Anion Dependent Particle Size Control of Platinum Nanoparticles Synthesized in Ethylene Glycol
Abstract
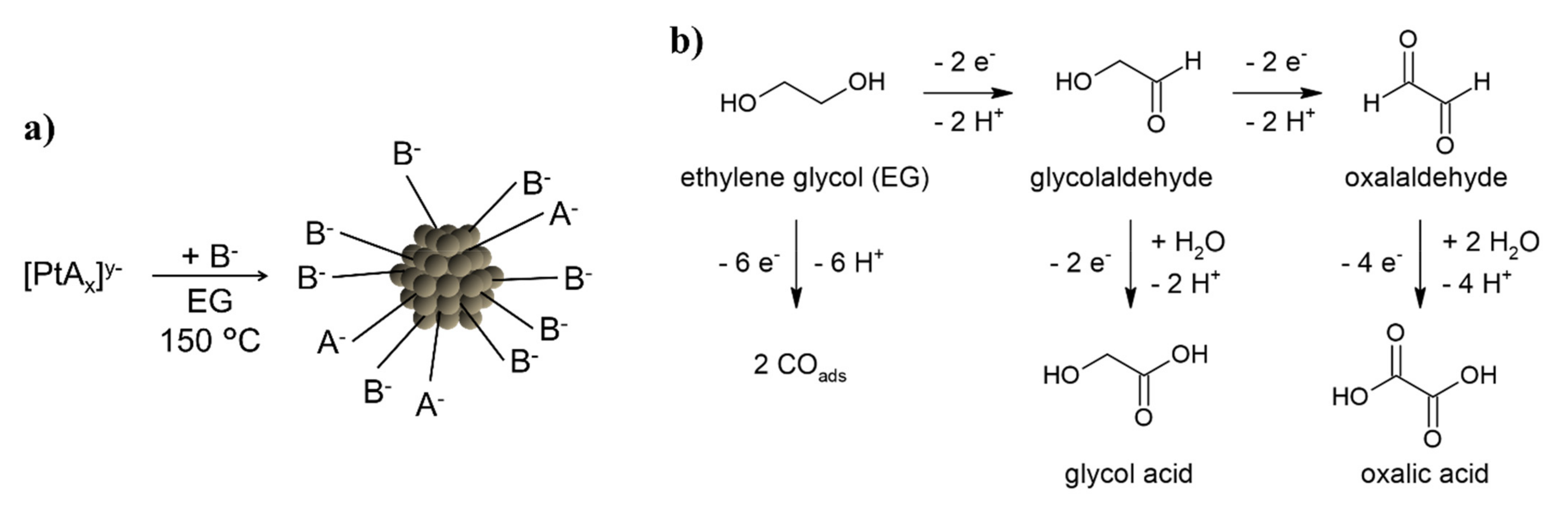
:1. Introduction
2. Experimental Section
2.1. NP Synthesis
2.1.1. Synthesis of “Surfactant-Free” Pt NPs by Thermal Reduction of H2PtCl6 in Presence of Na(acac)
2.1.2. Synthesis of “Surfactant-Free” Pt NPs by Thermal Reduction of H2Pt(OH)6 in Presence of NaOH or Na(acac)
2.1.3. Synthesis of “Surfactant-Free” Pt NPs by Thermal Reduction of Pt(acac)2 in Presence of Na(acac)
2.1.4. Cleaning of “Surfactant-Free” NPs
2.2. Characterization of Nanoparticles
Transmission Electron Spectroscopy (TEM)
3. Results and Discussion
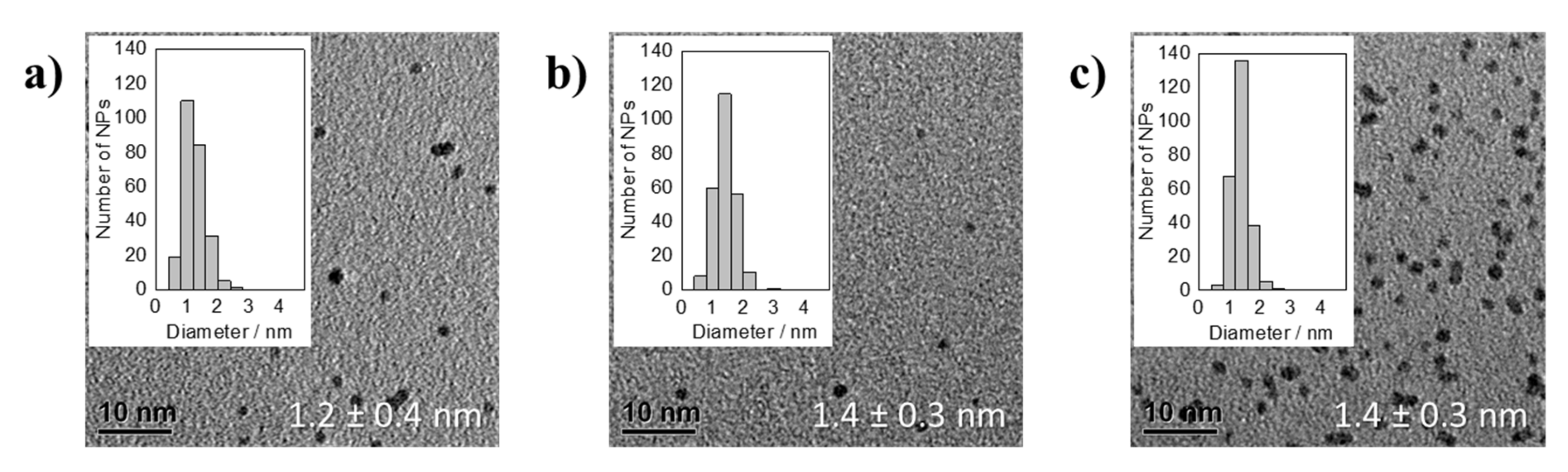
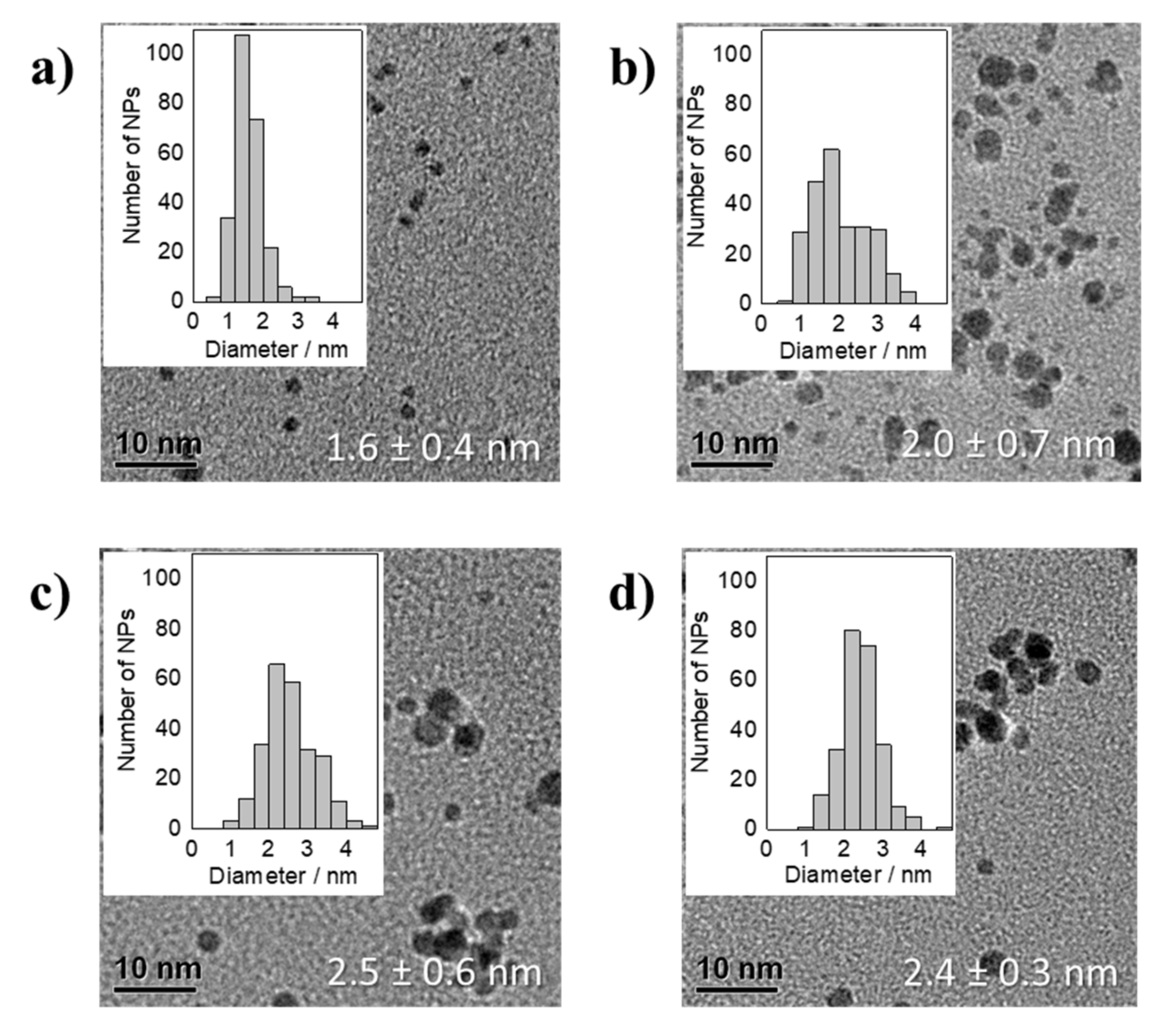
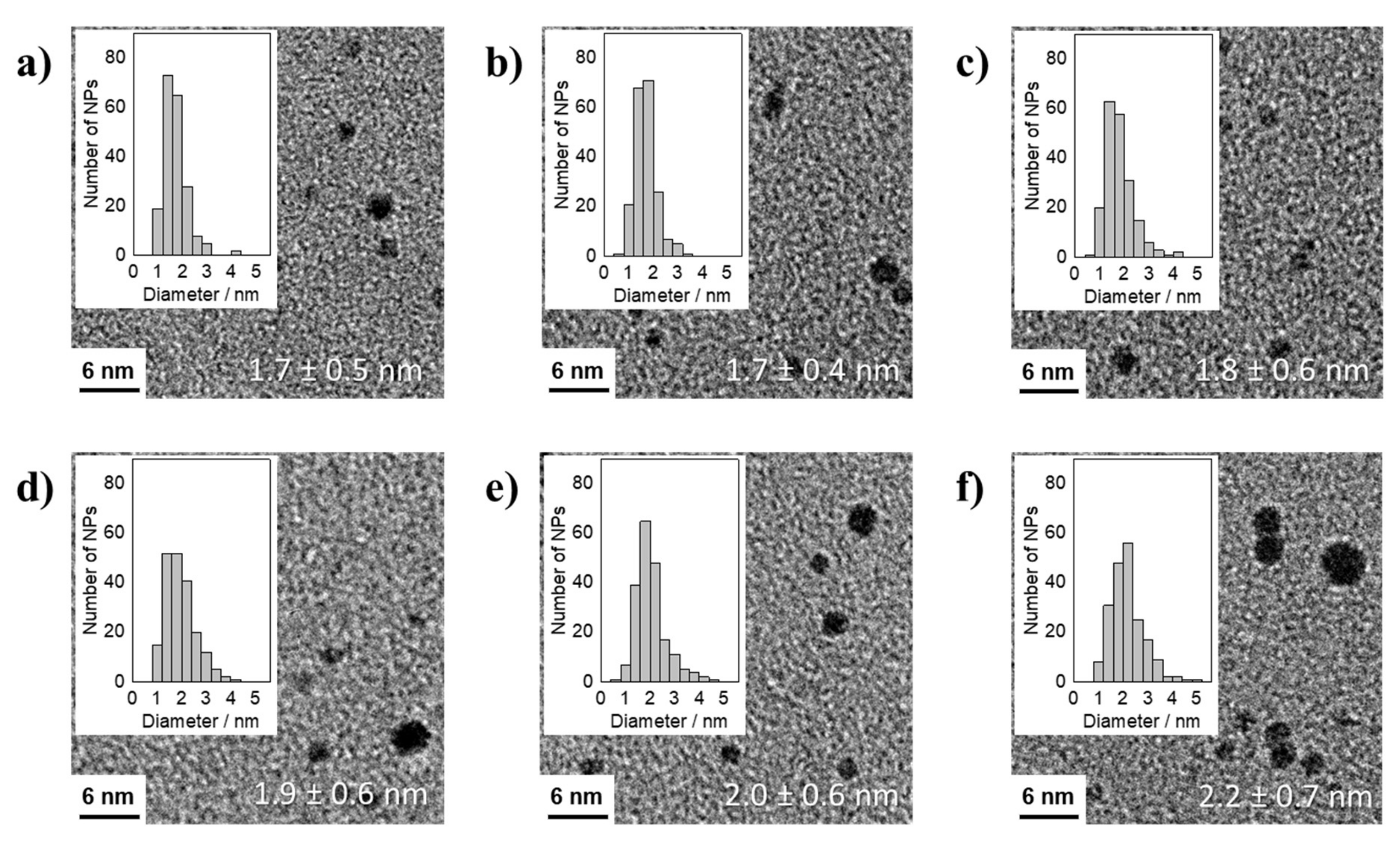
3.1. Thermal Reduction of Pt Precursors with Varying OH− Concentrations
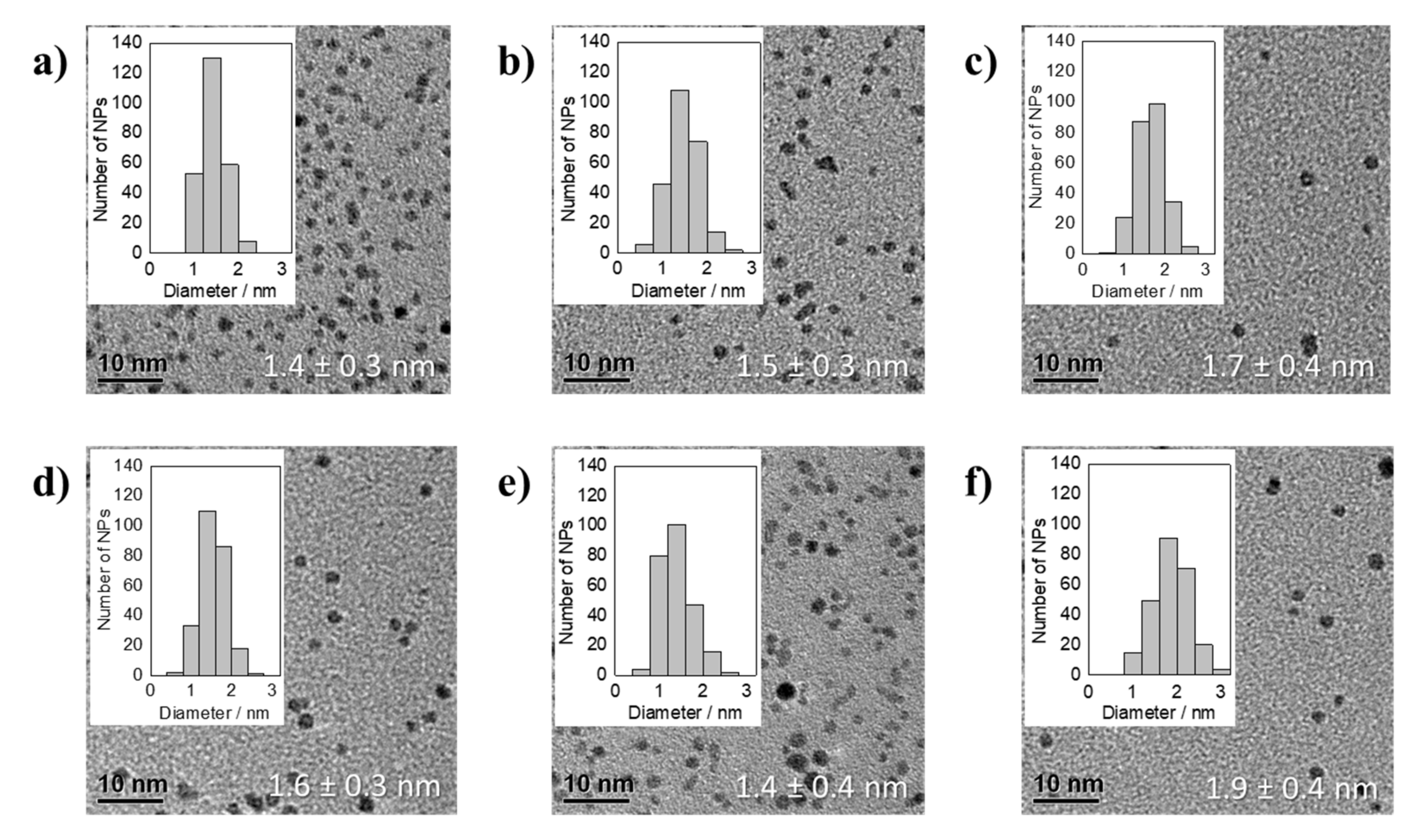
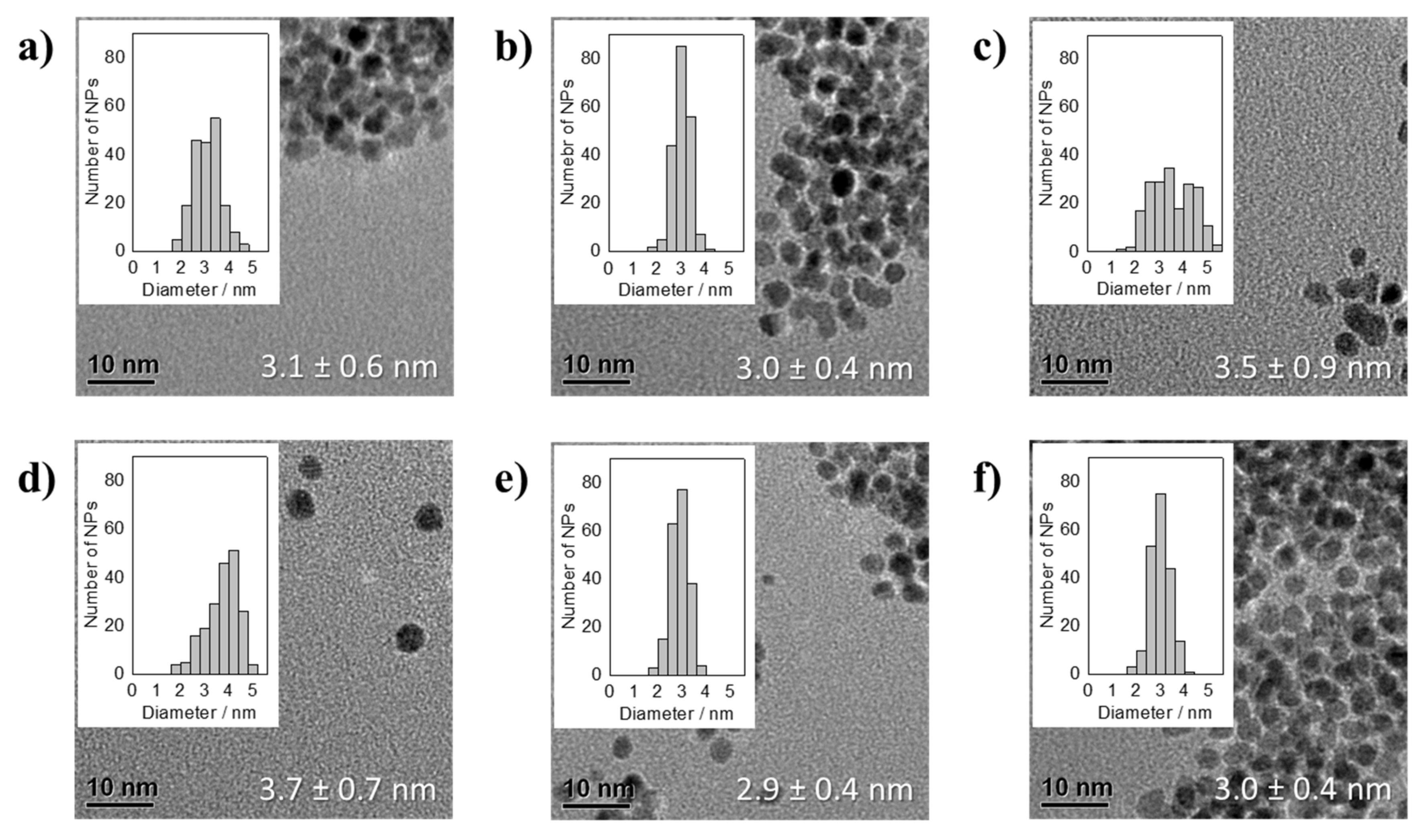
3.2. Thermal Reduction of Pt Precursors with Varying Acac− Concentrations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Zhang, J.; Wang, X.; Ren, J.; Zuo, B.; Tang, Y. Metal Nanoclusters Stabilized with Simple Ions and Solvents—Promising Building Blocks for Future Catalysts. Top. Catal. 2005, 35, 35–41. [Google Scholar] [CrossRef]
- Wang, X.; Sonström, P.; Arndt, D.; Stöver, J.; Zielasek, V.; Borchert, H.; Thiel, K.; Al-Shamery, K.; Bäumer, M. Heterogeneous Catalysis with Supported Platinum Colloids: A Systematic Study of the Interplay between Support and Functional Ligands. J. Catal. 2011, 278, 143–152. [Google Scholar] [CrossRef]
- Neumann, S.; Grotheer, S.; Tielke, J.; Schrader, I.; Quinson, J.; Zana, A.; Oezaslan, M.; Arenz, M.; Kunz, S. Nanoparticles in a Box: A Concept to Isolate, Store and Re-Use Colloidal Surfactant-Free Precious Metal Nanoparticles. J. Mater. Chem. A 2017, 5, 6140–6145. [Google Scholar] [CrossRef]
- Neumann, S.; Schröder, J.; Bizzotto, F.; Arenz, M.; Dworzak, A.; Oezaslan, M.; Bäumer, M.; Kunz, S. Halide-Induced Leaching of Pt Nanoparticles—Manipulation of Particle Size by Controlled Ostwald Ripening. ChemNanoMat 2019, 5, 462–471. [Google Scholar] [CrossRef]
- Neumann, S.; Gutmann, T.; Buntkowsky, G.; Paul, S.; Thiele, G.; Sievers, H.; Bäumer, M.; Kunz, S. Insights into the Reaction Mechanism and Particle Size Effects of CO Oxidation over Supported Pt Nanoparticle Catalysts. J. Catal. 2019, 377, 662–672. [Google Scholar] [CrossRef]
- Speder, J.; Altmann, L.; Roefzaad, M.; Bäumer, M.; Kirkensgaard, J.J.K.K.; Mortensen, K.; Arenz, M. Pt Based PEMFC Catalysts Prepared from Colloidal Particle Suspensions—A Toolbox for Model Studies. Phys. Chem. Chem. Phys. 2013, 15, 3602. [Google Scholar] [CrossRef] [PubMed]
- Quinson, J.; Inaba, M.; Neumann, S.; Swane, A.A.; Bucher, J.; Simonsen, S.B.; Theil Kuhn, L.; Kirkensgaard, J.J.K.; Jensen, K.M.Ø.; Oezaslan, M.; et al. Investigating Particle Size Effects in Catalysis by Applying a Size-Controlled and Surfactant-Free Synthesis of Colloidal Nanoparticles in Alkaline Ethylene Glycol: Case Study of the Oxygen Reduction Reaction on Pt. ACS Catal. 2018, 8, 6627–6635. [Google Scholar] [CrossRef]
- Schrader, I.; Warneke, J.; Backenköhler, J.; Kunz, S. Functionalization of Platinum Nanoparticles with L-Proline: Simultaneous Enhancements of Catalytic Activity and Selectivity. J. Am. Chem. Soc. 2015, 137, 905–912. [Google Scholar] [CrossRef] [PubMed]
- Schrader, I.; Neumann, S.; Himstedt, R.; Zana, A.; Warneke, J.; Kunz, S. The Effect of Particle Size and Ligand Configuration on the Asymmetric Catalytic Properties of Proline-Functionalized Pt-Nanoparticles. Chem. Commun. 2015, 51, 16221–16224. [Google Scholar] [CrossRef] [PubMed]
- Šulce, A.; Backenköhler, J.; Schrader, I.; Piane, M.D.; Müller, C.; Wark, A.; Ciacchi, L.C.; Azov, V.; Kunz, S. Ligand-Functionalized Pt Nanoparticles as Asymmetric Heterogeneous Catalysts: Molecular Reaction Control by Ligand-Reactant Interactions. Catal. Sci. Technol. 2018, 8, 6062–6075. [Google Scholar] [CrossRef]
- Šulce, A.; Flaherty, D.W.; Kunz, S. Kinetic Analysis of the Asymmetric Hydrogenation of SS-Keto Esters over $α$-Amino Acid-Functionalized Pt Nanoparticles. J. Catal. 2019, 374, 82–92. [Google Scholar] [CrossRef]
- Bock, C.; Paquet, C.; Couillard, M.; Botton, G.A.; MacDougall, B.R. Size-Selected Synthesis of PtRu Nano-Catalysts: Reaction and Size Control Mechanism. J. Am. Chem. Soc. 2004, 126, 8028–8037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrader, I.; Warneke, J.; Neumann, S.; Grotheer, S.; Swane, A.A.; Kirkensgaard, J.J.K.; Arenz, M.; Kunz, S. Surface Chemistry of “Unprotected” Nanoparticles: A Spectroscopic Investigation on Colloidal Particles. J. Phys. Chem. C 2015, 119, 17655–17661. [Google Scholar] [CrossRef]
- Kacenauskaite, L.; Quinson, J.; Schultz, H.; Kirkensgaard, J.J.K.; Kunz, S.; Vosch, T.; Arenz, M. UV-Induced Synthesis and Stabilization of Surfactant-Free Colloidal Pt Nanoparticles with Controlled Particle Size in Ethylene Glycol. ChemNanoMat 2017, 3, 89–93. [Google Scholar] [CrossRef]
- Schröder, J.; Neumann, S.; Kunz, S. Visible-Light-Induced Synthesis of “Surfactant-Free” Pt Nanoparticles in Ethylene Glycol as a Synthetic Approach for Mechanistic Studies on Nanoparticle Formation. J. Phys. Chem. C 2020, 124, 21798–21809. [Google Scholar] [CrossRef]
- Quinson, J.; Bucher, J.; Simonsen, S.B.; Kuhn, L.T.; Kunz, S.; Arenz, M. Monovalent Alkali Cations: Simple and Eco-Friendly Stabilizers for Surfactant-Free Precious Metal Nanoparticle Colloids. ACS Sustain. Chem. Eng. 2019, 7, 13680–13686. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schröder, J.; Neumann, S.; Quinson, J.; Arenz, M.; Kunz, S. Anion Dependent Particle Size Control of Platinum Nanoparticles Synthesized in Ethylene Glycol. Nanomaterials 2021, 11, 2092. https://doi.org/10.3390/nano11082092
Schröder J, Neumann S, Quinson J, Arenz M, Kunz S. Anion Dependent Particle Size Control of Platinum Nanoparticles Synthesized in Ethylene Glycol. Nanomaterials. 2021; 11(8):2092. https://doi.org/10.3390/nano11082092
Chicago/Turabian StyleSchröder, Johanna, Sarah Neumann, Jonathan Quinson, Matthias Arenz, and Sebastian Kunz. 2021. "Anion Dependent Particle Size Control of Platinum Nanoparticles Synthesized in Ethylene Glycol" Nanomaterials 11, no. 8: 2092. https://doi.org/10.3390/nano11082092
APA StyleSchröder, J., Neumann, S., Quinson, J., Arenz, M., & Kunz, S. (2021). Anion Dependent Particle Size Control of Platinum Nanoparticles Synthesized in Ethylene Glycol. Nanomaterials, 11(8), 2092. https://doi.org/10.3390/nano11082092






