Recyclable Graphene Sheets as a Growth Template for Crystalline ZnO Nanowires
Abstract
1. Introduction
2. Experimental Details
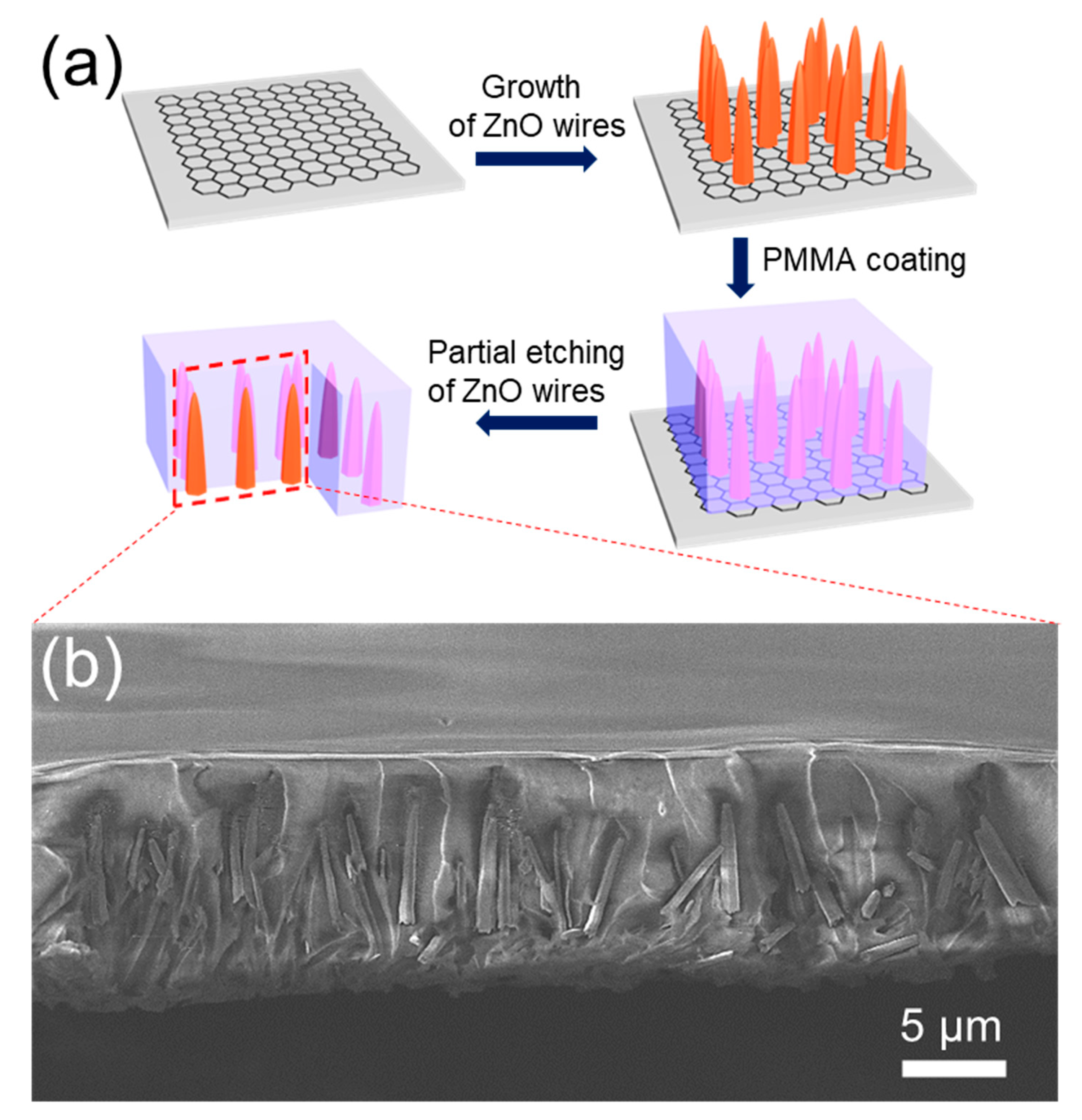
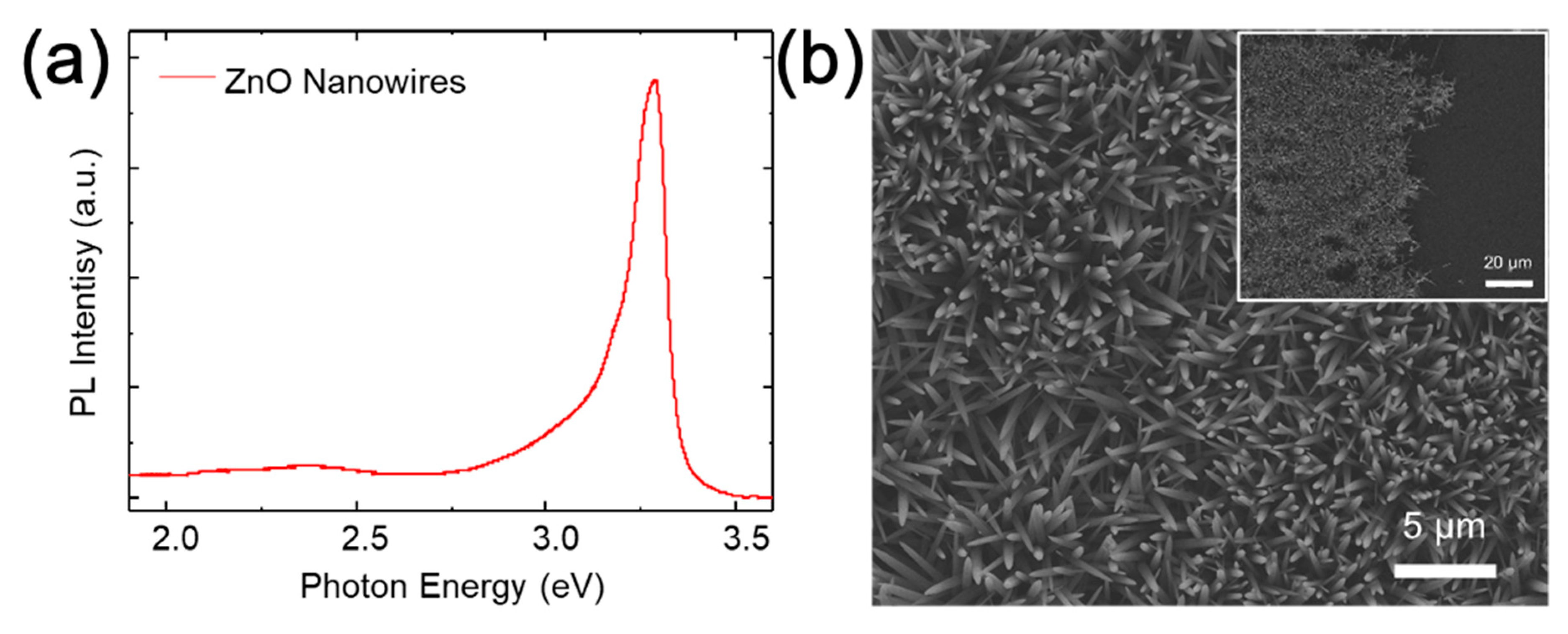
3. Results and Discussions
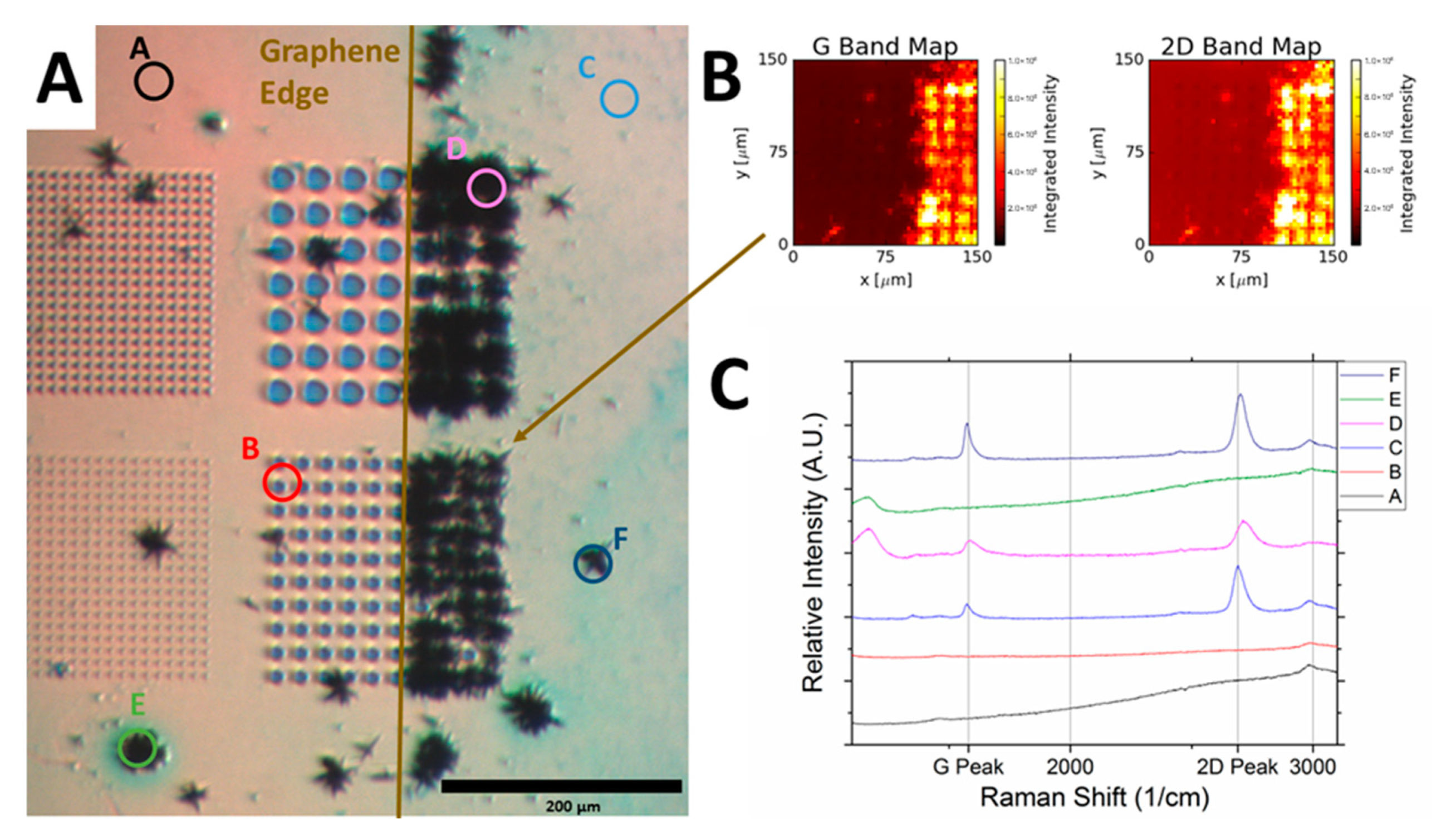
4. Raman Spectroscopy Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yoon, J.; Jo, S.; Chun, I.S.; Jung, I.; Kim, H.-S.; Meitl, M.; Menard, E.; Li, X.; Coleman, J.J.; Paik, U.; et al. GaAs photovoltaics and optoelectronics using releasable multilayer epitaxial assemblies. Nature 2010, 465, 329. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, K.; Jung, S.H.; Kim, C.Z.; Shin, H.-B.; Choi, H.; Kang, H.K. Internal stress-assisted epitaxial lift-off process for flexible thin film (In) GaAs solar cells on metal foil. Appl. Phys. Lett. 2017, 111, 233509. [Google Scholar] [CrossRef]
- Youtsey, C.; McCarthy, R.; Reddy, R.; Forghani, K.; Xie, A.; Beam, E.; Wang, J.; Fay, P.; Ciarkowski, T.; Carlson, E.; et al. Wafer-scale epitaxial lift-off of GaN using bandgap-selective photoenhanced wet etching. Phys. Status Solidi B 2017, 254, 1600774. [Google Scholar] [CrossRef]
- Woodhouse, M.; Goodrich, A. A Manufacturing Cost Analysis Relevant to Single- and Dual-Junction Photovoltaic Cells Fabricated with III-Vs and III-Vs Grown on Czochralski Silicon; NREL/PR-6A20-601267563; Technical Report; National Renewable Energy Lab. (NREL): Golden, CO, USA, 2013. [Google Scholar]
- Kim, Y.-J.; Lee, J.-H.; Yi, G.-C. Vertically aligned ZnO nanostructures grown on graphene layers. Appl. Phys. Lett. 2009, 95, 213101. [Google Scholar] [CrossRef]
- Hong, Y.J.; Lee, W.H.; Wu, Y.; Ruoff, R.S.; Fukui, T. van der Waals epitaxy of InAs nanowires vertically aligned on single-layer graphene. Nano Lett. 2012, 12, 1431. [Google Scholar] [CrossRef] [PubMed]
- Munshi, A.M.; Dheeraj, D.L.; Fauske, V.T.; Kim, D.-C.; van Helvoort, A.T.J.; Fimland, B.; Weman, H. Vertically aligned GaAs nanowires on graphite and few-layer graphene: Generic model and epitaxial growth. Nano Lett. 2012, 12, 4570. [Google Scholar] [CrossRef]
- Kim, J.; Bayram, C.; Park, H.; Cheng, C.-W.; Dimitrakopoulos, C.; Ott, J.A.; Reuter, K.B.; Bedell, S.W.; Sadana, D.K. Principle of direct van der Waals epitaxy of single-crystalline films on epitaxial graphene. Nat. Commun. 2014, 5, 1. [Google Scholar] [CrossRef]
- Kumaresan, V.; Largeau, L.; Madouri, A.; Glas, F.; Zhang, H.; Oehler, F.; Cavanna, A.; Babichev, A.; Travers, L.; Gogneau, N. Epitaxy of GaN nanowires on graphene. Nano Lett. 2016, 16, 4895. [Google Scholar] [CrossRef]
- Kim, Y.; Cruz, S.S.; Lee, K.; Alawode, B.O.; Choi, C.; Song, Y.; Johnson, J.M.; Heidelberger, C.; Kong, W.; Choi, S. Remote epitaxy through graphene enables two-dimensional material-based layer transfer. Nature 2017, 544, 340. [Google Scholar] [CrossRef]
- Jeong, J.; Min, K.-A.; Shin, D.H.; Yang, W.S.; Yoo, J.; Lee, S.W.; Hong, S.; Hong, Y.J. Remote homoepitaxy of ZnO microrods across graphene layers. Nanoscale 2018, 10, 22970. [Google Scholar] [CrossRef]
- Lin, L.; Peng, H.; Liu, Z. Synthesis challenges for graphene industry. Nat. Mater. 2019, 18, 520. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Robinson, J.T.; Diankov, G.; Dai, H. Nanocrystal growth on graphene with various degrees of oxidation. J. Am. Chem. Soc. 2010, 132, 3270–3271. [Google Scholar] [CrossRef] [PubMed]
- Kajbafvala, A.; Shayegh, M.R.; Mazloumi, M.; Zanganeh, S.; Lak, A.; Mohajerani, M.S.; Sadrnezhaad, S.K. Nanostructure sword-like ZnO wires: Rapid synthesis and characterization through a microwave-assisted route. J. Alloy. Compd. 2009, 469, 293–297. [Google Scholar] [CrossRef]
- Zhou, J.; Xu, N.S.; Wang, Z.L. Dissolving behavior and stability of ZnO wires in biofluids: A study on biodegradability and biocompatibility of ZnO nanostructures. Adv. Mater. 2006, 18, 2432–2435. [Google Scholar] [CrossRef]
- Jeong, J.; Jin, D.K.; Cha, J.; Kang, B.K.; Wang, Q.; Choi, J.; Lee, S.W.; Mikhailovskii, Y.V.; Neplokh, V.; Amador-Mendez, N.; et al. Selective-area remote epitaxy of ZnO microrods using multilayer–monolayer-patterned graphene for transferable and flexible device fabrications. ACS Appl. Nano. Mater. 2020, 3, 8920–8930. [Google Scholar] [CrossRef]
- Gao, Y.; Roslyak, O.; Dervishi, E.; Karan, S.N.; Ghosh, Y.; Sheehan, C.J.; Wang, F.; Gupta, G.; Mohite, A.; Dattelbaum, A.M.; et al. Hybrid graphene–giant nanocrystal quantum dot assemblies with highly efficient biexciton emission. Adv. Opt. Mater. 2015, 3, 39–43. [Google Scholar] [CrossRef]
- Auchter, E.; Marquez, J.; Yarbro, S.L.; Dervishi, E. A facile alternative technique for large-area graphene transfer via sacrificial polymer. AIP Adv. 2017, 7, 125306. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Yoon, A.; Kim, M.; Yi, G.-C.; Liu, C. Hydrothermally grown ZnO nanostructures on few-layer graphene sheets. Nanotechnology 2011, 22, 245603. [Google Scholar] [CrossRef]
- Choi, W.M.; Shin, K.-S.; Lee, H.S.; Choi, D.; Kim, K.; Shin, H.-J.; Yoon, S.-M.; Choi, J.-Y.; Kim, S.-W. Selective growth of ZnO nanorods on SiO 2/Si substrates using a graphene buffer layer. Nano Res. 2011, 4, 440. [Google Scholar] [CrossRef]
- Oh, H.; Park, J.; Choi, W.; Kim, H.; Tchoe, Y.; Agrawal, A.; Yi, G.-C. Vertical ZnO nanotube transistor on a graphene film for flexible inorganic electronics. Small 2018, 14, 1800240. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Yoo, H.; Lee, C.-H.; Park, J.B.; Baek, H.; Kim, M.; Yi, G.-C. Position-and morphology-controlled ZnO nanostructures grown on graphene layers. Adv. Mater. 2012, 24, 5565. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-J.; Yoo, J.; Kwon, B.-H.; Hong, Y.J.; Lee, C.-H.; Yi, G.-C. Position-controlled ZnO nanoflower arrays grown on glass substrates for electron emitter application. Nanotechnology 2008, 19, 315202. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Tang, Q.; Zhou, Z. Structural and electronic properties of graphene–ZnO interfaces: Dispersion-corrected density functional theory investigations. Nanotechnology 2013, 24, 305401. [Google Scholar] [CrossRef]
- Hong, Y.J.; Yoo, J.; Doh, Y.-J.; Kang, S.H.; Kong, K.; Kim, M.; Lee, D.R.; Oh, K.H.; Yi, G.-C. Controlled epitaxial growth modes of ZnO nanostructures using different substrate crystal planes. J. Mater. Chem. 2009, 19, 941. [Google Scholar] [CrossRef][Green Version]
- Reynolds, D.C.; Look, D.C.; Jogai, B. Optically pumped ultraviolet lasing from ZnO. Solid State Commun. 1996, 99, 873. [Google Scholar] [CrossRef]
- Vanheusden, K.; Seager, C.H.; Warren, W.L.; Tallant, D.R.; Voigt, J.A. Mechanisms behind green photoluminescence in ZnO phosphor powders. Appl. Phys. Lett. 1996, 68, 403. [Google Scholar] [CrossRef]
- Manfrinato, V.R.; Stein, A.; Zhang, L.; Nam, C.-Y.; Yager, K.G.; Stach, E.A.; Black, C.T. Aberration-corrected electron beam lithography at the one nanometer length scale. Nano Lett. 2017, 17, 4562. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Resonant Raman spectroscopy of disordered, amorphous, and diamondlike carbon. Phys. Rev. B 2001, 64, 075414. [Google Scholar] [CrossRef]
- Dervishi, E.; Ji, Z.; Htoon, H.; Sykora, M.; Doorn, S.K. Raman spectroscopy of bottom-up synthesized graphene quantum dots: Size and structure dependence. Nanoscale 2019, 11, 16571. [Google Scholar] [CrossRef] [PubMed]
- Dresselhaus, M.S.; Jorio, A.; Hofmann, M.; Dresselhaus, G.; Saito, R. Perspectives on carbon nanotubes and graphene Raman spectroscopy. Nano Lett. 2010, 10, 751. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, A.; Meyer, J.C.; Scardaci, V.; Casiraghi, C.; Lazzeri, M.; Mauri, F.; Piscanec, S.; Jiang, D.; Novoselov, K.S.; Roth, S. Raman spectrum of graphene and graphene layers. Phys. Rev. Lett. 2006, 97, 187401. [Google Scholar] [CrossRef] [PubMed]
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially resolved Raman spectroscopy of single- and few-layer graphene. Nano Lett. 2007, 7, 238–242. [Google Scholar] [CrossRef]
- Auchter, E.; Marquez, J.; Stevens, G.; Silva, R.; Mcculloch, Q.; Guengerich, Q.; Blair, A.; Litchfield, S.; Li, N.; Sheehan, C.; et al. Ultra-thin and strong formvar-based membranes with controlled porosity for micro-and nano-scale systems. Nanotechnology 2018, 29, 215712. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.V.; Huang, N.M.; N Yusoff, N.; Lim, H.N. High performance magnetically separable graphene/zinc oxide nanocomposite. Mater. Lett. 2013, 93, 411. [Google Scholar] [CrossRef]
- Zhang, Y.; Ram, M.K.; Stefanakos, E.K.; Goswami, D.Y. Enhanced photocatalytic activity of iron doped zinc oxide nanowires for water decontamination. Surf. Coat. Technol. 2013, 217, 119. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Martins Ferreira, E.H.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 11, 3190. [Google Scholar] [CrossRef]
- Compaan, A.; Trodahl, H.J. Resonance Raman scattering in Si at elevated temperatures. Phys. Rev. B 1984, 29, 793. [Google Scholar] [CrossRef]
- Piscanec, S.; Cantoro, M.; Ferrari, A.C.; Zapien, J.A.; Lifshitz, Y.; Lee, S.T.; Hofmann, S.; Robertson, J. Raman spectroscopy of silicon nanowires. Phys. Rev. B 2003, 68, 241312. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.; Kim, D.; Auchter, E.; Marquez, J.; Tutchton, R.; Li, N.; Luk, T.S.; Dervishi, E.; Kim, Y.-J.; Zhu, J.-X.; et al. Recyclable Graphene Sheets as a Growth Template for Crystalline ZnO Nanowires. Nanomaterials 2021, 11, 2093. https://doi.org/10.3390/nano11082093
Kim Y, Kim D, Auchter E, Marquez J, Tutchton R, Li N, Luk TS, Dervishi E, Kim Y-J, Zhu J-X, et al. Recyclable Graphene Sheets as a Growth Template for Crystalline ZnO Nanowires. Nanomaterials. 2021; 11(8):2093. https://doi.org/10.3390/nano11082093
Chicago/Turabian StyleKim, Yeonhoo, Dongheun Kim, Eric Auchter, Justin Marquez, Roxanne Tutchton, Nan Li, Ting S. Luk, Enkeleda Dervishi, Yong-Jin Kim, Jian-Xin Zhu, and et al. 2021. "Recyclable Graphene Sheets as a Growth Template for Crystalline ZnO Nanowires" Nanomaterials 11, no. 8: 2093. https://doi.org/10.3390/nano11082093
APA StyleKim, Y., Kim, D., Auchter, E., Marquez, J., Tutchton, R., Li, N., Luk, T. S., Dervishi, E., Kim, Y.-J., Zhu, J.-X., & Yoo, J. (2021). Recyclable Graphene Sheets as a Growth Template for Crystalline ZnO Nanowires. Nanomaterials, 11(8), 2093. https://doi.org/10.3390/nano11082093








