Mechanism of Amyloid Gel Formation by Several Short Amyloidogenic Peptides
Abstract
:1. Introduction
2. Results
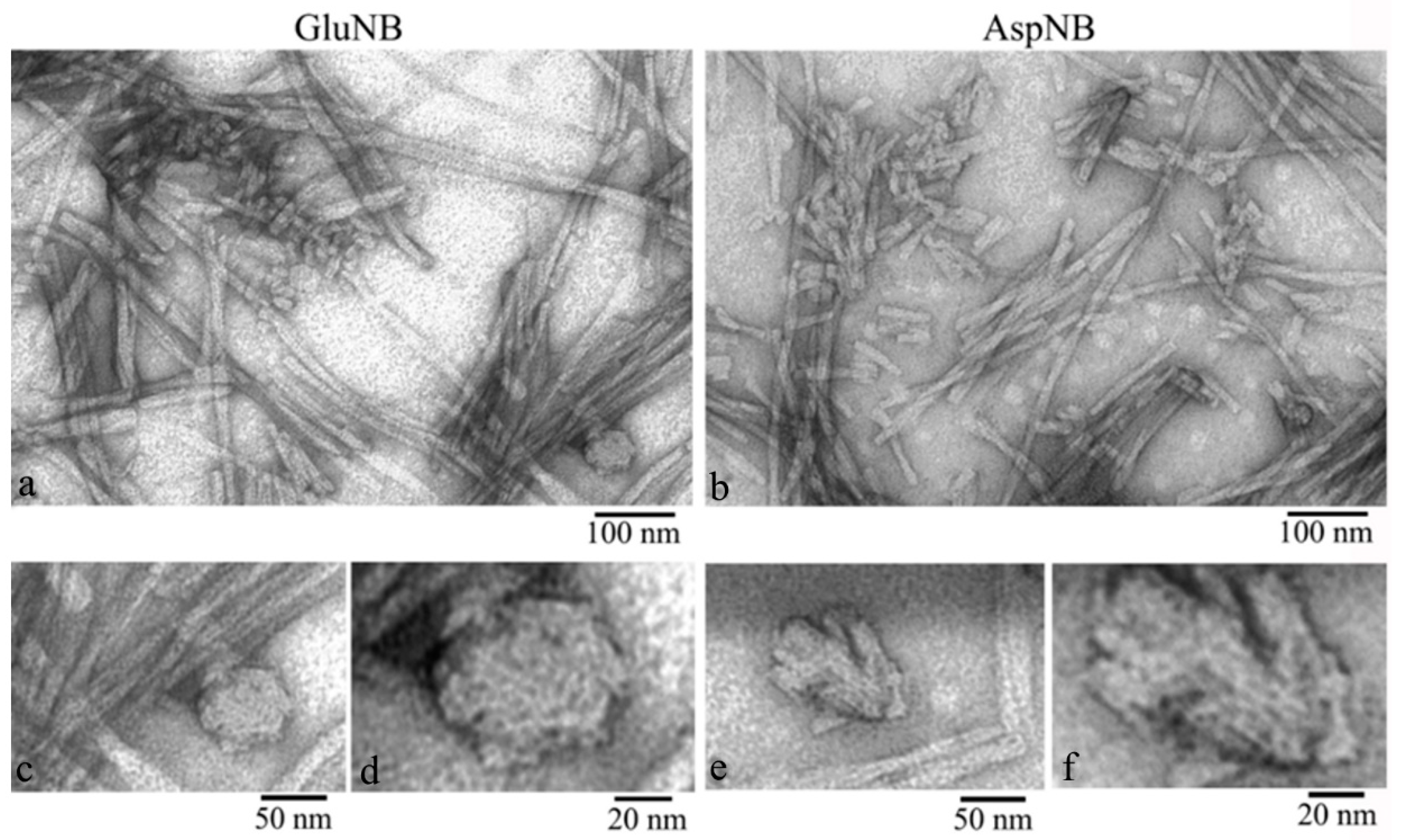
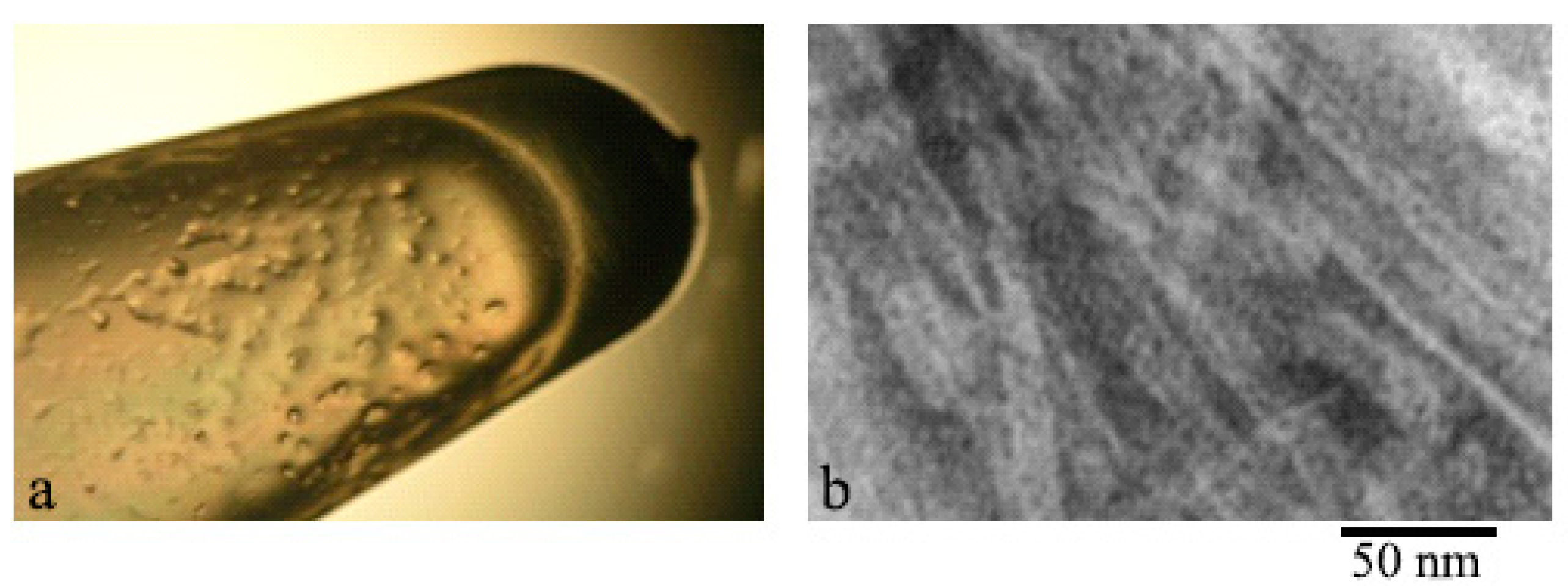
2.1. Electron Microscopy Analysis of Gels of GluNB and AspNB Peptides
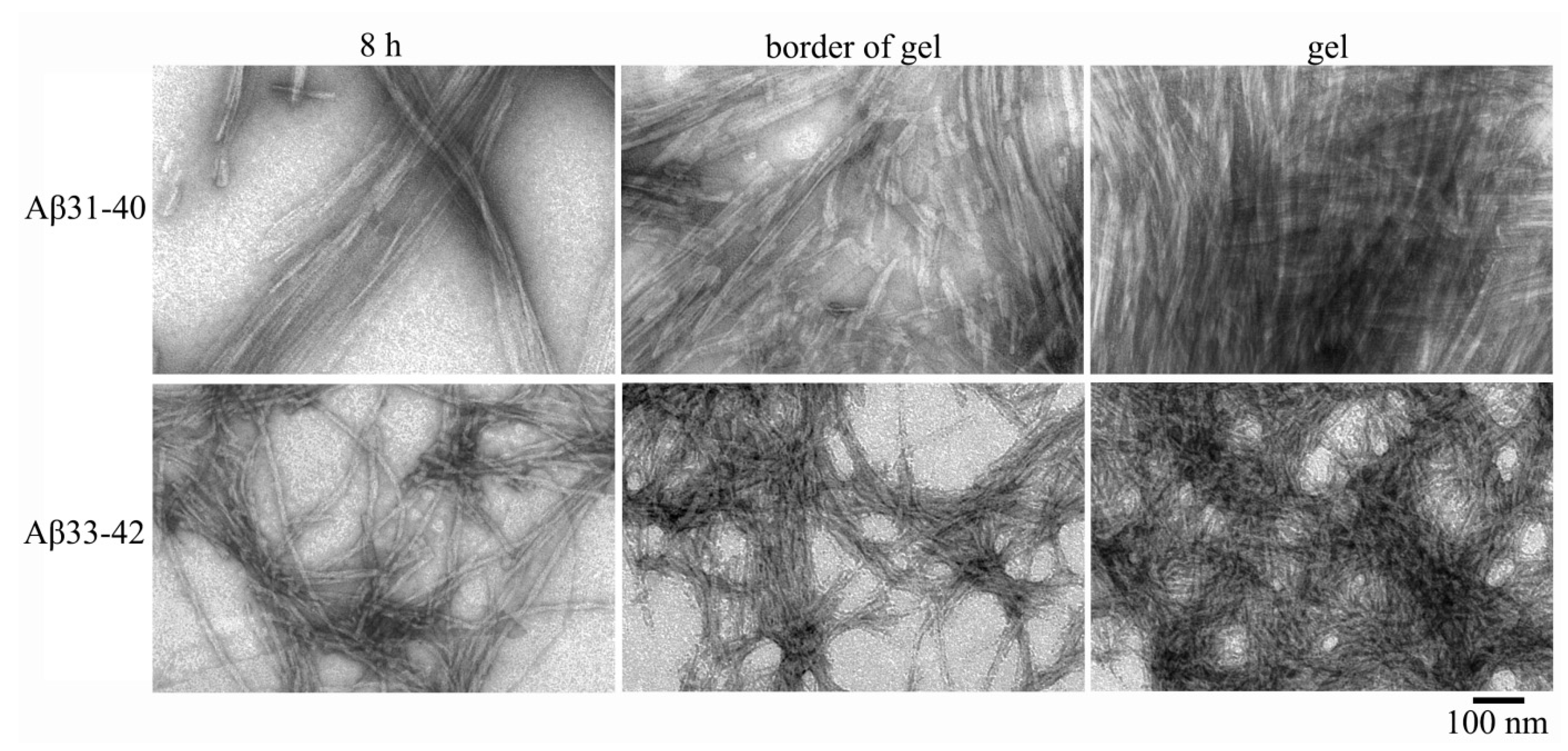
2.2. Electron Microscopy Analysis of Gels of Aβ(31–40) and Aβ(33–42) Peptides
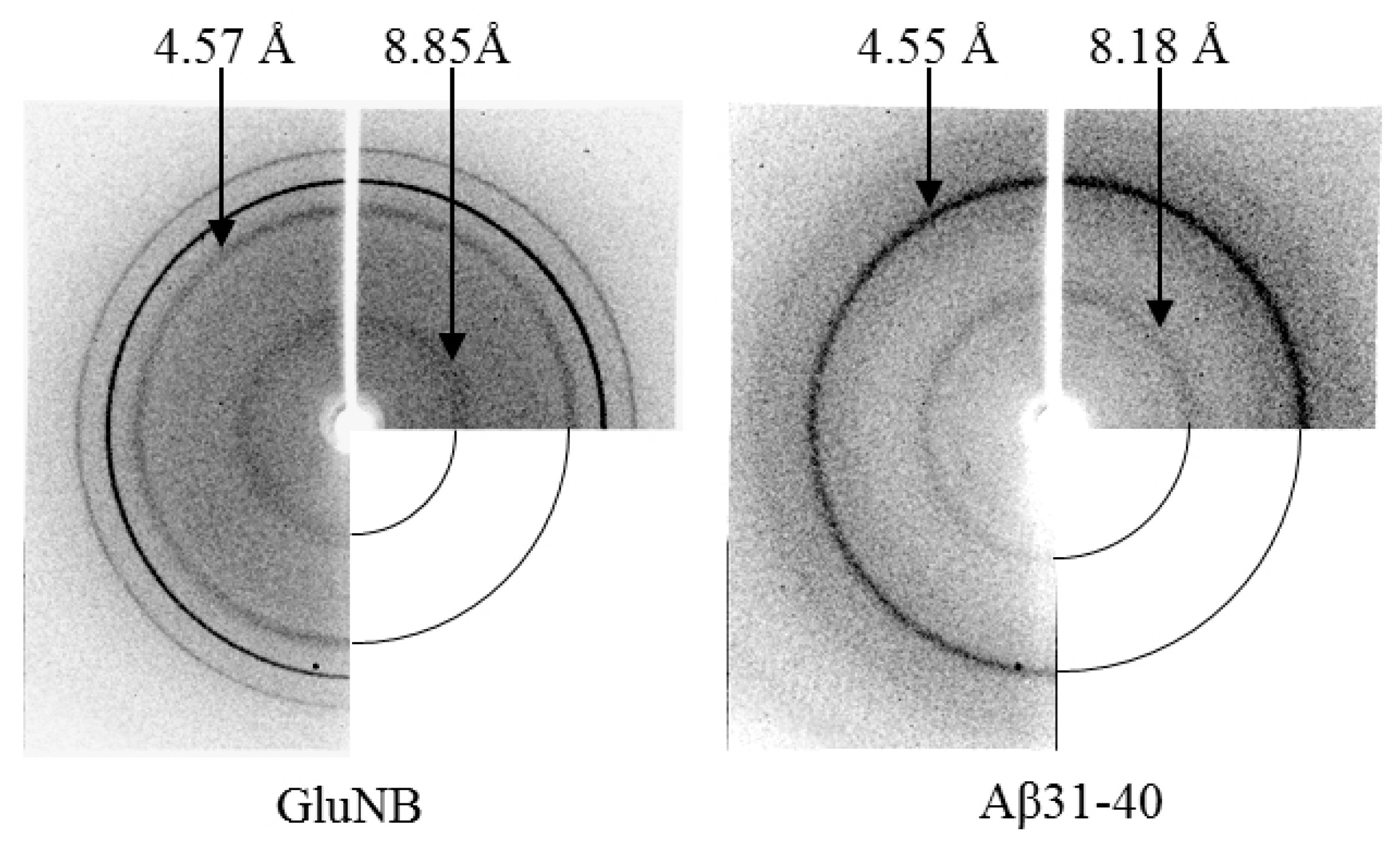
2.3. X-ray Diffraction Analysis
3. Discussion
4. Materials and Methods
4.1. Chemical Synthesis of Amyloidogenic Peptides: Aβ(31–40), Aβ(33–42), AspNB and GluNB
4.2. Samples
4.3. Electron Microscopy
4.4. X-ray Diffraction Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TEM | Transmission electron microscopy |
| AFM | Atom force microscopy |
| NMR | Nuclear magnetic resonance |
| CD | Circular dichroism |
| ThT | Thioflavin T |
| PADs | Potential amyloidogenic determinants |
References
- Chiti, F.; Dobson, C.M. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem. 2006, 75, 333–366. [Google Scholar] [CrossRef] [Green Version]
- Nizhnikov, A.A.; Antonets, K.S.; Inge-Vechtomov, S.G. Amyloids: From Pathogenesis to Function. Biochem. Biokhimiia 2015, 80, 1127–1144. [Google Scholar] [CrossRef] [PubMed]
- Maji, S.K.; Perrin, M.H.; Sawaya, M.R.; Jessberger, S.; Vadodaria, K.; Rissman, R.A.; Singru, P.S.; Nilsson, K.P.R.; Simon, R.; Schubert, D.; et al. Functional Amyloids As Natural Storage of Peptide Hormones in Pituitary Secretory Granules. Science 2009, 325, 328–332. [Google Scholar] [CrossRef] [Green Version]
- Chapman, M.R.; Robinson, L.S.; Pinkner, J.S.; Roth, R.; Heuser, J.; Hammar, M.; Normark, S.; Hultgren, S.J. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 2002, 295, 851–855. [Google Scholar] [CrossRef] [Green Version]
- Alteri, C.J.; Xicohténcatl-Cortes, J.; Hess, S.; Caballero-Olín, G.; Girón, J.A.; Friedman, R.L. Mycobacterium tuberculosis produces pili during human infection. Proc. Natl. Acad. Sci. USA 2007, 104, 5145–5150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cherny, I.; Gazit, E. Amyloids: Not only pathological agents but also ordered nanomaterials. Angew. Chem. Int. Ed. Engl. 2008, 47, 4062–4069. [Google Scholar] [CrossRef] [PubMed]
- Beaven, G.H.; Gratzer, W.B.; Davies, H.G. Formation and structure of gels and fibrils from glucagon. Eur. J. Biochem. 1969, 11, 37–42. [Google Scholar] [CrossRef]
- Aggeli, A.; Bell, M.; Boden, N.; Keen, J.N.; Knowles, P.F.; McLeish, T.C.; Pitkeathly, M.; Radford, S.E. Responsive gels formed by the spontaneous self-assembly of peptides into polymeric beta-sheet tapes. Nature 1997, 386, 259–262. [Google Scholar] [CrossRef]
- Aggeli, A.; Bell, M.; Carrick, L.M.; Fishwick, C.W.G.; Harding, R.; Mawer, P.J.; Radford, S.E.; Strong, A.E.; Boden, N. pH as a trigger of peptide beta-sheet self-assembly and reversible switching between nematic and isotropic phases. J. Am. Chem. Soc. 2003, 125, 9619–9628. [Google Scholar] [CrossRef]
- Corrigan, A.M.; Müller, C.; Krebs, M.R.H. The formation of nematic liquid crystal phases by hen lysozyme amyloid fibrils. J. Am. Chem. Soc. 2006, 128, 14740–14741. [Google Scholar] [CrossRef]
- de Groot, N.S.; Parella, T.; Aviles, F.X.; Vendrell, J.; Ventura, S. Ile-phe dipeptide self-assembly: Clues to amyloid formation. Biophys. J. 2007, 92, 1732–1741. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manno, M.; Giacomazza, D.; Newman, J.; Martorana, V.; San Biagio, P.L. Amyloid gels: Precocious appearance of elastic properties during the formation of an insulin fibrillar network. Langmuir ACS J. Surf. Colloids 2010, 26, 1424–1426. [Google Scholar] [CrossRef]
- Bhak, G.; Lee, S.; Park, J.W.; Cho, S.; Paik, S.R. Amyloid hydrogel derived from curly protein fibrils of alpha-synuclein. Biomaterials 2010, 31, 5986–5995. [Google Scholar] [CrossRef]
- Woodard, D.; Bell, D.; Tipton, D.; Durrance, S.; Cole, L.; Li, B.; Xu, S. Gel Formation in Protein Amyloid Aggregation: A Physical Mechanism for Cytotoxicity. PLoS ONE 2014, 9, e94789. [Google Scholar] [CrossRef] [Green Version]
- Shimanovich, U.; Efimov, I.; Mason, T.O.; Flagmeier, P.; Buell, A.K.; Gedanken, A.; Linse, S.; Åkerfeldt, K.S.; Dobson, C.M.; Weitz, D.A.; et al. Protein microgels from amyloid fibril networks. ACS Nano 2015, 9, 43–51. [Google Scholar] [CrossRef]
- Marchesan, S.; Vargiu, A.; Styan, K. The Phe-Phe Motif for Peptide Self-Assembly in Nanomedicine. Molecules 2015, 20, 19775–19788. [Google Scholar] [CrossRef] [Green Version]
- Medini, K.; Mansel, B.W.; Williams, M.A.K.; Brimble, M.A.; Williams, D.E.; Gerrard, J.A. Controlling gelation with sequence: Towards programmable peptide hydrogels. Acta Biomater. 2016, 43, 30–37. [Google Scholar] [CrossRef]
- Reches, M.; Gazit, E. Casting metal nanowires within discrete self-assembled peptide nanotubes. Science 2003, 300, 625–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitan, G.; Vollers, S.S.; Teplow, D.B. Elucidation of primary structure elements controlling early amyloid beta-protein oligomerization. J. Biol. Chem. 2003, 278, 34882–34889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malinchik, S.B.; Inouye, H.; Szumowski, K.E.; Kirschner, D.A. Structural analysis of Alzheimer’s beta(1-40) amyloid: Protofilament assembly of tubular fibrils. Biophys. J. 1998, 74, 537–545. [Google Scholar] [CrossRef] [Green Version]
- Jacob, R.S.; Ghosh, D.; Singh, P.K.; Basu, S.K.; Jha, N.N.; Das, S.; Sukul, P.K.; Patil, S.; Sathaye, S.; Kumar, A.; et al. Self healing hydrogels composed of amyloid nano fibrils for cell culture and stem cell differentiation. Biomaterials 2015, 54, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Selivanova, O.M.; Surin, A.K.; Marchenkov, V.V.; Dzhus, U.F.; Grigorashvili, E.I.; Suvorina, M.Y.; Glyakina, A.V.; Dovidchenko, N.V.; Galzitskaya, O.V. The Mechanism Underlying Amyloid Polymorphism is Opened for Alzheimer’s Disease Amyloid-β Peptide. J. Alzheimers Dis. JAD 2016, 54, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Galzitskaya, O.V.; Selivanova, O.M. Rosetta Stone for Amyloid Fibrils: The Key Role of Ring-Like Oligomers in Amyloidogenesis. J. Alzheimers Dis. JAD 2017, 59, 785–795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selivanova, O.M.; Surin, A.K.; Ryzhykau, Y.L.; Glyakina, A.V.; Suvorina, M.Y.; Kuklin, A.I.; Rogachevsky, V.V.; Galzitskaya, O.V. To Be Fibrils or To Be Nanofilms? Oligomers Are Building Blocks for Fibril and Nanofilm Formation of Fragments of Aβ Peptide. Langmuir ACS J. Surf. Colloids 2018, 34, 2332–2343. [Google Scholar] [CrossRef] [PubMed]
- Bezsonov, E.E.; Groenning, M.; Galzitskaya, O.V.; Gorkovskii, A.A.; Semisotnov, G.V.; Selyakh, I.O.; Ziganshin, R.H.; Rekstina, V.V.; Kudryashova, I.B.; Kuznetsov, S.A.; et al. Amyloidogenic peptides of yeast cell wall glucantransferase Bgl2p as a model for the investigation of its pH-dependent fibril formation. Prion 2013, 7, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Selivanova, O.M.; Glyakina, A.V.; Gorbunova, E.Y.; Mustaeva, L.G.; Suvorina, M.Y.; Grigorashvili, E.I.; Nikulin, A.D.; Dovidchenko, N.V.; Rekstina, V.V.; Kalebina, T.S.; et al. Structural model of amyloid fibrils for amyloidogenic peptide from Bgl2p-glucantransferase of S. cerevisiae cell wall and its modifying analog. New morphology of amyloid fibrils. Biochim. Biophys. Acta 2016, 1864, 1489–1499. [Google Scholar] [CrossRef] [PubMed]
- Dovidchenko, N.V.; Glyakina, A.V.; Selivanova, O.M.; Grigorashvili, E.I.; Suvorina, M.Y.; Dzhus, U.F.; Mikhailina, A.O.; Shiliaev, N.G.; Marchenkov, V.V.; Surin, A.K.; et al. One of the possible mechanisms of amyloid fibrils formation based on the sizes of primary and secondary folding nuclei of Aβ40 and Aβ42. J. Struct. Biol. 2016, 194, 404–414. [Google Scholar] [CrossRef]
- Galzitskaya, O.V.; Surin, A.K.; Glyakina, A.V.; Rogachevsky, V.V.; Selivanova, O.M. Should the Treatment of Amyloidosis Be Personified? Molecular Mechanism of Amyloid Formation by Aβ Peptide and Its Fragments. J. Alzheimers Dis. Rep. 2018, 2, 181–199. [Google Scholar] [CrossRef] [Green Version]
- Benditt, E.P.; Eriksen, N. Amyloid. 3. A protein related to the subunit structure of human amyloid fibrils. Proc. Natl. Acad. Sci. USA 1966, 55, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Glenner, G.G.; Keiser, H.R.; Bladen, H.A.; Cuatrecasas, P.; Eanes, E.D.; Ram, J.S.; Kanfer, J.N.; DeLellis, R.A. Amyloid. VI. A comparison of two morphologic components of human amyloid deposits. J. Histochem. Cytochem. Off. J. Histochem. Soc. 1968, 16, 633–644. [Google Scholar] [CrossRef] [Green Version]
- Bhak, G.; Lee, J.-H.; Hahn, J.-S.; Paik, S.R. Granular assembly of alpha-synuclein leading to the accelerated amyloid fibril formation with shear stress. PLoS ONE 2009, 4, e4177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dean, D.N.; Das, P.K.; Rana, P.; Burg, F.; Levites, Y.; Morgan, S.E.; Ghosh, P.; Rangachari, V. Strain-specific Fibril Propagation by an Aβ Dodecamer. Sci. Rep. 2017, 7, 40787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galzitskaya, O.V. Oligomers Are Promising Targets for Drug Development in the Treatment of Proteinopathies. Front. Mol. Neurosci. 2019, 12, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atherton, E.; Sheppard, R.C. Solid Phase Peptide Synthesis: A Practical Approach; The Practical Approach Series; IRL Press at Oxford University Press: Oxford, UK; New York, NY, USA, 1989; ISBN 978-0-19-963066-0. [Google Scholar]
- Fields, G.B.; Noble, R.L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int. J. Pept. Protein Res. 1990, 35, 161–214. [Google Scholar] [CrossRef]
- Chan, W.C.; White, P.D. (Eds.) Fmoc Solid Phase Peptide Synthesis: A Practical Approach; The Practical Approach Series; Oxford University Press: New York, NY, USA, 2000; ISBN 978-0-19-963725-6. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galzitskaya, O.V.; Selivanova, O.M.; Gorbunova, E.Y.; Mustaeva, L.G.; Azev, V.N.; Surin, A.K. Mechanism of Amyloid Gel Formation by Several Short Amyloidogenic Peptides. Nanomaterials 2021, 11, 3129. https://doi.org/10.3390/nano11113129
Galzitskaya OV, Selivanova OM, Gorbunova EY, Mustaeva LG, Azev VN, Surin AK. Mechanism of Amyloid Gel Formation by Several Short Amyloidogenic Peptides. Nanomaterials. 2021; 11(11):3129. https://doi.org/10.3390/nano11113129
Chicago/Turabian StyleGalzitskaya, Oxana V., Olga M. Selivanova, Elena Y. Gorbunova, Leila G. Mustaeva, Viacheslav N. Azev, and Alexey K. Surin. 2021. "Mechanism of Amyloid Gel Formation by Several Short Amyloidogenic Peptides" Nanomaterials 11, no. 11: 3129. https://doi.org/10.3390/nano11113129
APA StyleGalzitskaya, O. V., Selivanova, O. M., Gorbunova, E. Y., Mustaeva, L. G., Azev, V. N., & Surin, A. K. (2021). Mechanism of Amyloid Gel Formation by Several Short Amyloidogenic Peptides. Nanomaterials, 11(11), 3129. https://doi.org/10.3390/nano11113129






