Peroxymonosulfate Activation by Bi2WO6/BiOCl Heterojunction Nanocomposites under Visible Light for Bisphenol A Degradation
Abstract
:1. Introduction
2. Experimental Section
2.1. Preparation of Materials
2.2. Materials Characterization
2.3. Activity Evaluation
3. Results and Discussion
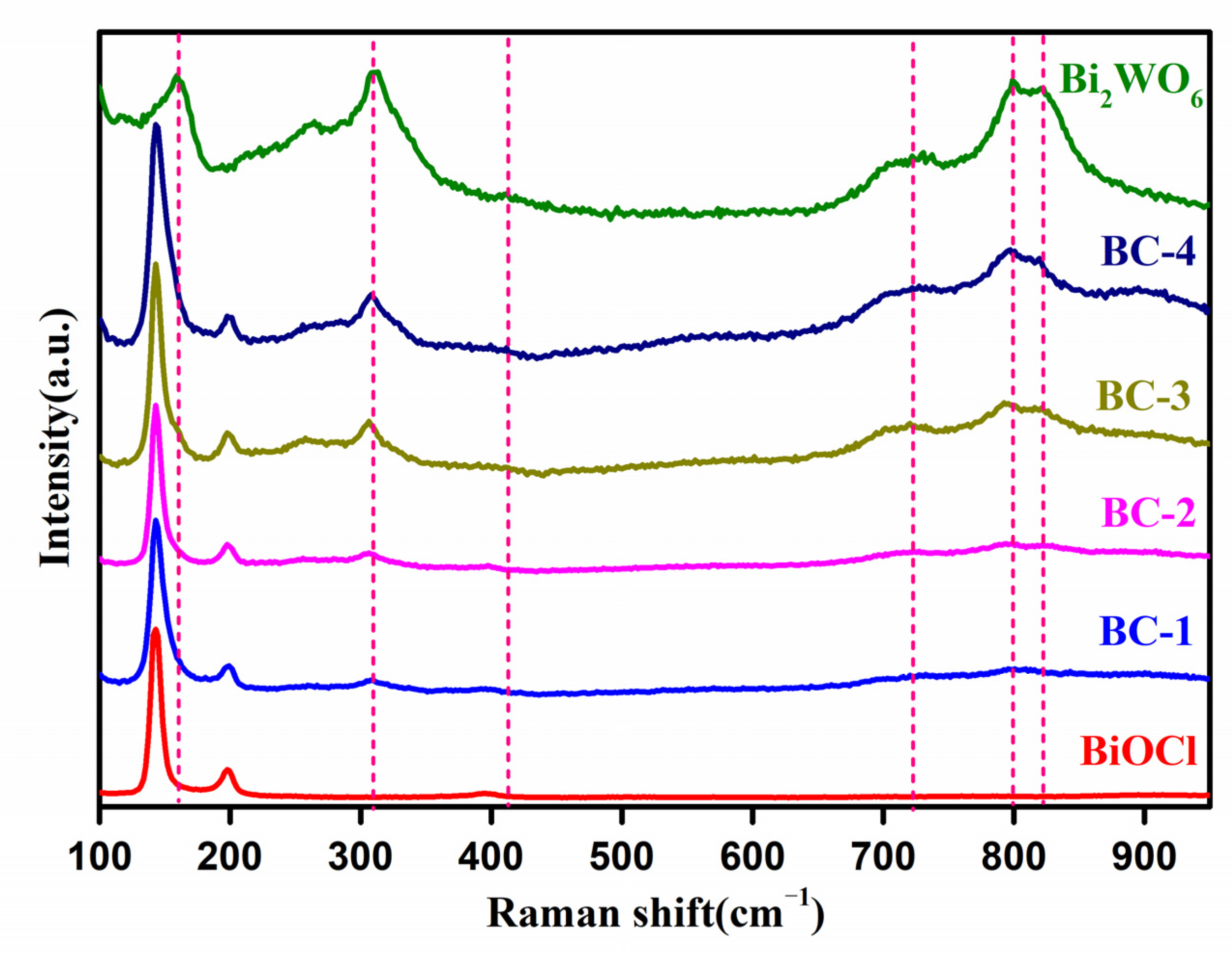
3.1. Characterizations of Catalysts
3.2. Photocatalytic Activity Evaluation
3.3. Photocatalytic Reaction Mechanisms
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.; von Gunten, U.; Kim, J.H. Persulfate-Based Advanced Oxidation: Critical Assessment of Opportunities and Roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Guo, N.; Xu, X.; Yu, Y.; Wang, Q.; Wang, N.; Han, X.; Yu, H. Degradation of bisphenol a using peroxymonosulfate activated by WO3@MoS2/Ag hollow nanotubes photocatalyst. Chemosphere 2019, 227, 589–597. [Google Scholar] [CrossRef]
- Deng, W.; Zhao, H.; Pan, F.; Feng, X.; Jung, B.; Abdel-Wahab, A.; Batchelor, B.; Li, Y. Visible-Light-Driven Photocatalytic Degradation of Organic Water Pollutants Promoted by Sulfite Addition. Environ. Sci. Technol. 2017, 51, 13372–13379. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Nengzi, L.C.; Wang, Z.; Zhang, X.; Li, B.; Cheng, X. Construction of Bi2O3/CuNiFe LDHs composite and its enhanced photocatalytic degradation of lomefloxacin with persulfate under simulated sunlight. J. Hazard. Mater. 2020, 383, 121236. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Cui, Y.; Huang, R.; Zhong, L.; Yan, P.; Zhang, S.; Zhao, Q.; Jiang, D.; Tang, A.; Yang, H. A heterogeneous Fenton reaction system of N-doped TiO2 anchored on sepiolite activates peroxymonosulfate under visible light irradiation. Chem. Eng. J. 2020, 383, 123142. [Google Scholar] [CrossRef]
- Li, R.; Huang, J.; Cai, M.; Huang, J.; Xie, Z.; Zhang, Q.; Liu, Y.; Liu, H.; Lv, W.; Liu, G. Activation of peroxymonosulfate by Fe doped g-C3N4/graphene under visible light irradiation for Trimethoprim degradation. J. Hazard. Mater. 2020, 384, 121435. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Li, S.; Li, X.; Li, J.; Fan, S.; Chen, X.; Yin, Z.; Tadé, M.; Liu, S. Visible-light-driven sonophotocatalysis and peroxymonosulfate activation over 3D urchin-like MoS2/C nanoparticles for accelerating levofloxacin elimination: Optimization and kinetic study. Chem. Eng. J. 2019, 378, 122039. [Google Scholar] [CrossRef]
- Lim, J.; Yang, Y.; Hoffmann, M.R. Activation of Peroxymonosulfate by Oxygen Vacancies-Enriched Cobalt-Doped Black TiO2 Nanotubes for the Removal of Organic Pollutants. Environ. Sci. Technol. 2019, 53, 6972–6980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, X.; Kang, J.; Tian, W.; Zhang, H.; Ho, S.-H.; Zhu, Y.-A.; Ao, Z.; Sun, H.; Wang, S. Interfacial-engineered cobalt@carbon hybrids for synergistically boosted evolution of sulfate radicals toward green oxidation. Appl. Catal. B Environ. 2019, 256, 117795. [Google Scholar] [CrossRef]
- He, R.; Xu, D.; Cheng, B.; Yu, J.; Ho, W. Review on nanoscale Bi-based photocatalysts. Nanoscale Horiz. 2018, 3, 464–504. [Google Scholar] [CrossRef]
- Zhang, N.; Li, L.; Shao, Q.; Zhu, T.; Huang, X.; Xiao, X. Fe-Doped BiOCl Nanosheets with Light-Switchable Oxygen Vacancies for Photocatalytic Nitrogen Fixation. ACS Appl. Energy Mater. 2019, 2, 8394–8398. [Google Scholar] [CrossRef]
- Zhang, L.; Han, Z.; Wang, W.; Li, X.; Su, Y.; Jiang, D.; Lei, X.; Sun, S. Solar-Light-Driven Pure Water Splitting with Ultrathin BiOCl Nanosheets. Chemistry 2015, 21, 18089–18094. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Wang, D.; Zhang, Y.; Feng, Z.; Liu, L.; Wang, W. Photoreductive BiOCl Ultrathin Nanosheets for Highly Efficient Photocatalytic Color Switching. ACS Appl. Mater. Interfaces 2020, 12, 8604–8613. [Google Scholar] [CrossRef]
- Al Marzouqi, F.; Al Farsi, B.; Kuvarega, A.T.; Al Lawati, H.A.J.; Al Kindy, S.M.Z.; Kim, Y.; Selvaraj, R. Controlled Microwave-Assisted Synthesis of the 2D-BiOCl/2D-g-C3N4 Heterostructure for the Degradation of Amine-Based Pharmaceuticals under Solar Light Illumination. ACS Omega 2019, 4, 4671–4678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Miao, P.; Zhang, Y.; Wu, J.; Zhang, B.; Du, Y.; Han, X.; Sun, J.; Xu, P. Recent Advances in Plasmonic Nanostructures for Enhanced Photocatalysis and Electrocatalysis. Adv. Mater. 2021, 33, e2000086. [Google Scholar] [CrossRef]
- Zhang, L.; Ran, J.; Qiao, S.Z.; Jaroniec, M. Characterization of semiconductor photocatalysts. Chem. Soc. Rev. 2019, 48, 5184–5206. [Google Scholar] [CrossRef]
- Yang, M.Q.; Gao, M.; Hong, M.; Ho, G.W. Visible-to-NIR Photon Harvesting: Progressive Engineering of Catalysts for Solar-Powered Environmental Purification and Fuel Production. Adv. Mater. 2018, 30, e1802894. [Google Scholar] [CrossRef] [PubMed]
- Li, F.-T.; Wang, Q.; Wang, X.-J.; Li, B.; Hao, Y.-J.; Liu, R.-H.; Zhao, D.-S. In-situ one-step synthesis of novel BiOCl/Bi24O31Cl10 heterojunctions via self-combustion of ionic liquid with enhanced visible-light photocatalytic activities. Appl. Catal. B Environ. 2014, 150–151, 574–584. [Google Scholar] [CrossRef]
- Sun, X.G.; Zhang, Y.Y.; Li, P.; Guo, D.; Zi, H.; Guo, J.Z.; Li, Y.T. Heterostructure nano-NiO/BiOCl composites with advanced adsorption and photocatalytic performance for organic dyes. J. Alloys Compd. 2018, 736, 22–28. [Google Scholar] [CrossRef]
- Waiskopf, N.; Ben-Shahar, Y.; Banin, U. Photocatalytic Hybrid Semiconductor-Metal Nanoparticles; from Synergistic Properties to Emerging Applications. Adv. Mater. 2018, 30, e1706697. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Lei, W.; Liu, G.; Liu, M. Visible- and NIR-Light Responsive Black-Phosphorus-Based Nanostructures in Solar Fuel Production and Environmental Remediation. Adv. Mater. 2018, 30, e1804770. [Google Scholar] [CrossRef]
- Chen, S.; Takata, T.; Domen, K. Particulate photocatalysts for overall water splitting. Nat. Rev. Mater. 2017, 2, 17050. [Google Scholar] [CrossRef]
- Xing, Z.; Hu, J.; Ma, M.; Lin, H.; An, Y.; Liu, Z.; Zhang, Y.; Li, J.; Yang, S. From One to Two: In Situ Construction of an Ultrathin 2D-2D Closely Bonded Heterojunction from a Single-Phase Monolayer Nanosheet. J. Am. Chem. Soc. 2019, 141, 19715–19727. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tu, W.; Chen, C.; Plewa, A.; Ye, H.; Sam Oh, J.A.; He, L.; Wu, T.; Zeng, K.; Lu, L. Chemical Bonding Construction of Reduced Graphene Oxide-Anchored Few-Layer Bismuth Oxychloride for Synergistically Improving Sodium-Ion Storage. Chem. Mater. 2019, 31, 7311–7319. [Google Scholar] [CrossRef]
- Cao, X.; Chen, Z.; Lin, R.; Cheong, W.-C.; Liu, S.; Zhang, J.; Peng, Q.; Chen, C.; Han, T.; Tong, X.; et al. A photochromic composite with enhanced carrier separation for the photocatalytic activation of benzylic C–H bonds in toluene. Nat. Catal. 2018, 1, 704–710. [Google Scholar] [CrossRef]
- Huang, Y.; Kou, S.; Zhang, X.; Wang, L.; Lu, P.; Zhang, D. Facile Fabrication of Z-Scheme Bi2WO6/WO3 Composites for Efficient Photodegradation of Bisphenol A with Peroxymonosulfate Activation. Nanomaterials 2020, 10, 724. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Kang, S.; Yang, Y.; Qin, H.; Ni, Z.; Yang, S.; Li, X. Facile synthesis of Bi/Bi2WO6 nanocomposite with enhanced photocatalytic activity under visible light. Appl. Catal. B Environ. 2016, 196, 89–99. [Google Scholar] [CrossRef]
- Mi, Y.; Wen, L.; Wang, Z.; Cao, D.; Xu, R.; Fang, Y.; Zhou, Y.; Lei, Y. Fe(III) modified BiOCl ultrathin nanosheet towards high-efficient visible-light photocatalyst. Nano Energy 2016, 30, 109–117. [Google Scholar] [CrossRef]
- Huang, Y.; Kou, S.; Zhang, X.; Wang, L.; Zhang, D. In-situ construction of WC/Bi2WO6 nanocomposites for efficient photodegradation of bisphenol A with peroxymonosulfate activation. Ceram. Int. 2021, 47, 20626–20637. [Google Scholar] [CrossRef]
- Zhou, H.; Wen, Z.; Liu, J.; Ke, J.; Duan, X.; Wang, S. Z-scheme plasmonic Ag decorated WO3/Bi2WO6 hybrids for enhanced photocatalytic abatement of chlorinated-VOCs under solar light irradiation. Appl. Catal. B Environ. 2019, 242, 76–84. [Google Scholar] [CrossRef]
- Cao, S.; Shen, B.; Tong, T.; Fu, J.; Yu, J. 2D/2D Heterojunction of Ultrathin MXene/Bi2WO6 Nanosheets for Improved Photocatalytic CO2 Reduction. Adv. Funct. Mater. 2018, 28, 1800136. [Google Scholar] [CrossRef]
- Huang, H.; Cao, R.; Yu, S.; Xu, K.; Hao, W.; Wang, Y.; Dong, F.; Zhang, T.; Zhang, Y. Single-unit-cell layer established Bi2WO6 3D hierarchical architectures: Efficient adsorption, photocatalysis and dye-sensitized photoelectrochemical performance. Appl. Catal. B Environ. 2017, 219, 526–537. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Chen, Z.; Zhou, L.; Xu, H.; Zhu, W. Fabrication of flower-like Bi2WO6 superstructures as high performance visible-light driven photocatalysts. J. Mater. Chem. 2007, 17, 2526–2532. [Google Scholar] [CrossRef]
- Wang, J.; Wei, Y.; Yang, B.; Wang, B.; Chen, J.; Jing, H. In situ grown heterojunction of Bi2WO6/BiOCl for efficient photoelectrocatalytic CO2 reduction. J. Catal. 2019, 377, 209–217. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Hashimoto, M.; Chishiro, K.; Moriyama, K.; Tanaka, S.; Hirai, T. Photocatalytic Dinitrogen Fixation with Water on Bismuth Oxychloride in Chloride Solutions for Solar-to-Chemical Energy Conversion. J. Am. Chem. Soc. 2020, 142, 7574–7583. [Google Scholar] [CrossRef]
- Yao, H.; Li, H.; Hu, T.; Hou, W. Solvent-Free Synthesis of Bismuth Oxychloride Microflower/Nanosheet Homojunctions for Photoactivity Enhancement. ChemCatChem 2018, 10, 3726–3735. [Google Scholar] [CrossRef]
- Phu, N.D.; Hoang, L.H.; Van Hai, P.; Huy, T.Q.; Chen, X.-B.; Chou, W.C. Photocatalytic activity enhancement of Bi2WO6 nanoparticles by Ag doping and Ag nanoparticles modification. J. Alloys Compd. 2020, 824, 153914. [Google Scholar] [CrossRef]
- Li, Q.; Lu, M.; Wang, W.; Zhao, W.; Chen, G.; Shi, H. Fabrication of 2D/2D g-C3N4/Au/Bi2WO6 Z-scheme photocatalyst with enhanced visible-light-driven photocatalytic activity. Appl. Surf. Sci. 2020, 508, 144182. [Google Scholar] [CrossRef]
- Ma, Q.; Hu, X.; Liu, N.; Sharma, A.; Zhang, C.; Kawazoe, N.; Chen, G.; Yang, Y. Polyethylene glycol (PEG)-modified Ag/Ag2O/Ag3PO4/Bi2WO6 photocatalyst film with enhanced efficiency and stability under solar light. J. Colloid Interface Sci. 2020, 569, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Dong, X.; Lei, B.; Li, J.; Chen, P.; Zhang, Y.; Dong, F. Graphene oxide mediated co-generation of C-doping and oxygen defects in Bi2WO6 nanosheets: A combined DRIFTS and DFT investigation. Nanoscale 2019, 11, 20562–20570. [Google Scholar] [CrossRef]
- Armelao, L.; Bottaro, G.; Maccato, C.; Tondello, E. Bismuth oxychloride nanoflakes: Interplay between composition-structure and optical properties. Dalton. Trans. 2012, 41, 5480–5485. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.X.; Di, J.; Yin, S.; Xu, H.; Zhang, J.; Xu, Y.G.; Xu, L.; Li, H.M.; Ji, M.X. Facile fabrication of the visible-light-driven Bi2WO6/BiOBr composite with enhanced photocatalytic activity. Rsc Adv. 2014, 4, 82–90. [Google Scholar] [CrossRef]
- Tian, Y.; Chang, B.; Lu, J.; Fu, J.; Xi, F.; Dong, X. Hydrothermal synthesis of graphitic carbon nitride-Bi2WO6 heterojunctions with enhanced visible light photocatalytic activities. ACS Appl. Mater. Interfaces 2013, 5, 7079–7085. [Google Scholar] [CrossRef]
- Wang, C.; Xue, Y.; Wang, P.F.; Ao, Y.H. Effects of water environmental factors on the photocatalytic degradation of sulfamethoxazole by AgI/UiO-66 composite under visible light irradiation. J. Alloys Compd. 2018, 748, 314–322. [Google Scholar] [CrossRef]
- Lee, C.G.; Javed, H.; Zhang, D.; Kim, J.H.; Westerhoff, P.; Li, Q.; Alvarez, P.J.J. Porous Electrospun Fibers Embedding TiO2 for Adsorption and Photocatalytic Degradation of Water Pollutants. Environ. Sci. Technol. 2018, 52, 4285–4293. [Google Scholar] [CrossRef]
- Huang, H.; Zhou, C.; Jiao, X.; Yuan, H.; Zhao, J.; He, C.; Hofkens, J.; Roeffaers, M.B.J.; Long, J.; Steele, J.A. Subsurface Defect Engineering in Single-Unit-Cell Bi2WO6 Monolayers Boosts Solar-Driven Photocatalytic Performance. ACS Catal. 2019, 10, 1439–1443. [Google Scholar] [CrossRef]
- Wang, T.; Liu, S.; Mao, W.; Bai, Y.; Chiang, K.; Shah, K.; Paz-Ferreiro, J. Novel Bi2WO6 loaded N-biochar composites with enhanced photocatalytic degradation of rhodamine B and Cr(VI). J. Hazard. Mater. 2020, 389, 121827. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Zhao, X.; Wang, Y.; Mao, R.; Wang, Y.; Qiao, M.; Zhao, S.; Zhu, Y. Synergetic activation of peroxymonosulfate by Co3O4 modified g-C3N4 for enhanced degradation of diclofenac sodium under visible light irradiation. Appl. Catal. B Environ. 2017, 218, 810–818. [Google Scholar] [CrossRef]














| Sample | SBET(m2∙g−1) | Average Pore Size (nm) |
|---|---|---|
| BiOCl | 5.394 | 3.292 |
| Bi2WO6 | 46.202 | 3.792 |
| BC-3 | 16.129 | 3.704 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Yin, X.; He, P.; Kou, S.; Zhang, X.; Wang, L.; Lu, P. Peroxymonosulfate Activation by Bi2WO6/BiOCl Heterojunction Nanocomposites under Visible Light for Bisphenol A Degradation. Nanomaterials 2021, 11, 3130. https://doi.org/10.3390/nano11113130
Huang Y, Yin X, He P, Kou S, Zhang X, Wang L, Lu P. Peroxymonosulfate Activation by Bi2WO6/BiOCl Heterojunction Nanocomposites under Visible Light for Bisphenol A Degradation. Nanomaterials. 2021; 11(11):3130. https://doi.org/10.3390/nano11113130
Chicago/Turabian StyleHuang, Yongkui, Xiangyang Yin, Pei He, Shuangwu Kou, Xiaoting Zhang, Lei Wang, and Peili Lu. 2021. "Peroxymonosulfate Activation by Bi2WO6/BiOCl Heterojunction Nanocomposites under Visible Light for Bisphenol A Degradation" Nanomaterials 11, no. 11: 3130. https://doi.org/10.3390/nano11113130
APA StyleHuang, Y., Yin, X., He, P., Kou, S., Zhang, X., Wang, L., & Lu, P. (2021). Peroxymonosulfate Activation by Bi2WO6/BiOCl Heterojunction Nanocomposites under Visible Light for Bisphenol A Degradation. Nanomaterials, 11(11), 3130. https://doi.org/10.3390/nano11113130







