Indocyanine Green Loaded Polymeric Nanoparticles: Physicochemical Characterization and Interaction Studies with Caco-2 Cell Line by Light and Transmission Electron Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Caco-2 Cell Culture and Maintenance
2.3. Preparation of ICG-Loaded NPs
2.4. Physicochemical and Ultrastructural Characterization of ICG-Loaded NPs
2.4.1. Dimensional Analysis
2.4.2. Evaluation of Physical Stability
2.4.3. Ultrastructural Analysis (TEM) of ICG-NPs1
2.4.4. Fluorescence Studies
2.5. Cytotoxicity Test
2.6. Microscopic and Ultrastructural Analysis of Interactions between ICG-Loaded NPs and Caco-2 Cell Culture In Vitro
2.7. Statistical Analysis
3. Results
3.1. Preparation of ICG-Loaded NPs
3.2. Physicochemical and Ultrastructural Characterization of ICG-Loaded NPs
3.2.1. Dimensional Analysis
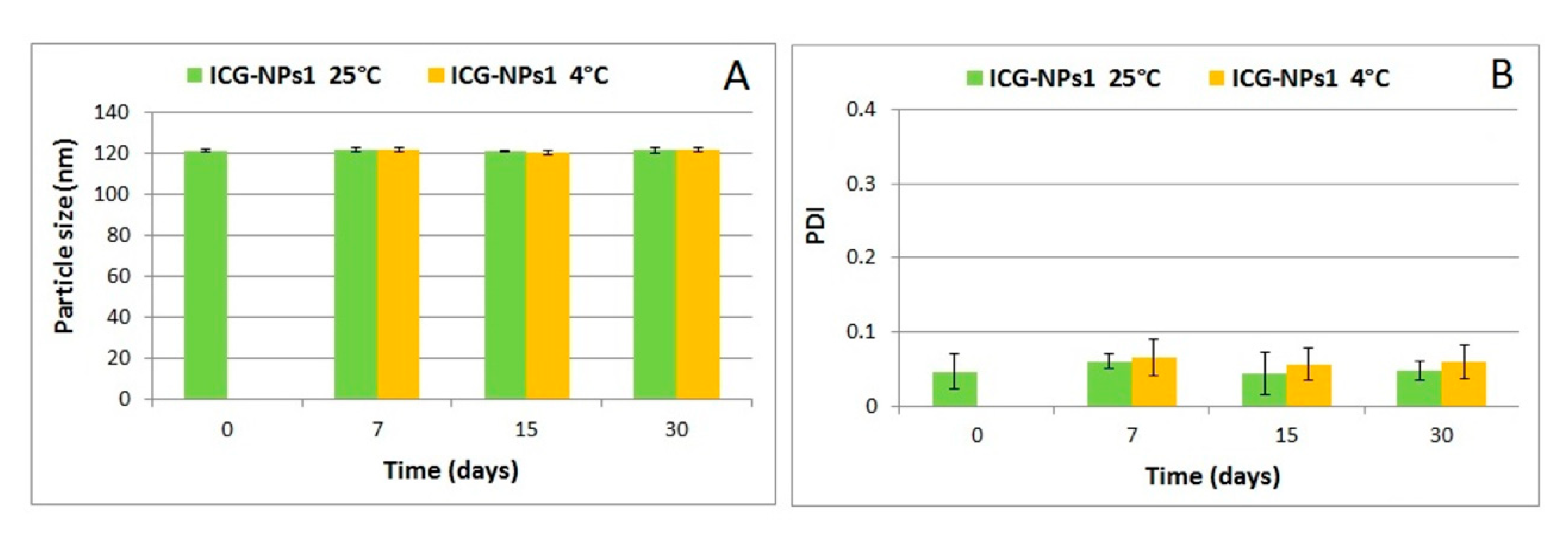
3.2.2. Evaluation of Physical Stability
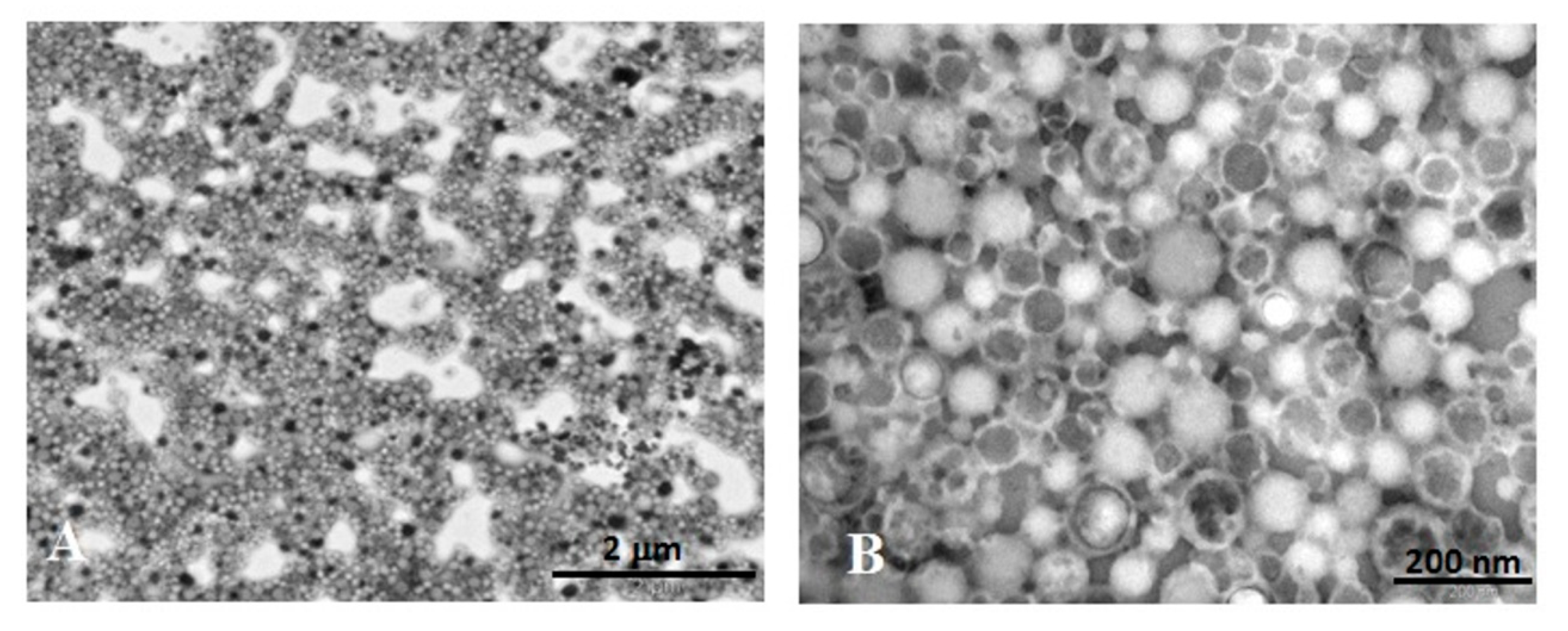
3.2.3. Ultrastructural Analysis (TEM) of ICG-NPs1
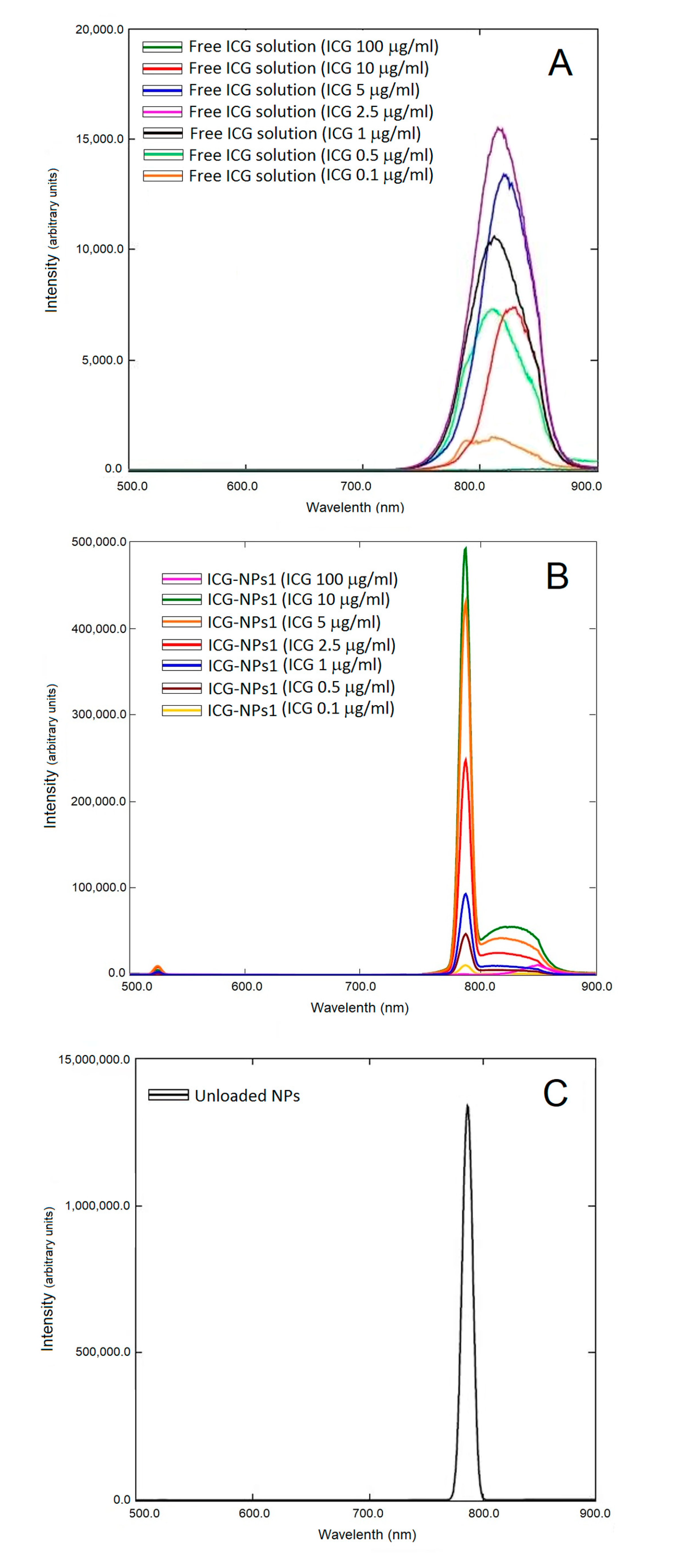
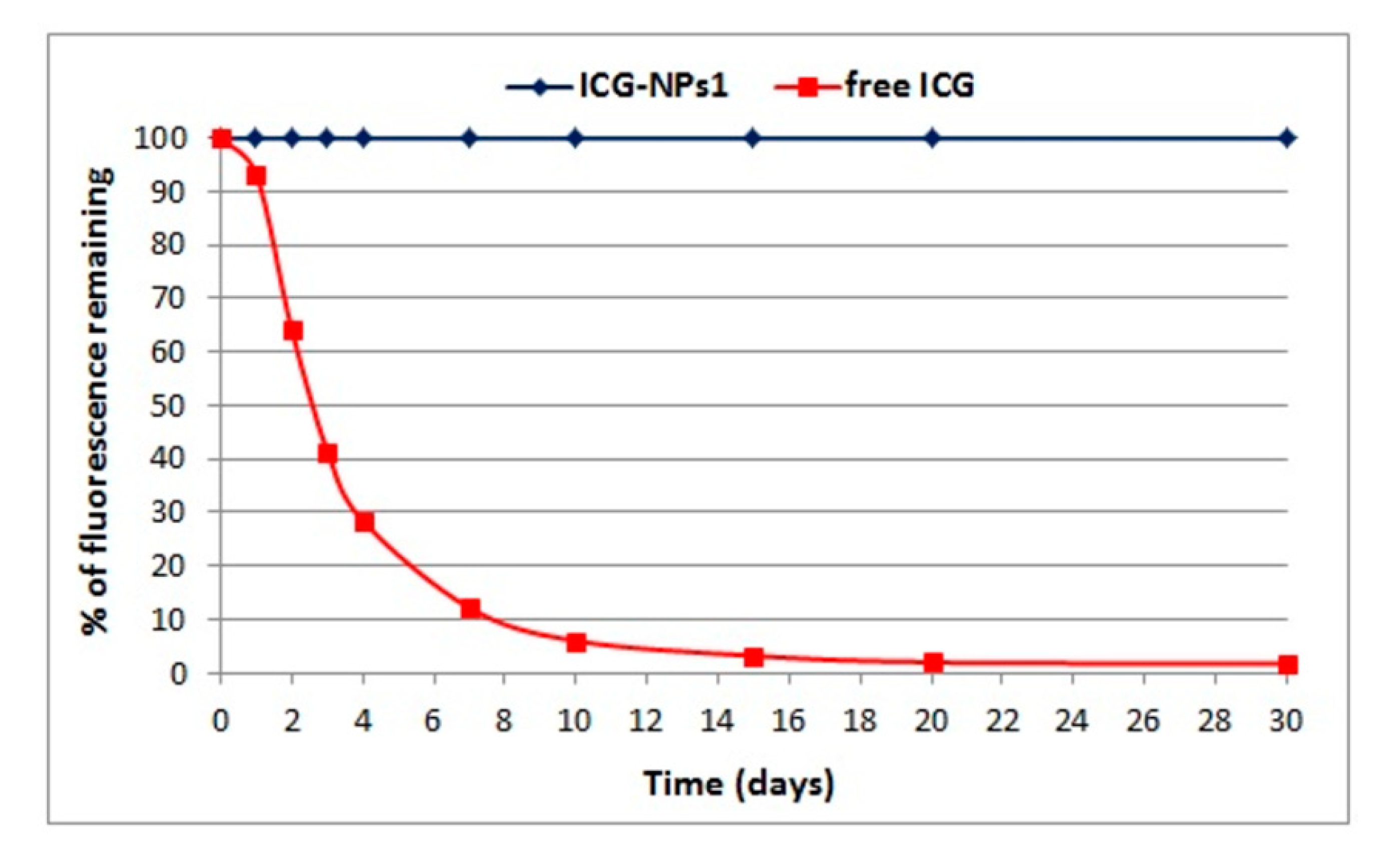
3.2.4. Fluorescence Studies
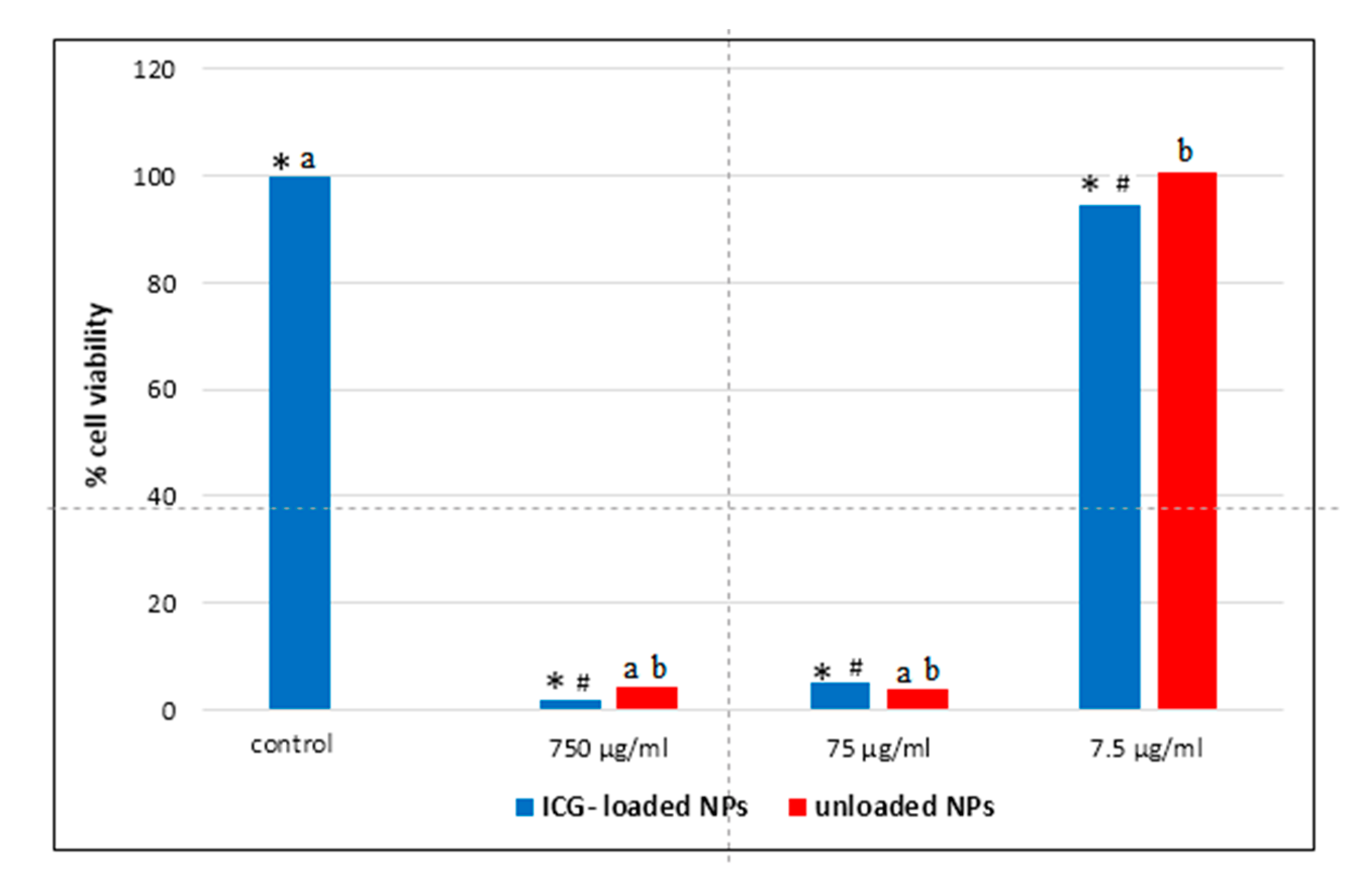
3.3. Cytotoxicity Assay
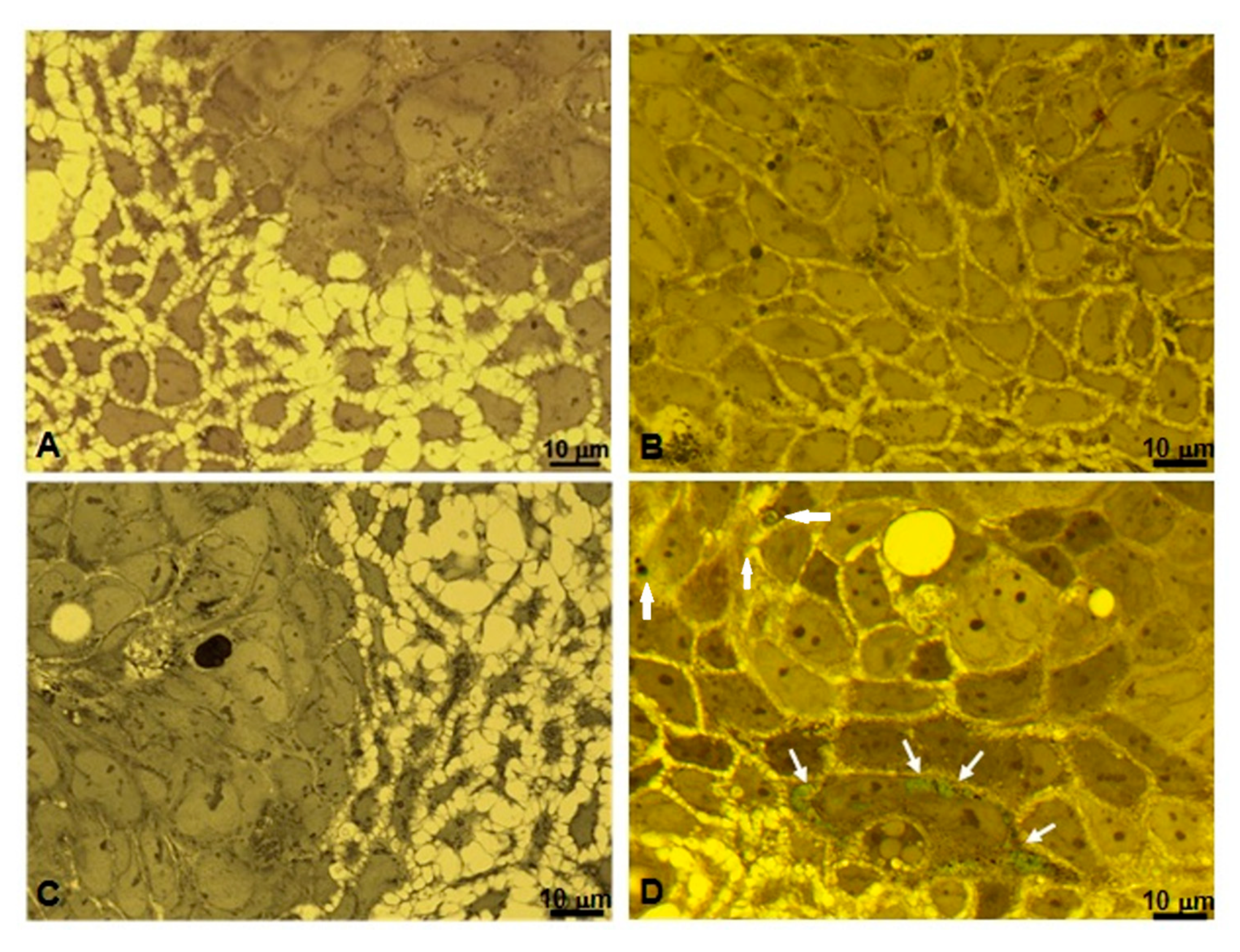
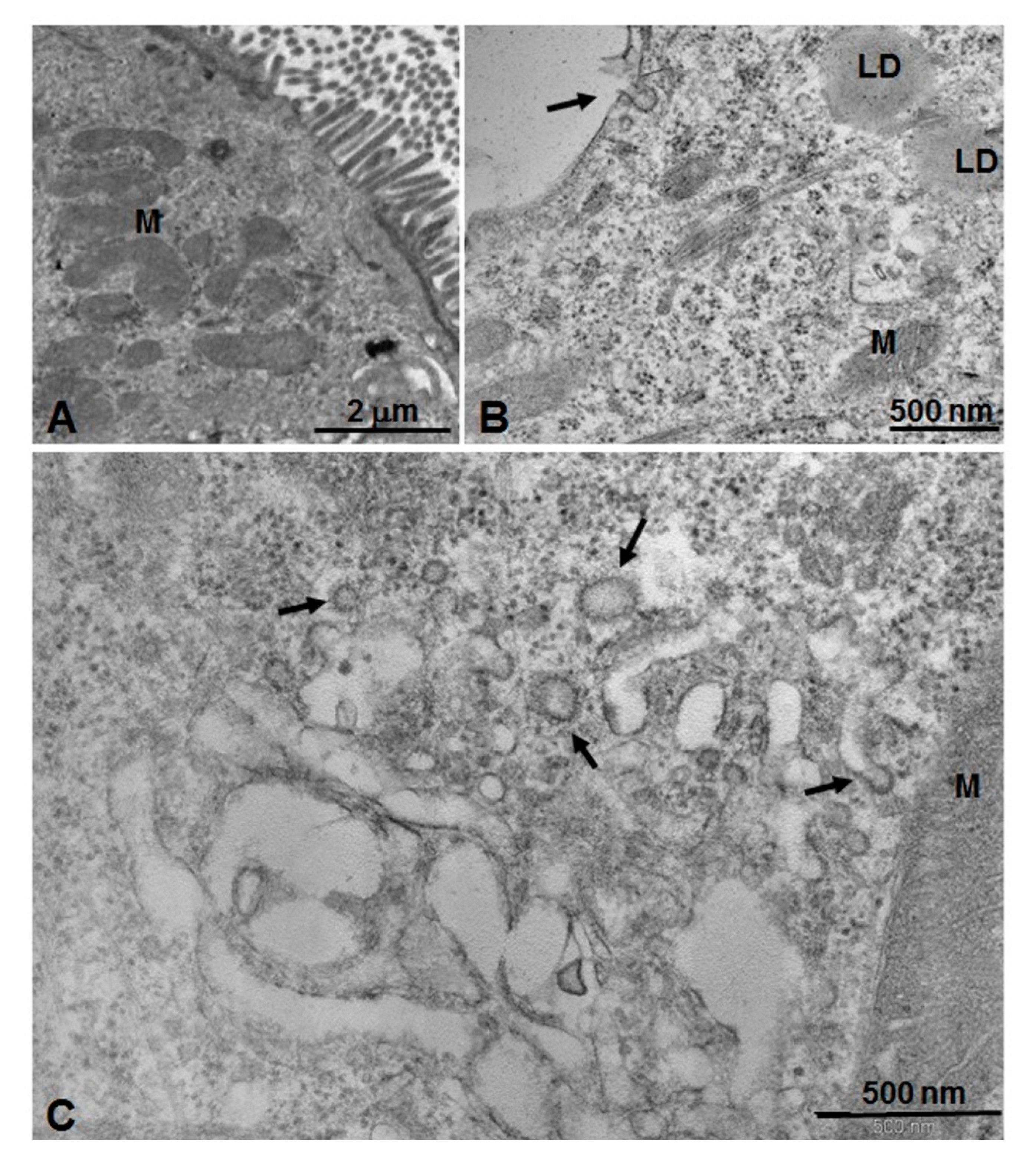
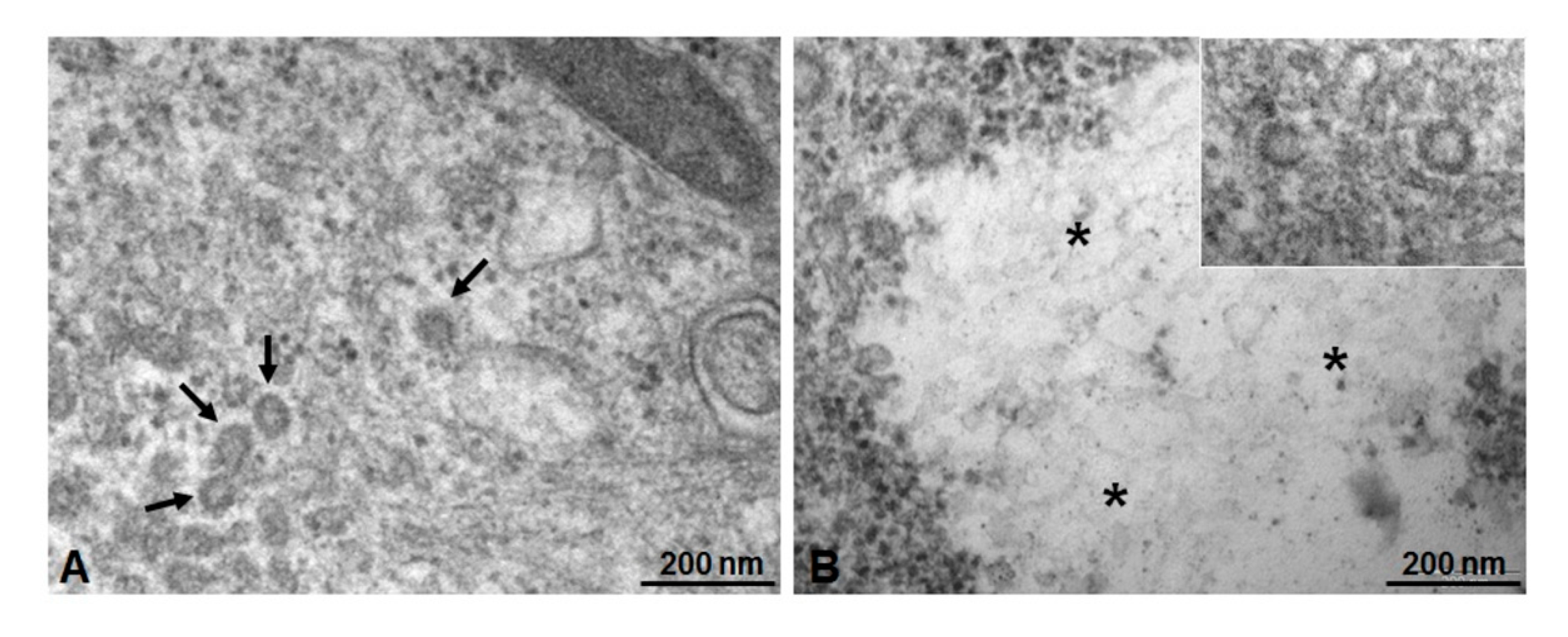
3.4. Microscopic and Ultrastructural Analysis of Interactions between ICG-Loaded NPs and Caco-2 Cell Culture In Vitro
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sulheim, E.; Baghirov, H.; von Haartman, E.; Bøe, A.; Åslund, A.K.; Mørch, Y.; de Lange Davies, C. Cellular uptake and intracellular degradation of poly (alkyl cyanoacrylate) nanoparticles. J. Nanobiotechnol. 2016, 14, 1. [Google Scholar] [CrossRef]
- Cabeza, L.; Ortiz, R.; Arias, J.L.; Prados, J.; Martínez, M.A.R.; Entrena, J.M.; Luque, R.; Melguizo, C. Enhanced antitumor activity of doxorubicin in breast cancer through the use of poly (butylcyanoacrylate) nanoparticles. Int. J. Nanomed. 2015, 10, 1291–1306. [Google Scholar]
- Obinu, A.; Rassu, G.; Corona, P.; Maestri, M.; Riva, F.; Miele, D.; Giunchedi, P.; Gavini, E. Poly (ethyl 2 cyanoacrylate) nanoparticles (PECA-NPs) as possible agents in tumor treatment. Colloids Surf. B Biointerfaces 2019, 177, 520–528. [Google Scholar] [CrossRef]
- Arias, J.L.; Gallardo, V.; Gómez-Lopera, S.A.; Plaza, R.C.; Delgado, A.V. Synthesis and characterization of poly (ethyl-2-cyanoacrylate) nanoparticles with a magnetic core. J. Control. Release 2001, 77, 309–321. [Google Scholar] [CrossRef]
- Kokudo, N.; Ishizawa, T. Clinical application of fluorescence imaging of liver cancer using indocyanine green. Liver Cancer 2012, 1, 15–21. [Google Scholar] [CrossRef]
- Ma, Y.; Tong, S.; Bao, G.; Gao, C.; Dai, Z. Indocyanine green loaded SPIO nanoparticles with phospholipid-PEG coating for dual-modal imaging and photothermal therapy. Biomaterials 2013, 34, 7706–7714. [Google Scholar] [CrossRef]
- Saxena, V.; Sadoqi, M.; Shao, J. Degradation kinetics of indocyanine green in aqueous solution. J. Pharm. Sci. 2003, 92, 2090–2097. [Google Scholar] [CrossRef]
- De Gasperi, A.; Mazza, E.; Prosperi, M. Indocyanine green kinetics to assess liver function: Ready for a clinical dynamic assessment in major liver surgery? World J. Hepatol. 2016, 8, 355–367. [Google Scholar] [CrossRef]
- Rao, J.; Dragulescu-Andrasi, A.; Yao, H. Fluorescence imaging in vivo: Recent advances. Curr. Opin. Biotechnol. 2007, 18, 17–25. [Google Scholar] [CrossRef]
- Abo, T.; Nanashima, A.; Tobinaga, S.; Hidaka, S.; Taura, N.; Takagi, K.; Arai, J.; Miyaaki, H.; Shibata, H.; Nagayasu, T. Usefulness of intraoperative diagnosis of hepatic tumors located at the liver surface and hepatic segmental visualization using indocyanine green-photodynamic eye imaging. Eur. J. Surg. Oncol. 2015, 41, 257–264. [Google Scholar] [CrossRef]
- Kaibori, M.; Ishizaki, M.; Matsui, K.; Kwon, A.H. Intraoperative indocyanine green fluorescent imaging for prevention of bile leakage after hepatic resection. Surgery 2011, 150, 91–98. [Google Scholar] [CrossRef]
- Tagaya, N.; Yamazaki, R.; Nakagawa, A.; Abe, A.; Hamada, K.; Kubota, K.; Oyama, T. Intraoperative identification of sentinel lymph nodes by near-infrared fluorescence imaging in patients with breast cancer. Am. J. Surg. 2008, 195, 850–853. [Google Scholar] [CrossRef]
- Degrauwe, N.; Hocquelet, A.; Digklia, A.; Schaefer, N.; Denys, A.; Duran, R. Theranostics in Interventional Oncology: Versatile Carriers for Diagnosis and Targeted Image-Guided Minimally Invasive Procedures. Front. Pharmacol. 2019, 10, 450. [Google Scholar] [CrossRef]
- Schaafsma, B.E.; van der Vorst, J.R.; Gaarenstroom, K.N.; Peters, A.A.W.; Verbeek, F.P.R.; de Kroon, C.D.; Trimbos, J.B.M.Z.; van Poelgeest, M.I.E.; Frangioni, J.V.; van de Velde, C.J.H.; et al. Randomized comparison of near-infrared fluorescence lymphatic tracers for sentinel lymph node mapping of cervical cancer. Gynecol. Oncol. 2012, 127, 126–130. [Google Scholar] [CrossRef]
- Ishizawa, T.; Fukushima, N.; Shibahara, J.; Masuda, K.; Tamura, S.; Aoki, T.; Hasegawa, K.; Beck, Y.; Fukayama, M.; Kokudo, N. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer 2009, 115, 2491–2504. [Google Scholar] [CrossRef]
- Ishizawa, T.; Masuda, K.; Urano, Y.; Kawaguchi, Y.; Satou, S.; Kaneko, J.; Hasegawa, K.; Shibahara, J.; Fukayama, M.; Tsuji, S.; et al. Mechanistic background and clinical applications of indocyanine green fluorescence imaging of hepatocellular carcinoma. Ann. Surg. Oncol. 2014, 21, 440–448. [Google Scholar] [CrossRef]
- Peloso, A.; Franchi, E.; Canepa, M.C.; Barbieri, L.; Briani, L.; Ferrario, J.; Bianco, C.; Quaretti, P.; Brugnatelli, S.; Dionigi, P.; et al. Combined use of intraoperative ultrasound and indocyanine green fluorescence imaging to detect liver metastases from colorectal cancer. HPB 2013, 15, 928–934. [Google Scholar] [CrossRef]
- Gomes, A.J.; Lunardi, L.O.; Marchetti, J.M.; Lunardi, C.N.; Tedesco, A.C. Indocyanine green nanoparticles useful for photomedicine. Photomed. Laser Surg. 2006, 24, 514–521. [Google Scholar] [CrossRef]
- Sheng, Z.; Hu, D.; Xue, M.; He, M.; Gong, P.; Cai, L. Indocyanine green nanoparticles for theranostic applications. Nano Micro Lett. 2013, 5, 145–150. [Google Scholar] [CrossRef]
- Porcu, E.P.; Salis, A.; Gavini, E.; Rassu, G.; Maestri, M.; Giunchedi, P. Indocyanine green delivery systems for tumour detection and treatments. Biotechnol. Adv. 2016, 34, 768–789. [Google Scholar] [CrossRef]
- Jheng, P.R.; Lu, K.Y.; Yu, S.H.; Mi, F.L. Free DOX and chitosan-N-arginine conjugate stabilized indocyanine green nanoparticles for combined chemophotothermal therapy. Colloids Surf. B Biointerfaces 2015, 136, 402–412. [Google Scholar] [CrossRef] [PubMed]
- Salis, A.; Rassu, G.; Budai-Szűcs, M.; Benzoni, I.; Csányi, E.; Berkó, S.; Maestri, M.; Dionigi, P.; Porcu, E.P.; Gavini, E.; et al. Development of thermosensitive chitosan/glicerophospate injectable in situ gelling solutions for potential application in intraoperative fluorescence imaging and local therapy of hepatocellular carcinoma: A preliminary study. Expert Opin. Drug. Deliv. 2015, 12, 1583–1596. [Google Scholar] [CrossRef] [PubMed]
- Giunchedi, P.; Gavini, E.; Dionigi, P.; Maestri, M. Opinion Paper: Selective Targeting of Liver Nodules. Present Situation and New Challenges to Enhance Indocyanine Green Captation from Colorectal Liver Metastases. Curr. Drug. Deliv. 2015, 12, 474–476. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.X.; Huang, J.; Xu, J.S.; Sun, D.; Hinkle, G.H.; Martin, E.W.; Povoski, S.P. Fabrication of indocyanine green encapsulated biodegradable microbubbles for structural and functional imaging of cancer. J. Biomed. Opt. 2009, 14, 034020. [Google Scholar] [CrossRef][Green Version]
- Porcu, E.P.; Salis, A.; Rassu, G.; Maestri, M.; Galafassi, J.; Bruni, G.; Giunchedi, P.; Gavini, E. Engineered polymeric microspheres obtained by multi-step method as potential systems for transarterial embolization and intraoperative imaging of HCC: Preliminary evaluation. Eur. J. Pharm. Biopharm. 2017, 117, 160–167. [Google Scholar] [CrossRef]
- Patel, R.H.; Wadajkar, A.S.; Patel, N.L.; Kavuri, V.C.; Nguyen, K.T.; Liu, H. Multifunctionality of indocyanine green-loaded biodegradable nanoparticles for enhanced optical imaging and hyperthermia intervention of cancer. J. Biomed. Opt. 2012, 17, 046003. [Google Scholar] [CrossRef]
- Bahmani, B.; Guerrero, Y.; Bacon, D.; Kundra, V.; Vullev, V.I.; Anvari, B. Functionalized polymeric nanoparticles loaded with indocyanine green as theranostic materials for targeted molecular near infrared fluorescence imaging and photothermal destruction of ovarian cancer cells. Lasers Surg. Med. 2014, 46, 582–592. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, L.; Zhao, Y.; Meng, D.; Li, D.; Li, H.; Zhang, B.; Shi, J.; Zhang, H.; Zhang, Z. Targeted Imaging and Chemo-Phototherapy of Brain Cancer by a Multifunctional Drug Delivery System. Macromol. Biosci. 2015, 15, 1571–1585. [Google Scholar] [CrossRef]
- Kiessling, F.; Mertens, M.E.; Grimm, J.; Lammers, T. Nanoparticles for imaging: Top or flop? Radiology 2014, 273, 10–28. [Google Scholar] [CrossRef]
- Baetke, S.C.; Lammers, T.; Kiessling, F. Applications of nanoparticles for diagnosis and therapy of cancer. Br. J. Radiol. 2015, 88, 20150207. [Google Scholar] [CrossRef]
- Heo, D.N.; Yang, D.H.; Moon, H.J.; Lee, J.B.; Bae, M.S.; Lee, S.C.; Lee, W.J.; Sun, I.C.; Kwon, I.K. Gold nanoparticles surface-functionalized with paclitaxel drug and biotin receptor as theranostic agents for cancer therapy. Biomaterials 2012, 33, 856–866. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Kiessling, F.; Hennink, W.E.; Storm, G. Nanotheranostics and Image-Guided Drug Delivery: Current Concepts and Future Directions. Mol. Pharm. 2010, 7, 1899–1912. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Li, Z.; Xie, Y.; Liu, L.; Cao, Y. Cyanidin chloride modestly protects Caco-2 cells from ZnO nanoparticle exposure probably through the induction of autophagy. Food Chem. Toxicol. 2019, 127, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Horne, R.W.; Ronchetti, I.P. A negative staining carbon film technique for studying viruses in the electron microscope: I. Preparative procedures for examining icosahedral and filamentous viruses. J. Ultrastruct. Res. 1974, 47, 361–383. [Google Scholar] [CrossRef]
- Klang, V.; Valenta, C.; Matsko, N.B. Electron microscopy of pharmaceutical systems. Micron 2013, 44, 45–74. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Onda, N.; Kimura, M.; Yoshida, T.; Shibutani, M. Preferential tumor cellular uptake and retention of indocyanine green for in vivo tumor imaging. Int. J. Cancer 2016, 139, 673–682. [Google Scholar] [CrossRef]
- Wang, H.; Li, X.; Tse, B.W.C.; Yang, H.; Thorling, C.A.; Liu, Y.; Touraud, M.; Chouane, J.B.; Liu, X.; Roberts, M.S.; et al. Indocyanine green-incorporating nanoparticles for cancer theranostics. Theranostics 2018, 8, 1227–1242. [Google Scholar] [CrossRef]
- Couvreur, P.; Kante, B.; Roland, M.; Guiot, P.; Bauduin, P.; Speiser, P. Polycyanoacrylate nanocapsules as potential lysosomotropic carriers: Preparation, morphological and absorptive properties. J. Pharm. Pharmacol. 1979, 31, 331–332. [Google Scholar] [CrossRef]
- Nicolas, J.; Couvreur, P. Synthesis of poly (alkyl cyanoacrylate)-based colloidal nanomedicines. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 111–127. [Google Scholar] [CrossRef]
- Owens, D.E., III; Peppas, N.A. Opsonization, biodistribution, and pharmacokinetics of polymeric nanoparticles. Int. J. Pharm. 2006, 307, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.J.; Yang, V.C.; David, A.E. Cancer theranostics: The rise of targeted magnetic nanoparticles. Trends Biotechn. 2011, 29, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Elsabahy, M.; Wooley, K.L. Design of polymeric nanoparticles for biomedical delivery applications. Chem. Soc. Rev. 2012, 41, 2545–2561. [Google Scholar] [CrossRef]
- Langasco, R.; Fancello, S.; Rassu, G.; Cossu, M.; Cavalli, R.; Galleri, G.; Giunchedi, P.; Migheli, R.; Gavini, E. Increasing protective activity of genistein by loading into transfersomes: A new potential adjuvant in the oxidative stress-related neurodegenerative diseases? Phytomedicine 2019, 52, 23–31. [Google Scholar] [CrossRef]
- He, C.; Hu, Y.; Yin, L.; Tang, C.; Yin, C. Effects of particle size and surface charge on cellular uptake and biodistribution of polymeric nanoparticles. Biomaterials 2010, 31, 3657–3666. [Google Scholar] [CrossRef]
- Saxena, V.; Sadoqi, M.; Shao, J. Indocyanine green-loaded biodegradable nanoparticles: Preparation, physicochemical characterization and in vitro release. Int. J. Pharm. 2004, 278, 293–301. [Google Scholar] [CrossRef]
- Saxena, V.; Sadoqi, M.; Shao, J. Polymeric nanoparticulate delivery system for Indocyanine green: Biodistribution in healthy mice. Int. J. Pharm. 2006, 308, 200–204. [Google Scholar] [CrossRef]
- Marshall, M.V.; Rasmussen, J.C.; Tan, I.C.; Aldrich, M.B.; Adams, K.E.; Wang, X.; Fife, C.E.; Maus, E.A.; Smith, L.A.; Sevick-Muraca, E.M. Near-infrared fluorescence imaging in humans with indocyanine green: A review and update. Open. Surg. Oncol. J. 2010, 2, 12–25. [Google Scholar] [CrossRef]
- Saxena, V.; Sadoqi, M.; Shao, J. Enhanced photo-stability, thermal-stability and aqueous-stability of indocyanine green in polymeric nanoparticulate systems. J. Photochem. Photobiol. B Biol. 2004, 74, 29–38. [Google Scholar] [CrossRef]
- El-Daly, S.M.; Gamal-Eldeen, A.M.; Abo-Zeid, M.A.M.; Borai, I.H.; Wafay, H.A.; Abdel-Ghaffar, A.R.B. Photodynamic therapeutic activity of indocyanine green entrapped in polymeric nanoparticles. Photodiagn. Photodyn. Ther. 2013, 10, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Lea, T. Caco-2 Cell line. In The Impact of Food Bioactives on Health: In Vitro and Ex Vivo Models; Verhoeckx, K., Cotter, P., López-Expósito, I., Kleiveland, C., Lea, T., Mackie, A., Requena, T., Swiatecka, D., Wichers, H., Eds.; Springer: Cham, Switzerland, 2015; Volume 10, pp. 103–111. [Google Scholar]
- Engür, S.; Dikmen, M. The evaluation of the anti-cancer activity of ixazomib on Caco2 colon solid tumor cells, comparison with bortezomib. Acta Clin. Belg. 2017, 72, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Yameen, B.; Choi, W.I.; Vilos, C.; Swami, A.; Shi, J.; Farokhzad, O.C. Insight into nanoparticle cellular uptake and intracellular targeting. J. Control. Release 2014, 28, 485–499. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brownf, D.; Alkilanyd, A.M.; Farokhzada, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef] [PubMed]
- Canton, I.; Battaglia, G. Endocytosis at the nanoscale. Chem. Soc. Rev. 2012, 41, 2718–2739. [Google Scholar] [CrossRef]
- Ehrlich, M.; Boll, W.; Van Oijen, A.; Hariharan, R.; Chandran, K.; Nibert, M.L.; Kirchhausen, T. Endocytosis by random initiation and stabilization of clathrin-coated pits. Cell 2004, 3, 591–605. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhu, D.; Li, S.; Yu, X.; Zhao, Y.; Ouyang, X.; Xie, Z.; Li, L. Transporting carriers for intracellular targeting delivery via non-endocytic uptake pathways. Drug Deliv. 2017, 24, 45–55. [Google Scholar] [CrossRef]
| Sample | Mean Diameter (nm) ± SD | PDI ± SD |
|---|---|---|
| ICG-NPs1 | 121.1 ± 0.8 | 0.047 ± 0.023 |
| ICG-NPs2 | 193.9 ± 5.1 * | 0.127 ± 0.062 # |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Obinu, A.; Gavini, E.; Rassu, G.; Riva, F.; Calligaro, A.; Bonferoni, M.C.; Maestri, M.; Giunchedi, P. Indocyanine Green Loaded Polymeric Nanoparticles: Physicochemical Characterization and Interaction Studies with Caco-2 Cell Line by Light and Transmission Electron Microscopy. Nanomaterials 2020, 10, 133. https://doi.org/10.3390/nano10010133
Obinu A, Gavini E, Rassu G, Riva F, Calligaro A, Bonferoni MC, Maestri M, Giunchedi P. Indocyanine Green Loaded Polymeric Nanoparticles: Physicochemical Characterization and Interaction Studies with Caco-2 Cell Line by Light and Transmission Electron Microscopy. Nanomaterials. 2020; 10(1):133. https://doi.org/10.3390/nano10010133
Chicago/Turabian StyleObinu, Antonella, Elisabetta Gavini, Giovanna Rassu, Federica Riva, Alberto Calligaro, Maria Cristina Bonferoni, Marcello Maestri, and Paolo Giunchedi. 2020. "Indocyanine Green Loaded Polymeric Nanoparticles: Physicochemical Characterization and Interaction Studies with Caco-2 Cell Line by Light and Transmission Electron Microscopy" Nanomaterials 10, no. 1: 133. https://doi.org/10.3390/nano10010133
APA StyleObinu, A., Gavini, E., Rassu, G., Riva, F., Calligaro, A., Bonferoni, M. C., Maestri, M., & Giunchedi, P. (2020). Indocyanine Green Loaded Polymeric Nanoparticles: Physicochemical Characterization and Interaction Studies with Caco-2 Cell Line by Light and Transmission Electron Microscopy. Nanomaterials, 10(1), 133. https://doi.org/10.3390/nano10010133









