Abstract
Transition metal oxide is one of the most promising anode materials for lithium-ion batteries. Generally, the electrochemical property of transition metal oxides can be improved by optimizing their element components and controlling their nano-architecture. Herein, we designed nonstoichiometric Cu0.6Ni0.4Co2O4 nanowires for high performance lithium-ion storage. It is found that the specific capacity of Cu0.6Ni0.4Co2O4 nanowires remain 880 mAh g−1 after 50 cycles, exhibiting much better electrochemical performance than CuCo2O4 and NiCo2O4. After experiencing a large current charge and discharge state, the discharge capacity of Cu0.6Ni0.4Co2O4 nanowires recovers to 780 mAh g−1 at 50 mA g−1, which is ca. 88% of the initial capacity. The high electrochemical performance of Cu0.6Ni0.4Co2O4 nanowires is related to their better electronic conductivity and synergistic effect of metals. This work may provide a new strategy for the design of multicomponent transition metal oxides as anode materials for lithium-ion batteries.
1. Introduction
Lithium-ion batteries (LIB) have many advantages, such as high energy density, excellent cycle stability, no memory effect, and so on [1]. They are widely used in many fields, such as electronic products, electric vehicles, and energy storage system, which have greatly improved modern humans’ lives [2]. The energy density of LIB is closely related to the lithium storage performance and voltage of the anode materials. The transition metal oxide Co3O4 used as a promising anode material for lithium-ion batteries, first reported by Poizot [3] in 2000, has attracted extensive interest of researchers with 890 mAh g−1 theoretical capacity, which is much higher than commercial graphite (372 mAh g−1). Different from lithium intercalation mechanism of graphite, Co3O4 is based on a conversion reaction as anode materials transforms into metallic cobalt, with great volume expansion and metal reunion during lithiation/deithiation [4].
Due to the inherent defects of the material, many researchers attempt to enhance the electrochemical performance of Co3O4 by controlling their microstructures and optimizing their components [5]. On the one hand, great efforts have been devoted to the morphological control of Co3O4, and therefore, many of the different shaped Co3O4 nanostructures, such as peapod-like [6], yolk-shell [7], hollow nanofibers [8], nanowires [9,10], other complex structures [11,12,13,14,15,16,17,18] have been developed and applied as anode materials in lithium-ion batteries. Compared with other nanostructures, nanowires have large open space, which allows electrolytes to diffuse into the electrode inner region and shorten the solid-phase diffusion path of lithium ions. Moreover, the mechanical flexibility and Young’s modulus of nanowires are significant for micro-flexible electronic components [19]. For example, Guan [9] synthesized 1D Co3O4 mesoporous nanowires, grown on Ni foam, whose specific capacity was approximately 1600 mAh g−1 at current density of 0.5 A g−1. Zeng [20] designed quasi-single-crystalline mesoporous Co3O4 and CoO nanowires from precursor Co(Co3)0.5(OH) and the CoO nanowires remain nearly 100% capacity retention after 70 cycles.
On the other hand, it is a viable strategy to design transition-metal oxide composites for enhancing lithium storage performance [21]. It has been reported that the composites of bimetal oxide showed much higher electrochemical performance than the single ones due to low activation energy for electron transfer between cations [10,22,23]. MCo2O4 (M = Ni [24,25,26,27], Cu [28], Fe [29], Zn [30], Mn [31]) have been widely used as anode materials over the last few years. Lou [26] synthesized NiCo2O4 complex hollow spheres as anode material in lithium-ion battery and its specific capacity was 1400 mAh g−1 at 150 mA g−1 with great cycling stability. Jiang [32] reported CuCo2O4 nanoparticle showed high reversible capacity of 1040 mAh g−1 at 0.1 C. Cao [33] designed ultrathin ZnCo2O4 nanosheets for lithium anode with excellent long life (about 900 mAh g−1 after 200 cycles at 200 mA g−1 current density).
Herein, we report nonstoichiometric Cu0.6Ni0.4Co2O4 nanowires, which can be regarded as a composite of CuCo2O4 and NiCo2O4, as an anode material in lithium-ion battery. As we know, such composites, as an anode material in lithium-ion area has not been reported yet. Owing to the relatively low activation energy for electron transfer between Cu2+/Cu, Ni2+/Ni3+ and Co2+/Co3+, the electronic conductivity and cycling stability for Cu0.6Ni0.4Co2O4 nanowires are significantly improved. In the first cycle, discharge and charge capacities are 1120 mAh g−1 and 972 mAh g−1 at 50 mA g−1, respectively. After 50 cycles, the specific capacity of Cu0.6Ni0.4Co2O4 nanowires remain 880 mAh g−1 and the coulombic efficiency is approximately 100%.
2. Experimental
2.1. Synthesis of Anode Materials
All the reagents (Tianjin Damao Chemical Reagent Factory, Tianjin, China) were analytically pure without further treatment. Similar preparation methods could be referred to our previous work [34]. To prepare the Cu0.6Ni0.4Co2O4 nanowires, 4 mmol cobalt acetate was dissolved in 30 mL ultrapure water. Subsequently, 4 mmol lauryl sodium sulfate, 1.2 mmol copper chloride, and the 0.8 mmol nickel chloride were added to the above cobalt acetate solution in sequence. Then, 48 mmol hexamethylenetetramine was dissolved in 30 mL ultrapure water to form a transparent solution which was dropped into the above mixed solution slowly. After stirring for 1 h, the resultant solution was poured into a 100 mL Teflon-lined stainless autoclave. Then the autoclave was heated at 120 °C for 12 h in oven. The obtained sediment was filtered, rinsed and dried. Finally, after calcination of the powder at 600 °C for 2 h in muffle, Cu0.6Ni0.4Co2O4 was obtained. Similarly, NiCo2O4 and CuCo2O4 were obtained by adjusting the addition amount of copper chloride and nickel chloride.
2.2. Characterizations
Crystal information of the samples was acquired by Bruker D8 Discover X-ray diffractometer (XRD, Tokyo, Japan) with Cu Kα radiation (λ = 1.5406Å). Microstructures, particle sizes and element mapping were analyzed by using a JEOL-7100F field-emission scanning electron microscope (FESEM, Hitachi, Japan). Interior structure was observed by a JEOL JEM-2100F transmission electron microscope (TEM, FEI, Hillsboro, OR, USA). The elements and chemical states of the sample were analyzed by a Kratos Axis Ultra DLD X-ray photoelectron spectrometer (XPS, VG, Manchester, UK). The molar ratios were determined with an Agilent 7800 Inductively Coupled Plasma (ICP) Mass Spectrometry (Thermo Fisher, Waltham, MA, US).
2.3. Electrochemical Performance Test
The 2032-coin battery is assembled in a glove box filled with inert atmosphere for electrochemical performance test. The fabricated electrode consisted of active materials, acetylene black, polyvinylidene fluoride (PVDF) with a mass ratio of 7:2:1. Each component was well mixed to form a paste, which was coated on copper foil current collector. The loading on the electrode is approximately 1 mg cm−2. The electrolyte used was 1.0 M LiPF6 in a 50:50 (w/w) mixture of ethylene carbonate (EC) and diethyl carbonate (DEC). The specific capacity of the battery was tested by cyclic charge and discharge between 0–3 V (Land CT2001A, Hubei, China). The cyclic voltammetry and electrochemical impedance test were obtained by using an electrochemical workstation (CHI 760E, CH Instrument Ins., Shanghai, China).
3. Results and Discussion
The X-ray diffraction (XRD) patterns of the NiCo2O4, CuCo2O4, and Cu0.6Ni0.4Co2O4 powders are shown in Figure 1. It is discovered that the characteristic peaks of the NiCo2O4 and CuCo2O4 are very similar. All the diffraction peaks of the NiCo2O4, CuCo2O4 powders correspond to the (220), (311), (222), (400), (422), (511), and (440) plane reflections of cubic spinel NiCo2O4 or CuCo2O4. Cu0.6Ni0.4Co2O4 has similar characteristic peaks as NiCo2O4 [35] and CuCo2O4. We can regard Cu0.6Ni0.4Co2O4 as a composite of NiCo2O4 and CuCo2O4 [36]. The peak at 25° for anode materials is attributed to the disorderedly stacked carbon, derived from incomplete decomposition of organic ingredients [37]. There is a small peak in NiCo2O4 at 44° indexing as NiO (JCPDS NO. 65-2901), which is also found in previous literature [38]. The peak at 33° for CuCo2O4 may be indexed as CuO (JCPDS NO. 44-0706). According to the Scherer’s formula, the average crystal size of a material can be calculated [39,40],
where D is the average gain size, λ is X-ray wavelength and constant of 0.154 nm, β is half-height width of diffraction peak, and θ is Bragg diffraction angle. The average crystalline sizes of NiCo2O4 and CuCo2O4 are 16.5, and 24.9 nm, respectively. Meanwhile, that of Cu0.6Ni0.4Co2O4 is 18.0 nm, which is between 16.5 nm and 24.9 nm, indicating that the formation of composite of NiCo2O4 and CuCo2O4 will not result in a pronounced change in crystalline size.
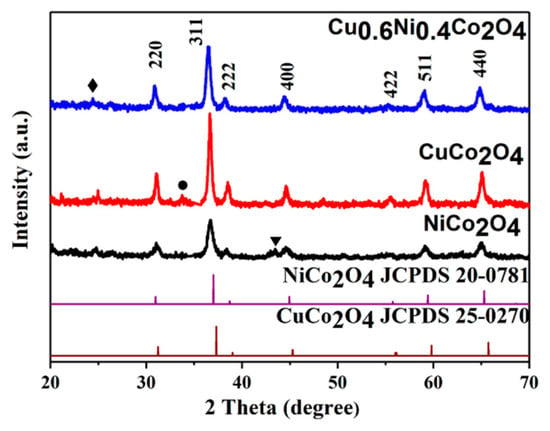
Figure 1.
X-ray diffraction (XRD) pattern of the NiCo2O4, CuCo2O4, and Cu0.6Ni0.4Co2O4 powders.
Scanning electron microscopy (SEM) images are used to observe the morphology and structures of materials, as shown in Figure 2. Apparently, the samples of the NiCo2O4 and Cu0.6Ni0.4Co2O4 consist of nanowires with an average diameter of ca. 50 nm. Interestingly, all these nanowires are assembled by small nanoparticles. The structure of CuCo2O4 is nanoparticles with an average diameter of ca. 30 nm. The TEM images of three anode materials in Figure 3, present the inner structures for NiCo2O4, Cu0.6Ni0.4Co2O4, and CuCo2O4. The anode materials are solid instead of hollow. Besides, inner structure of Cu0.6Ni0.4Co2O4 is similar with NiCo2O4 rather than CuCo2O4. Figure 3g is high resolution transmission electron microscope (HRTEM) images of the Cu0.6Ni0.4Co2O4. The lattice spacing of ca. 0.23 nm, 0.2 nm, 0.28 nm, and 0.24 nm can be seen, corresponding to the crystal surfaces (222), (400), (220), and (311) planes of cubic spinel Cu0.6Ni0.4Co2O4. Element mapping of Cu0.6Ni0.4Co2O4 is measured in Figure 4. The elements of Co, Ni, Cu, and O can be found in the mapping picture, indicating that these elements are uniformly distributed. The result of ICP measure shows the ratio of Cu, Ni, and Co is 0.61:0.41:1.99, which are well match with the designed values.
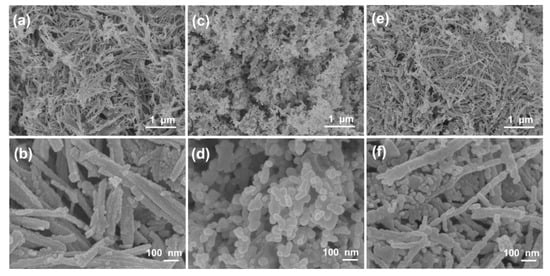
Figure 2.
Scanning electron microscopy (SEM) images of (a,b) NiCo2O4, (c,d), CuCo2O4 and (e,f) Cu0.6Ni0.4Co2O4.
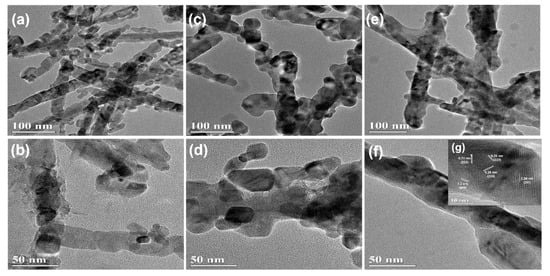
Figure 3.
Transmission electron microscopy (TEM) image of (a,b) NiCo2O4, (c,d) CuCo2O4 and (e,f) Cu0.6Ni0.4Co2O4; (g) the inset showing the HRTEM image of Cu0.6Ni0.4Co2O4.
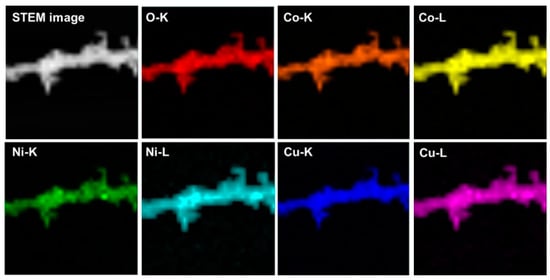
Figure 4.
Elementary mapping of Cu0.6Ni0.4Co2O4.
In order to investigate the elements and chemical states on the surface of the anode materials, XPS analysis is carried out and the results are shown in Figure 5. In the XPS survey spectrum of Cu0.6Ni0.4Co2O4 (Figure 5c), the signals of elements of Co, Ni, and Cu can be observed, which was different from those of NiCo2O4 and CuCo2O4 (Figure 5a,b), as the former has both Cu and Ni elements, and the three transition elements Cu, Ni, and Co will affect the outer electronic structure of each other. As shown in Figure 5a,b, no signal of Ni is found in CuCo2O4, while the Cu signal was absent in NiCo2O4, which are as expected. Figure 5d–f is the Ni 2p, Cu 2p, and Co 2p spectrum of the anode materials, respectively. There are six peaks in Ni 2p spectrum for NiCo2O4 and Cu0.6Ni0.4Co2O4. The peaks are at 854.2 eV and 871.5 eV of Ni2+, and 855.8 eV and 872.8 eV of Ni3+ for Cu0.6Ni0.4Co2O4. Comparing to the peaks for NiCo2O4, binding energy of Ni 2p for Cu0.6Ni0.4Co2O4 are stronger. The peak areas mean the relative content of element valence. It’s noted that the peak area of them is various, which shows the relative content of Ni2+ is lower in Cu0.6Ni0.4Co2O4. It indicates the amount of Ni2+ decreases in the presence of Cu2+. There are four peaks in Cu 2p spectrum for CuCo2O4 and Cu0.6Ni0.4Co2O4, indicating copper is divalent. Six peaks of Co 2p for the three materials can be found, manifesting the cobalt is trivalent and divalent. Similar, binding energy of Cu 2p, Co 2p for Cu0.6Ni0.4Co2O4 are stronger than CuCo2O4, and NiCo2O4, respectively. The peak positions are corresponding to previous reports [32,39,41], indicating that Cu0.6Ni0.4Co2O4 is prepared successfully and Cu2+ reduces the content of Ni2+. The electron density of Cu0.6Ni0.4Co2O4 in the outermost shell is affected by doping elements.
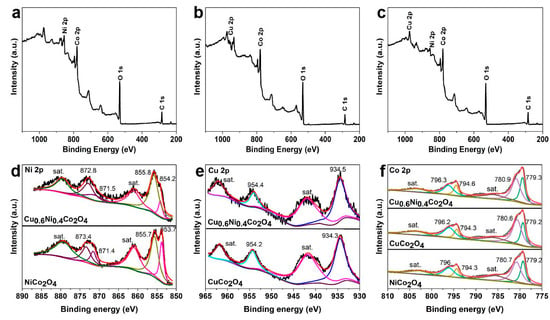
Figure 5.
XPS spectra of (a) NiCo2O4, (b) CuCo2O4, (c) Cu0.6Ni0.4Co2O4 and (d) Ni 2p of NiCo2O4 and Cu0.6Ni0.4Co2O4, (e) Cu 2p of CuCo2O4 and Cu0.6Ni0.4Co2O4, (f) Co 2p of the three materials.
Transition metal oxides undergo a conversion reaction in the lithiation, with the change in metal valence and the formation of solid electrolyte interface (SEI) layer and Li2O [3]. Figure 6 shows the CV test of NiCo2O4, CuCo2O4, and Cu0.6Ni0.4Co2O4 anode electrodes for the first three curves at a scan of 0.2 mV s−1 between 0.01 and 3 V. For the NiCo2O4 anode material, during the first scan, the main cathodic peaks locate in 0.7 V corresponding to the reduction of NiCo2O4 to metallic Ni and Co (Equation (2)), and the broad peaks at ca. 0.37 V corresponds to SEI layer [42]. The anode peaks are at the 1.63 V and 2.3 V, corresponding to oxidation of metallic Ni0 to Ni2+ and Co to Co3+, respectively. (Equations (5), (7) and (8)). For the CuCo2O4, during the initial scan, the main cathodic peaks locate in 0.75 V, corresponding to the reduction of CuCo2O4 to metallic Cu and Co (Equation (3)). It is mentioned that there are two peaks at ~0.5 V and ~0.6 V, revealing formation of SEI layer. The anode peaks were at the 1.1 V, 1.5 V and 2.2 V, corresponding to oxidation of metallic Cu and Co to CuCo2O4 (Equations (6), (7) and (8), respectively). For Cu0.6Ni0.4Co2O4, during the first scan, the main cathodic peaks locate in 1.2 V and 0.8 V, assigning to reduction of metal oxide and formation of SEI film (Equation (4)). The anode peaks are at the 1.63 V and 2.3 V, corresponding to oxidation of metallic Ni, Cu and Co to Cu0.6Ni0.4Co2O4 (Equations (5)–(8)). At the same time, the peaks of Cu0.6Ni0.4Co2O4 are narrower and stronger than those of NiCo2O4 and CuCo2O4. This can be due to co-work of Co, Cu, and Ni, which stabilizes the structure and enhance the electronic conductivity in the process of electrochemical reaction, resulting in a longer cycle life and a lower potential polarization. The different positions of the peaks reveal that the REDOX potential can be changed slightly by composition and content of metal oxide in the chemical reaction. The first cathodic scan of the three anode materials, in addition to the reduction of metals, the formation of SEI film and Li2O were occurred, so the second anode scan is quite different from the first one [43,44]. While, the second cycle anode scan of the three materials is roughly the same as the first cycle, demonstrating good cycle performance. The total reaction equations during charging and discharging are described as following:
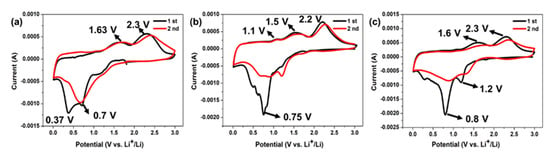
Figure 6.
Cyclic voltammetry measure of (a) NiCo2O4, (b) CuCo2O4, and (c) Cu0.6Ni0.4Co2O4.
Figure 7 shows specific capacities of the NiCo2O4, CuCo2O4, Cu0.6Ni0.4Co2O4 at the current density of 50 mA g−1 between 0.01 and 3 V (vs. Li+/Li). The first discharge specific capacities of NiCo2O4, CuCo2O4, and Cu0.6Ni0.4Co2O4 are 950, 901, and 1120 mAh g−1, respectively (Figure 7a). The first charge specific capacities of NiCo2O4, CuCo2O4, and Cu0.6Ni0.4Co2O4 are 785, 633, and 972 mAh g−1, there are large specific capacity losses in the first charge/discharge cycle. We attribute them to some irreversible electrochemical reactions, including the SEI film formation, electrolyte decomposition, and so on. This phenomenon can be observed for most anode materials, which leads to the low coulombic efficiency in the first cycle and explains a large difference in the peak position of the reduction peak between the first and the second cycles in the CV test. The NiCo2O4 electrode shows a specific discharge capacity of 743 mAh g−1 in second cycle, and the specific capacity declines to 245 mAh g−1 after 50 cycles. The CuCo2O4 electrode shows a specific discharge capacity of 690 mAh g−1 at the second cycle, while the specific capacity is attenuated to 375 mAh g−1 after 50 cycles. The Cu0.6Ni0.4Co2O4, which can be regarded as a compound of CuCo2O4 and NiCo2O4, exhibits the excellent electrochemical performance. The specific discharge capacity of Cu0.6Ni0.4Co2O4 is maintained 880 mAh g−1 from the second to the fiftieth cycle, and the coulombic efficiency close to 100%. As shown in Figure 7b, the change of the specific capacity of the three samples with the number of cycles is clearly visible. The capacities of NiCo2O4 and CuCo2O4 material go through a huge decay after 20 cycles, which is due to material pulverization. Meanwhile, Cu0.6Ni0.4Co2O4′s capacity has been well-maintained. The electrochemical stability is significantly improved because of better electronic conductivity of Cu0.6Ni0.4Co2O4 and the synergistic effect of the elements. Due to the various insertion voltages for the metal oxides, the other inactive metal oxides can be “volume-buffering reservoir” to release volume stress when the active metal oxide is in a lithiation/delithiation process at a certain voltage [45]. The capacity rate performance of Cu0.6Ni0.4Co2O4 was also studied and the results are showed in Figure 7c. The charge/discharge current density gradually increases to 2000 mA g−1 and then returns to 50 mA g−1. The charge-discharge voltage platform was found to be almost kept same. The stable reversible discharge capacities decrease from 830 to 150 mAh g−1 as the current density increases from 200 mA g−1 to 2000 mA g−1. Furthermore, when the current density is restored to 50 mA g−1, the discharge capacity is 780 mAh g−1, which is ca. 88% of the initial discharge capacity. These results demonstrate that the Cu0.6Ni0.4Co2O4 has a better rate performance and a higher cycling stability.
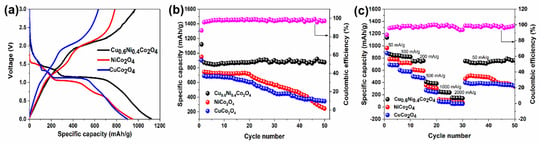
Figure 7.
Electrochemical tests of the three samples: (a) Initial charge and discharge curves profiles for NiCo2O4, CuCo2O4, Cu0.6Ni0.4Co2O4 at the current of 50 mA g−1 between 0.01 and 3 V (vs. Li+/Li). (b) Cycling performance of the three powders at a current of 50 mA g−1. (c) Capacity rate performance of the anode materials.
To further compare the properties of the materials and the modification effects. The charge transfer behaviors of NiCo2O4, CuCo2O4, and Cu0.6Ni0.4Co2O4 are measured according to electrochemical impedance spectroscopies (EIS). The Nyquist plots of the electrodes, with frequency range between 0.01 Hz to 100 kHz are shown in Figure 8. All plots make up a semicircle and a sloped line, the fitting circuit is shown insert. In the high intermediate frequency region, the diameter of the semicircle indicates charge transfer resistance (Rct) in the interface between the electrolyte and grains of active material [46]. It is easy to see that the diameter of the semicircle of Cu0.6Ni0.4Co2O4 is much smaller than the diameter of NiCo2O4 and CuCo2O4. The electron transfer resistance of Cu0.6Ni0.4Co2O4 (120 Ω) is less than of NiCo2O4 (300 Ω) and CuCo2O4 (380 Ω), which shows Cu0.6Ni0.4Co2O4 sample has the lowest charge-transfer resistance. The low-frequency line stands for the Warburg resistance (Wo) related to the lithium ions diffusion in electrode materials. The line slope of Cu0.6Ni0.4Co2O4 is larger than of NiCo2O4 and CuCo2O4, indicating faster solid-state diffusion of lithium ion is in the Cu0.6Ni0.4Co2O4. This evidence further proves that Cu0.6Ni0.4Co2O4 has better electrochemical performance and lower charge transfer resistance due to co-work of Cu2+, Ni2+/Ni3+ and Co2+/Co3+.
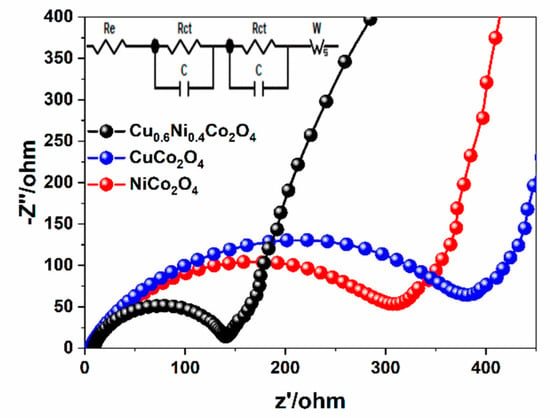
Figure 8.
Nyquist plots for the three electrodes in the frequency range from 100 kHz to 0.01 Hz.
4. Conclusions
In this work, Cu0.6Ni0.4Co2O4 nanowires, with high electrochemical performance, have been synthesized by a simple and scalable hydrothermal approach. The material shows initial specific discharge and charge capacities of 1120 and 972 mAh g−1 at current density of 50 mA g−1. After 50 cycles, its discharge capacity maintains 880 mAh g−1, and the coulombic efficiency is close to 100%. After a high current charge/discharge test, the reversible specific capacity can still be restored to 780 mAh g−1 at 50 mA g−1, which is ca. 88% of the initial capacity. EIS and CV tests have proved that co-doping with appropriate amount of Cu and Ni could significantly enhance ion diffusion and electron conduction during charge-discharge process. We attribute the high performance to relatively low activation energy for electron transfer between Cu2+, Ni2+/Ni3+ and Co2+/Co3+, the electronic conductivity and structure stability for Cu0.6Ni0.4Co2O4 nanowires are improved greatly. Therefore, it is important to further research into other multi-component transition metal oxides as anode materials in lithium battery.
Author Contributions
Synthesis of the sample, writing—original draft preparation, J.L. (Junhao Li); investigation of the electrochemical performance, N.J.; characterization and analysis of the sample, J.L. (Jinyun Liao) and Y.F.; Supervision, funding acquisition and writing—review and editing, Q.L. and H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (Nos. 21606050, U1801257, 21975056), the Natural Science Foundation of Guangdong Province (No. 2018A030313859), Pearl River Science and Technology New Star Project (No. 201806010039), the Major Project of Fundamental and Application Research of the Department of Education of Guangdong Province (No. 2017KZDXM079), the Science and Technology Project of Huizhou City (No. 2017C0412028), the Natural Science Foundation of Huizhou University (No. 20180927172750326).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: A perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Poizot, P.; Laruelle, S.; Grugeon, S.; Dupont, L.; Tarascon, J.-M. Nano-sized transition-metal oxides as negative-electrode materials for lithium-ion batteries. Nature 2000, 407, 496–499. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba, R.G.V.; Chowdari, B.V. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef]
- Lou, X.W.; Deng, D.; Lee, J.Y.; Archer, L.A. Thermal formation of mesoporous single-crystal Co3O4 nano-needles and their lithium storage properties. J. Mater. Chem. 2008, 18, 4397. [Google Scholar] [CrossRef]
- Gu, D.; Li, W.; Wang, F.; Bongard, H.; Spliethoff, B.; Schmidt, W.; Weidenthaler, C.; Xia, Y.; Zhao, D.; Schüth, F. Controllable Synthesis of Mesoporous Peapod-like Co3O4@Carbon Nanotube Arrays for High-Performance Lithium-Ion Batteries. Angew. Chem. Int. Ed. 2015, 54, 7060–7064. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Meng, J.; Li, Q.; Niu, C.; Wang, X.; Yang, W.; Li, W.; Mai, L. Interface-modulated fabrication of hierarchical yolk–shell Co3O4/C dodecahedrons as stable anodes for lithium and sodium storage. Nano Res. 2017, 10, 2364–2376. [Google Scholar] [CrossRef]
- Yan, C.; Chen, G.; Zhou, X.; Sun, J.; Lv, C. Template-Based Engineering of Carbon-Doped Co3O4 Hollow Nanofibers as Anode Materials for Lithium-Ion Batteries. Adv. Funct. Mater. 2016, 26, 1428–1436. [Google Scholar] [CrossRef]
- Ma, Y.; He, J.; Kou, Z.; Elshahawy, A.M.; Hu, Y.; Guan, C.; Li, X.; Wang, J. MOF-Derived Vertically Aligned Mesoporous Co3O4 Nanowires for Ultrahigh Capacity Lithium-Ion Batteries Anodes. Adv. Mater. Interfaces 2018, 5, 1800222. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Li, X.; Xu, D.; Wang, Z.; Guo, H.; Zhang, K. Three-dimensional hierarchical Co3O4/CuO nanowire heterostructure arrays on nickel foam for high-performance lithium ion batteries. Nano Energy 2014, 6, 19–26. [Google Scholar] [CrossRef]
- Guan, B.Y.; Yu, X.Y.; Wu, H.B.; Lou, X.W. Complex Nanostructures from Materials based on Metal-Organic Frameworks for Electrochemical Energy Storage and Conversion. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef]
- Dou, Y.; Xu, J.; Ruan, B.; Ruan, B.; Liu, Q.; Pan, Y.; Sun, Z.; Dou, S. Atomic Layer-by-Layer Co3O4/Graphene Composite for High Performance Lithium-Ion Batteries. Adv. Energy Mater. 2016, 6, 1501835. [Google Scholar] [CrossRef]
- Huang, G.; Zhang, F.; Du, X.; Qin, Y.; Yin, D.; Wang, L. Metal Organic Frameworks Route to in Situ Insertion of Multiwalled Carbon Nanotubes in Co3O4 Polyhedra as Anode Materials for Lithium-Ion Batteries. ACS Nano. 2015, 9, 1592–1599. [Google Scholar] [CrossRef]
- Hou, Y.; Li, J.; Wen, Z.; Cui, S.; Yuan, C.; Chen, J. Co3O4 nanoparticles embedded in nitrogen-doped porous carbon dodecahedrons with enhanced electrochemical properties for lithium storage and water splitting. Nano Energy 2015, 12, 1–8. [Google Scholar] [CrossRef]
- Li, Y.; Li, X.; Wang, Z.; Guo, H.; Li, T. Distinct impact of cobalt salt type on the morphology, microstructure, and electrochemical properties of Co3O4 synthesized by ultrasonic spray pyrolysis. J. Alloy. Compd. 2017, 696, 836–843. [Google Scholar] [CrossRef]
- Xu, G.L.; Li, J.-T.; Huang, L.; Lin, W.; Sun, S.G. Synthesis of Co3O4 nano-octahedra enclosed by {111} facets and their excellent lithium storage properties as anode material of lithium ion batteries. Nano Energy 2013, 2, 394–402. [Google Scholar] [CrossRef]
- Wang, D.; Yu, Y.; He, H.; Wang, J.; Zhou, W.; Abruna, H.D. Template-free synthesis of hollow-structured Co3O4 nanoparticles as high-performance anodes for lithium-ion batteries. ACS Nano 2015, 9, 1775–1781. [Google Scholar] [CrossRef]
- Wu, R.; Qian, X.; Rui, X.; Liu, H.; Yadian, B.; Zhou, K.; Weri, J.; Yan, Q.; Feng, X.Q.; Long, Y.; et al. Zeolitic imidazolate framework 67-derived high symmetric porous Co3O4 hollow dodecahedra with highly enhanced lithium storage capability. Small 2014, 10, 1932–1938. [Google Scholar] [CrossRef]
- Yu, K.; Pan, X.; Zhang, G.; Liao, X.; Zhou, X.; Yan, M.; Xu, L.; Mai, L. Nanowires in Energy Storage Devices: Structures, Synthesis, and Applications. Adv. Energy Mater. 2018, 8, 1802369. [Google Scholar] [CrossRef]
- Xiong, S.; Chen, J.S.; Lou, X.W.; Zeng, H.C. Mesoporous Co3O4 and CoO@C Topotactically Transformed from Chrysanthemum-like Co(CO3)0.5(OH)0.11H2O and Their Lithium-Storage Properties. Adv. Funct. Mater. 2012, 22, 861–871. [Google Scholar] [CrossRef]
- Yuan, C.; Wu, H.B.; Xie, Y.; Lou, X.W. Mixed transition-metal oxides: Design, synthesis, and energy-related applications. Angew. Chem. Int. Ed. 2014, 53, 1488–1504. [Google Scholar] [CrossRef]
- Wei, T.Y.; Chen, C.H.; Chien, H.C.; Lu, S.Y.; Hu, C.C. A cost-effective supercapacitor material of ultrahigh specific capacitances: Spinel nickel cobaltite aerogels from an epoxide-driven sol-gel process. Adv. Mater. 2010, 22, 347–351. [Google Scholar] [CrossRef]
- Hu, L.; Wu, L.; Liao, M.; Hu, X.; Fang, X. Electrical Transport Properties of Large, Individual NiCo2O4 Nanoplates. Adv. Funct. Mater. 2012, 22, 998–1004. [Google Scholar] [CrossRef]
- Alcntara, R.; Jaraba, M.; Lavela, P.; Tirado, J.L. NiCo2O4 Spinel: First Report on a Transition Metal Oxide for the Negative Electrode of Sodium-Ion Batteries. Chem. Mater. 2002, 14, 2847–2848. [Google Scholar] [CrossRef]
- Ma, F.-X.; Yu, L.; Xu, C.-Y.; Lou, X.W. Self-supported formation of hierarchical NiCo2O4 tetragonal microtubes with enhanced electrochemical properties. Energy Environ. Sci. 2016, 9, 862–866. [Google Scholar] [CrossRef]
- Shen, L.; Yu, L.; Yu, X.-Y.; Zhang, X.; Lou, X.W. Self-Templated Formation of Uniform NiCo2O4 Hollow Spheres with Complex Interior Structures for Lithium-Ion Batteries and Supercapacitors. Angew. Chem. Int. Ed. 2015, 127, 1868–1872. [Google Scholar] [CrossRef]
- Hu, H.; Guan, B.; Xia, B.; Lou, X.W. Designed Formation of Co3O4/NiCo2O4 Double-Shelled Nanocages with Enhanced Pseudocapacitive and Electrocatalytic Properties. J. Am. Chem. Soc. 2015, 137, 5590–5595. [Google Scholar] [CrossRef]
- Sharma, Y.; Sharma, N.; Rao, G.V.S.; Chowdari, B.V.R. Lithium recycling behaviour of nano-phase-CuCo2O4 as anode for lithium-ion batteries. J. Power Sources 2007, 173, 495–501. [Google Scholar] [CrossRef]
- Sharma, Y.; Sharma, N.; Subbarao, G.; Chowdari, B. Studies on spinel cobaltites, FeCo2O4 and MgCo2O4 as anodes for Li-ion batteries. Solid State Ionics 2008, 179, 587–597. [Google Scholar] [CrossRef]
- Bai, J.; Li, X.; Liu, G.; Qian, Y.; Xiong, S. Unusual Formation of ZnCo2O4 3D Hierarchical Twin Microspheres as a High-Rate and Ultralong-Life Lithium-Ion Battery Anode Material. Adv. Funct. Mater. 2014, 24, 3012–3020. [Google Scholar] [CrossRef]
- Lavela, P.; Tirado, J.L.; Vidal-Abarca, C. Sol–gel preparation of cobalt manganese mixed oxides for their use as electrode materials in lithium cells. Electrochim. Acta 2007, 52, 7986–7995. [Google Scholar] [CrossRef]
- Jiang, F.; Su, Q.; Li, H.; Yao, L.; Deng, H.; Du, G. Growth of ultrafine CuCo2O4 nanoparticle on graphene with enhanced lithium storage properties. Chem. Eng. J. 2017, 314, 301–310. [Google Scholar] [CrossRef]
- Zhu, Y.; Cao, C.; Zhang, J.; Xu, X. Two-dimensional ultrathin ZnCo2O4 nanosheets: General formation and lithium storage application. J. Mater. Chem. A 2015, 3, 9556–9564. [Google Scholar] [CrossRef]
- Lu, D.; Liao, J.; Zhong, S.; Leng, Y.; Ji, S.; Wang, H.; Wang, R.; Li, H. Cu0.6Ni0.4Co2O4 nanowires, a novel noble-metal-free catalyst with ultrahigh catalytic activity towards the hydrolysis of ammonia borane for hydrogen production. Int. J. Hydrogen Energ. 2018, 43, 5541–5550. [Google Scholar] [CrossRef]
- Liu, L.; Wang, J.; Hou, Y.; Chen, J.; Liu, H.K.; Wang, J.; Wu, Y. Self-Assembled 3D Foam-Like NiCo2O4 as Efficient Catalyst for Lithium Oxygen Batteries. Small 2016, 12, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Wen, Z.; Jin, J.; Cui, Y.; Lu, Y. Synthesis of ordered mesoporous CuCo2O4 with different textures as anode material for lithium ion battery. Microporous Mesoporous Mater. 2013, 169, 242–247. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, J.; Qu, B.; Lu, B.; Xu, Z. Graphene improving lithium-ion battery performance by construction of NiCo2O4/graphene hybrid nanosheet arrays. Nano Energy 2014, 3, 88–94. [Google Scholar] [CrossRef]
- Li, J.; Xiong, S.; Liu, Y.; Ju, Z.; Qian, Y. High Electrochemical Performance of Monodisperse NiCo2O4 Mesoporous Microspheres as an Anode Material for Li-Ion Batteries. ACS Appl. Mater. Inter. 2013, 5, 981–988. [Google Scholar] [CrossRef]
- Xu, J.; He, L.; Xu, W.; Tang, H.; Liu, H.; Han, T.; Zhang, C.; Zhang, Y. Facile synthesis of porous NiCo2O4 microflowers as high-performance anode materials for advanced lithium-ion batteries. Electrochim. Acta. 2014, 145, 185–192. [Google Scholar] [CrossRef]
- Wu, Z.; Pu, X.; Zhu, Y.; Jing, M.; Chen, Q.; Jia, X.; Ji, X. Uniform porous spinel NiCo2O4 with enhanced electrochemical performances. J. Alloy. Compd. 2015, 632, 208–217. [Google Scholar] [CrossRef]
- Lu, D.; Li, J.; Lin, C.; Liao, J.; Feng, Y.; Ding, Z.; Li, W.; Liu, Q.; Li, H. A Simple and Scalable Route to Synthesize CoxCu1-xCo2O4@CoyCu1-yCo2O4 Yolk-Shell Microspheres, A High-Performance Catalyst to Hydrolyze Ammonia Borane for Hydrogen Production. Small 2019, 15, e1805460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, J.-S. Morphology-Tuned Synthesis of NiCo2O4-Coated 3D Graphene Architectures Used as Binder-Free Electrodes for Lithium-Ion Batteries. Chem. Eur. J. 2016, 22, 4422–4430. [Google Scholar] [CrossRef] [PubMed]
- Gao, G.; Wu, H.B.; Lou, X.W. Citrate-Assisted Growth of NiCo2O4 Nanosheets on Reduced Graphene Oxide for Highly Reversible Lithium Storage. Adv. Energy Mater. 2014, 4, 1400422. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Z.; Lu, B. Ultrafine Au nanoparticles decorated NiCo2O4 nanotubes as anode material for high-performance supercapacitor and lithium-ion battery applications. Nano Energy 2014, 7, 114–123. [Google Scholar] [CrossRef]
- Guo, W.; Sun, W.; Wang, Y. Multi-Layer CuO@NiO Hollow Spheres: Microwave-Assisted Metal-Organic-Framework Derivation and Highly Reversible Structure-Matched Stepwise Lithium Storage. ACS Nano 2015, 9, 11462–11471. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhao, X.; Yang, M.; Wu, L.; Wen, Z.; Shen, X. Hierarchically ordered mesoporous Co3O4 materials for high performance Li-ion batteries. Sci. Rep. 2016, 6, 19564. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).