Carbonized Dehydroascorbic Acid: Aim for Targeted Repair of Graphene Defects and Bridge Connection of Graphene Sheets with Small Size
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Raw Materials
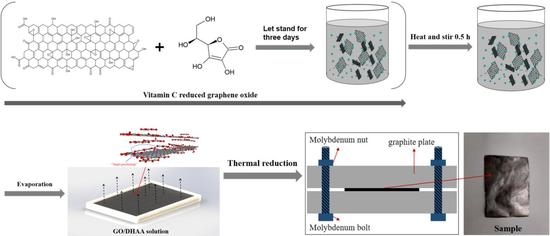
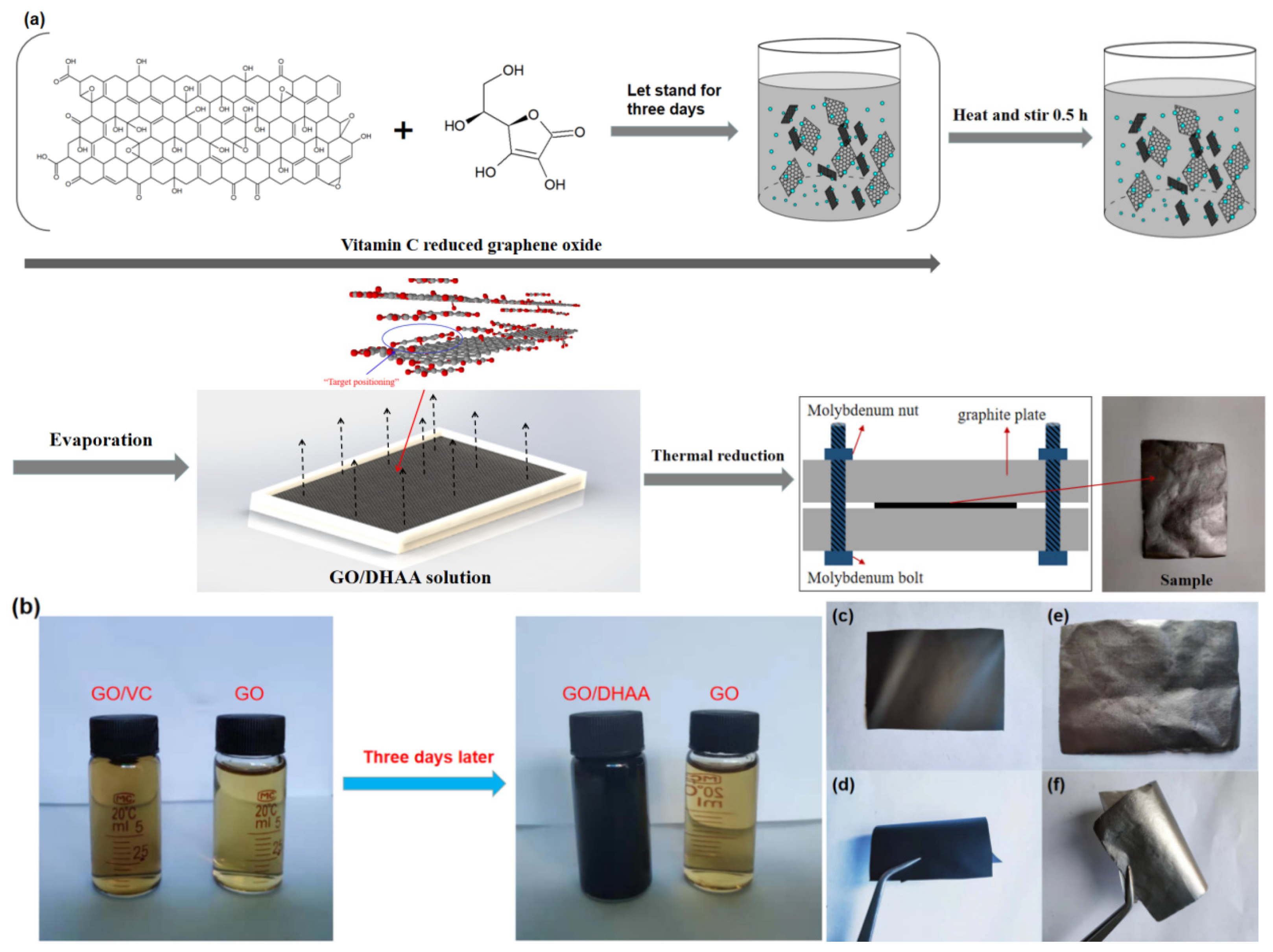
2.2. Preparation of GO/VC and rGO/VC-x% Films
2.3. Characterization
2.4. Measurement of Thermal Conductivity
3. Results and Discussion
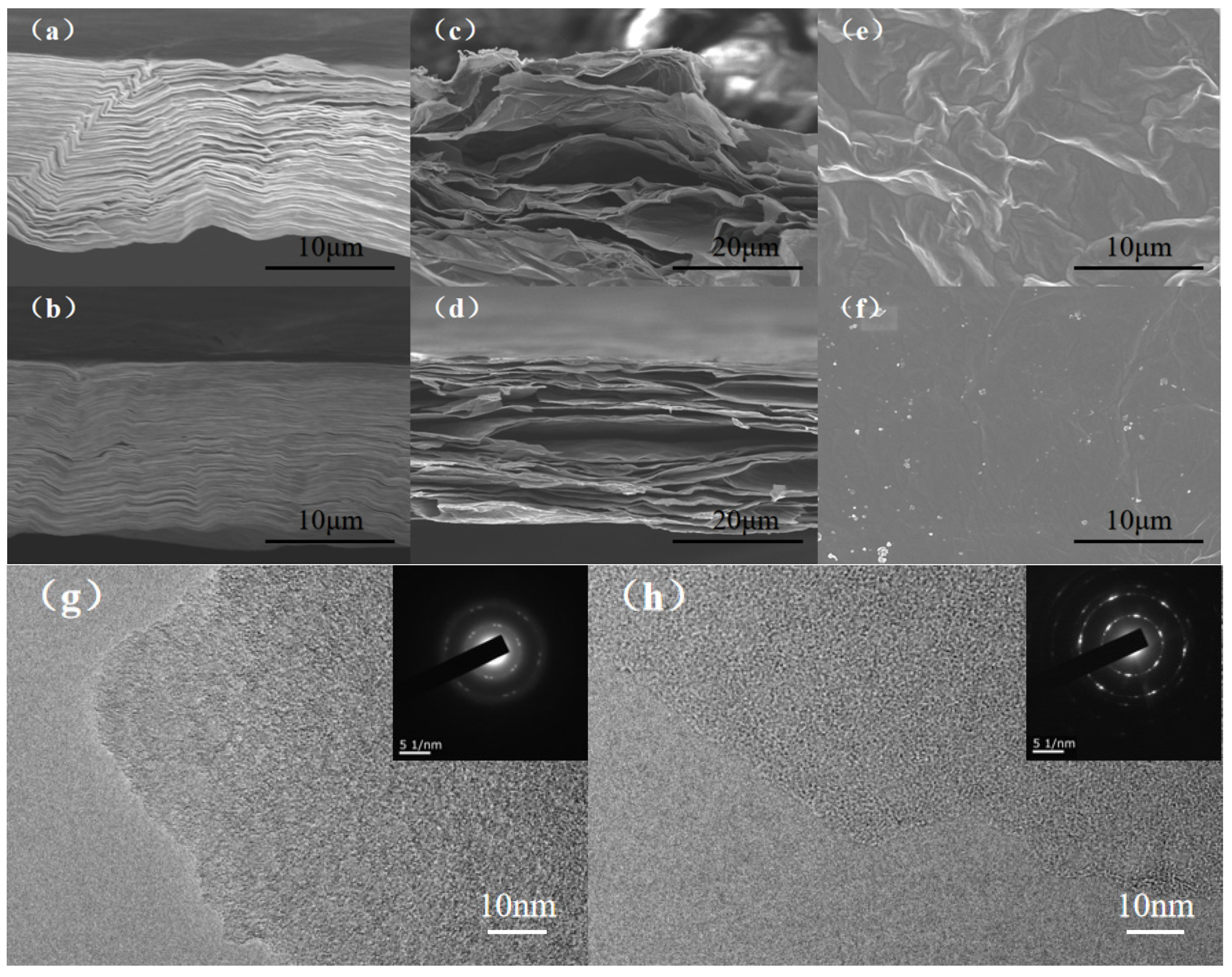
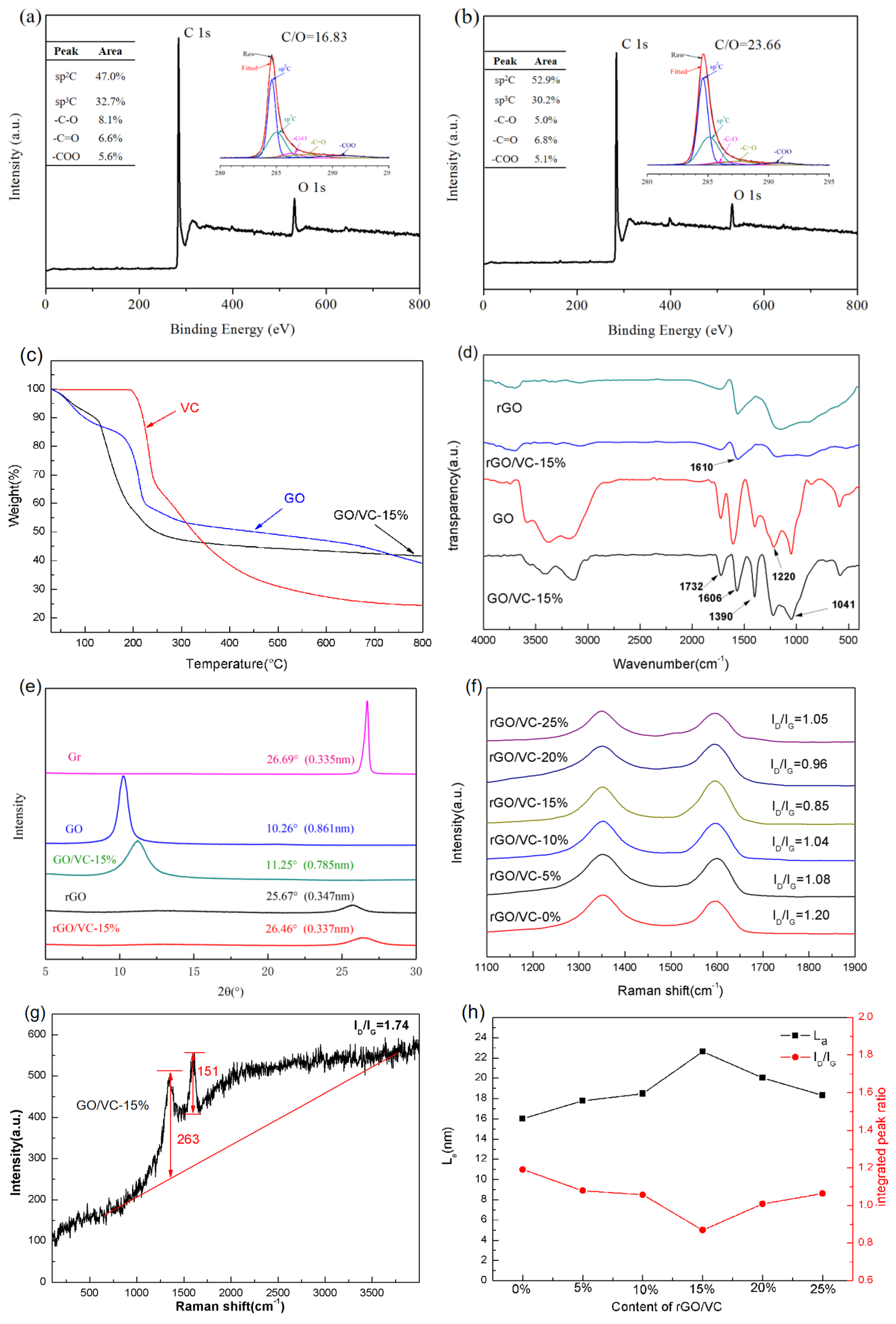
3.1. Morphology and Structural Characterizations
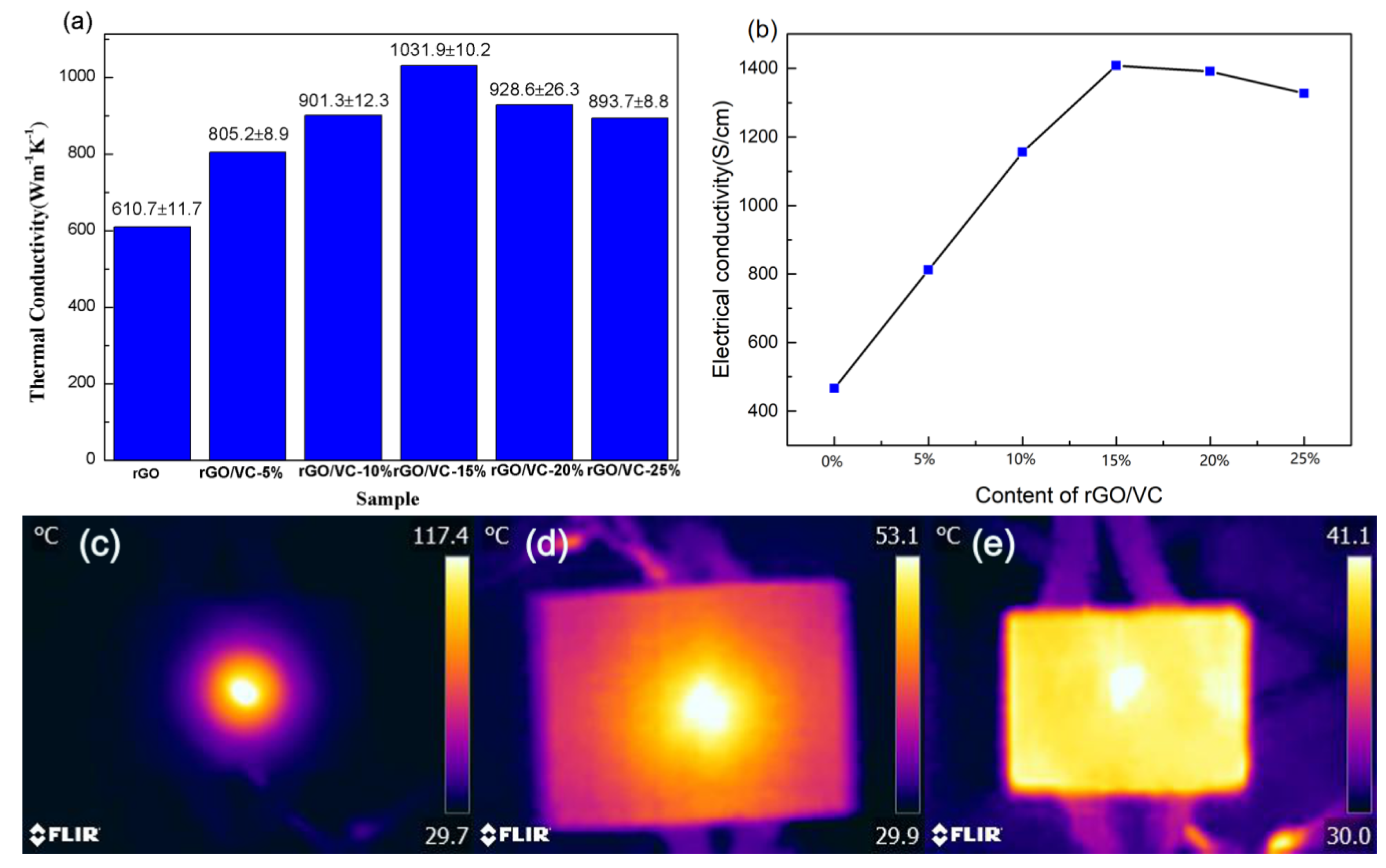
3.2. Thermal Conductivity and Infrared Surface Thermography of Modified Graphene Films
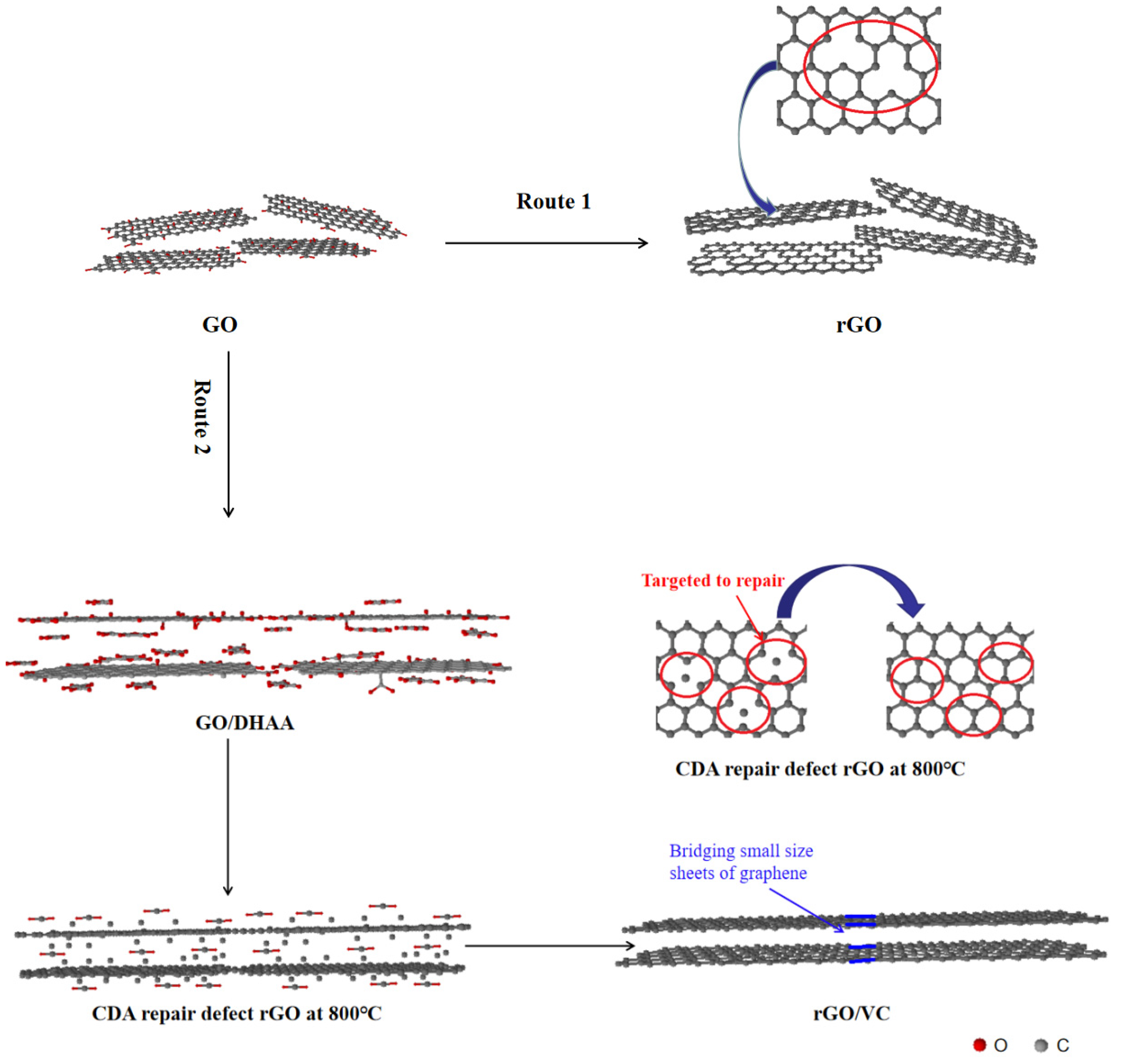
3.3. The Mechanism for the Function of Carbonized Dehydroascorbic acid for Targeted Repair of Graphene Defects and Bridge Connection of Graphene Sheets with Small Size
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Song, Y.M.; Xie, Y.; Malyarchuk, V.; Xiao, J.; Jung, I.; Choi, K.J.; Liu, Z.; Park, H.; Lu, C.; Kim, R.H. Digital cameras with designs inspired by the arthropod eye. Nature 2013, 497, 95–99. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S. Flexible electronics:sophisticated skin. Nat. Mater. 2013, 12, 871–872. [Google Scholar] [CrossRef] [PubMed]
- Belhardj, S.; Mimouni, S.; Saidane, A.; Benzohra, A. Using microchannels to cool microprocessors: A transmission-line-matrix study. Microelectron. J. 2003, 34, 247–253. [Google Scholar] [CrossRef]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Nirmalraj, P.N.; Lutz, T.; Kumar, S.; Duesberg, G.S.; Boland, J.J. Nanoscale mapping of electrical resistivity and connectivity in graphene strips and networks. Nano Lett. 2011, 11, 16–22. [Google Scholar] [CrossRef]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of single-layer graphene. Nano Lett. 2008, 8, 902–907. [Google Scholar] [CrossRef]
- Kargar, F.; Barani, Z.; Balinskiy, M.; Magana, A.S.; Lewis, J.S.; Balandin, A.A. Dual-functional graphene composites for electromagnetic shielding and thermal management. Adv. Electron. Mater. 2019, 5, 1800558. [Google Scholar] [CrossRef]
- Teng, C.; Xie, D.; Wang, J.F.; Yang, Z.; Ren, G.Y.; Zhu, Y. Ultrahigh Conductive Graphene Paper Based on Ball-Milling Exfoliated Graphene. Adv. Funct. Mater. 2017, 27. [Google Scholar] [CrossRef]
- Peng, L.; Xu, Z.; Liu, Z.; Guo, Y.; Li, P.; Gao, C. Ultrahigh Thermal Conductive yet Super Flexible Graphene Films. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Paredes, J.I.; Villar-Rodil, S.; Martinez-Alonso, A.; Tascón, J.M.D. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Bai, H.; Lu, G.; Li, C.; Shi, G. Flexible Graphene Films via the Filtration of Water-Soluble Noncovalent Functionalized Graphene Sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Shen, X.; Zheng, Q.; Yousefi, N.; Ye, L.; Mai, Y.W.; Kim, J.K. Fabrication of Highly-Aligned, Conductive, and Strong Graphene Papers Using Ultra Large Graphene Oxide Sheets. ACS Nano 2012, 6, 10708–10719. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Zhai, W.T.; Zheng, W.G. Ultrathin flexible graphene film: An excellent thermal conducting material with efficient EMI shielding. Adv. Funct. Mater. 2014, 24, 4542–4548. [Google Scholar] [CrossRef]
- Huang, Y.; Gong, Q.; Zhang, Q.; Shao, Y.; Wang, J.; Jiang, Y.; Zhao, M.; Zhuang, D.; Liang, J. Fabrication and molecular dynamics analyses of highly thermal conductive reduced graphene oxide films at ultra-high temperatures. Nanoscale 2017, 9, 2340–2347. [Google Scholar] [CrossRef]
- Nika, D.L.; Balandin, A.A. Two-dimensional phonon transport in graphene. J. Phys. Condens. Matter. 2012, 24, 233203. [Google Scholar] [CrossRef]
- Zhao, W.; Wang, Y.; Wu, Z.; Wang, W.; Bi, K.; Liang, Z.; Yang, J.; Chen, Y.; Xu, Z.; Ni, Z. Defect-engineered heat transport in graphene: A route to high efficient thermal rectification. Sci. Rep. 2015, 5, 11962. [Google Scholar] [CrossRef]
- Banhart, F.; Kotakoski, J.; Krasheninnikov, A.V. Structural defects in graphene. ACS Nano 2011, 5, 26–41. [Google Scholar] [CrossRef]
- Hu, S.; Chen, J.; Yang, N. Thermal transport in graphene with defect and doping: Phonon modes analysis. Carbon 2017, 116, 139–144. [Google Scholar] [CrossRef]
- Li, H.; Dai, S.; Miao, J.; Wu, X.; Chandrasekharan, N.; Qiu, H.; Yang, J.H. Enhanced thermal conductivity of graphene/polyimide hybrid film via a novel “molecular welding” strategy. Carbon 2018, 126, 319–327. [Google Scholar] [CrossRef]
- Wang, K.; Li, M.; Zhang, J.; Lu, H. Polyacrylonitrile coupled graphite oxide film with improved heat dissipation ability. Carbon 2019, 144, 249–258. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.H.; Yoshimura, M. Progress of reduction of graphene oxide by ascorbic acid. Appl. Surf. Sci. 2018, 447, 338–346. [Google Scholar] [CrossRef]
- Kang, D.; Shin, H.S. Control of size and physical properties of graphene oxide by changing the oxidation temperature. Carbon Lett. 2012, 13, 39–43. [Google Scholar] [CrossRef]
- Chun, K.C.; Martin, P. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Xu, Y.X.; Sheng, K.X.; Li, C.; Shi, G.Q. Highly conductive chemically converted graphene prepared from mildly oxidized graphene oxide. J. Mater. Chem. 2011, 217, 7376–7380. [Google Scholar] [CrossRef]
- Wang, N.; Samani, M.K.; Li, H.; Dong, L.; Zhang, Z.W.; Su, P.; Chen, S.J.; Chen, J.; Huang, S.R.; Yuan, G.J.; et al. Tailoring the Thermal and Mechanical Properties of Graphene Film by Structural Engineering. Small 2018, 14, 1801346. [Google Scholar] [CrossRef]
- Yamada, Y.; Yasuda, H.; Murota, K.; Nakamura, M.; Sodesawa, T.; Sato, S. Analysis of heat-treated graphite oxide by X-ray photoelectron spectroscopy. J. Mater. Sci. 2013, 48, 8171–8198. [Google Scholar] [CrossRef]
- Nika, D.L.; Balandin, A.A. Phonons and thermal transport in graphene and graphene-based materials. Rep. Prog. Phys. 2017, 80, 036502. [Google Scholar] [CrossRef]
- Lindsay, L.; Broido, D.A.; Mingo, N. Flexural phonons and thermal transport in multilayer graphene and graphite. Phys. Rev. B 2011, 83, 235428. [Google Scholar] [CrossRef]
- Peng, X.F.; Chen, K.Q. Thermal transport for flexural and in-plane phonons in graphene nanoribbons. Carbon 2014, 77, 360–365. [Google Scholar] [CrossRef]
- Chang, Y.Z.; Han, G.Y.; Xiao, Y.M.; Zhou, H.H.; Dong, J.H. A comparative study of graphene oxide reduction in vapor and liquid phases. New Carbon Mater. 2017, 32, 21–26. [Google Scholar] [CrossRef]
- Chen, W.; Yan, L. Preparation of graphene by a low-temperature thermal reduction at atmosphere pressure. Nanoscale 2010, 2, 559–563. [Google Scholar] [CrossRef]
- Chen, C.M.; Zhang, Q.; Yang, M.G.; Huang, C.H.; Yang, Y.G.; Wang, M.Z. Structural evolution during annealing of thermally reduced graphene nanosheets for application in supercapacitors. Carbon 2012, 50, 3572–3584. [Google Scholar] [CrossRef]
- Yue, L.; Li, W.S.; Sun, F.Q.; Zhao, L.Z.; Xing, L.D. Highly hydroxylated carbon fibres as electrode materials of all-vanadium redox flow battery. Carbon 2010, 48, 3079–3090. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Tan, W.K.; Kar, K.K.; Matsuda, A. Recent progress in the synthesis of graphene and derived materials for next generation electrodes of high performance lithium ion batteries. Prog. Energy Combust. Sci. 2019, 75, 100786. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, S.; Joanni, E.; Singh, R.K.; Yadav, R.M.; Verma, R.K.; Matsuda, A. A review on synthesis of graphene, h-BN and MoS2 for energy storage applications: Recent progress and perspectives. Nano Res. 2019, 12, 2655–2694. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Orfao, J.J.M. Modification of the surface chemistry of activated carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Tao, L.; Qiu, C.; Yu, F.; Yang, H.; Chen, M.; Wang, G.; Sun, L. Modification on Single-Layer Graphene Induced by Low-EnergyElectron-Beam Irradiation. J. Phys. Chem. C 2013, 117, 10079–10085. [Google Scholar] [CrossRef]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Ruoff, R.S. Chemical analysis of graphene oxide films after heat and chemical treatmentsby X-ray photoelectron and Micro-Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Tuinstra, F.; Koenig, J.L. Raman spectrum of graphite. J. Chem. Phys. 1970, 53, 1126–1130. [Google Scholar] [CrossRef]
- Cancado, L.G.; Takai, K.; Enoki, T.; Endo, M.; Kim, Y.A.; Mizusaki, H.; Jorio, A.; Coelho, L.N.; Magalhaes-Paniago, R.; Pimenta, M.A. General equation for the determination of the crystallite size La of nanographite by Raman spectroscopy. Appl. Phys. Lett. 2006, 88, 163106. [Google Scholar] [CrossRef]
- Kudin, K.N.; Ozbas, B.; Schniepp, H.C.; Prud’homme, R.K.; Aksay, I.A.; Car, R. Raman Spectra of Graphite Oxide and Functionalized Graphene Sheets. Nano Lett. 2008, 8, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Bagri, A.; Mattevi, C.; Acik, M.; Chabal, Y.J.; Chhowalla, M.; Shenoy, V.B. Structural evolution during the reduction of chemically derived graphene oxide. Nat. Chem. 2010, 2, 581–587. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Yang, R.; Zhang, L.C.; Shi, Z.W.; Yang, W.; Wang, D.M.; Xie, G.B.; Shi, D.X.; Zhang, G.Y. Restoration of graphene from graphene oxide by defect repair. Carbon 2012, 50, 2581–2587. [Google Scholar] [CrossRef]
- Ma, T.; Liu, Z.B.; Wen, J.X.; Gao, Y.; Ren, X.B.A.; Chen, H.J.; Jin, C.H.; Ma, X.L.; Xu, N.S.; Cheng, H.M.; et al. Tailoring the thermal and electrical transport properties of graphene films by grain size engineering. Nat. Commun. 2017, 8, 14486. [Google Scholar] [CrossRef]
- Balandin, A.A. Thermal properties of graphene and nanostructured carbon materials. Nat. Mater. 2011, 10, 569–581. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, G.; Liu, C.H.; Fan, S.S. High-density carbon nanotube buckypapers with superior transport and mechanical properties. Nano Lett. 2012, 12, 4848–4852. [Google Scholar] [CrossRef]
- Renteria, J.D.; Ramirez, S.; Malekpour, H.; Alonso, B.; Centeno, A.; Zurutuza, A.; Cocemasov, A.I.; Nika, D.L.; Balandin, A.A. Strongly anisotropic thermal conductivity of free-standing reduced graphene oxide films annealed at high temperature. Adv. Funct. Mater. 2015, 25, 4664–4672. [Google Scholar] [CrossRef]
- Gao, J.; Liu, F.; Liu, Y.L.; Ma, N.; Wang, Z.Q.; Zhang, X. Environment-friendly method to produce graphene that employs vitamin C and amino acid. Chem. Mater. 2010, 22, 2213–2218. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Lai, J.; Liu, J.; Lei, R.; Chen, Y. Carbonized Dehydroascorbic Acid: Aim for Targeted Repair of Graphene Defects and Bridge Connection of Graphene Sheets with Small Size. Nanomaterials 2020, 10, 531. https://doi.org/10.3390/nano10030531
Li J, Lai J, Liu J, Lei R, Chen Y. Carbonized Dehydroascorbic Acid: Aim for Targeted Repair of Graphene Defects and Bridge Connection of Graphene Sheets with Small Size. Nanomaterials. 2020; 10(3):531. https://doi.org/10.3390/nano10030531
Chicago/Turabian StyleLi, Jing, Jinfeng Lai, Jialiang Liu, Rubai Lei, and Yuxun Chen. 2020. "Carbonized Dehydroascorbic Acid: Aim for Targeted Repair of Graphene Defects and Bridge Connection of Graphene Sheets with Small Size" Nanomaterials 10, no. 3: 531. https://doi.org/10.3390/nano10030531
APA StyleLi, J., Lai, J., Liu, J., Lei, R., & Chen, Y. (2020). Carbonized Dehydroascorbic Acid: Aim for Targeted Repair of Graphene Defects and Bridge Connection of Graphene Sheets with Small Size. Nanomaterials, 10(3), 531. https://doi.org/10.3390/nano10030531