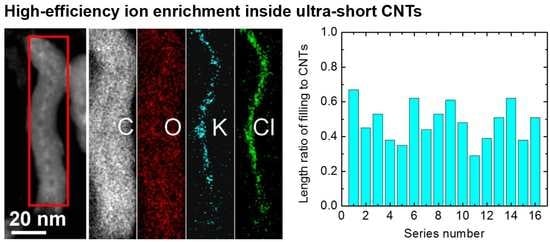
High-Efficiency Ion Enrichment inside Ultra-Short Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
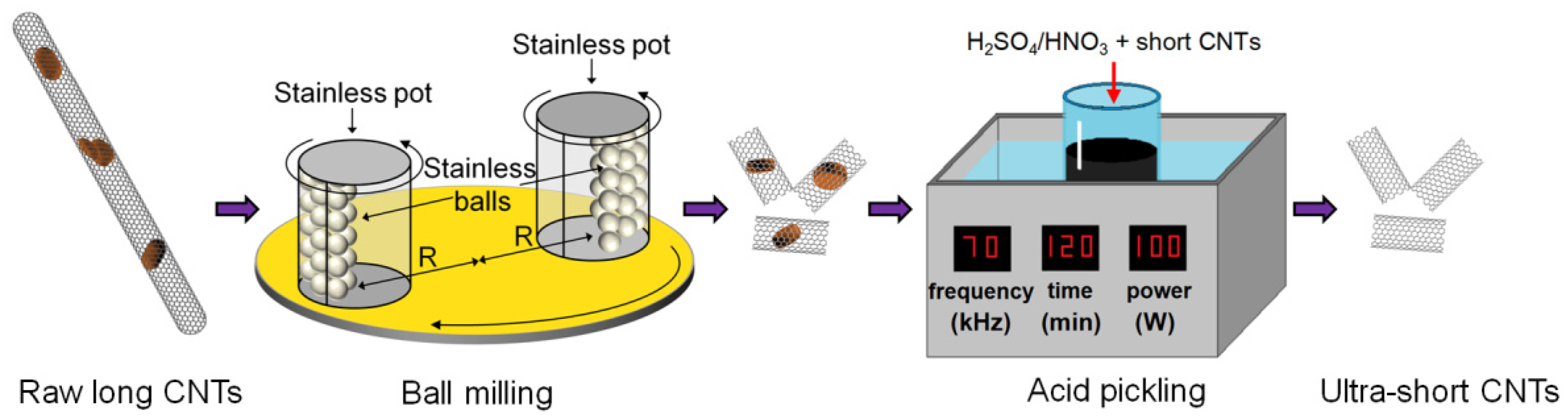
2.1. Preparation of Ultra-Short CNTs
2.2. CNT Filling Experiments
2.3. Characterization
3. Results and Discussion
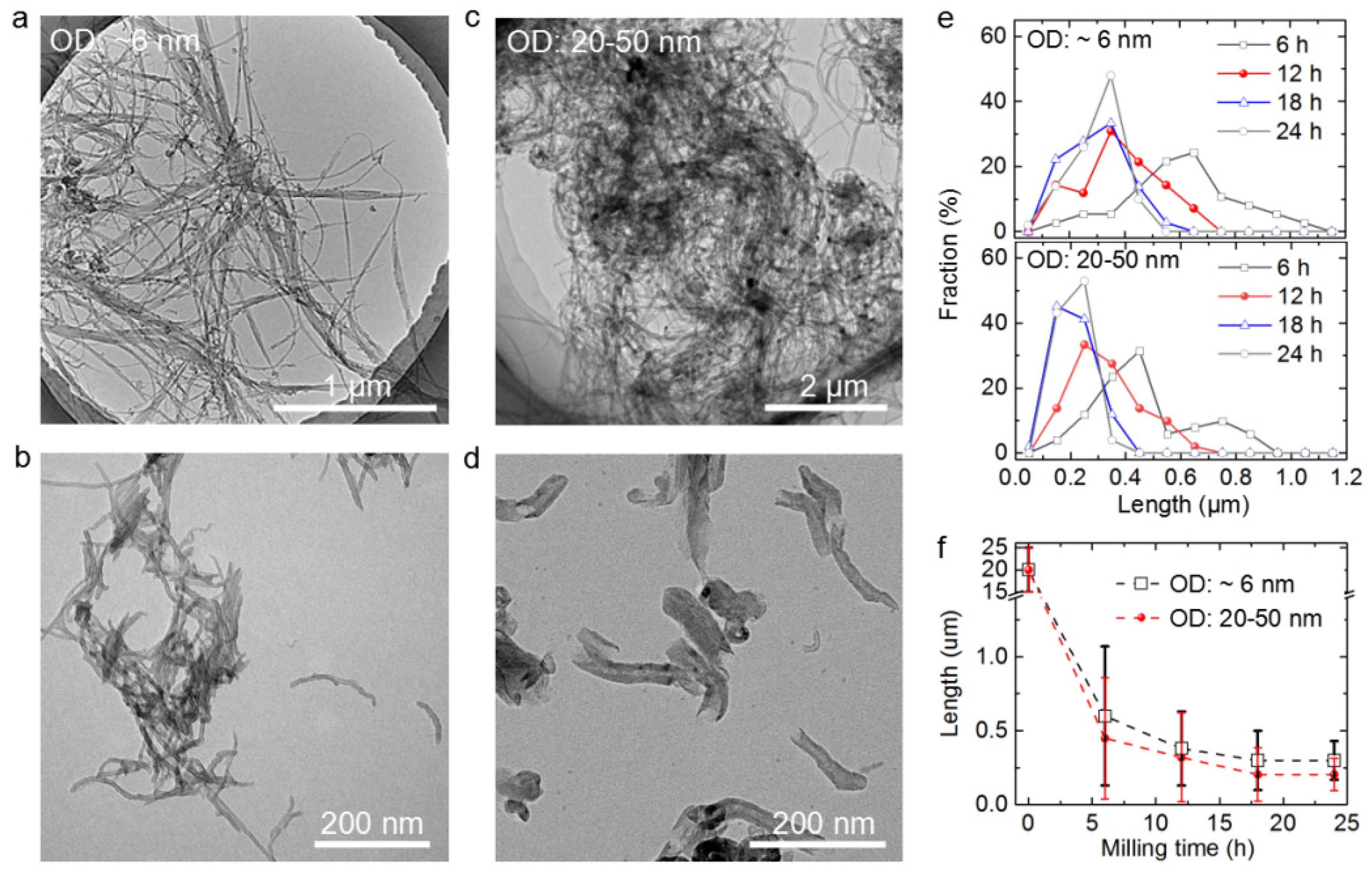
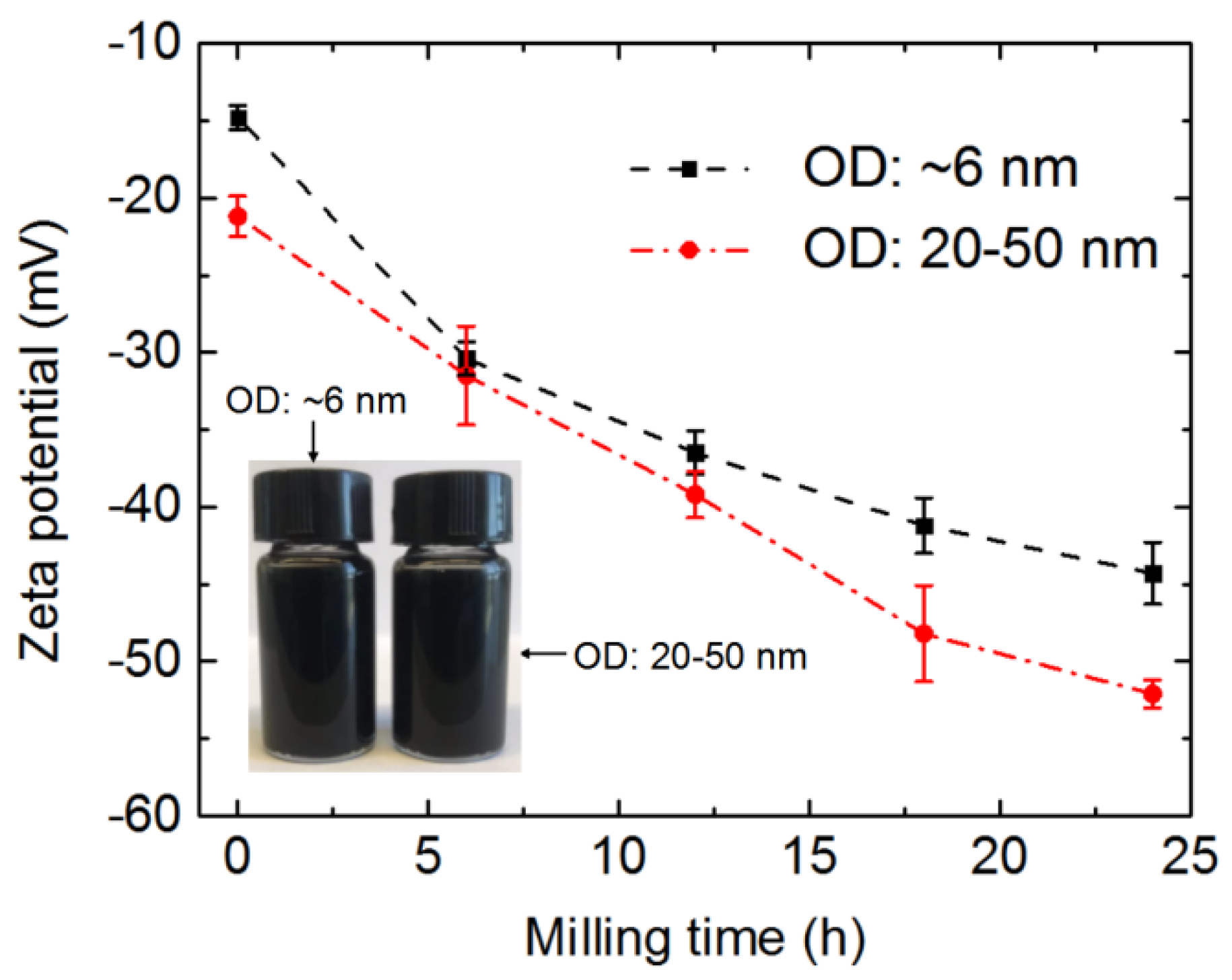
3.1. Shortening of CNTs
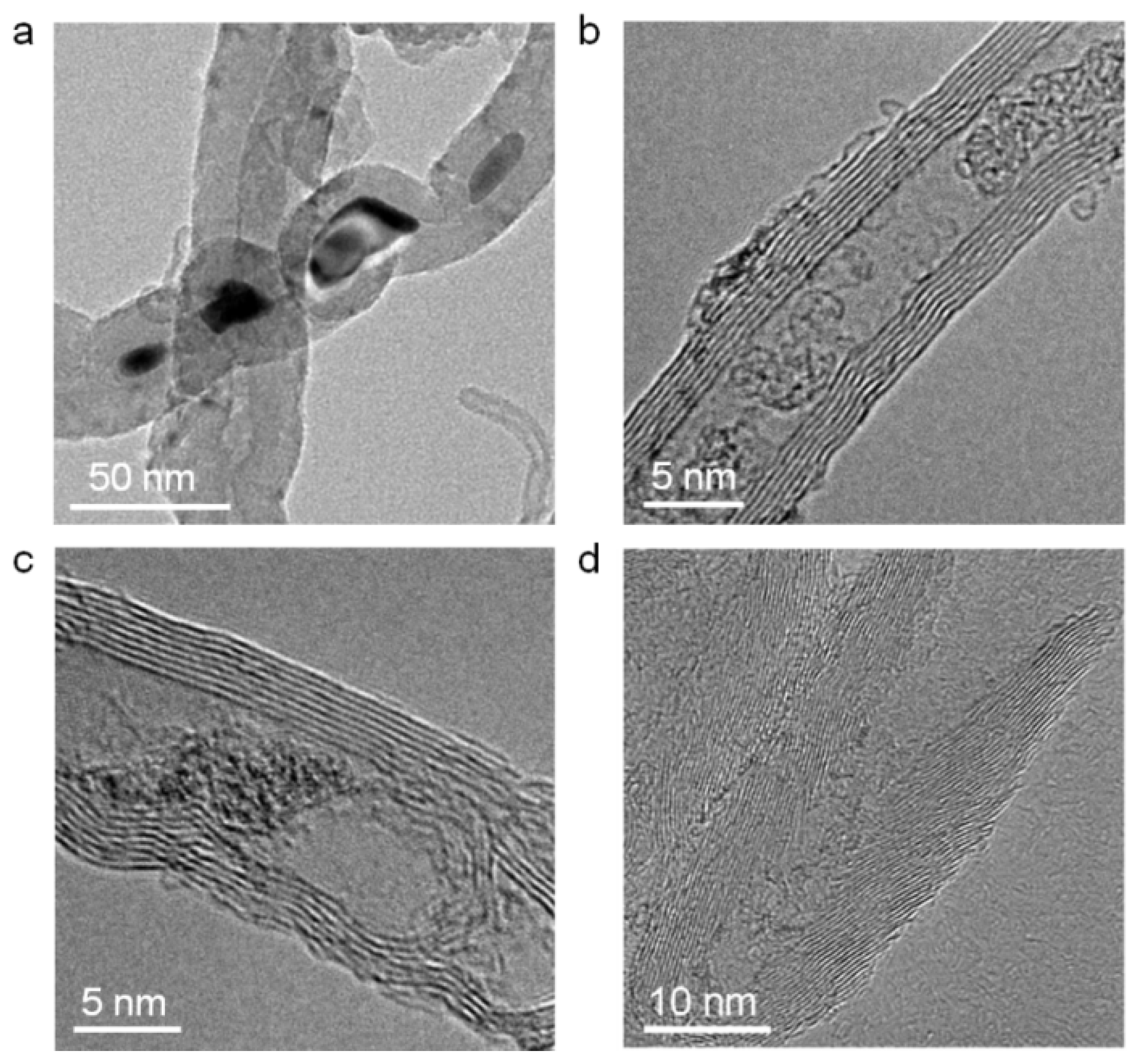
3.2. KCl Enrichment inside Ultra-Short CNTs
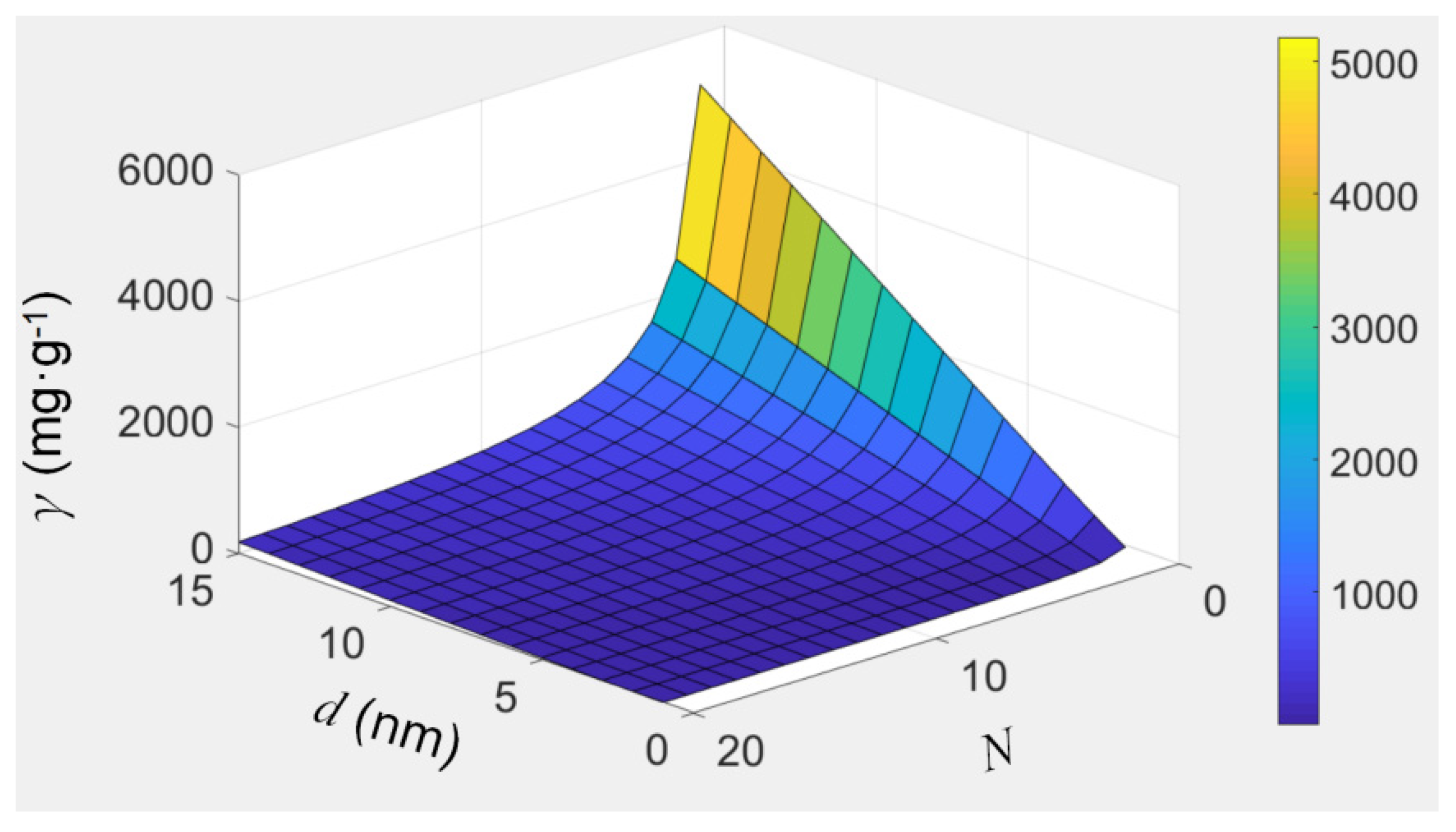
3.3. Discussion of Filling Capacity of CNTs
3.4. Enrichment of Other Ions by Ultra-Short CNTs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56. [Google Scholar] [CrossRef]
- Dresselhaus, M.S.; Dresselhaus, G.; Avouris, P. Carbon Nanotubes: Synthesis, Structures, Properties and Applications; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar]
- Yue, C.L.; Sun, H.M.; Liu, W.J.; Guan, B.B.; Deng, X.D.; Zhang, X.; Yang, P. Environmentally benign, rapid, and selective extraction of gold from ores and waste electronic materials. Angew. Chem. Int. Ed. 2017, 56, 9459–9463. [Google Scholar] [CrossRef]
- Yu, W.J.; Liu, C.; Zhang, L.L.; Hou, P.X.; Li, F.; Zhang, B.; Cheng, H.M. Synthesis and electrochemical lithium storage behavior of carbon nanotubes filled with iron sulfide nanoparticles. Adv. Sci. 2016, 3, 1600113. [Google Scholar] [CrossRef] [PubMed]
- Su, Q.M.; Du, G.H.; Zhang, J.; Zhong, Y.J.; Xu, B.S.; Yang, Y.H. In Situ Transmission Electron Microscopy Investigation of the Electrochemical Lithiation-Delithiation of Individual Co9S8/Co-Filled Carbon Nanotubes. ACS Nano 2013, 7, 11379–11387. [Google Scholar] [CrossRef] [PubMed]
- Cava, C.E.; Possagno, R.; Schnitzler, M.C.; Roman, P.C.; Oliveira, M.M.; Lepiensky, C.M. Iron- and iron oxide-filled multiwalled carbon nanotubes: Electrical properties and memory devices. Chem. Phys. Lett. 2007, 444, 304–308. [Google Scholar] [CrossRef]
- Cao, X.; He, J.; Li, H.; Kang, L.P.; He, X.X.; Sun, J.; Jiang, R.B.; Xu, H.; Lei, Z.B.; Liu, Z.H. CoNi2S4 nanoparticle/carbon nanotube sponge cathode with ultrahigh capacitance for highly compressible asymmetric supercapacitor. Small 2018, 14, 1800998. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Lethien, C.; Pinaud, S.; Brousse, K.; Laloo, R.; Turq, V.; Respaud, M.; Demortière, A.; Daffos, B.; Taberna, P.L.; et al. On-chip and freestanding elastic carbon films for micro-supercapacitors. Science 2016, 351, 691. [Google Scholar] [CrossRef] [PubMed]
- Mohan, M.K.C.; Shim, S.K.; Lee, J.K.; Jang, N.; Lee, N.; Tawfik, W.Z. Optimized Aluminum Reflector for Enhancement of UVC Cathodoluminescence Based-AlGaN Materials with Carbon Nanotube Field Emitters. Molecules 2021, 26, 4025. [Google Scholar] [CrossRef]
- Shim, S.K.; Tawfik, W.Z.; Kumar, C.M.M.; Liu, S.F.; Wang, X.Q.; Lee, N.; Lee, J.K. Nanopatterned sapphire substrate to enhance the efficiency of AlGaN-based UVC light source tube with CNT electron-beam. J. Mater. Chem. C 2020, 8, 17336–17341. [Google Scholar] [CrossRef]
- Tawfik, W.Z.; Kumar, C.M.M.; Park, J.; Shim, S.K.; Lee, H.S.; Lee, J.; Han, J.H.; Ryu, S.W.; Lee, N.; Lee, J.K. Cathodoluminescence of a 2 inch ultraviolet-light-source tube based on the integration of AlGaN materials and carbon nanotube field emitters. J. Mater. Chem. C 2019, 7, 11540–11548. [Google Scholar] [CrossRef]
- Salas-Treviño, D.; Saucedo-Cárdenas, O.; Loera-Arias, M.D.J.; Rodríguez-Rocha, H.; García-García, A.; Montes-de-Oca-Luna, R.; Piña-Mendoza, E.I.; Contreras-Torres, F.F.; García-Rivas, G.; Soto-Domínguez, A. Hyaluronate functionalized multi-wall carbon nanotubes filled with carboplatin as a novel drug nanocarrier against Murine Lung cancer cells. Nanomaterials 2019, 9, 1572. [Google Scholar] [CrossRef] [PubMed]
- Poudel, Y.R.; Li, W.Z. Syntheses, properties, and applications of carbon nanotubes filled with foreign materials: A review. Mater. Today Phys. 2018, 7, 7–34. [Google Scholar] [CrossRef]
- Marega, R.; Bonifazi, D. Filling carbon nanotubes for nanobiotechnological applications. New J. Chem. 2014, 38, 22–27. [Google Scholar] [CrossRef]
- Chatzichristos, A.; Hassan, J. Current understanding of water properties inside carbon nanotubes. Nanomaterials 2022, 12, 174. [Google Scholar] [CrossRef] [PubMed]
- Kharlamova, M.V.; Kramberger, C. Metal cluster size-dependent activation energies of growth of single-chirality single-walled carbon nanotubes inside metallocene-filled single-walled carbon nanotubes. Nanomaterials 2021, 11, 2649. [Google Scholar] [CrossRef] [PubMed]
- Cambre, S.; Campo, J.; Beirnaert, C.; Verlackt, C.; Cool, P.; Wenseleers, W. Asymmetric dyes align inside carbon nanotubes to yield a large nonlinear optical response. Nat. Nanotechnol. 2015, 10, 248–252. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.M.; Qian, S.Z.; Bau, H.H. Filling carbon nanotubes with particles. Nano Lett. 2005, 5, 873–878. [Google Scholar] [CrossRef]
- Morales, N.J.; Goyanes, S.; Chiliotte, C.; Bekeris, V.; Candal, R.J.; Rubiolo, G.H. One-step chemical vapor deposition synthesis of magnetic CNT-hercynite (FeAl2O4) hybrids with good aqueous colloidal stability. Carbon 2013, 61, 515–524. [Google Scholar] [CrossRef]
- Kozhuharova, R.; Ritschel, M.; Elefant, D.; Graff, A.; Leonhardt, A.; Mönch, I.; Mühl, T.; Groudeva-Zotova, S.; Schneider, C.M. Well-aligned Co-filled carbon nanotubes: Preparation and magnetic properties. Appl. Surf. Sci. 2004, 238, 355–359. [Google Scholar] [CrossRef]
- Ramachandran, K.; Raj kumar, T.; Babu, K.J.; Kumar, G.G. Ni-Co bimetal nanowires filled multiwalled carbon nanotubes for the highly sensitive and selective non-enzymatic glucose sensor applications. Sci. Rep. 2016, 6, 36583. [Google Scholar] [CrossRef] [PubMed]
- Fedotov, P.V.; Tonkikh, A.A.; Obraztsova, E.A.; Nasibulin, A.G.; Kauppinen, E.I.; Chuvilin, A.L.; Obraztsova, E.D. Optical properties of single-walled carbon nanotubes filled with CuCl by gas-phase technique. Phys. Status Solidi 2014, 251, 2466–2470. [Google Scholar] [CrossRef]
- Soldano, C.; Rossella, F.; Bellani, V.; Giudicatti, S.; Kar, S. Cobalt nanoclusterfilled carbon nanotube Arrays: Engineered photonic bandgap and optical reflectivity. ACS Nano 2010, 4, 6573–6578. [Google Scholar] [CrossRef]
- Zhao, D.L.; Zhang, J.M.; Li, X.; Shen, Z.M. Electromagnetic and microwave absorbing properties of Co-filled carbon nanotubes. J. Alloys Comp. 2010, 505, 712–716. [Google Scholar] [CrossRef]
- Zou, T.C.; Li, H.P.; Zhao, N.Q.; Shi, C.S. Electromagnetic and microwave absorbing properties of multi-walled carbon nanotubes filled with Ni nanowire. J. Alloys Comp. 2010, 496, L22–L24. [Google Scholar] [CrossRef]
- Zhang, S.L.; Zhang, Y.; Jiang, W.J.; Liu, X.; Xu, S.L.; Huo, R.J.; Zhang, F.Z.; Hu, J.S. Co@N-CNTs derived from triple-role CoAl-layered double hydrocide as an efficient catalyst for oxygen reduction reaction. Carbon 2016, 107, 162–170. [Google Scholar] [CrossRef]
- Wang, T.; Fu, Y.C.; Chai, L.Y.; Chao, L.; Bu, L.J.; Meng, Y.; Chen, C.; Ma, M.; Xie, Q.J.; Yao, S.Z. Filling carbon nanotubes with Prussian blue nanoparticles of high peroxidase-like catalytic activity for colorimetric chemo- and biosensing. Chemistry 2014, 20, 2623–2630. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Kramberger, C. Application of filled single-walled carbon nanotubes: Progress, challenges, and perspectives. Nanomaterials 2021, 11, 2863. [Google Scholar] [CrossRef]
- Gautam, U.K.; Costa, P.M.; Bando, Y.; Fang, X.S.; Li, L.; Imura, M.; Golerg, D. Recent developments in inorganically filled carbon nanotubes: Successes and challenges. Sci. Technol. Adv. Mater. 2010, 11, 054501. [Google Scholar] [CrossRef]
- Monthioux, M.; Flahaut, E.; Cleuziou, J.P. Hybrid carbon nanotubes: Strategy, progress, and perspectives. J. Mater. Res. 2006, 21, 2774–2793. [Google Scholar] [CrossRef]
- Guerret-Piecourt, C.; Bouar, Y.L.; Lolseau, A.; Pascard, H. Relation between metal electronic structure and morphology of metal compounds inside carbon nanotubes. Nature 1994, 372, 761. [Google Scholar] [CrossRef]
- Seraphin, S.; Zhou, D.; Jiao, J.; Withers, J.C.; Loutfy, R. Yttrium carbide in nanotubes. Nature 1993, 362, 503. [Google Scholar] [CrossRef]
- Setlur, A.A.; Lauerhaas, J.M.; Dai, J.Y.; Chang, R.P.H. A method for synthesizing large quantities of carbon nanotubes and encapsulated copper nanowires. Appl. Phys. Lett. 1996, 69, 345–347. [Google Scholar] [CrossRef]
- Ajayan, P.M.; Iijima, S. Capillarity-induced filling of carbon nanotubes. Nature 1993, 361, 333–334. [Google Scholar] [CrossRef]
- Tsang, S.C.; Chen, Y.K.; Harris, P.J.F.; Green, M.L.H. A simple chemical method of opening and filling carbon nanotubes. Nature 1994, 372, 159–162. [Google Scholar] [CrossRef]
- Ugarte, D.; Stockli, T.; Bonard, J.M.; Chatelain, A.; De Heer, W.A. Filling carbon nanotubes. Appl. Phys. A Mater. 1998, 67, 101–105. [Google Scholar] [CrossRef]
- Qiang, F.U.; Weinberg, G.; Dang-sheng, S.U. Selective filling of carbon nanotubes with metals by selective washing. New Carbon Mater. 2008, 23, 17–20. [Google Scholar]
- Tsang, S.C.; Harris, P.J.F.; Green, M.L.H. Thinning and opening of carbon nanotubes by oxidation using carbon-dioxide. Nature 1993, 362, 520–522. [Google Scholar] [CrossRef]
- Hou, P.X.; Liu, C.; Cheng, H.M. Purification of carbon nanotubes. Carbon 2008, 46, 2003–2025. [Google Scholar] [CrossRef]
- Satishkumar, B.C.; Govindaraj, A.; Mofokeng, J.; Subbanna, G.N.; Rao, C.N.R. Novel Experiments with Carbon Nanotubes: Opening, Filling, Closing and Functionalizing Nanotubes. J. Phys. B At. Mol. Opt. Phys. 1996, 29, 4925. [Google Scholar] [CrossRef]
- Ugarte, D.; Chatelain, A.; De Heer, W.A. Nanocapillarity and chemistry in carbon nanotubes. Science 1996, 274, 1897–1899. [Google Scholar] [CrossRef]
- Wang, X.L.; Shi, G.S.; Liang, S.S.; Liu, J.; Li, D.Y.; Fang, G.R.L.; Yan, L.; Fang, H.P. Unexpectedly High Salt Accumulation inside Carbon Nanotubes Soaked in Dilute Salt Solutions. Phys. Rev. Lett. 2018, 121, 22610220. [Google Scholar] [CrossRef] [PubMed]
- Francke, M.; Hermann, H.; Wenzel, R.; Seifert, G.; Wetzig, K. Modification of carbon nanostructures by high energy ball-milling under argon and hydrogen atmosphere. Carbon 2005, 43, 1204–1212. [Google Scholar] [CrossRef]
- Munkhbayar, B.; Nine, M.J.; Jeoun, J.; Bat-Erdene, M.; Chung, H.; Jeong, H. Influence of dry and wet ball milling on dispersion characteristics of the multi-walled carbon nanotubes in aqueous solution with and without surfactant. Powder Techno. 2013, 134, 132–140. [Google Scholar] [CrossRef]
- Kukovecz, Á.; Kanyó, T.; Kónya, Z.; Kiricsi, I. Long-time low-impact ball milling of multi-wall carbon nanotubes. Carbon 2005, 43, 994–1000. [Google Scholar] [CrossRef]
- Aramwit, P.; Kanokpanont, S.; Nakpheng, T.; Srichana, T. The effect of sericin from various extraction methods on cell viability and collagen production. Int. J. Mol. Sci. 2010, 11, 2200–2211. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, G.; Guo, P.; Yang, J.; Fang, H.P. Blockage of Water Flow in Carbon Nanotubes by Ions Due to Interactions between Cations and Aromatic Rings. Phys. Rev. Lett. 2015, 115, 164502. [Google Scholar] [CrossRef]
- Shi, G.S.; Liu, J.; Wang, C.L.; Song, B.; Tu, Y.S.; Hu, J.; Fang, H.P. Ion Enrichment on the Hydrophobic Carbon-based Surface in Aqueous Salt Solutions due to Cation-π Interactions. Sci. Rep. 2013, 3, 3436. [Google Scholar] [CrossRef] [PubMed]
- Tomo, Y.; Askounis, A.; Ikuta, T.; Takata, Y.; Sefiane, K.; Takahashi, K. Superstable ultra-thin water film confined in a hydrophilized carbon nanotube. Nano Lett. 2018, 18, 1869–1874. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, K.V.; Shimizu, S.; Drahushuk, L.W.; Kilcoyne, D.; Strano, M.S. Observation of extreme phase transition temperatures of water confined inside isolated carbon nanotubes. Nat. Nanotechnol. 2017, 12, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Fiyadh, S.S.; Alsaadi, M.A.; Jaafar, W.Z.; Alomar, M.K.; Fayaed, S.S.; Mohd, N.S.; Hin, L.S.; El-Shafie, A. Review on heavy metal adsorption processes by carbon nanotubes. J. Clean. Prod. 2019, 230, 783–793. [Google Scholar] [CrossRef]
- Breitwieser, A.; Sleytr, U.B.; Pum, D. A new method for dispersing pristine carbon nanotubes using regularly arranged S-Layer proteins. Nanomaterials 2021, 11, 1346. [Google Scholar] [CrossRef]
- Iijima, S.; Ajayan, P.M.; Ichihashi, T. Growth model for carbon nanotubes. Phys. Rev. Lett. 1992, 69, 3100–3103. [Google Scholar] [CrossRef]
- Li, Y.H.; Ding, J.; Luan, Z.K.; Di, Z.C.; Zhu, Y.F.; Xu, C.L. Competitive adsorption of Pb2+, Cu2+ and Cd2+ ions from aqueous solutions by multiwalled carbon nanotubes. Carbon 2003, 41, 2787–2792. [Google Scholar] [CrossRef]
- Li, Z.; Chen, J.; Ge, Y. Removal of lead ion and oil droplet from aqueous solution by lignin-grafted carbon nanotubes. Chem. Eng. J. 2017, 308, 809–817. [Google Scholar] [CrossRef]
- Yang, P.F.; Li, F.X.; Wang, B.H.; Niu, Y.F.; Wei, J.X.; Yu, Q.J. In situ synthesis of carbon nanotube-steel slag composite for Pb(II) and Cu(II) removal from aqueous solution. Nanomaterials 2022, 12, 1199. [Google Scholar] [CrossRef] [PubMed]
- Vesali-Naseh, M.; Vesali Naseh, M.R.; Ameri, P. Adsorption of Pb(II) ions from aqueous solutions using carbon nanotubes: A systematic review. J. Clean. Prod. 2021, 291, 125917. [Google Scholar] [CrossRef]
- Oliveira, A.R.; Correia, A.A.; Rasteiro, M.G. Heavy metals removal from aqueous solutions by multiwall carbon nanotubes: Effect of MWCNTs dispersion. Nanomaterials 2021, 11, 2082. [Google Scholar] [CrossRef]
- Gong, Z.Y.; Chan, H.T.; Chen Q., L.; Chen, H.B. Application of nanotechnology in analysis and removal of heavy metals in food and water resources. Nanomaterials 2021, 11, 1792. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbentsea review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.J.; Luan, Z.K.; Di, Z.C.; Zhang, Z.G.; Zhu, C.L. Carbon nanotubes-iron oxides magnetic composites as adsorbent for removal of Pb(II) and Cu(II) from water. Carbon 2005, 43, 880–883. [Google Scholar] [CrossRef]
- Wang, Y.G.; Zhao, G.H.; Zhang, Q.; Wang, H.; Zhang, Y.; Cao, W. Electrochemical aptasensor based on gold modified graphene nanocomposite with different morphologies for ultrasensitive detection of Pb2+. Sensor. Actuator. B Chem. 2019, 288, 325–331. [Google Scholar] [CrossRef]
- Mubarak, N.M.; Sahu, J.N.; Abdullahet, E.C.; Jayakumar, N.S. Rapid adsorption of toxic Pb(II) ions from aqueous solution using multiwall carbon nanotubes synthesized by microwave chemical vapor deposition technique. J. Environ. Sci. 2016, 45, 143–155. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiang, Y.; Wang, X.; Ying, Z.; Zhou, Y.; Liu, R.; Gao, S.; Yan, L. High-Efficiency Ion Enrichment inside Ultra-Short Carbon Nanotubes. Nanomaterials 2022, 12, 3528. https://doi.org/10.3390/nano12193528
Qiang Y, Wang X, Ying Z, Zhou Y, Liu R, Gao S, Yan L. High-Efficiency Ion Enrichment inside Ultra-Short Carbon Nanotubes. Nanomaterials. 2022; 12(19):3528. https://doi.org/10.3390/nano12193528
Chicago/Turabian StyleQiang, Yu, Xueliang Wang, Zhemian Ying, Yuying Zhou, Renduo Liu, Siyan Gao, and Long Yan. 2022. "High-Efficiency Ion Enrichment inside Ultra-Short Carbon Nanotubes" Nanomaterials 12, no. 19: 3528. https://doi.org/10.3390/nano12193528
APA StyleQiang, Y., Wang, X., Ying, Z., Zhou, Y., Liu, R., Gao, S., & Yan, L. (2022). High-Efficiency Ion Enrichment inside Ultra-Short Carbon Nanotubes. Nanomaterials, 12(19), 3528. https://doi.org/10.3390/nano12193528