim, T.-T.; Dong, Z. Application of layered double hydroxides for removal of oxyanions: A review. Water Res. 2008, 42, 1343–1368. [Google Scholar] [CrossRef]
- Xu, S.-M.; Pan, T.; Dou, Y.-B.; Yan, H.; Zhang, S.-T.; Ning, F.-Y.; Shi, W.-Y.; Wei, M. Theoretical and Experimental Study on MIIMIII-Layered Double Hydroxides as Efficient Photocatalysts toward Oxygen Evolution from Water. J. Phys. Chem. C 2015, 119, 18823–18834. [Google Scholar] [CrossRef]
- Guo, J.; Mao, C.; Zhang, R.; Shao, M.; Wei, M.; Feng, P. Reduced titania@layered double hydroxide hybrid photoanodes for enhanced photoelectrochemical water oxidation. J. Mater. Chem. A 2017, 5, 11016–11025. [Google Scholar] [CrossRef]
- Zhu, Y.; Ren, J.; Yang, X.; Chang, G.; Bu, Y.; Wei, G.; Han, W.; Yang, D. Interface engineering of 3D BiVO4/Fe-based layered double hydroxide core/shell nanostructures for boosting photoelectrochemical water oxidation. J. Mater. Chem. A 2017, 5, 9952–9959. [Google Scholar] [CrossRef]
- Parida, K.; Satpathy, M.; Mohapatra, L. Incorporation of Fe3+ into Mg/Al layered double hydroxide framework: Effects on textural properties and photocatalytic activity for H2 generation. J. Mater. Chem. 2012, 22, 7350–7357. [Google Scholar] [CrossRef]
- Guo, J.; Yang, X.; Bai, S.; Xiang, X.; Luo, R.; He, J.; Chen, A. Effect of Mo Doping and Nife-LDH Cocatalyst on PEC Water Oxidation Efficiency. J. Colloid Interface Sci. 2018, 540, 9–19. [Google Scholar] [CrossRef]
- Hunter, B.M.; Hieringer, W.; Winkler, J.R.; Gray, H.B.; Müller, A.M. Effect of interlayer anions on [NiFe]-LDH nanosheet water oxidation activity. Energy Environ. Sci. 2016, 9, 1734–1743. [Google Scholar] [CrossRef]
- Zheng, S.; Lu, J.; Yan, D.; Qin, Y.; Li, H.; Evans, D.G.; Duan, X. An Inexpensive Co-Intercalated Layered Double Hydroxide Composite with Electron Donor-Acceptor Character for Photoelectrochemical Water Splitting. Sci. Rep. 2015, 5, 12170. [Google Scholar] [CrossRef]
- Chong, R.; Wang, G.; Du, Y.; Jia, Y.; Wang, X.; Li, C.; Chang, Z.; Zhang, L. Anion engineering of exfoliated CoAl layered double hydroxides on hematite photoanode toward highly efficient photoelectrochemical water splitting. Chem. Eng. J. 2019, 366, 523–530. [Google Scholar] [CrossRef]
- Xiong, X.; Zhao, Y.; Shi, R.; Yin, W.; Zhao, Y.; Waterhouse, G.I.; Zhang, T. Selective photocatalytic CO2 reduction over Zn-based layered double hydroxides containing tri or tetravalent metals. Sci. Bull. 2020, 65, 987–994. [Google Scholar] [CrossRef]
- Zhao, X.-J.; Xu, S.-M.; Zhong, Y.; Chen, Z.-R.; Yin, P.; Miao, Y.-C.; Guo, J.-Y.; Zhang, W.; Jie, Y.; Yan, H. Theoretical Study on Photocatalytic CO2 Reduction to CO and CH4 over M(II)2M(III/IV)-Layered Double Hydroxides. J. Phys. Chem. C 2022, 126, 1356–1365. [Google Scholar] [CrossRef]
- Hongo, T.; Wakasa, H.; Yamazaki, A. Synthesis and adsorption properties of nanosized Mg-Al layered double hydroxides with Cl−, NO3− or SO42− as interlayer anion. Mater. Sci. Pol. 2011, 29, 86–91. [Google Scholar] [CrossRef]
- Jawad, A.; Liao, Z.; Zhou, Z.; Khan, A.; Wang, T.; Ifthikar, J.; Shahzad, A.; Chen, Z.; Chen, Z. Fe-MoS4: An Effective and Stable LDH-Based Adsorbent for Selective Removal of Heavy Metals. ACS Appl. Mater. Interfaces 2017, 9, 28451–28463. [Google Scholar] [CrossRef]
- Suárez-Quezada, M.; Romero-Ortiz, G.; Samaniego-Benítez, J.; Suárez, V.; Mantilla, A. H2 production by the water splitting reaction using photocatalysts derived from calcined ZnAl LDH. Fuel 2018, 240, 262–269. [Google Scholar] [CrossRef]
- Mostafa, M.S.; Lan, C.; Selim, M.S.; Ruiyi, Z.; Ya, G.; Shuai, Z.; Ge, G. Synthesis of novel CoBiTi LDH and fabrication of LDH-LDO 3D-Heterojunction for enhanced infrared induced water splitting to hydrogen. J. Clean. Prod. 2022, 340, 130663. [Google Scholar] [CrossRef]
- Li, Z.; Yang, B.; Zhang, S.; Wang, B.; Xue, B. A novel approach to hierarchical sphere-like ZnAl-layered double hydroxides and their enhanced adsorption capability. J. Mater. Chem. A 2014, 2, 10202–10210. [Google Scholar] [CrossRef]
- Kim, B.-K.; Gwak, G.-H.; Okada, T.; Oh, J.-M. Effect of particle size and local disorder on specific surface area of layered double hydroxides upon calcination-reconstruction. J. Solid State Chem. 2018, 263, 60–64. [Google Scholar] [CrossRef]
- Toma, M.; Toma, K.; Michioka, K.; Ikezoe, Y.; Obara, D.; Okamoto, K.; Tamada, K. Collective plasmon modes excited on a silver nanoparticle 2D crystalline sheet. Phys. Chem. Chem. Phys. 2011, 13, 7459–7466. [Google Scholar] [CrossRef]
- Peng, F.; Wang, D.; Cao, H.; Liu, X. Loading 5-Fluorouracil into calcined Mg/Al layered double hydroxide on AZ31 via memory effect. Mater. Lett. 2018, 213, 383–386. [Google Scholar] [CrossRef]
- Puttaswamy, N.S.; Kamath, P.V. Reversible thermal behaviour of layered double hydroxides:a thermogravimetric study. J. Mater. Chem. 1997, 7, 1941–1945. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Liu, Z.; Xie, C.; Feng, S.; Liu, D.; Shao, M.; Wang, S. Layered Double Hydroxide Nanosheets with Multiple Vacancies Obtained by Dry Exfoliation as Highly Efficient Oxygen Evolution Electrocatalysts. Angew. Chem. Int. Ed. Engl. 2017, 56, 5867–5871. [Google Scholar] [CrossRef]
- Song, F.; Hu, X. Exfoliation of layered double hydroxides for enhanced oxygen evolution catalysis. Nat. Commun. 2014, 5, 4477. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, C.; Zhang, Z.; Liu, D.; Chen, R.; Wang, S. In Situ Exfoliated, N-Doped, and Edge-Rich Ultrathin Layered Double Hydroxides Nanosheets for Oxygen Evolution Reaction. Adv. Funct. Mater. 2018, 28, 1703363. [Google Scholar] [CrossRef]
- Jiang, D.; Liu, Z.; Fu, L.; Jing, H.; Yang, H. Efficient Nanoclay-Based Composite Photocatalyst: The Role of Nanoclay in Photogenerated Charge Separation. J. Phys. Chem. C 2018, 122, 25900–25908. [Google Scholar] [CrossRef]
- Shakeel, M.; Arif, M.; Yasin, G.; Li, B.; Khan, H.D. Layered by layered Ni-Mn-LDH/g-C3N4 nanohybrid for multi-purpose photo/electrocatalysis: Morphology controlled strategy for effective charge carriers separation. Appl. Catal. B Environ. 2018, 242, 485–498. [Google Scholar] [CrossRef]
- Boumeriame, H.; Da Silva, E.S.; Cherevan, A.S.; Chafik, T.; Faria, J.L.; Eder, D. Layered double hydroxide (LDH)-based materials: A mini-review on strategies to improve the performance for photocatalytic water splitting. J. Energy Chem. 2021, 64, 406–431. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Wahid, M.A. Hydrogen from solar energy, a clean energy carrier from a sustainable source of energy. Int. J. Energy Res. 2019, 44, 4110–4131. [Google Scholar] [CrossRef]
- Li, Y.; Taghizadeh-Hesary, F. The economic feasibility of green hydrogen and fuel cell electric vehicles for road transport in China. Energy Policy 2021, 160, 112703. [Google Scholar] [CrossRef]
- Liu, W.; Zuo, H.; Wang, J.; Xue, Q.; Ren, B.; Yang, F. The production and application of hydrogen in steel industry. Int. J. Hydrogen Energy 2021, 46, 10548–10569. [Google Scholar] [CrossRef]
- Iida, A.; Nosaka, N.; Yumoto, T.; Knaup, E.; Naito, H.; Nishiyama, C.; Yamakawa, Y.; Tsukahara, K.; Terado, M.; Sato, K.; et al. The Clinical Application of Hydrogen as a Medical Treatment. Acta Med. Okayama 2016, 70, 331–337. [Google Scholar] [CrossRef]
- Nayak, S.; Parida, K. Recent Progress in LDH@Graphene and Analogous Heterostructures for Highly Active and Stable Photocatalytic and Photoelectrochemical Water Splitting. Chem. Asian J. 2021, 16, 2211–2248. [Google Scholar] [CrossRef]
- Chen, W.; Wang, T.; Xue, J.; Li, S.; Wang, Z.; Sun, S. Cobalt-Nickel Layered Double Hydroxides Modified on TiO2 Nanotube Arrays for Highly Efficient and Stable PEC Water Splitting. Small 2016, 13, 1602420. [Google Scholar] [CrossRef]
- Zhang, R.; Shao, M.; Xu, S.; Ning, F.; Zhou, L.; Wei, M. Photo-assisted synthesis of zinc-iron layered double hydroxides/TiO2 nanoarrays toward highly-efficient photoelectrochemical water splitting. Nano Energy 2017, 33, 21–28. [Google Scholar] [CrossRef]
- Li, H.; Kang, Z.; Liu, Y.; Lee, S.-T. Carbon nanodots: Synthesis, properties and applications. J. Mater. Chem. 2012, 22, 24230–24253. [Google Scholar] [CrossRef]
- Lv, X.; Xiao, X.; Cao, M.; Bu, Y.; Wang, C.; Wang, M.; Shen, Y. Efficient carbon dots/NiFe-layered double hydroxide/BiVO4 photoanodes for photoelectrochemical water splitting. Appl. Surf. Sci. 2018, 439, 1065–1071. [Google Scholar] [CrossRef]
- Yang, R.; Zhou, Y.; Xing, Y.; Li, D.; Jiang, D.; Chen, M.; Shi, W.; Yuan, S. Synergistic coupling of CoFe-LDH arrays with NiFe-LDH nanosheet for highly efficient overall water splitting in alkaline media. Appl. Catal. B Environ. 2019, 253, 131–139. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Hähnel, A.; Naumann, V.; Lin, C.; Azimi, S.; Schweizer, S.L.; Maijenburg, A.W.; Wehrspohn, R.B. Bifunctional Heterostructure Assembly of NiFe LDH Nanosheets on NiCoP Nanowires for Highly Efficient and Stable Overall Water Splitting. Adv. Funct. Mater. 2018, 28, 1706847. [Google Scholar] [CrossRef]
- Yang, M.; Wang, K.; Li, Y.; Yang, K.; Jin, Z. Pristine hexagonal CdS assembled with NiV LDH nanosheet formed p-n heterojunction for efficient photocatalytic hydrogen evolution. Appl. Surf. Sci. 2021, 548, 149212. [Google Scholar] [CrossRef]
- Sahoo, D.P.; Nayak, S.; Reddy, K.H.; Martha, S.; Parida, K. Fabrication of a Co(OH)2/ZnCr LDH "p-n" Heterojunction Photocatalyst with Enhanced Separation of Charge Carriers for Efficient Visible-Light-Driven H2 and O2 Evolution. Inorg. Chem. 2018, 57, 3840–3854. [Google Scholar] [CrossRef]
- Rhodes, C.J. The 2015 Paris Climate Change Conference: Cop21. Sci. Prog. 2016, 99, 97–104. [Google Scholar] [CrossRef]
- Nicoletti, G.; Arcuri, N.; Nicoletti, G.; Bruno, R. A technical and environmental comparison between hydrogen and some fossil fuels. Energy Convers. Manag. 2015, 89, 205–213. [Google Scholar] [CrossRef]
- Landrigan, P.J.; Fuller, R.; Acosta, N.J.R.; Adeyi, O.; Arnold, R.; Basu, N.; Baldé, A.B.; Bertollini, R.; Bose-O'Reilly, S.; Boufford, J.I.; et al. The Lancet Commission on pollution and health. Lancet 2018, 391, 462–512. [Google Scholar] [CrossRef]
- Wang, T.; Sang, X.; Zheng, W.; Yang, B.; Yao, S.; Lei, C.; Li, Z.; He, Q.; Lu, J.; Lei, L.; et al. Gas Diffusion Strategy for Inserting Atomic Iron Sites into Graphitized Carbon Supports for Unusually High-Efficient CO2 Electroreduction and High-Performance Zn-CO2 Batteries. Adv. Mater. 2020, 32, e2002430. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, Z.; Wu, J.; Yi, X.; Zheng, A.; Umar, A.; O'Hare, D.; Wang, Q. Comprehensive investigation of CO2 adsorption on Mg–Al–CO3 LDH-derived mixed metal oxides. J. Mater. Chem. A 2013, 1, 12782–12790. [Google Scholar] [CrossRef]
- Ren, J.; Ouyang, S.; Xu, H.; Meng, X.; Wang, T.; Wang, D.; Ye, J. Photothermal Catalysis: Targeting Activation of CO2 and H2 over Ru-Loaded Ultrathin Layered Double Hydroxides to Achieve Efficient Photothermal CO2 Methanation in Flow-Type System. Adv. Energy Mater. 2017, 7, 1601657. [Google Scholar] [CrossRef]
- Saliba, D.; Ezzeddine, A.; Sougrat, R.; Khashab, N.M.; Hmadeh, M.; Al-Ghoul, M. Cadmium-Aluminum Layered Double Hydroxide Microspheres for Photocatalytic CO2 Reduction. ChemSusChem 2016, 9, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Durndell, L.J.; Manayil, J.C.; Isaacs, M.A.; Parlett, C.M.A.; Karthikeyan, S.; Douthwaite, R.E.; Coulson, B.; Wilson, K.; Lee, A.F. Delaminated CoAl-Layered Double Hydroxide@TiO2 Heterojunction Nanocomposites for Photocatalytic Reduction of CO2. Part. Part. Syst. Char. 2018, 35, 1700317. [Google Scholar] [CrossRef]
- Liu, L.; Pitts, D.T.; Zhao, H.; Zhao, C.; Li, Y. Silver-incorporated bicrystalline (anatase/brookite) TiO2 microspheres for CO2 photoreduction with water in the presence of methanol. Appl. Catal. A Gen. 2013, 467, 474–482. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, H.; He, T. Study on nanoporous CuBi2O4 photocathode coated with TiO2 overlayer for photoelectrochemical CO2 reduction. Chemosphere 2021, 264, 128508. [Google Scholar] [CrossRef]
- Wang, R.; Qiu, Z.; Wan, S.; Wang, Y.; Liu, Q.; Ding, J.; Zhong, Q. Insight into mechanism of divalent metal cations with different d-bands classification in layered double hydroxides for light-driven CO2 reduction. Chem. Eng. J. 2021, 427, 130863. [Google Scholar] [CrossRef]
- Rao, H.; Schmidt, L.; Bonin, J.; Robert, M. Visible-light-driven methane formation from CO2 with a molecular iron catalyst. Nature 2017, 548, 74–77. [Google Scholar] [CrossRef]
- Tan, L.; Xu, S.; Wang, Z.; Xu, Y.; Wang, X.; Hao, X.; Bai, S.; Ning, C.; Wang, Y.; Zhang, W.; et al. Highly Selective Photoreduction of CO2 with Suppressing H2 Evolution over Monolayer Layered Double Hydroxide under Irradiation above 600 nm. Angew. Chem. Int. Ed. 2019, 58, 11860–11867. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, S.-M.; Tan, L.; Liu, G.; Shen, T.; Yu, C.; Wang, H.; Tao, Y.; Cao, X.; Zhao, Y.; et al. 600 nm-driven photoreduction of CO2 through the topological transformation of layered double hydroxides nanosheets. Appl. Catal. B Environ. 2020, 270, 118884. [Google Scholar] [CrossRef]
- Lin, Z.D.; Guo, R.T.; Yuan, Y.; Ji, X.Y.; Hong, L.F.; Pan, W.G. Fabrication of flower spherical-like Z-scheme FeWO4/NiAl-LDH photocatalysts with excellent activity for CO2 photoreduction under visible light. Appl. Surf. Sci. 2021, 567, 150805. [Google Scholar] [CrossRef]
- Song, Q.; Zhou, Y.; Hu, J.; Zhou, C.; Shi, X.; Li, D.; Jiang, D. Synergistic effects of surface Lewis Base/Acid and nitrogen defect in MgAl layered double Oxides/Carbon nitride heterojunction for efficient photoreduction of carbon dioxide. Appl. Surf. Sci. 2021, 563, 150369. [Google Scholar] [CrossRef]
- Liu, X.; Tao, S.; Zhang, J.; Zhu, Y.; Ma, R.; Lu, J. Ultrathin p–n type Cu2O/CuCoCr-layered double hydroxide heterojunction nanosheets for photo-assisted aqueous Zn–CO2 batteries. J. Mater. Chem. A 2021, 9, 26061–26068. [Google Scholar] [CrossRef]
- Fu, Y.; Chen, L.; Xiong, Y.; Chen, H.; Xie, R.; Wang, B.; Zhang, Y.; Liu, T.; Zhang, P. NiFe-CN catalysts derived from the solid-phase exfoliation of NiFe-layered double hydroxide for CO2 electroreduction. New J. Chem. 2022, 46, 16019–16024. [Google Scholar] [CrossRef]
- Iwase, K.; Hirano, T.; Honma, I. Copper Aluminum Layered Double Hydroxides with Different Compositions and Morphologies as Electrocatalysts for the Carbon Dioxide Reduction Reaction. ChemSusChem 2021, 15, e202102340. [Google Scholar] [CrossRef]
- Gupta, V.K. Suhas Application of low-cost adsorbents for dye removal—A review. J. Environ. Manag. 2009, 90, 2313–2342. [Google Scholar] [CrossRef]
- Mohapatra, L.; Parida, K. Zn–Cr layered double hydroxide: Visible light responsive photocatalyst for photocatalytic degradation of organic pollutants. Sep. Purif. Technol. 2011, 91, 73–80. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, N.; Fan, Z.; Hu, Z.-T.; Fan, L.; Zhou, J.; Huang, X. On-site H2O2 electro-generation process combined with ultraviolet: A promising approach for odorous compounds purification in drinking water system. Chem. Eng. J. 2021, 430, 132829. [Google Scholar] [CrossRef]
- Gao, H.; Cao, R.; Xu, X.; Xue, J.; Zhang, S.; Hayat, T.; Alharbi, N.S.; Li, J. Surface Area- and Structure-Dependent Effects of LDH for Highly Efficient Dye Removal. ACS Sustain. Chem. Eng. 2018, 7, 905–915. [Google Scholar] [CrossRef]
- Chen, S.; Yang, F.; Cao, Z.; Yu, C.; Wang, S.; Zhong, H. Enhanced photocatalytic activity of molybdenum disulfide by compositing ZnAl–LDH. Colloids Surf. A Physicochem. Eng. Asp. 2019, 586, 124140. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, F.; Zhang, C.; Zeng, G.; Tan, X.; Yu, Z.; Zhong, Y.; Wang, H.; Cui, F. Utilization of LDH-based materials as potential adsorbents and photocatalysts for the decontamination of dyes wastewater: A review. RSC Adv. 2016, 6, 79415–79436. [Google Scholar] [CrossRef]
- Gao, R.; Zhu, J.; Yan, D. Transition metal-based layered double hydroxides for photo(electro)chemical water splitting: A mini review. Nanoscale 2021, 13, 13593–13603. [Google Scholar] [CrossRef]
- Baliarsingh, N.; Parida, K.M.; Pradhan, G.C. Effects of Co, Ni, Cu, and Zn on Photophysical and Photocatalytic Properties of Carbonate Intercalated MII/Cr LDHs for Enhanced Photodegradation of Methyl Orange. Ind. Eng. Chem. Res. 2014, 53, 3834–3841. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, S.; Li, B.; Yan, H.; He, S.; Tian, L.; Shi, W.; Ma, J.; Wei, M.; Evans, D.G.; et al. A family of visible-light responsive photocatalysts obtained by dispersing CrO6 octahedra into a hydrotalcite matrix. Chem. A Eur. J. 2011, 17, 13175–13181. [Google Scholar] [CrossRef]
- Megala, S.; Silambarasan, A.; Kanagesan, S.; Selvaraj, M.; Maadeswaran, P.; Ramesh, R.; Alam, M.M.; Assiri, M.A. Interfacial coupling of CuWO4 nanoparticles on NiAl LDH as a novel photoctalyst for dissolved organic dye degradation. J. Mol. Struct. 2021, 1252, 132149. [Google Scholar] [CrossRef]
- Ma, J.; Ding, J.; Yu, L.; Li, L.; Kong, Y.; Komarneni, S. BiOCl dispersed on NiFe–LDH leads to enhanced photo-degradation of Rhodamine B dye. Appl. Clay Sci. 2015, 109–110, 76–82. [Google Scholar] [CrossRef]
- Abd-Ellatif, W.R.; Mahmoud, N.G.; Hashem, A.A.; El-Aiashy, M.K.; Ezzo, E.M.; Mahmoud, S.A. Efficient photodegradation of E124 dye using two-dimensional Zn-Co LDH: Kinetic and thermodynamic studies. Environ. Technol. Innov. 2022, 27, 102393. [Google Scholar] [CrossRef]
- Pirkarami, A.; Rasouli, S.; Ghasemi, E. CdS@NiCo-LDH hybrid photoelectrocatalyst with enhanced photocatalytic activity: A convenient and stable hybrid for wastewater treatment. J. Alloys Compd. 2022, 911, 164736. [Google Scholar] [CrossRef]
- Fei, W.; Song, Y.; Li, N.; Chen, D.; Xu, Q.; Li, H.; He, J.; Lu, J. Fabrication of visible-light-active ZnO/ZnFe-LDH heterojunction on Ni foam for pollutants removal with enhanced photoelectrocatalytic performance. Sol. Energy 2019, 188, 593–602. [Google Scholar] [CrossRef]
- Argote-Fuentes, S.; Feria-Reyes, R.; Ramos-Ramírez, E.; Gutiérrez-Ortega, N.; Cruz-Jiménez, G. Photoelectrocatalytic Degradation of Congo Red Dye with Activated Hydrotalcites and Copper Anode. Catalysts 2021, 11, 211. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).