Abstract
Corrosion-induced iron rust causes severe danger, pollution, and economic problems. In this work, nanopowders of Fe2O3 and Fe2O3/zeolite are synthesized for the first time using rusted iron waste and natural zeolite heulandite by chemical precipitation. The chemical composition, nanomorphologies, structural parameters, and optical behaviors are investigated using different techniques. The Fe2O3/zeolite nanocomposite showed smaller sizes and greater light absorption capability in visible light than Fe2O3 nanopowder. The XRD pattern shows crystalline hematite (α-Fe2O3) with a rhombohedral structure. The crystallite sizes for the plane (104) of the Fe2O3 and Fe2O3/zeolite are 64.84 and 56.53 nm, respectively. The Fe2O3 and Fe2O3/zeolite have indirect bandgap values of 1.87 and 1.91 eV and direct bandgap values of 2.04 and 2.07 eV, respectively. Fe2O3 and Fe2O3/zeolite nanophotocatalysts are used for solar photoelectrochemical (PEC) hydrogen production. The Fe2O3/zeolite exhibits a PEC catalytic hydrogen production rate of 154.45 mmol/g.h @ 1 V in 0.9 M KOH solution, which is the highest value yet for Fe2O3-based photocatalysts. The photocurrent density of Fe2O3/zeolite is almost two times that of Fe2O3 catalyst, and the IPCE (incident photon-to-current conversion efficiency) reached ~27.34%@307 nm and 1 V. The electrochemical surface area (ECSA) values for Fe2O3 and Fe2O3/zeolite photocatalysts were 7.414 and 21.236 m2/g, respectively. The rate of hydrogen production for Fe2O3/zeolite was 154.44 mmol h−1/g. This nanophotocatalyst has a very low PEC corrosion rate of 7.6 pm/year; it can retain ~97% of its initial performance. Therefore, the present research can be applied industrially as a cost-effective technique to address two issues at once by producing solar hydrogen fuel and recycling the rusted iron wires.
1. Introduction
Fossil fuel burning is the major source of COx emissions (CO2 and CO) in atmospheric air, which causes global warming. The resulting air pollution can have catastrophic effects on humans and animals alike [1,2]. Hydrogen fuel is a carbon-free, renewable, and environmentally friendly source of energy that can be used as an ideal alternative to fossil fuels.
Therefore, the developments of effective techniques for large hydrogen fuel production at reasonable cost are important research areas. The photoelectrochemical (PEC) hydrogen production utilizing semiconductor-based catalysts is a promising technique to meet these requirements. In the PEC process, the photocatalyst produces an electron/hole pair after absorbing a photon, which is then isolated, transported, and contributed to the cathodic hydrogen evolution/anodic oxygen evolution reactions at applied voltage [3,4]. Under incident light with a suitable wavelength, electron/hole pairs are created in the semiconductor. The holes reacted with H2O to generate hydroxyl radical (OH). The electrons can react with O2 to produce superoxide radicals (). These reactive species are primarily responsible for the water splitting and hydrogen production [5]. There are several semiconductor materials such as WO3, ZrO2, In2O3, SnO2, Fe2O3, TiO2, ZnO, CuO, and CdS that were applied to upgrade the PEC performance. Among them, Fe2O3 is used as a photocatalyst for the PEC due to its hard solubility, high chemical stability, low cost, and massive abundance [6,7]. Additionally, it is a non-toxic and ecologically benign substance, all of which are required for large-scale solar energy conversion at a reasonable cost. Fe2O3 has semiconducting properties with a narrow bandgap (∼2.1 eV). This low bandgap enables it to be a good photocatalyst in the visible region. However, this material has many drawbacks that limit its application in practical photocatalytic such as low diffusion lengths of holes, poor conductivity, fast electron–hole recombination, poor adsorption property, agglomeration, and difficulty in being recovered [8]. Several studies immobilized the Fe2O3 nanoparticles on different supports, such as activated carbon, silica, alumina, clay, and zeolite to overcome these disadvantages. Among them, zeolite is of particular interest because, besides its semiconducting nature, it has a high adsorption capacity against organic contaminants. Zeolite possesses ionic exchange properties that are idyllic for the adsorption/degradation of organic dyes [9,10]. It also has enormous unique areas, adjustable hydrophobicity/hydrophilicity, and photochemical stability [11,12]. In addition, zeolite is low-cost, abundant, and bio-compatible. Zeolite is a monocrystal mineral composed of Si and Al atoms in a tetrahedral arrangement (TO4; T = Si, Al) [13]. It can be used in many applications such as cement, porcelain, electronics, and water splitting for the production of hydrogen. When a semiconductor is supported on a suitable support, such as zeolites, the semiconductor particles are evenly dispersed, preventing them from aggregating.
In the past few years, zeolite was used as a support for semiconductor-based PEC catalysts to enhance the hydrogen production rate. The ZnCo/CdS/zeolite heterostructure was prepared and optimized by Jia-Hui et al. to achieve photocatalytic hydrogen activity 59 times greater than that of pristine CdS, which is ascribed to zeolite’s role in improving the separation and transportation capacity of photo-generated charge carriers [14]. Yue and Khan reported the formation of vacant sites on the zeolite surface due to the exchange of ions in titano-zeolites, which assists the hydrogen photoproduction [15]. Additionally, Pt/zeolite and Cu/zeolite were prepared and applied for the hydrogen [16,17]. Owing to its large use in many applications, iron has been considered one of the primary manufacturing materials over the past decades. Iron corrosion happens after the iron contacts the air moisture. The corrosion of iron structures causes millions of tons of rusty waste to form, resulting in danger, environmental pollution, and economic issues. Therefore, considering the worldwide vast use of iron wires, the recycling/reuse of rusty waste is predicted to substantially decrease the wastes amounts, leading to the creation of recycling-oriented societies.
Hence, the Fe2O3 nanoparticles production from rusted iron wastes can thus be considered in many fields as a viable alternative to synthetic and natural iron supplies. Previously, different techniques have been used to prepare Fe2O3 nanostructures such as sol-gel, spray pyrolysis, hydrothermal, chemical vapor deposition, and thermal evaporation [18,19]. Most of these methods require complicated reactions, high energy intakes, and poor product yield. Since no special additives or equipment are needed, chemical precipitation is considered the most effective and low-cost technique for the production of Fe2O3.
The objective of this work is to replace the iron precursors with rust wastes as a source of iron for the synthesis of Fe2O3 and Fe2O3/zeolite nanopowder by chemical precipitation. The prepared Fe2O3/zeolite nanocomposite is applied for the PEC production of solar hydrogen fuel. The photon-to-electron and photon-to-hydrogen conversion efficiencies are calculated for Fe2O3 and Fe2O3/zeolite.
2. Materials and Experimental Procedures
2.1. Materials
Natural zeolite was delivered from a zeolite mine located southwest of Taiz (Al-Ahyuq region, Taiz City, Yemen). HCl and KOH were received from El-Nasr Company (Cairo, Egypt). All chemicals were at least 99 percent pure, and they were utilized just as they were bought, with no further purification. Rusted iron wires were collected from construction sites.
2.2. Preparation of the Zeolite, Fe2O3 and Fe2O3/Zeolite
Rusted iron wire fragments were collected from construction sites in Egypt’s Beni-Suef City. The average length of wires is about 30 cm with a diameter of about 1 cm. The color of the wires is dark red. Upon cutting to small fragments, the rusted wires were washed using deionized (DI) water. A total of 10 g of these pieces was dissolved in 80 mL of HCl (37%) and 170 mL DI water under magnetic stirring at 85 °C. The solution was filtered, and 20 mL of H2O2 (30%) was added to the obtained pale green-colored solution. Under intense 60 min-stirring, the ammonia solution was dropped to the iron solution. In a glass beaker with a volume of 200 mL, the iron was precipitated. Varying volumes of ammonium hydroxide solution (10, 15, and 20 mL) were used to prepare Fe2O3 powders with different crystallite sizes. The samples were labeled as Fe2O3 (I), Fe2O3 (II), and Fe2O3 (III), where I, II, and III refer to the 10, 15, and 20 mL of ammonia supplied to the reaction, respectively. Then, the resulting precipitated iron powder was filtrated before washing and drying. Then, the collected powder was heated for 3 h at 500 °C. A total of 15 g of raw zeolite mine was washed with DI water and dried in the air. Then, it was triggered mechanically by ball milling. Table 1 shows the conditions for ball milling parameters.

Table 1.
The ball milling conditions for preparing zeolite.
For preparing Fe2O3/zeolite nanocomposite with optimized composition, different weight ratios of activated zeolite and iron powder (Fe2O3 (III)) were added to 100 mL of DI water under ultrasonication for 3 h. The total weight of Fe2O3/zeolite nanocomposite is kept at 2 g. The weight ratios were 0.2/1.8, 0.6/1.4, 0.8/1.2, 1.0/1.0, 1.2/0.8, 1.4/0.6, and 1.8/0.2. The resulting mixtures were dried at 80 °C for 12 h. Finally, the Fe2O3/zeolite nanocomposites were calcinated at 550 °C for 240 min. The nanopowders were recorded as xFe2O3/yzeolite, where x and y were denoted to the adding weight of Fe2O3 and zeolite, respectively. The synthesis steps of Fe2O3/zeolite nanocomposite are illustrated by a schematic in Figure 1.

Figure 1.
Schematic of the synthesis steps of Fe2O3/zeolite.
2.3. Characterizations
A Philips X’Pert Pro MRD diffractometer (XRD, λ = 0.154 nm, Philips X’Pert Pro MRD, Royston, UK) was utilized to obtain the X-ray diffraction (XRD) patterns of the samples with an operating voltage of 40 kV in the range from 5° to 80°. The samples nanomorphologies were examined using a JEOL JSM-5400LV scanning electron microscope (SEM, JEOL, Tokyo, Japan). The chemical compositions were investigated by Energy Dispersive X-ray spectrometry (EDX, JEOL JED-2300 SEM, Tokyo, Japan). FT-IR (Fourier transform-infrared) spectra of Fe2O3 and Fe2O3/zeolite nanocomposite were examined through Vertex 70 FTIR-FT Raman spectrometer (Billerica, MA, USA). The UV/Vis optical properties of the samples were scanned in the range 250–900 nm with an increment of 1 nm by UV-Vis double beam spectrophotometer (LAMBDA 950, PerkinElmer Inc., Waltham, MA, USA). About 0.05 g nanopowder is dispersed in 10 mL of dimethylformamide by ultrasonic for 3 h. Then, 3 mL of the prepared suspension is used for UV-Vis spectroscopy scanning in a standard quartz cuvette.
2.4. PEC Water Splitting Measurements
The PEC behaviors in 0.9 M KOH (100 mL, pH 13.5) were measured at room temperature (20 °C) utilizing a Keithly measuring-source unit (Tektronix Company, model: 2400, Beaverton, OR, USA) with LabTracer software and a 400 W metal-halide lamp (New-port, 66926-500HX-R07, Newport, UK) with a set of linear optical filters (307–636 nm). The sweeping scan rate was 1 mV/s. Fe2O3 and Fe2O3/zeolite doses of 1 g were used. The PEC current density–voltage (J–V) curves were quantified in darkness, monochromatic, and white light exposure conditions. In addition, the Fe2O3/zeolite stability was investigated using current density–time (J–t) measurements. All PEC measurements were carried out in a quartz cell of volume 150 mL.
3. Results and Discussion
3.1. Photocatalysts Characterization
3.1.1. Structural of Fe2O3 and Fe2O3/Zeolite
The crystallinity and phase of the Fe2O3, zeolite, and Fe2O3/zeolite nanocomposite were identified using XRD analysis as seen in Figure 2A. Zeolite’s distinctive XRD peaks, in Figure 2A, are noted at 2θ ~9.68°, 11.00°, 17.16°, 18.87°, 22.24°, 26.01°, 27.97, 29.84°, 31.83°, 35.88°, 47.58°, 61.76°, and 67.31°. Such peaks correspond to the crystallographic plane (020), (200), (111), (−131), (−222), (−422), (−351), (−530), (−202), (005), (311), and (223), based on PDF card No. 00-053-1176, respectively. Based on the XRD card, the type of zeolite is heulandite.
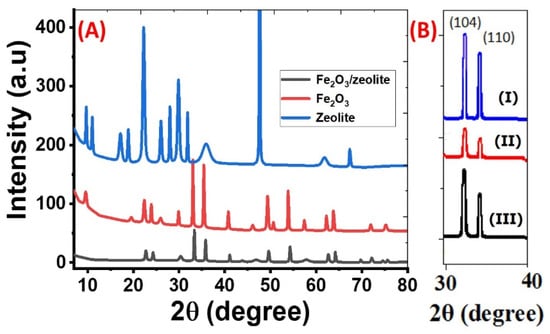
Figure 2.
(A) XRD patterns of zeolite, Fe2O3, and Fe2O3/zeolite nanocomposite; and (B) XRD (104) and (110) peaks of Fe2O3 (I), Fe2O3 (II), and Fe2O3 (III).
For iron oxide, the XRD pattern in Figure 2A suggests that crystalline hematite (α-Fe2O3) with rhombohedral structure (space group: R-3c) was formed according to the standard card No. 01-089-0597. This agrees with the previously reported data for Fe2O3 [20]. The pattern of Fe2O3 nanoparticles displays the core α-Fe2O3 feature peaks. These peaks are found at 33.00°, 35.39°, 49.32°, 53.84°, and 63.74° and correspond to the planes (104), (110), (024), (116), and (300). The sharp and intensive peaks indicate the high purity and crystallinity of the synthesized hematite nanoparticles using bulk Fe-based rust. These XRD data are similar to previously synthesized iron oxide in many works using synthetic precursors [21,22,23]. From the estimated FWHM of the strongest (104) and (110), the crystallite sizes of the Fe2O3 nanoparticles were estimated based on the Debye–Scherrer relation to be ~64.84 and 50.46 nm, respectively.
For zeolite, many distinct peaks are observed at 22.72° (101), 41.05° (210), and 54.24° (221), corresponding to tetragonal zeolite (Al0.05Si0.95O2) according to card No. 04-002-8520. As illustrated in Figure 2A, the main core features of XRD patterns of Fe2O3 and Fe2O3/zeolite are very close, indicating that the introduction of zeolite did not affect the structural properties of the Fe2O3 photocatalyst. However, the coupling of Fe2O3 with zeolite leads to an increase in the FWHM and a slight shift in the plane position of the Fe2O3 toward higher angles after coupling. Hence, the crystallites sizes of (104) and (110) peaks for Fe2O3 nanoparticles were decreased to 56.53 and 47.85 nm for Fe2O3/zeolite nanocomposite. Similar behavior was reported for hydrothermally prepared 4A-zeolite supported alpha-Fe2O3 [24]. In addition, the relative intensities of the diffraction peaks of Fe2O3/zeolite nanocomposite became weaker than the peaks of Fe2O3, indicating a change in the crystallinity of the photocatalyst due to the distribution of Fe2O3 on the surface of the zeolite [25]. The structural parameters such as crystallite size (D), interplanar distance (d), dislocation density (δ), and microstrain (ε) are calculated for the highest two peaks, (104) and (110), utilizing the XRD patterns of Fe2O3 and Fe2O3/zeolite nanopowders. Besides peak position, peak height, and relative intensity, the obtained values are displayed in Table 2. For the two planes (104) and (110), the value of microstrain increases while d-spacing decreases after loading the zeolite with Fe2O3. The strongest peak corresponds to the plane (104), which indicates the preferred growth orientation of hematite. This growth orientation is beneficial to carrier transport [26]. The number of lattice defects was estimated depending on the dislocation density, δ, which refers to the dislocation lines length per unit volume of the crystal. The δ value is estimated using the relation; δ = 1/D2. The values of δ for the Fe2O3 and Fe2O3/zeolite at the preferred orientation (104) are 2.378 × 10−4 and 3.129 × 10−4 dislocation/nm2, respectively. The increase in dislocation density proposes the decrease of Fe2O3/zeolite crystallinity [27], which strongly influences the photocatalytic properties of the fabricated nanomaterials. This is also confirmed by the decreasing of the XRD peaks intensities after loading Fe2O3 on zeolite, as seen in Table 2. The existence of a high density of the defects in the Fe2O3/zeolite nanocrystallites can contribute positively to the photocatalytic properties as a result of the active surface area increase and the formation of a high density of the active centers [28]. These active centers may result from the formation of static charge fields about the dislocation lines [29].

Table 2.
Values of the crystallographic parameters of Fe2O3 and Fe2O3/zeolite nanohybrid.
Figure 2B shows the XRD (104) and (110) peaks of Fe2O3 (I), Fe2O3 (II), and Fe2O3 (III) that were prepared using different amounts of ammonium hydroxide solution (10, 15, and 20 mL). From Figure 2B, the average crystallite size (D) for the highest two planes (104) and (110) were calculated by the Debye–Scherer equation at different amounts of ammonia solution. The average values of D for Fe2O3 (I), Fe2O3 (II), and Fe2O3 (III) are found to be 57.65, 44.12, and 36.42 nm respectively. Then, the average crystallite size of Fe2O3 depends on the volume of used ammonium hydroxide. According to the effective mass model, when particle size is reduced at the nanoscale, quantum confinement has an influence on electrons in nanoparticles. Changing the quantum (crystallite) size can alter the optical characteristics. As a result, the crystallite size is critical to the generation of hydrogen.
3.1.2. Surface Morphology
It is well-known that the photocatalytic activity of the photocatalyst is strongly related to its surface morphology. The morphologies of natural zeolite, Fe2O3, and Fe2O3/zeolite nanopowders are examined utilizing the SEM technique as shown in Figure 3.
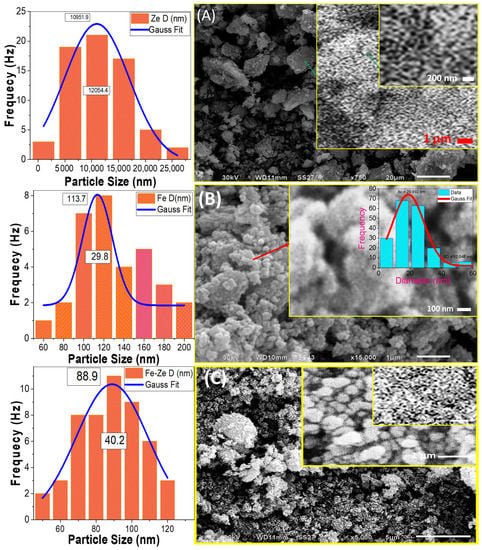
Figure 3.
SEM micrographs and the corresponding particle size distribution for (A) natural zeolite, (B) Fe2O3, and (C) Fe2O3/zeolite. The inset of (B) shows the pore diameter distribution.
The SEM images of natural zeolite, Figure 3A, show micro/nano-stones in nonuniform shapes of various sizes. The sizes of stones for zeolite are changed from 21.6 to 3.2 µm, as seen in the corresponding particle size distribution (left of Figure 4A) of the particle size distribution. The mean particle size is 10.951 ± 0.820 μm with a standard deviation of 6.027 ± 1.647 μm. A close look at the image reveals the existence of many small nanoprotrusions/nanograins over zeolite particle surfaces with an average size of ~115 nm. Additionally, there are many small nanopores with a diameter of ~71 nm on the surface of zeolite with irregular shapes as seen in high magnification Figure 3A. The high surface area due to the porous framework provides a chance to incorporate iron oxide nanoclusters inside the pore cavity of zeolite [30]. Additionally, these pores can adsorb organic pollutants, which can increase photodegradation efficiency.
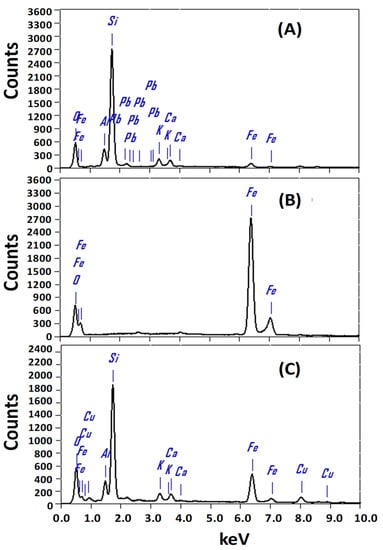
Figure 4.
EDX spectrum of (A) zeolite, (B) Fe2O3, and (C) Fe2O3/zeolite nanocomposite.
The Fe2O3 nanopowder was composed of many nanoparticles with semi-spherical shapes. The SEM image of Fe2O3 nanoparticles shows that the nanoparticles are small in size, seen in Figure 3B. The corresponding particle size distribution is shown on the left of Figure 3B. Based on Gaussian fitting; the mean size of Fe2O3 nanoparticle is 113.65 ± 4.67 nm with a standard deviation of 14.92 ± 5.95 nm. These nanoparticles are self-assembled and aggregated to form nanopores of average diameter ~20.99 nm with a standard deviation of ±6.02 nm, as shown from the inset pore-diameter distribution of Figure 3B.
Fine spherical Fe2O3 nanoparticles coated the zeolite surface and appeared as homogeneous distributions that produced a nano-sized Fe2O3 coating surface over zeolite stones after loading zeolite with the intended Fe2O3 photo-catalyst, Figure 3C. It is also possible that the Fe2O3 coating was quite homogeneous, with no obvious uncoated zeolite sites. The size of the Fe2O3 nanoparticles seems to be decreased after loading on zeolite compared to the free-standing Fe2O3 nanopowder. The size distribution of the supported Fe2O3 nanoparticles on the surface of zeolite, left of Figure 3C, indicates an average value of 88.94 ± 1.67 nm. Additionally, the high magnification SEM image, inset of Figure 3C, shows a more homogeneous pore-diameter distribution with a mean value of 35.50 ± 2.25 nm.
The interlock between Fe2O3 nanoparticles and their precipitation over the zeolite is expected to be beneficial for PEC activity. Haileyesus et al. reported that similar interlock structures can offer a rapid migration of the induced electrons and holes to the catalyst surface, which leads to a low probability of recombination [31]. Additionally, the decrease of the particle size to the nanoscale and the widening of the pores can offer a huge effective surface area of Fe2O3 nanocatalyst. This can offer intensive absorption of the incident light.
3.1.3. Chemical Compositions of the Photocatalysts
To identify the chemical compositions of the designed photocatalysts and atomic ratios of the elements, the EDX spectra of zeolite, Fe2O3, and Fe2O3/zeolite nanocomposite were measured and presented in Figure 4. The chemical composition for the zeolite shows the main three elements (O, Al, and Si) as revealed by EDX analysis. Additionally, small signals for K, Ca, and Fe are observed, in addition to a small trace from Cu. These signals are similar to previously reported signals for the zeolite [32].
The EDX analysis of Fe2O3, Figure 4B, indicated the presence of O (37.62%) and Fe (62.38%) signals as the main components at around 0.525 and 6.398 keV. The atomic ratios of Fe to O suit the stoichiometry ratios of Fe2O3 well. This confirms the high purity of the prepared Fe2O3 nanopowder, which coincides with the XRD results. After loading Fe2O3 onto zeolite, there are main four characteristic peaks for O, Al, Si, and Fe with atomic ratios of 53.12%, 6.30%, 26.63%, and 9.01%, respectively. This indicates the successive loading of Fe2O3 onto the surface of the zeolite.
3.1.4. The Photocatalysts’ Optical Properties
Nanomaterials’ optical properties are important characteristics that influence their uses [33,34]. The absorption (A) and transmittance (T) spectra from 250 to 850 nm of zeolite, Fe2O3, and Fe2O3/zeolite are shown in Figure 5. The zeolite sample has a sharp peak corresponding to a strong absorption band at the UV region (below λ = 300 nm), as seen in Figure 5A. Then, the absorbance decreases sharply with increasing the wavelength from 280 up to 850 nm. Therefore, the zeolite sample displayed a very low spectral response in the visible region.
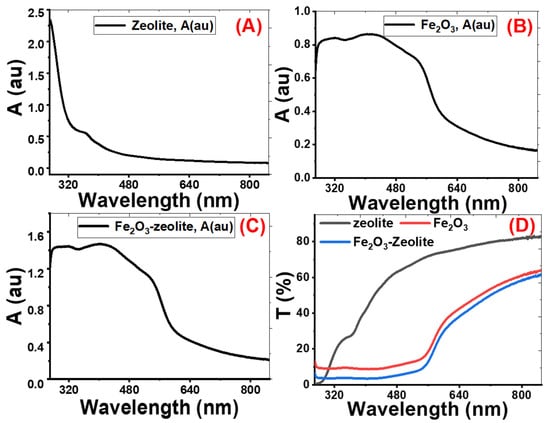
Figure 5.
Absorbance (A%) for zeolite (A), Fe2O3 (B), and Fe2O3/zeolite (C), and transmittance (T%) for all samples (D).
The absorbance spectra for Fe2O3 and Fe2O3/zeolite show similar optical behaviors, as seen in Figure 5B,C. The Fe2O3 has strong photoabsorption in the UV and visible spectral regions [35]. Fe2O3 shows an absorption band edges up to 580 nm. The wide absorption band of Fe2O3 in the visible region is due to the direct transition (O2−2p→Fe3+3d) and the spin-forbidden-excitations (Fe3+3d→3d), which rises the indirect transitions [36,37,38].
For the Fe2O3/zeolite, Figure 5C, the right edge of the photons uptake band shifts to a longer λ compared with that of Fe2O3, Figure 5B. This is correlated with the size of the nanoparticles of the Fe2O3 formed in the zeolite matrix. Hence, a broad and intense visible absorption range was observed for the Fe2O3/zeolite in Figure 5B. This would be better to achieve a massive electron–hole pair generation through electron transportation between the valence and conduction bands.
The absorbance values at λ = 500 nm are 0.185, 0.765 and 1.219 for zeolite, Fe2O3, and Fe2O3/zeolite, respectively, as seen in Figure 5D. This means more photons in the visible region, the concentrated portion of the solar light, can be absorbed by Fe2O3/zeolite than Fe2O3. This high absorbance refers to the dispersion of the Fe2O3 aggregates within the zeolite mesoporous structure and the modification of the electronic structure of Fe2O3/zeolite. Hence, zeolite has effectively enhanced the visible light absorption capability of the loaded Fe2O3 nanostructures. From Figure 5D, the general behavior of the transmittance spectrum of zeolite is the increase of transmittance% with the wavelength from UV to the visible region. The low transmittance for zeolite in the UV region is due to the existence of a strong absorption band in this region. The transmittance spectra for Fe2O3 and Fe2O3/zeolite (Figure 5D) can be divided into two regions. At wavelengths from 250 to 550 nm, the transmittance is nearly constant below 12%. Above 550 nm, the transmittance of Fe2O3 and Fe2O3/zeolite varies linearly with wavelength. The transmission of Fe2O3/zeolite is higher than that of Fe2O3 in the whole range of wavelengths.
The diffuse reflectance spectra (DRS) of the photocatalysts were measured to estimate the bandgap energies of the Fe2O3 and Fe2O3/zeolite. For this purpose, the Kubelka–Munk (K–M) model was used. Based on the following equation, this approach allows the absorption coefficient to be calculated by measuring diffuse light reflectance from a powdered mixture comprising absorbing and scattering components [39].
where F(R), R, S, and α indicate the K–M function, diffuse reflectance of the sample, the scattering coefficient, and the absorption coefficient, respectively. The K–M function is directly proportional to the absorption coefficient. Therefore, the direct and/or indirect band gaps of Fe2O3 and Fe2O3/zeolite were estimated by the following equation
where , Eg, and refer to the photon energy, bandgap energy, and independent constant. For indirect bandgaps, n = 1/2, while for direct bandgaps, n = 2 [40]. The absorption bandgaps energies (direct or indirect) can be calculated from the straight-line portions of ()n versus curve that intersects the energy axis, as shown in Figure 6.
F(R) = (1 − R)2/2R = α/S
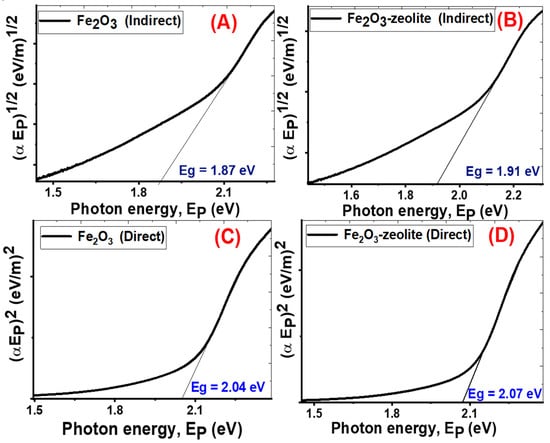
Figure 6.
Indirect energy gap (A,B) for Fe2O3, and Fe2O3/zeolite and direct energy gap (C,D) for Fe2O3, and Fe2O3/zeolite, respectively.
The Fe2O3 and Fe2O3/zeolite have indirect bandgap values of 1.87 and 1.91 eV and direct bandgap values of 2.04 and 2.07 eV, respectively (Figure 6), which demonstrates the formation and incorporation of Fe2O3 nanoparticles in the zeolite. These values are consistent with the reported values for Fe2O3 prepared by different techniques in the [22,41]. Based on the quantization effect, the bandgap is proportional inversely to the crystallite size due to the confinement of the movement of electrons. Therefore, the increase in the bandgap of Fe2O3/zeolite compared to Fe2O3 can be understood based on the decrease in the crystallite size as seen in XRD data. This behavior is similar to that reported for many nanomaterials such as ZnO and ITO [42,43]. The studied optical properties suggest that the produced Fe2O3 from the rusted iron and its loading on zeolite as a host can greatly improve its semiconducting performance toward the massive absorption of the visible light. This suggests that the prepared Fe2O3/zeolite can be used for solar energy applications.
3.1.5. FT-IR Study
FT-IR data of Fe2O3, zeolite and Fe2O3/zeolite nanocomposite are shown in Figure S1 (Supplementary Materials). The FT-IR spectrum of Fe2O3 nanoparticles was observed in the 4000–400 cm−1 wavenumber range, Figure S1. The bands of Fe2O3 appear at 1641 and 3415 cm−1, owing to the bending vibrations of the absorbed H2O and surface hydroxyl, and O–H stretch modes [20]. The appeared absorption modes at 2920 and 2850 cm−1 are assigned to the symmetric and asymmetric −CH2−groups stretch modes. A strong Fe–O asymmetric stretching mode was detected around 1040 cm−1 [44]. The located bands at 461, 537, and 790 cm−1 were attributed to the Fe–O stretch mode of Fe2O3 as confirmed in the literature [45]. A strong Fe–O asymmetric stretching mode was detected around 1040 cm−1 [44]. The located bands at 461, 537, and 790 cm−1 were attributed to the Fe−O stretch mode of Fe2O3 [45]. For zeolite, the bands at 3620 and 3446 cm−1 were attributable to Si–OH groups with H-bonding. The absorption mode at 1640 cm−1 was attributed to the OH bending mode [46]. The strong 1040, 790, and 600 cm−1 modes were significant to the internal asymmetric stretch and external symmetric stretch of X–O–X (X = Al or Si), and the internal X–O bending mode of AlO4/SiO4 tetrahedral [46]. The modes at 600 and 470 cm−1 authorize the existence of double five-membered rings of the pentasil zeolite [46]. For Fe2O3/zeolite, there are mixed bands between Fe2O3 and zeolite. The presence of broadband at 3429 cm−1 can be certified the O–H stretch mode, while the mode at 1650 cm−1 can be referred to as the O–H bending [47]. Bands of the zeolite appear at 1000 cm−1 in the nanocomposite, and the shift of these bands relative to that of zeolite refers to the break of H-bonds as a result of the existence of Fe on zeolite SiO4/AlO4 surfaces. Strong bands at 720, 598, 530, and 460 cm−1 were attributed to the symmetric vibration of (Al or Si)–O due to the internal vibration of zeolite.
3.2. Photoelectrocatalytic (PEC) H2 Generation
3.2.1. PEC Characteristics and Conversion Efficiencies
PEC technology for converting solar energy to hydrogen via the water-splitting cycle was aided by the catalysts Fe2O3 and Fe2O3/zeolite. When Fe2O3 is subjected to light, the electron (e−) can be excited from the valence band, leaving a hole (h+) to the conduction band. The rate of hydrogen production depends on the lifetime of the carrier charge. The limitations of bare α-Fe2O3 faces in use as a PEC photoanode arise from the electronic structure of the material. The α-Fe2O3 suffers from a high density of mid bandgap trap states arising from closely spaced d levels that result in closely spaced optical transitions spanning the visible and into the near-ultraviolet regions. This leads to low carriers’ mobility and short lifetimes. In the Fe2O3/zeolite nanocomposite, the electrons can be trapped on the surface of the mesoporous zeolite. The zeolitic network can inhibit recombination of e/h pairs due to strong electric field strength through the distribution of photogenerated electrons inside zeolite [48]. Hence, the effective e−/h+ separation occur over robust interfacial interactions in Fe2O3/zeolite. This causes a decrease in e−/h+ recombination rates, which results in an efficient photoelectrocatalytic performance of Fe2O3-zeolite. Additionally, the Fe2O3/zeolite has a large effective surface area due to the porous framework of zeolite, which can increase PEC efficiency and allow for more intense absorption of incident light.
The optimized content of Fe2O3 and zeolite is highly desirable to reach high PEC performance. The photocurrent density is measured for Fe2O3 (III), Fe2O3 (II), and Fe2O3 (I) at an applied voltage of 1 V in 0.9 M KOH under light illumination, as seen in Figure S2 (Supplementary Materials). The photocurrent density is found to be 57.5, 48.82, and 42.64 mA/cm2 Fe2O3 (III), Fe2O3 (II), and Fe2O3 (I), respectively. Therefore, Fe2O3 (III) photoelectrode produces the highest photocurrent, which considers the optimized PEC photoelectrode. Additionally, nanocomposites of varied Fe2O3 (III)/zeolite weight ratios (0.2/1.8, 0.6/1.4, 0.8/1.2, 1.0/1.0, 1.2/0.8, 1.4/0.6, and 1.8/0.2) are utilized to manufacture Fe2O3/zeolite photoelectrodes for hydrogen production in order to optimize the nanocomposite composition. The photocurrent densities for all electrodes are measured under light illumination and at 1 V, as seen in Figure S3 (Supplementary Materials). The highest photocurrent density is found to be 57.93 mA/cm2 for Fe2O3/zeolite with a weight ratio of 1:1.
Figure 7 shows the PEC performance of the optimized electrode. The variation of the current density (J) in darkness and white lighting from a metal-halide lamp versus the applied voltage (E) is presented in Figure 7A at 25 °C with a sweep rate of 0.1 mV/s. Using the Fe2O3 and Fe2O3/zeolite photo-electrocatalysts and in white lighting, the value of J is greatly enhanced vs. the positive applied voltage. By switching from the dark status to white light illumination status, the current density of Fe2O3 is increased from 1.14 to 29.1 mA/cm2 at +1 V, which refers to the PEC effect of Fe2O3. As shown in Figure 7A, J is increased by loading Fe2O3 on zeolite from 29.1 to 57.6 mA/cm2 at +1 V. This is due to the extending of the bandgap to the Vis/NIR range, which speeds up the redox reactions and then facilitates the PEC reaction. This also suggests a ~2-fold enhancement of the J-value relative to the Fe2O3 photocatalyst, which agrees with the increase of the surface charge, the extension of Eg, and the strong absorptions in the Vis/NIR because of the loading of Fe2O3. In addition, it is very well-associated with the size variation of the Fe2O3 nanoparticles. Reduction in the size of Fe2O3 nanoparticles after loading on zeolite compared to Fe2O3 nanopowder, Figure 3, leads to greater surface areas and enhanced active surface spots that improve hydrogen generation activity. Additionally, Fe2O3/zeolite’s quantum confinement raises the reduction potentials to transfer the bound protons to H2 molecules. The quantum containment of Fe2O3/zeolite allows for further effective absorption in the Vis/NIR region (Figure 5). Note that Fe2O3 and Fe2O3/zeolite photoelectrocatalysts exhibit light-harvesting with J-values of 0.58 and 1.01 mA/cm2 at 0 V, and photocurrent onset at −0.098 and −0.056 V, correspondingly. It shows that, after loading Fe2O3 on the zeolite matrix, the interfacial transport resistances decrease, emphasizing the importance of the loading process in improving PEC efficiency. As a result of ions’ exchange ability, vacant sites in the zeolite surface also photoassisted hydrogen production [49]. Simultaneously, zeolite’s aluminosilicate frame is contributing to delayed charge carriers’ separations [50]. Since the control processes of electron/hole transfer are very important in photocatalytic reactions, zeolite can play an active role in electron transfer processes as an electron acceptor or electron donor. [51,52]. The Z-scheme mechanism for the nanocomposite can maintain photogenerated charge carriers with strong redox ability. The spatial isolation of charge carriers is providing a large driving force for the photocatalytic water reduction reaction [53]. To assess the photoelectrocatalysts’ performances as a tiny outer voltage is introduced between the electrodes of the PEC cell, the electrical energies introduced to the cell have to be deducted. This may be accomplished using the applied bias photon to current conversion efficiency (ABPE). The following Equation (3) is used to compute ABPE [54]:
where Eapp is the externally applied bias and p refers to the illuminating light power density (75 mW/cm2). Figure 7B demonstrates how ABPE varies with applied voltage at various wavelengths. The two highest ABPE% values are de-convoluted under white light illumination; (3.37% at 0.464 V and at 8.78% at 0.997 V) for Fe2O3 and (12.05% at 0.430 V and 20.01% at 0.882 V) for Fe2O3/zeolite. This indicates a ~3-fold improvement along with a decrease of the applied voltage, which can be beneficial for PEC cell operation. Additionally, Fe2O3/zeolite photocatalyst displays ABPE% of 1.64%at 0 V. This demonstrates that interfacial transport resistances have been reduced and photocatalytic performance has improved [54].
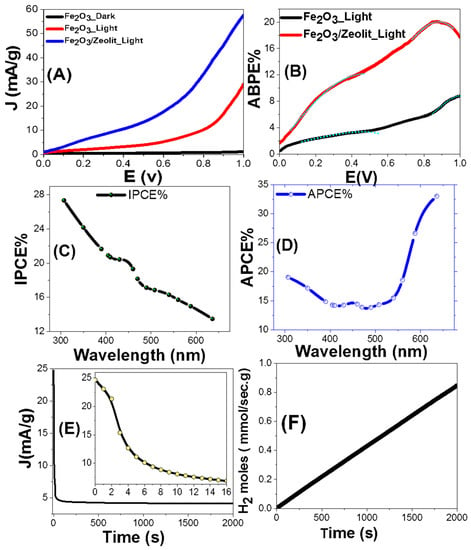
Figure 7.
(A) The current density (J) vs. the applied potential for Fe2O3 and Fe2O3/zeolite under darkness and white light exposure, (B) APBE%, (C) IPCE%(λ), and (D) APCE%(λ)@1V vs. the incident wavelengths; variation of (E) J and (F) the number of H2 moles versus the exposure time.
The enhanced solar absorption of the Fe2O3/zeolite photocatalyst is verified by estimating the photon-to-current incident efficiency (IPCE) at various wavelengths (λ) of the incident photons and constant potential (+1 V). The IPCE is calculated using the following Equation (4) [40]:
where λ is in nm. The variation of IPCE% with the wavelength of the monochromatic light for Fe2O3/zeolite photocatalyst is represented in Figure 7C. The highest IPCE% is ~27.34% @307 nm, in addition to another peak of 20.37% centered at ~440 nm corresponding to the highest absorption seen in Figure 5.
In the IPCE calculations, optical losses including transmittance (T) or reflectance (R) of incident photons were neglected. To compensate the optical losses, the absorbed photon to current conversion efficiency (APCE) is measured. APCE represents the number of photogenerated carriers that participate per absorbed photon in the generated photocurrent. The APCE is computed using the following Equation (5) [55]:
Here, A represents the optical absorbance. Figure 7D displays the behavior of APCE% as a function of the wavelength. As noted, APCE% is 19.1%@307 nm; then, it decreases to reach 13.8%@490 nm, followed by a successive increase to reach a maximum value of 33.0%@636 nm.
The stability of the Fe2O3/zeolite photocatalyst, for H2 generation, is studied for a prolonged time in 0.9 M KOH under white light and an applied voltage of +1 V Figure 7E shows the evolution of the J throughout time. The J-value dropped dramatically within the first 16 s, reaching roughly 6.9 mA/g. Then, limited photocorrosion processes occur between the PEC catalyst and the redox electrolyte, which account for the dramatic fall in the J-value [3]. For time > 16 s, before achieving a steady value of roughly 4.63 mA/g for 60 s, there is a slight reduction in J-value. This demonstrates that, in spite of the early decline in J-value, the Fe2O3/zeolite photocatalyst has high photochemical stability and a long lifespan as an active photocatalyst for the PEC H2 generation.
The full amount of hydrogen energy generated to the overall input sunlight energy (AM 1.5 G, 100 mW/cm2) is the solar-to-hydrogen conversion efficiency (STH). It can be used to calculate the total efficiency of the PEC cell [56]:
where Ptotal, ECSA, and H2/S refer to the total light power density in mW cm−2, the electrochemical surface area in cm2, and the rate of hydrogen generation/s, respectively. Applying Faraday’s law, the number of generated H2 moles by the PEC cell can be calculated using Equation (7).
Here, F refers to the Faraday constant (9.65 104 C/mol), and t is the period of generation. Figure 7F shows the variation of H2 (moles) versus the production time. The creation rate of H2 is 154.44 mmol h−1 g−1. Zeolite plays an effective role in the rapid spread of hydrogen bubbles which escape from the photocatalyst. This paves the way for higher current and additional H2 creation over the same period [57].
The ECSA of Fe2O3 and Fe2O3/zeolite photocatalysts were obtained utilizing the Randles–Sevcik equation,
ECSA= I(RT)0.5 (C n F)−1.5(v D)−0.5/0.4463
Here, n, C, and T stand for the number of electrons in a redox reaction (n = 1), analyte concentration, and temperature, correspondingly, while F, R, and D stand for Faraday, gas-molar, and analyte diffusion constants [26]. Utilizing Figure 7A, the ECSAs of the photocatalysts were found using ECSA = Q·m−1/C, whereas Q, m, and C indicate the negative-scan hydrogen-adsorption charges after double-layer charge modification, photocatalyst mass, and complete monolayer charges of the electrode-cover H-atoms, respectively [26]. The Q value was estimated by integrating the curve of the photocatalyst, Figure 7A, divided by the scan rate. The values of ECSA for the photocatalysts are determined and presented in Table 3. For Fe2O3 and Fe2O3/zeolite, the values were 7.414 and 21.236 m2/g, respectively. The 3-fold improvement in the ECSA explains the improved PEC performance, Figure 7A, of Fe2O3/zeolite photocatalyst versus the Fe2O3. Then, the estimated STH value was 12.74% for the Fe2O3/zeolite photocatalyst.

Table 3.
ECSA values and corrosion and Tafel parameters for Fe2O3 and Fe2O3/zeolite photocatalysts.
3.2.2. Corrosion and Tafel Parameters of Fe2O3 and Fe2O3/Zeolite Photocatalysts
The Tafel relationship, V = β log(J) + C, was used to quantify combined anodic and cathodic Tafel or polarization parameters to determine the mechanism of the H2 generation reaction(HGR) and the rate-limiting phase [58]. Low Tafel slopes, high current exchange rates, and good HGR performances are all characteristics of the ideal photocatalyst. Figure 8A shows the Tafel plots for Fe2O3 and Fe2O3/zeolite. Figure 8B,C displays the main characteristics: corrosion potential and current (Ecorr and Icorr) and anodic (βa) and cathodic (βc) Tafel slopes for the Fe2O3 and Fe2O3/zeolite. The values of βa and βc for Fe2O3 and Fe2O3/zeolite are found using the slopes of the curves’ linear segments, as shown in Figure 8D,E [26,59]. The obtained values of Ecorr, Icorr, βa, and βc were presented in Table 3 for Fe2O3 and Fe2O3/zeolite. For Fe2O3/ zeolite, the βa and βc values are 139.9 and 5.5 mV dec−1, respectively, and 63.4 and 6.8 mV dec−1 for Fe2O3. The PEC HGR mechanism and rate-limiting phases are indicated by the Tafel slopes. The Volmer–Tafel mechanism is predominant when the recombination phase is a rate limit and the Tafel slope is 30 mV dec−1. The Volmer–Heyrovsky H2 generation process could be presumed to be dominant when PEC desorption is a rate limit and the Tafel slope is 40 mV dec−1. The reaction pathways are dependent on the surfaces covered with adsorbed hydrogen if the Tafel slope is 120 mV dec−1. The βc-value denotes the needed over-potential to enhance the HGR rate by a factor of ten [26,59]. The low values of βc refer to the low optical band gaps of the designed Fe2O3 and Fe2O3/zeolite photocatalysts. This means that small amounts of energy (low overpotentials) are needed to achieve efficient HGR.
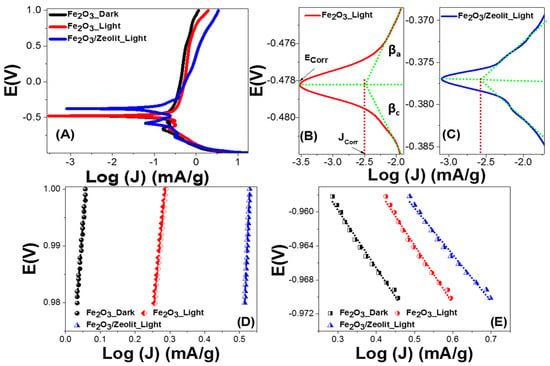
Figure 8.
Combined anodic and cathodic polarization of Fe2O3 and Fe2O3/zeolite (A), Ecorr and Jcorr of (B) Fe2O3 and (C) Fe2O3/zeolite; calculation of (D) anodic (βa) and (E) cathodic (βc) Tafel slopes.
The corrosion rate is directly dependent on Icorr, where Ecorr offers aspects about the solution’s corrosion propensity. From Figure 8A–C, the Fe2O3/zeolite presents nobler behavior. The Fe2O3/zeolite has a smaller Ecorr (376.7 mV) than Fe2O3 (478.3 mV). Generally, the Ecorr values revealed in this work are greater than any earlier stated values for Fe2O3-based photocatalysts and are moved to more noble behaviors when compared to commercial Fe2O3 [60].
To verify the relative ability of the electrode to resist corrosions; the values of Icorr, polarization resistance (RP), and corrosion rate (CR) could be determined. The CR is related to the kinetic value Icorr directly, while Rp is inversely proportional. From Table 2, the loading of Fe2O3 on the zeolite host reduces Icorr from 3.15 to 2.66 μA cm−2, which is much smaller than any previously reported Fe2O3 photoelectrode’s corrosion current. For example, Kim et al. reported 5.31 μA/cm2 for Fe2O3 and 8.69 μA/cm2 for Fe3O4 [60]. The values of Rp are determined by the Stern–Geary equation, Rp = βa βc/[2.303 Icorr (βa + βc)], utilizing the straight segments near to Ecorr of the curves. The values of CR (n year−1) are determined by CR = 3272 [Icorr × W/(ECSA × d)], whereas EW and d represent the equivalent weight (g eq−1) and density (g cm−3). For Fe2O3 and Fe2O3/zeolite, the values of Rp and CR are reported in Table 3. The Rp values are increased from 847.66 to 864.98 Ω cm2, whereas CR is decreased from 15.02 to 7.61 pm Year−1 by loading Fe2O3 on zeolite host. Therefore, photocorrosion is suppressed by the loading of the Fe2O3 photocatalyst into zeolite [61]. This is because zeolite can provide specific photophysical properties such as preventing the Fe2O3 nanoparticles from aggregating and improving their stability against sinterisation. The above-mentioned corrosion metrics show a significant improvement of the Fe2O3 photocatalyst’s stability through the use of zeolite as catalyst support. The obtained CR values outperform any prior Fe2O3-based PEC electrode results [62,63].
4. Conclusions
A highly effective recycling technique for rusted iron wastes and a scalable method for the preparation of Fe2O3 and Fe2O3/zeolite nanocomposite have been reported. The Fe2O3/zeolite nanocomposite showed smaller sizes, more homogeneous nanopore diameter distribution, greater Vis/NIR light absorption capability, and a wider bandgap than Fe2O3 nanopowder. Fe2O3/zeolite nanocomposite was applied successfully as a low-cost nanophotocatalyst. The application of Fe2O3/zeolite for photoelectrocatalytic hydrogen production showed a production rate of 154.45 mmol g−1 h−1 at 1 V in 0.9 M KOH solution, which is the highest value yet for Fe2O3-based photocatalysts. The photocurrent density of Fe2O3/zeolite is almost 2-fold that of the Fe2O3 catalyst, and the IPCE% reached ~27.34%@307 nm and 1 V nm. This nanophotocatalyst has also shown remarkable stability with a very low PEC corrosion rate of 7.6 pm/year. Additionally, it can retain ~97% of its initial performance.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/nano11123445/s1, Figure S1: FT-IR spectra of Fe2O3, zeolite, and Fe2O3/zeolite nanocomposite. Figure S2: Variation of current density (J) for Fe2O3 (I), (II), and (III) under white light illumination and at 1 V. Figure S3: Variation of current density (J) for Fe2O3 (III)/zeolite photoelectrodes with different Fe2O3 (III)/zeolite weight ratios at 1 V under white light illumination.
Author Contributions
Conceptualization, F.M., A.M.A. and M.S.; methodology, F.M., A.M.A. and M.S.; validation, F.M., A.M.A. and M.S.; formal analysis, F.M., A.M.A. and M.S.; investigation, F.M., A.M.A. and M.S.; resources, F.M., A.M.A., M.B. and M.S.; data curation, F.M., A.M.A. and M.S.; writing—original draft preparation, F.M., A.M.A. and M.S.; writing—review and editing, F.M., A.M.A., M.B. and M.S.; visualization, F.M., A.M.A. and M.S.; project administration, F.M., A.M.A., M.B. and M.S.; funding acquisition, F.M., A.M.A., M.B. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mehaney, A.; Shehatah, A.A.; Ahmed, A.M. Modeling of phononic crystal cavity for sensing different biodiesel fuels with high sensitivity. Mater. Chem. Phys. 2021, 257, 123774. [Google Scholar] [CrossRef]
- Mehaney, A.; Ahmed, A.M. Theoretical design of porous phononic crystal sensor for detecting CO2 pollutions in air. Phys. E Low-Dimens. Syst. Nanostructures 2020, 124, 114353. [Google Scholar] [CrossRef]
- Zayed, M.; Ahmed, A.M.; Shaban, M. Synthesis and characterization of nanoporous ZnO and Pt/ZnO thin films for dye degradation and water splitting applications. Int. J. Hydrog. Energy 2019, 44, 17630–17648. [Google Scholar] [CrossRef]
- Rabia, M.; Mohamed, S.H.; Zhao, H.; Shaban, M.; Lei, Y.; Ahmed, A.M. TiO2/TiOxNY hollow mushrooms-like nanocomposite photoanode for hydrogen electrogeneration. J. Porous Mater. 2020, 27, 133–139. [Google Scholar] [CrossRef]
- Pourtaheri, A.; Nezamzadeh-Ejhieh, A. Enhancement in photocatalytic activity of NiO by supporting onto an Iranian clinoptilolite nano-particles of aqueous solution of cefuroxime pharmaceutical capsule. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 137, 338–344. [Google Scholar] [CrossRef]
- Lassoued, A.; Lassoued, M.S.; Dkhil, B.; Ammar, S.; Gadri, A. Photocatalytic degradation of methylene blue dye by iron oxide (α-Fe2O3) nanoparticles under visible irradiation. J. Mater. Sci. Mater. Electron. 2018, 29, 8142–8152. [Google Scholar] [CrossRef]
- Khedr, M.H.; Abdel Halim, K.S.; Soliman, N.K. Synthesis and photocatalytic activity of nano-sized iron oxides. Mater. Lett. 2009, 63, 598–601. [Google Scholar] [CrossRef]
- Mishra, M.; Chun, D.M. α-Fe2O3 as a photocatalytic material: A review. Appl. Catal. A Gen. 2015, 498, 126–141. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Kockler, J.; Motti, C.A.; Glass, B.D.; Oelgemöller, M. Titanium dioxide/zeolite integrated photocatalytic adsorbents for the degradation of amoxicillin. Appl. Catal. B Environ. 2015, 166–167, 45–55. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Mohamed, A.S. Adsorption Removal of Safranin Dye Contaminants from Water Using Various Types of Natural Zeolite. Silicon 2019, 11, 1635–1647. [Google Scholar] [CrossRef]
- Wang, S.; Li, H.; Xie, S.; Liu, S.; Xu, L. Physical and chemical regeneration of zeolitic adsorbents for dye removal in wastewater treatment. Chemosphere 2006, 65, 82–87. [Google Scholar] [CrossRef]
- Shaban, M.; AbuKhadra, M.R.; Nasief, F.M.; Abd El-Salam, H.M. Removal of Ammonia from Aqueous Solutions, Ground Water, and Wastewater Using Mechanically Activated Clinoptilolite and Synthetic Zeolite-A: Kinetic and Equilibrium Studies. Water Air Soil Pollut. 2017, 228, 1–16. [Google Scholar] [CrossRef]
- Shaban, M.; Abukhadra, M.R.; Shahien, M.G.; Ibrahim, S.S. Novel bentonite/zeolite-NaP composite efficiently removes methylene blue and Congo red dyes. Environ. Chem. Lett. 2018, 16, 275–280. [Google Scholar] [CrossRef]
- Zhao, J.H.; Wang, Y.; Tang, X.; Li, Y.H.; Liu, F.T.; Zhang, Y.; Li, K. Enhanced photocatalytic hydrogen evolution over bimetallic zeolite imidazole framework-encapsulated CdS nanorods. Dalt. Trans. 2019, 48, 3560–3565. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Khan, F. Methods for increasing photo-assisted production of hydrogen over titanium exchanged zeolites. Int. J. Hydrogen Energy 1991, 16, 609–613. [Google Scholar] [CrossRef]
- Mendes, P.; Lapisardi, G.; Bouchy, C.; Rivallan, M.; Silva, J.M.; Ribeiro, M.F. Hydrogenating activity of Pt/zeolite catalysts focusing acid support and metal dispersion influence. Appl. Catal. A Gen. 2015, 504, 17–28. [Google Scholar] [CrossRef][Green Version]
- Zahmakiran, M.; Durap, F.; Özkar, S. Zeolite confined copper(0) nanoclusters as cost-effective and reusable catalyst in hydrogen generation from the hydrolysis of ammonia-borane. Int. J. Hydrogen Energy 2010, 35, 187–197. [Google Scholar] [CrossRef]
- Satsangi, V.R.; Dass, S.; Shrivastav, R. Nanostructured α-Fe2O3 in PEC Generation of Hydrogen. In On Solar Hydrogen & Nanotechnology; John Wiley & Sons, Ltd.: Chichester, UK, 2010; pp. 349–397. [Google Scholar]
- Tamirat, A.G.; Rick, J.; Dubale, A.A.; Su, W.N.; Hwang, B.J. Using hematite for photoelectrochemical water splitting: A review of current progress and challenges. Nanoscale Horiz. 2016, 1, 243–267. [Google Scholar] [CrossRef]
- Norouzi, A.; Nezamzadeh-Ejhieh, A. α-Fe2O3/Cu2O heterostructure: Brief characterization and kinetic aspect of degradation of methylene blue. Phys. B Condens. Matter 2020, 599, 412422. [Google Scholar] [CrossRef]
- Keerthana, S.P.; Yuvakkumar, R.; Ravi, G.; Kumar, P.; Elshikh, M.S.; Hussein, H.A.; Abdulwahed, F.A.; Velauthapillai, D.A. Strategy to enhance the photocatalytic efficiency of α-Fe2O3. Chemosphere 2021, 270, 129498. [Google Scholar] [CrossRef]
- Jaafar, N.F.; Abdul Jalil, A.; Triwahyono, S.; Muhd Muhid, M.N.; Sapawe, N.; Satar, M.A.H.; Asaari, H. Photodecolorization of methyl orange over α-Fe2O3-supported HY catalysts: The effects of catalyst preparation and dealumination. Chem. Eng. J. 2012, 191, 112–122. [Google Scholar] [CrossRef]
- Mhamane, D.; Kim, H.K.; Aravindan, V.; Roh, K.C.; Srinivasan, M.; Kim, K.B. Rusted iron wire waste into high performance anode (α-Fe2O3) for Li-ion batteries: An efficient waste management approach. Green Chem. 2016, 18, 1395–1404. [Google Scholar] [CrossRef]
- Chen, F.; Li, Y.; Cai, W.; Zhang, J. Preparation and sono-Fenton performance of 4A-zeolite supported α-Fe2O3. J. Hazard. Mater. 2010, 177, 743–749. [Google Scholar] [CrossRef]
- An, N.; Yu, Q.; Liu, G.; Li, S.; Jia, M.; Zhang, W. Complete oxidation of formaldehyde at ambient temperature over supported Pt/Fe2O3 catalysts prepared by colloid-deposition method. J. Hazard. Mater. 2011, 186, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.; Rabia, M.; Shaban, M. Synthesis and characterization of biogenic iron oxides of different nanomorphologies from pomegranate peels for efficient solar hydrogen production. J. Mater. Res. Technol. 2020, 9, 4255–4271. [Google Scholar] [CrossRef]
- Ravishankar, S.; Balu, A.R.; Usharani, K.; Balamurugan, S.; Prabha, D.; Nagarethinam, V.S. Optical and magnetic properties of PbS thin films doped with Fe2+ ions. Optik 2017, 134, 121–127. [Google Scholar] [CrossRef]
- Parmar, V.; Changela, K.; Srinivas, B.; Sankar, M.M.; Mohanty, S.; Panigrahi, S.K.; Hariharan, K.; Kalyanasundaram, D. Relationship between dislocation density and antibacterial activity of cryo-rolled and cold-rolled copper. Materials 2019, 12, 200. [Google Scholar] [CrossRef]
- Van Vlack, L.H. Elements of Materials Science and Engineering, 6th ed.; Addison Wesley: Boston, MA, USA, 1989; Volume 156. [Google Scholar]
- Na, K.; Somorjai, G.A. Hierarchically Nanoporous Zeolites and Their Heterogeneous Catalysis: Current Status and Future Perspectives. Catal. Lett. 2015, 145, 193–213. [Google Scholar] [CrossRef]
- Tedla, H.; Díaz, I.; Kebede, T.; Taddesse, A.M. Synthesis, characterization and photocatalytic activity of zeolite supported ZnO/Fe2O3/MnO2 nanocomposites. J. Environ. Chem. Eng. 2015, 3, 1586–1591. [Google Scholar] [CrossRef]
- Garay-Rodríguez, M.E.; Gutiérrez-Arzaluz, M.; Mejía-Saavedra, J.; Carrizales-Yánez, L.; Mugica-Álvarez, V.; Torres-Rodríguez, M. Natural Mexican Zeolite Modified with Iron to Remove Arsenic Ions from Water Sources. Proceedings 2018, 2, 1312. [Google Scholar] [CrossRef]
- Elsayed, H.A.; Sayed, H.; Taha, T.A.; Alharbi, A.G.; Alenad, A.M.; Alshammari, B.A.; Ahmed, A.M.; Mehaney, A.; Aly, A.H. Simple and efficient design towards a significant improvement of the optical absorption of amorphous silicon solar cell. J. Quant. Spectrosc. Radiat. Transf. 2021, 275, 107890. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Mehaney, A.; Elsayed, H.A. Detection of toluene traces in exhaled breath by using a 1D PC as a biomarker for lung cancer diagnosis. Eur. Phys. J. Plus 2021, 136, 1–14. [Google Scholar] [CrossRef]
- Cao, Z.; Qin, M.; Jia, B.; Gu, Y.; Chen, P.; Volinsky, A.A.; Qu, X. One pot solution combustion synthesis of highly mesoporous hematite for photocatalysis. Ceram. Int. 2015, 41, 2806–2812. [Google Scholar] [CrossRef]
- Mahadik, M.; Shinde, S.; Mohite, V.; Kumbhar, S.; Rajpure, K.; Moholkar, A.; Kim, J.; Bhosale, C. Photoelectrocatalytic oxidation of Rhodamine B with sprayed α-Fe2O3 photocatalyst. Mater. Express 2013, 3, 247–255. [Google Scholar] [CrossRef]
- Duret, A.; Grätzel, M. Visible light-induced water oxidation on mesoscopic α-Fe2O3 films made by ultrasonic spray pyrolysis. J. Phys. Chem. B 2005, 109, 17184–17191. [Google Scholar] [CrossRef]
- Souza, F.L.; Lopes, K.P.; Nascente, P.A.P.; Leite, E.R. Nanostructured hematite thin films produced by spin-coating deposition solution: Application in water splitting. Sol. Energy Mater. Sol. Cells 2009, 93, 362–368. [Google Scholar] [CrossRef]
- Derikvandi, H.; Nezamzadeh-Ejhieh, A. Designing of experiments for evaluating the interactions of influencing factors on the photocatalytic activity of NiS and SnS2: Focus on coupling, supporting and nanoparticles. J. Colloid Interface Sci. 2017, 490, 628–641. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Mohamed, F.; Ashraf, A.M.; Shaban, M.; Aslam Parwaz Khan, A.; Asiri, A.M. Enhanced photoelectrochemical water splitting activity of carbon nanotubes@TiO2 nanoribbons in different electrolytes. Chemosphere 2020, 238, 124554. [Google Scholar] [CrossRef]
- Souza, F.L.; Lopes, K.P.; Longo, E.; Leite, E.R. The influence of the film thickness of nanostructured α-Fe2O3 on water photooxidation. Phys. Chem. Chem. Phys. 2009, 11, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, D.; Leung, K.T. Controlled growth of two-dimensional and one-dimensional ZnO nanostructures on indium tin oxide coated glass by direct electrodeposition. Langmuir 2008, 24, 9707–9716. [Google Scholar] [CrossRef]
- Khosroabadi, A.A.; Gangopadhyay, P.; Duong, B.; Thomas, J.; Sigdel, A.K.; Berry, J.J.; Gennett, T.; Peyghambarian, N.; Norwood, R.A. Fabrication, electrical and optical properties of silver, indium tin oxide (ITO), and indium zinc oxide (IZO) nanostructure arrays. Phys. Status Solidi 2013, 210, 831–838. [Google Scholar] [CrossRef]
- Kalska-Szostko, B.; Wykowska, U.; Piekut, K.; Zambrzycka, E. Stability of iron (Fe) nanowires. Colloids Surfaces A Physicochem. Eng. Asp. 2013, 416, 66–72. [Google Scholar] [CrossRef]
- Saharan, P.; Chaudhary, G.R.; Mehta, S.K.; Umar, A. Removal of water contaminants by iron oxide nanomaterials. J. Nanosci. Nanotechnol. 2014, 14, 627–643. [Google Scholar] [CrossRef]
- Ismail, A.A.; Mohamed, R.M.; Fouad, O.A.; Ibrahim, I.A. Synthesis of nanosized ZSM-5 using different alumina sources. Cryst. Res. Technol. 2006, 41, 145–149. [Google Scholar] [CrossRef]
- Mohapatra, M.; Mohapatra, L.; Singh, P.; Anand, S.; Mishra, B. A comparative study on Pb(II), Cd(II), Cu(II), Co(II) adsorption from single and binary aqueous solutions on additive assisted nano-structured goethite. Int. J. Eng. Sci. Technol. 2011, 2, 89–103. [Google Scholar] [CrossRef]
- Derikvandi, H.; Nezamzadeh-Ejhieh, A. Increased photocatalytic activity of NiO and ZnO in photodegradation of a model drug aqueous solution: Effect of coupling, supporting, particles size and calcination temperature. J. Hazard. Mater. 2017, 321, 629–638. [Google Scholar] [CrossRef] [PubMed]
- Dutta, P.K.; Turbeville, W. Intrazeolitic photoinduced redox reactions between Ru(bpy)32+ and methylviologen. J. Phys. Chem. 1992, 96, 9410–9416. [Google Scholar] [CrossRef]
- Dubey, N.; Rayalu, S.S.; Labhsetwar, N.K.; Devotta, S. Visible light active zeolite-based photocatalysts for hydrogen evolution from water. Int. J. Hydrogen Energy 2008, 33, 5958–5966. [Google Scholar] [CrossRef]
- Corma, A.; Garcia, H. Zeolite-based photocatalysts. Chem. Commun. 2004, 4, 1443–1459. [Google Scholar] [CrossRef]
- Chica, A. Zeolites: Promised Materials for the Sustainable Production of Hydrogen. ISRN Chem. Eng. 2013, 2013, 1–19. [Google Scholar] [CrossRef]
- Ghattavi, S.; Nezamzadeh-Ejhieh, A. GC-MASS detection of methyl orange degradation intermediates by AgBr/g-C3N4: Experimental design, bandgap study, and characterization of the catalyst. Int. J. Hydrogen Energy 2020, 45, 24636–24656. [Google Scholar] [CrossRef]
- Aboud, A.A.; Shaban, M.; Revaprasadu, N. Effect of Cu, Ni and Pb doping on the photo-electrochemical activity of ZnO thin films. RSC Adv. 2019, 9, 7729–7736. [Google Scholar] [CrossRef]
- Jiang, C.; Moniz, S.J.A.; Wang, A.; Zhang, T.; Tang, J. Photoelectrochemical devices for solar water splitting-materials and challenges. Chem. Soc. Rev. 2017, 46, 4645–4660. [Google Scholar] [CrossRef]
- Choudhary, S.; Upadhyay, S.; Kumar, P.; Singh, N.; Satsangi, V.R.; Shrivastav, R.; Dass, S. Nanostructured bilayered thin films in photoelectrochemical water splitting—A review. Int. J. Hydrogen Energy 2012, 37, 18713–18730. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R. Electrochemical water splitting using nano-zeolite Y supported tungsten oxide electrocatalysts. J. Nanoparticle Res. 2018, 20, 1–11. [Google Scholar] [CrossRef]
- Patel, M.; Park, W.H.; Ray, A.; Kim, J.; Lee, J.H. Photoelectrocatalytic sea water splitting using Kirkendall diffusion grown functional Co3O4 film. Sol. Energy Mater. Sol. Cells 2017, 171, 267–274. [Google Scholar] [CrossRef]
- Shaban, M.; Kholidy, I.; Ahmed, G.M.; Negem, M.; Abd El-Salam, H.M. Cyclic voltammetry growth and characterization of Sn-Ag alloys of different nanomorphologies and compositions for efficient hydrogen evolution in alkaline solutions. RSC Adv. 2019, 9, 22389–22400. [Google Scholar] [CrossRef]
- Kim, Y.-S.; Kim, J.-G. Corrosion Behavior of Pipeline Carbon Steel under Different Iron Oxide Deposits in the District Heating System. Metals 2017, 7, 182. [Google Scholar] [CrossRef]
- White, J.C.; Dutta, P.K. Assembly of nanoparticles in zeolite y for the photocatalytic generation of hydrogen from water. J. Phys. Chem. C 2011, 115, 2938–2947. [Google Scholar] [CrossRef]
- Otani, K.; Sakairi, M. Effects of metal cations on corrosion of mild steel in model fresh water. Corros. Sci. 2016, 111, 302–312. [Google Scholar] [CrossRef]
- Kleiman-Shwarsctein, A.; Hu, Y.S.; Forman, A.J.; Stucky, G.D.; McFarland, E.W. Electrodeposition of α-Fe2O3 doped with Mo or Cr as photoanodes for photocatalytic water splitting. J. Phys. Chem. C 2008, 112, 15900–15907. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).