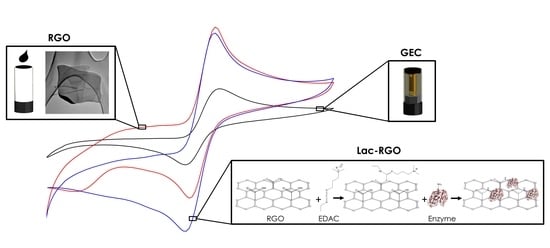
Graphene for the Building of Electroanalytical Enzyme-Based Biosensors. Application to the Inhibitory Detection of Emerging Pollutants
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical and Reagents
2.2. Electrochemical Measurements
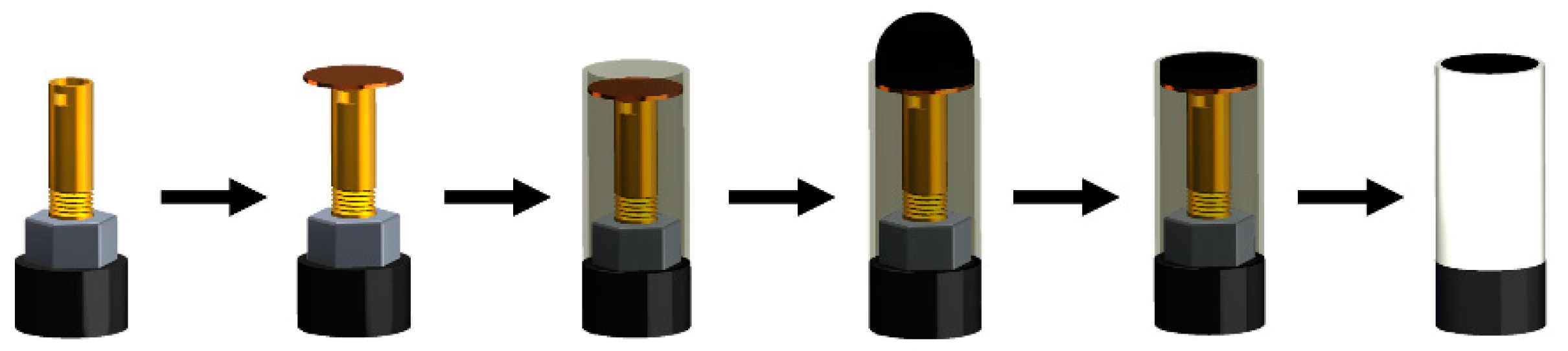
2.3. Sensor Characterization
2.4. Graphene Synthesis and Characterization
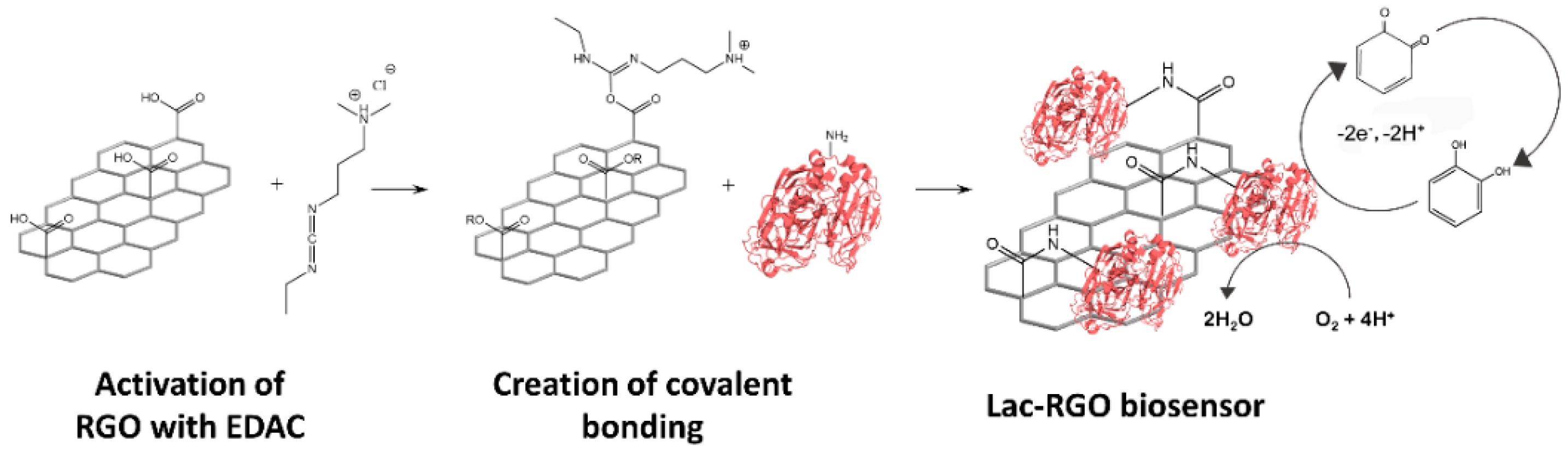
2.5. Graphene Oxide Modification and Immobilization of the Enzyme for the Biosensor Building
3. Results
3.1. Study of the Synthesized Graphene Oxide
3.2. Modification and Characterization of the Graphene Sensor
3.3. Characterization and Application of the Biosensor
3.3.1. Electrochemical Response of the Laccase Biosensor
3.3.2. Analytical Characterization of the Biosensor
3.3.3. Kinetic and Inhibition Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hulanicki, A.; Glab, S.; Ingman, F. Chemical sensors: Definitions and classification. Pure Appl. Chem. 1991, 63, 1247–1250. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Tung, T.T.; Nine, M.J.; Krebsz, M.; Pasinszki, T.; Coghlan, C.J.; Tran, D.N.H.; Losic, D. Recent advances in sensing applications of graphene assemblies and their composites. Adv. Funct. Mater. 2017, 27, 1702891. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M.; Tung, T.T.; Losic, D. Carbon nanomaterial based biosensors for non-invasive detection of cancer and disease biomarkers for clinical diagnosis. Sensors 2017, 17, 1919. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; García-Hernández, C.; de Saja, J.A.; Fernández-Escudero, J.A.; Barajas, E.; Medrano, G.; García-Cabezón, C.; Martin-Pedrosa, F.; Rodriguez-Mendez, M.L. The advantages of disposable screen-printed biosensors in a bioelectronic tongue for the analysis of grapes. LWT Food Sci. Technol. 2015, 62, 940–947. [Google Scholar] [CrossRef]
- Zhou, M.; Zhai, Y.; Dong, S. Electrochemical sensing and biosensing platform based on chemically reduced graphene oxide. Anal. Chem. 2009, 81, 5603–5613. [Google Scholar] [CrossRef] [PubMed]
- Suhito, I.R.; Koo, K.M.; Kim, T.H. Recent advances in electrochemical sensors for the detection of biomolecules and whole cells. Biomedicines 2021, 9, 15. [Google Scholar] [CrossRef]
- Ocaña, C.; Lukic, S.; del Valle, M. Aptamer-antibody sandwich assay for cytochrome c employing an MWCNT platform and electrochemical impedance. Microchim. Acta 2015, 182, 2045–2053. [Google Scholar] [CrossRef]
- Serrano, N.; Prieto-Simón, B.; Cetó, X.; Del Valle, M. Array of peptide-modified electrodes for the simultaneous determination of Pb(II), Cd(II) and Zn(II). Talanta 2014, 125, 159–166. [Google Scholar] [CrossRef]
- Bélanger, D.; Pinson, J. Electrografting: A powerful method for surface modification. Chem. Soc. Rev. 2011, 40, 3995–4048. [Google Scholar] [CrossRef]
- Sun, C.-L.; Su, C.-H.; Wu, J.-J. Synthesis of short graphene oxide nanoribbons for improved biomarker detection of Parkinson’s disease. Biosens. Bioelectron. 2015, 67, 327–333. [Google Scholar] [CrossRef]
- Martín, A.; Batalla, P.; Hernández-Ferrer, J.; Martínez, M.T.; Escarpa, A. Graphene oxide nanoribbon-based sensors for the simultaneous bio-electrochemical enantiomeric resolution and analysis of amino acid biomarkers. Biosens. Bioelectron. 2015, 68, 163–167. [Google Scholar] [CrossRef]
- Barua, S.; Geng, X.; Chen, B. Graphene-based nanomaterials for healthcare applications. In Photonanotechnology for Therapeutics and Imaging; Choi, S.-K., Ed.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2020; pp. 45–81. ISBN 978-0-12-817840-9. [Google Scholar]
- Pottathara, Y.B.; Grohens, Y.; Kokol, V.; Kalarikkal, N.; Thomas, S. Synthesis and processing of emerging two-dimensional nanomaterials. In Nanomaterials Synthesis; Pottathara, B.Y., Thomas, S., Kalarikkal, N., Grohens, Y., Kokol, V., Eds.; Micro and Nano Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–25. ISBN 978-0-12-815751-0. [Google Scholar]
- Smith, A.T.; La Chance, A.M.; Zeng, S.; Liu, B.; Sun, L. Synthesis, properties, and applications of graphene oxide/reduced graphene oxide and their nanocomposites. Nano Mater. Sci. 2019, 1, 31–47. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Aceta, Y.; del Valle, M. Graphene electrode platform for impedimetric aptasensing. Electrochim. Acta 2017, 229, 458–466. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Hu, S. Nanocomposites of graphene and graphene oxides: Synthesis, molecular functionalization and application in electrochemical sensors and biosensors. A review. Microchim. Acta 2017, 184, 1–44. [Google Scholar] [CrossRef]
- Toh, S.Y.; Loh, K.S.; Kartom Kamarudin, S.; Ramli, W.; Daud, W. Graphene production via electrochemical reduction of graphene oxide: Synthesis and characterisation. Chem. Eng. J. 2014, 251, 422–434. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Pumera, M. Graphene-based nanomaterials and their electrochemistry. Chem. Soc. Rev. 2010, 39, 4146–4157. [Google Scholar] [CrossRef]
- Jian, X.; Liu, X.; Yang, H.M.; Guo, M.M.; Song, X.L.; Dai, H.Y.; Liang, Z.H. Graphene quantum dots modified glassy carbon electrode via electrostatic self-assembly strategy and its application. Electrochim. Acta 2016, 190, 455–462. [Google Scholar] [CrossRef]
- Bonet-San-Emeterio, M.; Algarra, M.; Petković, M.; del Valle, M. Modification of electrodes with N-and S-doped carbon dots. Evaluation of the electrochemical response. Talanta 2020, 212, 120806. [Google Scholar] [CrossRef]
- Algarra, M.; González-Calabuig, A.; Radotić, K.; Mutavdzic, D.; Ania, C.O.; Lázaro-Martínez, J.M.; Jiménez-Jiménez, J.; Rodríguez-Castellón, E.; del Valle, M. Enhanced electrochemical response of carbon quantum dot modified electrodes. Talanta 2018, 178, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Shereema, R.M.; Rao, T.P.; Sameer Kumar, V.B.; Sruthi, T.V.; Vishnu, R.; Prabhu, G.R.D.; Sharath Shankar, S. Individual and simultaneous electrochemical determination of metanil yellow and curcumin on carbon quantum dots based glassy carbon electrode. Mater. Sci. Eng. C 2018, 93, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Merkoçi, A.; Pumera, M.; Llopis, X.; Pérez, B.; Del Valle, M.; Alegret, S. New materials for electrochemical sensing VI: Carbon nanotubes. TrAC Trends Anal. Chem. 2005, 24, 826–838. [Google Scholar] [CrossRef]
- Cipri, A.; Del Valle, M. Pd nanoparticles/multiwalled carbon nanotubes electrode system for voltammetric sensing of tyrosine. J. Nanosci. Nanotechnol. 2014, 14, 6692–6698. [Google Scholar] [CrossRef]
- Moore, R.R.; Banks, C.E.; Compton, R.G. Basal plane pyrolytic graphite modified electrodes: Comparison of carbon nanotubes and graphite powder as electrocatalysts. Anal. Chem. 2004, 76, 2677–2682. [Google Scholar] [CrossRef]
- Fusco, G.; Bollella, P.; Mazzei, F.; Favero, G.; Antiochia, R.; Tortolini, C. Catalase-based modified graphite electrode for hydrogen peroxide detection in different beverages. J. Anal. Methods Chem. 2016, 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Montañez, J.L.; Ramos, E.G.; Alegret, S.; Delgado, R.J. Biosensor de glucosa basado en un biocompósito disperso de grafito-epoxi-platino-glucosa oxidasa. Inf. Technol. 2011, 22, 29–40. [Google Scholar] [CrossRef][Green Version]
- Zacco, E.; Pividori, M.I.; Alegret, S.; Galve, R.; Marco, M.P. Electrochemical magnetoimmunosensing strategy for the detection of pesticides residues. Anal. Chem. 2006, 78, 1780–1788. [Google Scholar] [CrossRef]
- Tuomela, M.; Hatakka, A. Oxidative fungal enzymes for bioremediation. In Comprehensive Biotechnology; Moo-Young, M., Ed.; Pergamon: Oxford, UK, 2019; pp. 224–239. ISBN 978-0-444-64047-5. [Google Scholar]
- Rodríguez-Couto, S. Solid-state fermentation for laccases production and their applications. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Larroche, C., Soccol, C.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 211–234. ISBN 978-0-444-63990-5. [Google Scholar]
- Zandaryaa, S. Chemical contaminants: Those invisible additives in our drink. A World Sci. 2011, 9, 18–21. [Google Scholar]
- Frye, C.; Bo, E.; Calamandrei, G.; Calzà, L.; Dessì-Fulgheri, F.; Fernández, M.; Fusani, L.; Kah, O.; Kajta, M.; Le Page, Y.; et al. Endocrine disrupters: A review of some sources, effects, and mechanisms of actions on behaviour and neuroendocrine systems. J. Neuroendocrinol. 2012, 24, 144–159. [Google Scholar] [CrossRef]
- Monneret, C. What is an endocrine disruptor? C. R. Biol. 2017, 340, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Bourguignon, J.P.; Giudice, L.C.; Hauser, R.; Prins, G.S.; Soto, A.M.; Zoeller, R.T.; Gore, A.C. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr. Rev. 2009, 30, 293–342. [Google Scholar] [CrossRef] [PubMed]
- Céspedes, F.; Martínez-Fàbregas, E.; Alegret, S. New materials for electrochemical sensing I. Rigid conducting composites. TrAC Trends Anal. Chem. 1996, 15, 296–304. [Google Scholar] [CrossRef]
- Alegret, S.; Alonso, J.; Bartrolí, J.; Céspedes, F.; Martínez-Fàbregas, E.; del Valle, M. Amperometric biosensors based on bulk-modified epoxy graphite biocomposites. Sens. Mater. 1996, 8, 147–153. [Google Scholar]
- Kalinke, C.; de Oliveira, P.R.; Bonet-San-Emeterio, M.; González-Calabuig, A.; del Valle, M.; Salvio Mangrich, A.; Humberto Marcolino Junior, L.; Bergamini, M.F. Voltammetric electronic tongue based on carbon paste electrodes modified with biochar for phenolic compounds stripping detection. Electroanalysis 2019, 31, 2238–2245. [Google Scholar] [CrossRef]
- Eng, A.Y.S.; Ambrosi, A.; Chua, C.K.; Šaněk, F.; Sofer, Z.; Pumera, M. Unusual inherent electrochemistry of graphene oxides prepared using permanganate oxidants. Chem. A Eur. J. 2013, 19, 12673–12683. [Google Scholar] [CrossRef]
- Bonet-San-Emeterio, M.; González-Calabuig, A.; del Valle, M. Artificial neural networks for the resolution of dopamine and serotonin complex mixtures using a graphene-modified carbon electrode. Electroanalysis 2019, 31, 1–9. [Google Scholar] [CrossRef]
- Ortiz-Aguayo, D.; del Valle, M. Label-free aptasensor for lysozyme detection using electrochemical impedance spectroscopy. Sensors 2018, 18, 354. [Google Scholar] [CrossRef]
- Ocaña, C.; Hayat, A.; Mishra, R.; Vasilescu, A.; Del Valle, M.; Marty, J.-L. A novel electrochemical aptamer-antibody sandwich assay for lysozyme detection. Analyst 2015, 140, 4153. [Google Scholar] [CrossRef]
- Cattaruzza, F.; Cricenti, A.; Flamini, A.; Girasole, M.; Longo, G.; Prosperi, T.; Andreano, G.; Cellai, L.; Chirivino, E. Controlled loading of oligodeoxyribonucleotide monolayers onto unoxidized crystalline silicon; fluorescence-based determination of the surface coverage and of the hybridization efficiency; parallel imaging of the process by atomic force microscopy. Nucleic Acids Res. 2006, 34, 2–13. [Google Scholar] [CrossRef][Green Version]
- Tortolini, C.; Rea, S.; Carota, E.; Cannistraro, S.; Mazzei, F. Influence of the immobilization procedures on the electroanalytical performances of trametes versicolor laccase based bioelectrode. Microchem. J. 2011, 100, 8–13. [Google Scholar] [CrossRef]
- Khoo, W.Y.H.; Pumera, M.; Bonanni, A. Graphene platforms for the detection of caffeine in real samples. Anal. Chim. Acta 2013, 804, 92–97. [Google Scholar] [CrossRef]
- Hui, K.H.; Ambrosi, A.; Pumera, M.; Bonanni, A. Improving the analytical performance of graphene oxide towards the assessment of polyphenols. Chem. Eur. J. 2016, 22, 3830–3834. [Google Scholar] [CrossRef]
- Shang, N.G.; Papakonstantinou, P.; McMullan, M.; Chu, M.; Stamboulis, A.; Potenza, A.; Dhesi, S.S.; Marchetto, H. Catalyst-free efficient growth, orientation and biosensing properties of multilayer graphene nanoflake films with sharp edge planes. Adv. Funct. Mater. 2008, 18, 3506–3514. [Google Scholar] [CrossRef]
- Haditale, M.; Dariani, R.S.; Ghasemian Lemraski, E. Electrical behavior of graphene under temperature effect and survey of I–T curve. J. Theor. Appl. Phys. 2019, 13, 351–356. [Google Scholar] [CrossRef]
- Ambrosi, A.; Bonanni, A.; Sofer, Z.; Cross, J.S.; Pumera, M. Electrochemistry at chemically modified graphenes. Chem. A Eur. J. A Eur. J. 2011, 17, 10763–10770. [Google Scholar] [CrossRef] [PubMed]
- Anonymous. Organic Electrochemistry: Revised and Expanded, 5th ed.; Hammerich, O., Speiser, B., Eds.; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Palanisamy, S.; Ramaraj, S.K.; Chen, S.M.; Yang, T.C.K.; Pan, Y.F.; Chen, T.W.; Velusamy, V.; Selvam, S. A novel laccase biosensor based on laccase immobilized graphene-cellulose microfiber composite modified screen-printed carbon electrode for sensitive determination of catechol. Sci. Rep. 2017, 7, 41214. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Kashanian, S.; Rafipour, R. Laccase immobilization on the electrode surface to design a biosensor for the detection of phenolic compound such as catechol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Sánchez, C.; Tzanov, T.; Gübitz, G.M.; Cavaco-Paulo, A. Voltammetric monitoring of laccase-catalysed mediated reactions. Bioelectrochemistry 2002, 58, 149–156. [Google Scholar] [CrossRef]
- Harris, D.C. Quantitative Chemical Analysis, 7th ed.; Freeman and Co.: New York, NY, USA, 1948. [Google Scholar]
- McNaught, A.D.; Wilkinson, A. The IUPAC Compendium of Chemical Terminology, 2nd ed.; Blackwell Scientific Publications: Oxford, UK, 1997; ISBN 0-9678550-9-8. [Google Scholar]
- Borman, P.; Elder, D. Q2(R1) Validation of analytical procedures. In ICH Quality Guidelines; John Wiley & Sons: Hoboken, NJ, USA, 2017; pp. 127–166. ISBN 9781118971147. [Google Scholar]
- Tembe, S.; Inamdar, S.; Haram, S.; Karve, M.; D’Souza, S.F. Electrochemical biosensor for catechol using agarose–guar gum entrapped tyrosinase. J. Biotechnol. 2007, 128, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.H.; Liu, L.H.; Bai, X.; Shi, H.C. A reduced graphene oxide based biosensor for high-sensitive detection of phenols in water samples. Sens. Actuators B Chem. 2013, 181, 661–667. [Google Scholar] [CrossRef]
- Medina-Plaza, C.; de Saja, J.A.; Rodriguez-Mendez, M.L. Bioelectronic tongue based on lipidic nanostructured layers containing phenol oxidases and lutetium bisphthalocyanine for the analysis of grapes. Biosens. Bioelectron. 2014, 57, 276–283. [Google Scholar] [CrossRef]
- Nazari, M.; Kashanian, S.; Moradipour, P.; Maleki, N. A novel fabrication of sensor using ZnO-Al2O3 ceramic nanofibers to simultaneously detect catechol and hydroquinone. J. Electroanal. Chem. 2018, 812, 122–131. [Google Scholar] [CrossRef]
- Bin, Z.; Yanhong, C.; Jiaojiao, X. Biomimetic oxidase sensor based on functionalized surface of carbon nanotubes and iron prophyrins for catechol detection. Bioprocess Biosyst. Eng. 2018, 42, 279–290. [Google Scholar] [CrossRef]
- Guo, M.; Wang, H.; Huang, D.; Han, Z.; Li, Q.; Wang, X.; Chen, J. Amperometric catechol biosensor based on laccase immobilized on nitrogen-doped ordered mesoporous carbon (N-OMC)/PVA matrix. Sci. Technol. Adv. Mater. 2014, 15, 1–9. [Google Scholar] [CrossRef]
- Zhao, Q.-L.; Zhang, Z.-L.; Huang, B.-H.; Peng, J.; Zhang, M.; Pang, D.-W. Facile preparation of low cytotoxicity fluorescent carbon nanocrystals by electrooxidation of graphite. Chem. Commun. 2008, 5116. [Google Scholar] [CrossRef]
- Albayati, S.A.R.; Kashanian, S.; Nazari, M.; Rezaei, S. Novel fabrication of a laccase biosensor to detect phenolic compounds using a carboxylated multiwalled carbon nanotube on the electropolymerized support. Bull. Mater. Sci. 2019, 42, 1–8. [Google Scholar] [CrossRef]
- Hu, F.; Chen, S.; Wang, C.; Yuan, R.; Yuan, D.; Wang, C. Study on the application of reduced graphene oxide and multiwall carbon nanotubes hybrid materials for simultaneous determination of catechol, hydroquinone, p-cresol and nitrite. Anal. Chim. Acta 2012, 724, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Umasankar, Y.; Periasamy, A.P.; Chen, S.M. Electrocatalysis and simultaneous determination of catechol and quinol by poly(malachite green) coated multiwalled carbon nanotube film. Anal. Biochem. 2011, 411, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhao, J.; Liu, H.; Liu, R.; Wang, H.; Liu, J. Electrochemical determination of diphenols and their mixtures at the multiwall carbon nanotubes/poly (3-methylthiophene) modified glassy carbon electrode. Microchim. Acta 2010, 169, 277–282. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. The Michaelis-Menten model accounts for the kinetic properties of many enzymes. In Biochemistry; W H Freeman: New York, NY, USA, 2002. [Google Scholar]
- Roskoski, R. Michaelis-Menten Kinetics; Elsevier: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Hazan, R.; Levine, A.; Abeliovich, H. Benzoic acid, a weak organic acid food preservative, exerts specific effects on intracellular membrane trafficking pathways in saccharomyces cerevisiae. Appl. Environ. Microbiol. 2004, 70, 4449–4457. [Google Scholar] [CrossRef] [PubMed]
- Nair, B. Final report on the safety assessment of benzyl alcohol, benzoic acid, and sodium benzoate. Int. J. Toxicol. 2001, 20, 23–50. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Gilbert, P.A. EDTA—Ethylenediaminetetraacetic acid. In Detergents. Anthropogenic Compounds; de Oude, N.T., Ed.; Springer: Berlin/Heidelberg, Germany, 1992; Volume 3, pp. 243–259. [Google Scholar]
- Oviedo, C.; Rodríguez, J. EDTA: The chelating agent under environmental scrutiny. Quim. Nova 2003, 26, 901–905. [Google Scholar] [CrossRef]
- World Health Organization & International Programme on Chemical Safety. Benzoic Acid and Sodium Benzoate; Concise International Chemical Assessment Document, 26; World Health Organization: Geneva, Switzerland, 2000. [Google Scholar]
- World Health Organization & International Programme on Chemical Safety. Health criteria and other supporting information. In Guidelines for Drinking-Water Quality; World Health Organization: Geneva, Switzerland, 1996; ISBN 9241544805. [Google Scholar]
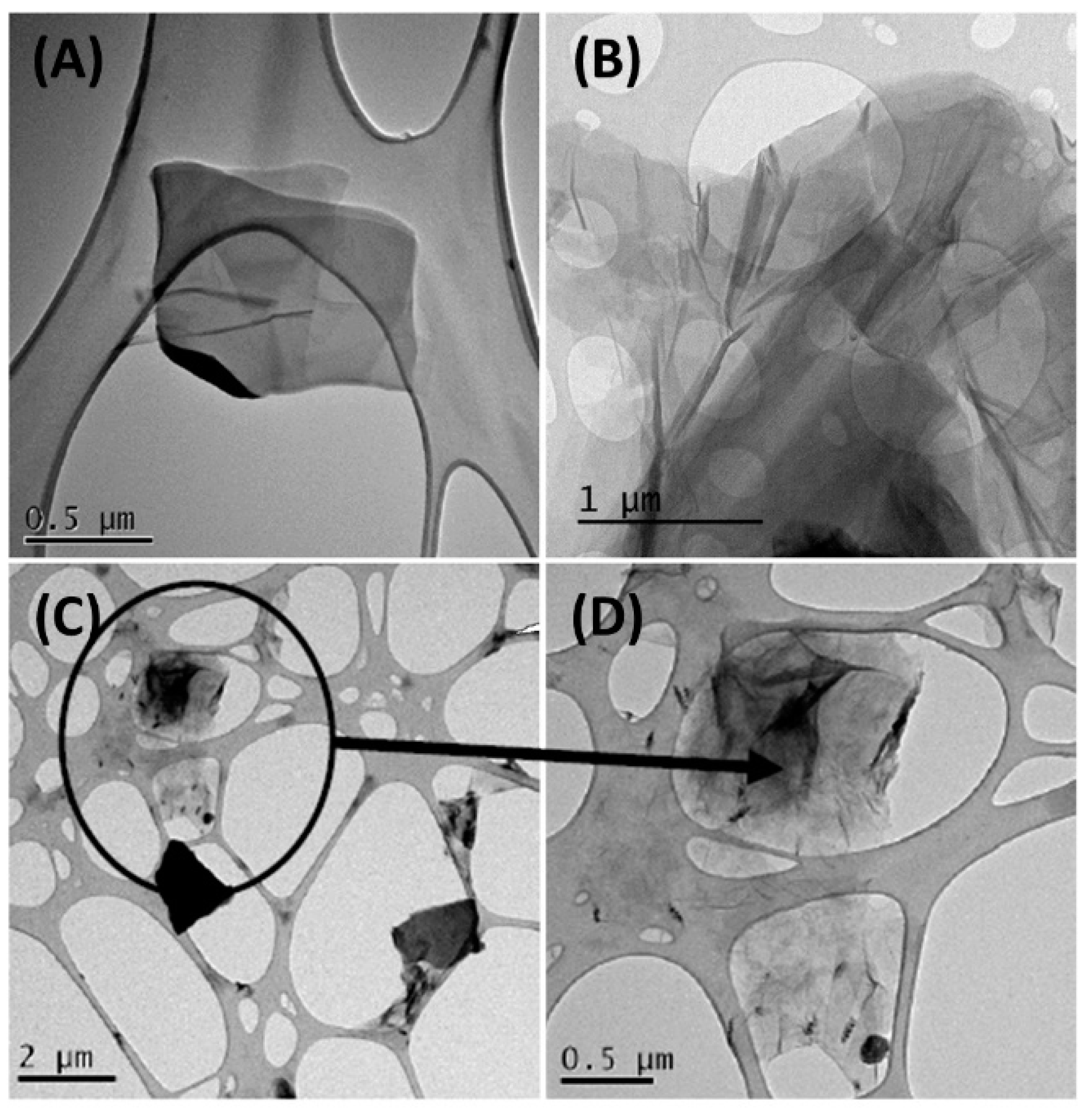
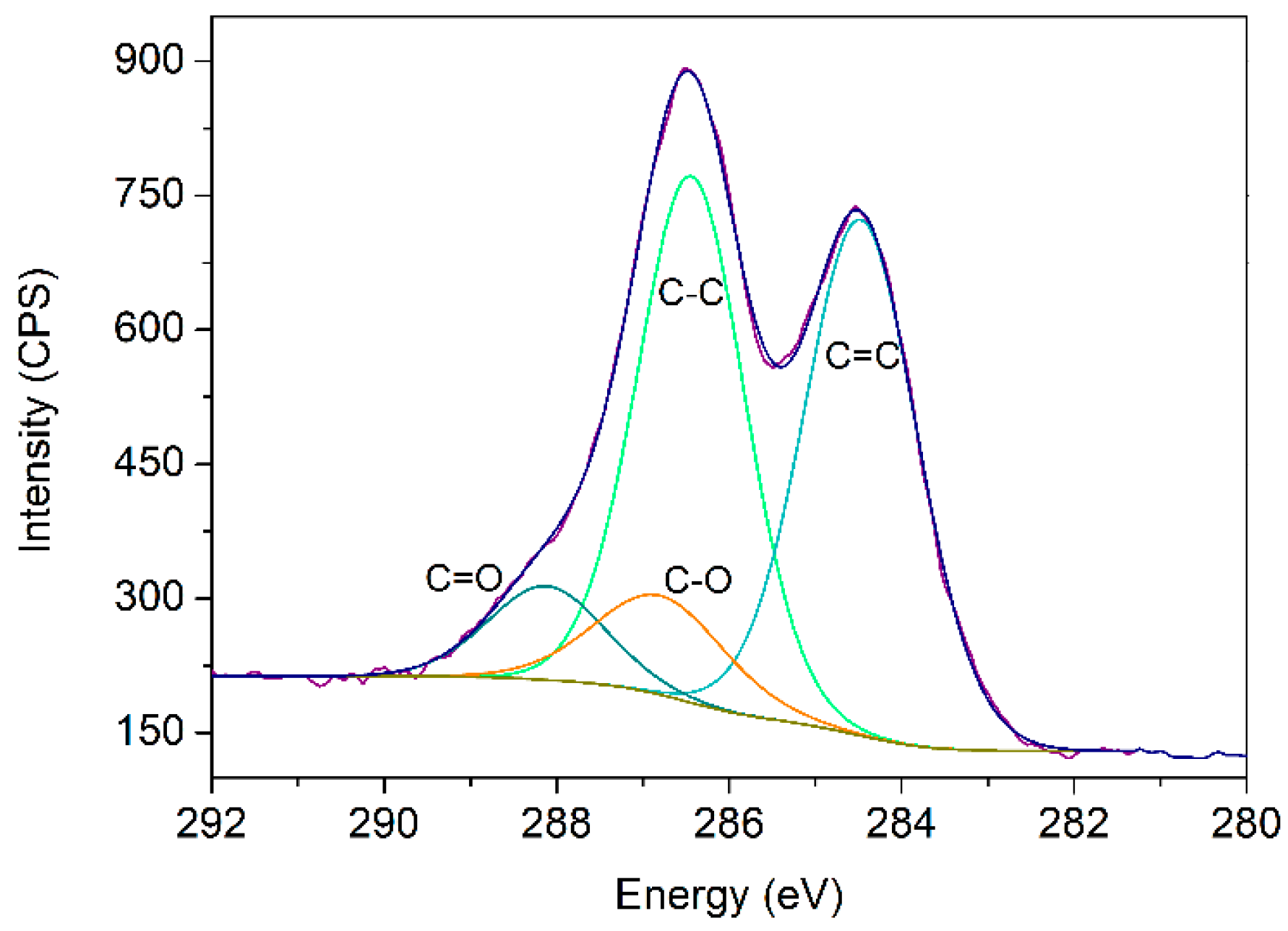
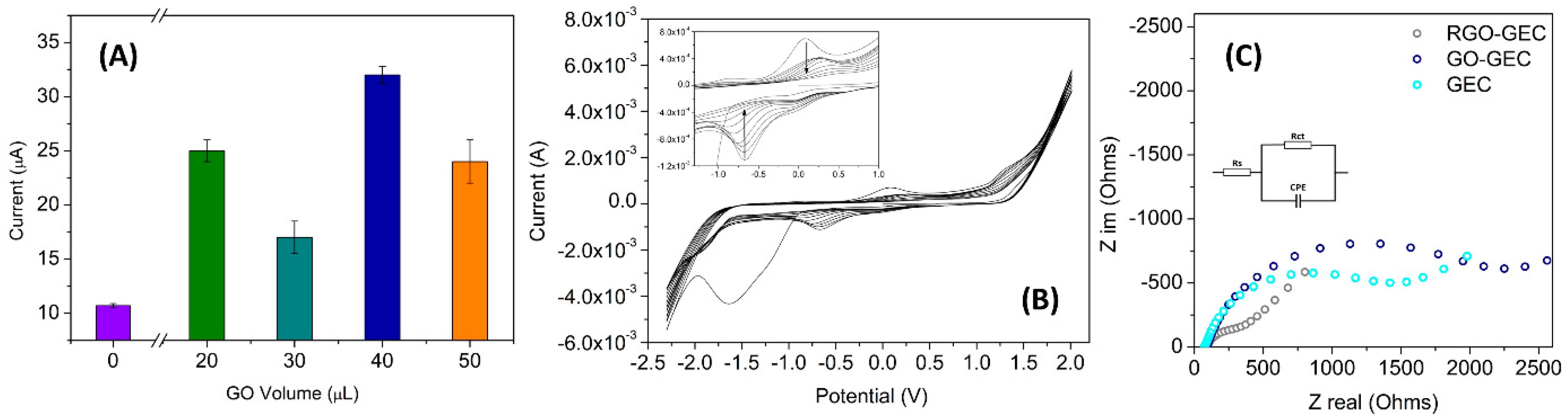
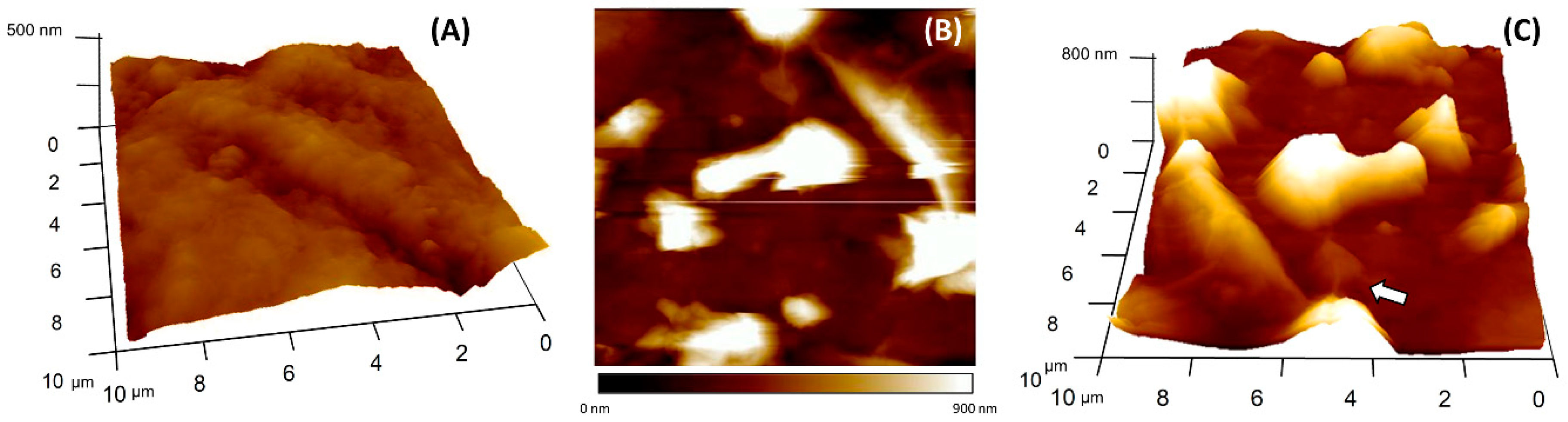
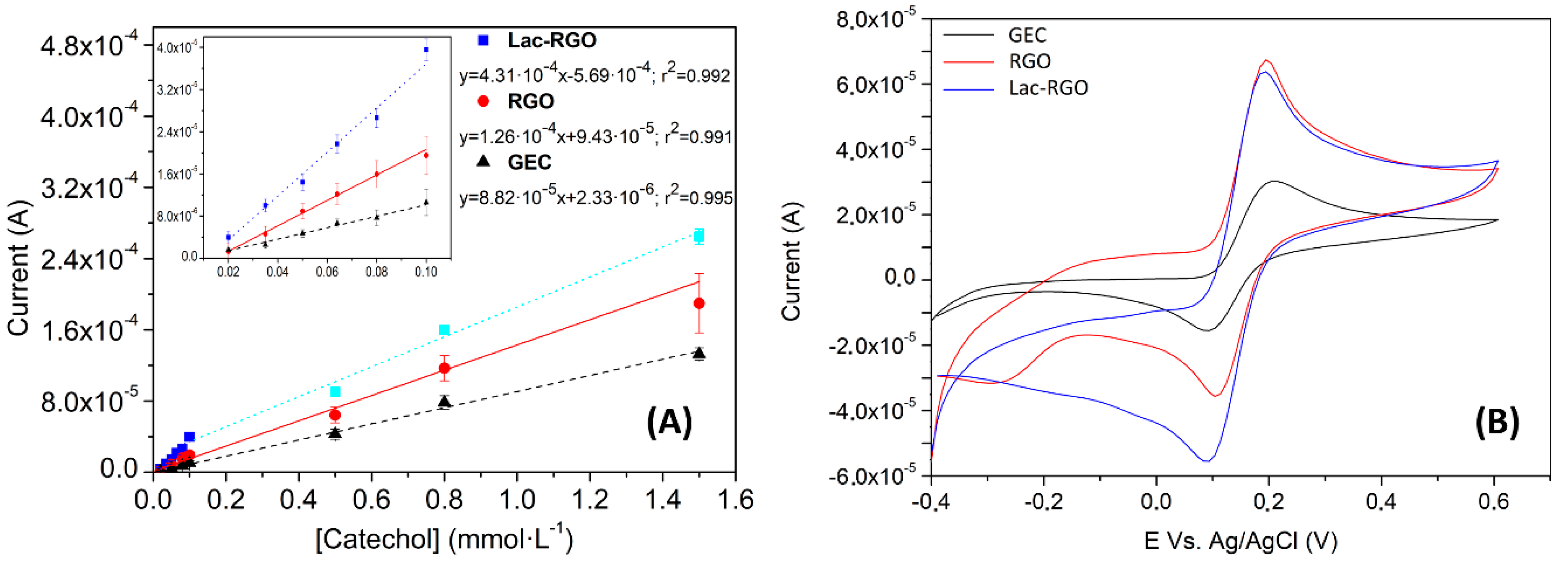



| Bond | Energy (eV) | % Area (RSD) * |
|---|---|---|
| C=C | 284.5 | 42.81 (1.30%) |
| C-C | 286.4 | 40.39 (2.1%) |
| C-O | 286.9 | 10.24 (8.3%) |
| C=O | 288.2 | 6.56 (9.5%) |
| Electrode | Rs (Ω) | Rct (Ω) | CPE (F) |
|---|---|---|---|
| GEC | 240.7 ± 4 | 1638 ± 62 | 7.9 × 10−5 ± 0.7 × 10−5 |
| RGO | 252.0 ± 3 | 206 ± 142 | 4.2 × 10−5 ± 0.5 × 10−5 |
| Laccase-RGO | 252.0 ± 2 | 231 ± 138 | 4.3 × 10−5 ± 0.4 × 10−5 |
| Method | Electrode | LOD (µM) | Sensitivity (µA·µM−1) | Linear Range (µM) | Ref |
|---|---|---|---|---|---|
| CV | Lac/rGO/GEC | 2.1 | 0.431 (15.39 µA µM−1 cm−2) | 2–100 | - |
| CV | Tyrosinase-polysaccharide | 6 | 0.001 | 60–800 | [60] |
| CV | Lac/PANI/GCE 1 | 2.07 | 0.23 | 3.2–19.6 | [55] |
| CV | Lac/AP-rGOs/GCE 2 | 7 | 15.79 | 15–700 | [61] |
| CV | Lac/AA/LuPc2 3 | 0.58 | 0.36 | 4–150 | [62] |
| CV | AuNPs/ZnO/Al2O3/GO/chitosan/GCE | 3.1 | - | 0.5–40 | [63] |
| CV | FePP/MWCNT/Nafion/GCE 4 | 3.75 | 0.1867 | 65–1600 | [64] |
| Amp. 11 | N-OMC/PVA/Lac/AuE 5 | 0.31 | 0.29 | 0.39–8.98 | [65] |
| Amp. | Lac/CNTs–CS/GCE 6 | 0.66 | - | 1.2–30 | [66] |
| Amp | Lac/PAP/MWCNTs/SPE 7 | 0.20 | 5.8 × 104 | 0.63–20.7 | [47] |
| Amp | Lac/Nafion/MWCNTs/SPE | 0.45 | 1.6 × 104 | 1.4–65.4 | [47] |
| Amp | Lac/MWCNTs/SPE | 0.73 | 1.5 × 104 | 2.4–134.4 | [47] |
| DPV | Lac/MWCNT-COOH/AuNPs/SDBS/PEDOT/GCE 8 | 12.26 | 0.012 | 12.0–94.1 | [67] |
| DPV | RGO-MWCNT/GCE | 1.8 | 0.07 | 5.5–540 | [68] |
| DPV | MWCNT-PMG/GCE 9 | 5.8 | 1.3 µA µM−1 cm−2 | 30–1190 | [69] |
| DPV | MWCNT/PMT/GCE 10 | 0.05 | 1.895 | 0.5–150 | [70] |
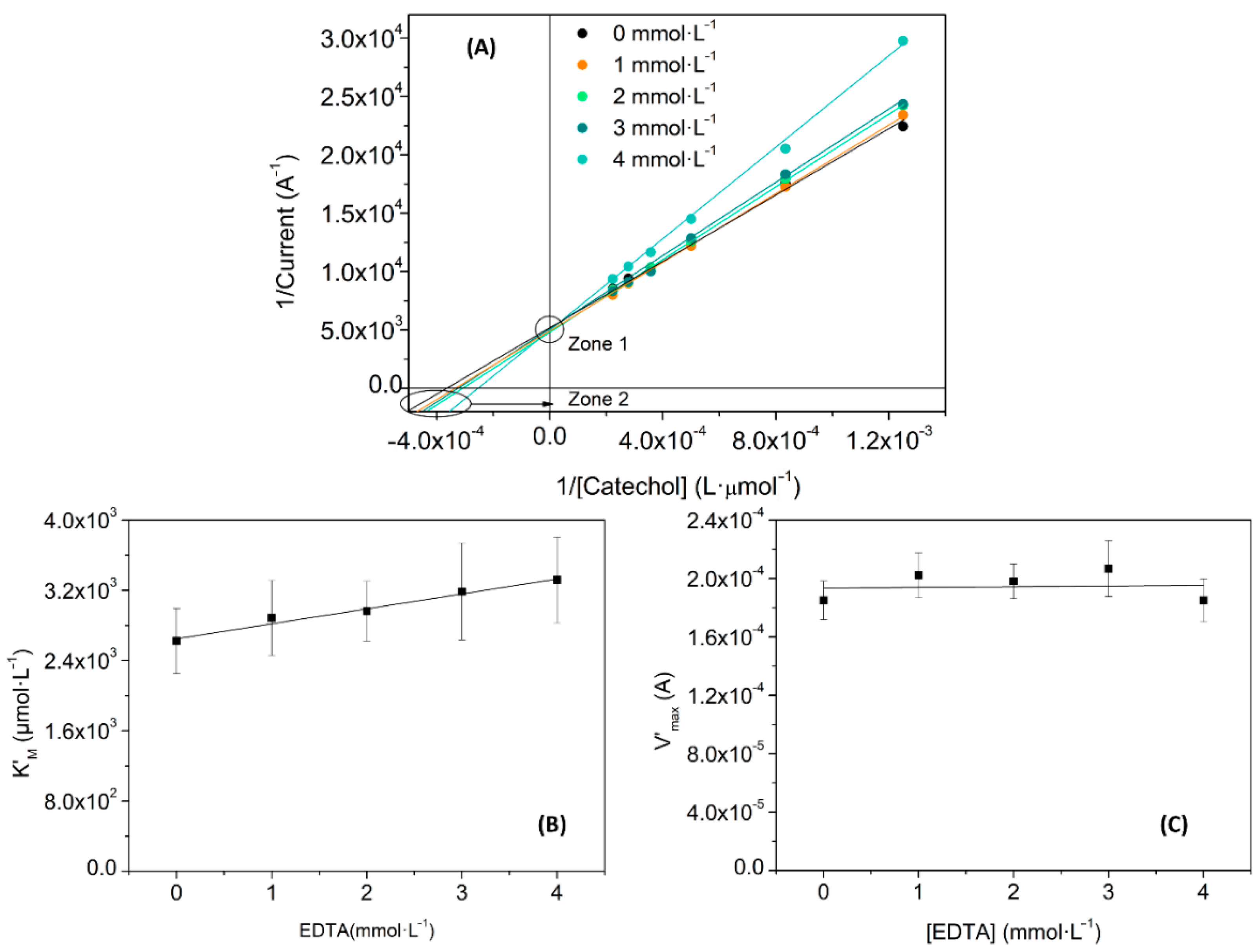
| Substance | Slope of K’M (µmol·L−1) vs. [I] | Slope of V’max (A·L·mmol−1) vs. [I] | KI (mmol·L−1) |
|---|---|---|---|
| Benzoic acid | 0.11 ± 0.04 | 3·10−6 ± 6·10−6 | 25 ± 19 |
| EDTA | 0.2 ± 0.1 | 0 ± 1·10−5 | 17 ± 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonet-San-Emeterio, M.; Felipe Montiel, N.; del Valle, M. Graphene for the Building of Electroanalytical Enzyme-Based Biosensors. Application to the Inhibitory Detection of Emerging Pollutants. Nanomaterials 2021, 11, 2094. https://doi.org/10.3390/nano11082094
Bonet-San-Emeterio M, Felipe Montiel N, del Valle M. Graphene for the Building of Electroanalytical Enzyme-Based Biosensors. Application to the Inhibitory Detection of Emerging Pollutants. Nanomaterials. 2021; 11(8):2094. https://doi.org/10.3390/nano11082094
Chicago/Turabian StyleBonet-San-Emeterio, Marta, Noelia Felipe Montiel, and Manel del Valle. 2021. "Graphene for the Building of Electroanalytical Enzyme-Based Biosensors. Application to the Inhibitory Detection of Emerging Pollutants" Nanomaterials 11, no. 8: 2094. https://doi.org/10.3390/nano11082094
APA StyleBonet-San-Emeterio, M., Felipe Montiel, N., & del Valle, M. (2021). Graphene for the Building of Electroanalytical Enzyme-Based Biosensors. Application to the Inhibitory Detection of Emerging Pollutants. Nanomaterials, 11(8), 2094. https://doi.org/10.3390/nano11082094