Abstract
Tooth extraction induces changes in both hard and soft tissues, which may compromise implant placement. Leukocyte- and platelet-rich fibrin (L-PRF) is used to promote tissue healing, either alone or in combination with other grafting materials. Objective: This study aimed to compare post-extraction socket healing using L-PRF alone or combined with a biphasic calcium phosphate graft (HA/β-TCP) after eight weeks. Materials and Methods: 15 patients, both sexes, mean age 56.7 ± 8.2 years, requiring alveolar ridge preservation after single-rooted tooth extraction for subsequent implant placement, were included. Sockets were randomly assigned to four groups: control with blood clot only (CTR), autogenous bone graft (AB), L-PRF membrane (LPRF), and L-PRF combined with HA/β-TCP (LPRFHA). The protocol consisted of tooth extraction and immediate graft placement, followed by bone biopsy at 8 weeks for histomorphometric analysis and implant installation. New Bone Formation (NBF) was quantified from ten photomicrographs per sample using ImageJ software (version 1.54, 5 February 2025). One-way ANOVA with Bonferroni post hoc tests was applied, with statistical significance set at p ≤ 0.05. Results: A significant difference in NBF (%) was observed between the control and LPRFHA groups (p = 0.014), with greater bone formation in the control group (62.4 ± 18.6%) compared with LPRFHA (55.8 ± 17.2%; p = 0.012). No significant differences were found among AB, LPRF, and LPRFHA groups. LPRF and AB showed comparable bone formation (60.2 ± 17.5% and 60.1 ± 20.0%, respectively). Conclusions: L-PRF, either alone or combined with HA/β-TCP, can be used for alveolar ridge preservation in maxillary sockets. L-PRF, alone or with synthetic HA/β-TCP graft, effectively preserves the anterior maxillary ridge for early loading at eight weeks. All treatments achieved bone formation for implant placement, with the blood clot alone showing superior results.
1. Introduction
Tooth extraction initiates a complex metabolic process regulated by growth factors, which promotes new bone formation through osteogenesis, while simultaneously inducing resorption of the preexisting tissue and its replacement by mature bone during remodeling [1]. In the case of alveolar ridge remodeling, a reduction of 3–5 mm in horizontal dimensions and 1–3 mm in vertical dimensions occurs within the first three to six months, which may compromise implant rehabilitation [2]. To address this challenge, several procedures have been proposed to optimize bone regeneration, including socket grafting techniques using autologous, allogeneic, xenogeneic, or alloplastic bone substitutes, as well as biologically active materials such as growth factors [3,4].
Autogenous bone is considered the gold standard for bone grafting due to its osteogenic, osteoinductive, and osteoconductive properties. However, alternatives are necessary because of surgical morbidity and the limited amount of graft material available for reconstruction [5]. Current alternatives such as allografts, xenografts, and synthetic biomaterials act as structural scaffolds that facilitate osteoconduction [6]. Nevertheless, both allografts and xenografts may trigger immune responses that modulate bone regeneration, in which acute inflammation plays a critical role in resorption and the reduction of bone volume [7]. In contrast, alloplastic grafts do not induce inflammatory responses, which provides volumetric stability but limits degradation and bone remodeling within their internal surface, ultimately leading to reduced biomechanical response and an increased risk of fracture [8].
Over the past decades, research in biomaterial-based tissue engineering has gained increasing relevance in both medical and dental fields. The application of platelet-enriched materials has shown positive outcomes, particularly leukocyte- and platelet-rich fibrin (L-PRF), which stands out as an osteoinductive alternative through cytokines and growth factors with the potential to stimulate tissue regeneration [9,10,11,12,13] (Figure 1).

Figure 1.
Stages of particularly leukocyte- and platelet-rich fibrin (L-PRF) processing: (a) L-PRF matrix; (b) fibrin clot extraction after centrifugation; (c) drainage of excess exudate in the L-PRF box; (d) membrane ready for surgical use; (e) fraction fluid liquid (FFL) used as a polymerizing agent for LPRFHA preparation.
Although L-PRF is not considered an osteoinductive material on its own, its combination with bone grafts and other biomaterials has shown favorable outcomes in enhancing bone regeneration and soft tissue healing, acting as a biological connector that promotes cell recruitment, osteoprogenitor migration, and neoangiogenesis [14,15].
The objective of this study was to compare the healing response of L-PRF alone and in combination with the synthetic graft HA/β-TCP (Figure 2) in bone regeneration of human maxillary sockets after an eight-week post-extraction period.
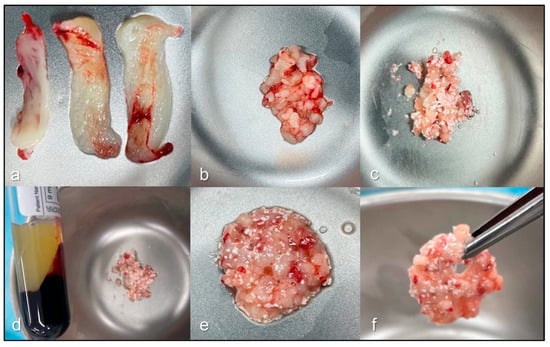
Figure 2.
Preparation process of leukocyte- and platelet-rich fibrin and hydroxyapatite (LPRFHA). Initially, leukocyte- and platelet-rich fibrin (L-PRF) membranes are obtained after the dehydration process (a), ideally providing four units according to the established protocol. One of these membranes is finely chopped (b) and mixed with the bioceramic biomaterial (c). Next, fraction fluid liquid (FFL) is added to this mixture (d), initiating the agglutination process (e). Finally, a cohesive and homogeneous material, known as sticky bone (f), is obtained, ready for use in bone regeneration procedures.
2. Materials and Methods
A controlled, prospective, double-blind clinical trial registration number U1111-1312-1819 (Brazilian Registration of Clinical Trials—ReBEC) (Figure 3), was conducted to evaluate the efficacy of L-PRF, used alone or in combination with a synthetic HA/β-TCP graft, in the bone healing of fresh human maxillary sockets after eight weeks. The protocol was approved by the Ethics Committee of the Federal University of Juiz de Fora (Approval No. 4,875,915, 30 July 2021). The alveoli were randomized using a simple random sequence into four groups using Microsoft Excel® (Microsoft Office Home 2019). The random allocation was concealed from the participants and the involved professionals, including the surgeon responsible for biopsy collection and the investigator in charge of slide reading. The randomization sequence was revealed only to the surgeon, who performed the extractions and managed the alveoli (control and experimental groups). After image acquisition and processing, the randomization was revealed to the investigators to assign the data to the corresponding groups and subsequently quantify the New Bone Formation (BRA).
Figure 3.
CONSORT flow diagram.
Patients classified as ASA I or II requiring extraction of at least four single-rooted teeth in the maxillary region were included. All participants presented a bone classification suitable for type 2 implant placement [16], with sufficient height and width to achieve primary stability. The Extraction Defect Sounding (EDS) classification assesses the extent of alveolar bone loss following tooth extraction, helping to determine the necessity of regenerative interventions. In this study, the sockets showed moderate bone defects, typically involving partial loss of one alveolar wall, and required only minimal bone regeneration procedures for implant placement (EDS-2/EDS-3) [17].
Exclusion criteria included uncontrolled systemic diseases, smoking >10 cigarettes/day, use of drugs affecting bone metabolism, hematologic disorders, local infections, or the need for extraction of multi-rooted teeth. Extractions were performed using an atraumatic, flapless technique, and sockets were randomly assigned to four groups: (a) control without graft (CTR), (b) particulate autogenous bone (AB), (c) L-PRF alone (LPRF), and (d) sticky bone (LPRFHA) a mixture of L-PRF and HA/β-TCP (150–425 µm, Osteosynt® (Teknimed, Toulouse, France). Randomization was kept blinded for both participants and evaluators. L-PRF was obtained from peripheral blood collected in additive-free tubes and centrifuged at 2700 rpm for 12 min (750 g).
The clots were processed into standardized membranes, and the fraction fluid liquid (FFL) was used to mix with the biomaterial in the sticky bone group. The ratio was one membrane per 0.5 g of biomaterial, resulting in a cohesive graft. Following socket grafting, sutures were placed using polyglactin 910. Patients received standard postoperative medication, including analgesics, antibiotics, anti-inflammatories, and 0.12% chlorhexidine for six weeks.
After satisfactory healing without relevant complications, the final sample comprised 15 patients and 60 sockets. At eight weeks, a customized guide was fabricated for bone biopsy collection and subsequent implant placement. Samples were fixed in 10% formalin and processed for light microscopy using routine preparation (10% EDTA decalcification, paraffin embedding, 3 µm sections, and H&E staining). NBF was measured in μm2 using ImageJ (National Institutes of Health, Bethesda, MD, USA), analyzing only particles within a size range defined by the software’s “Size” command.
A pilot study involving 24 alveoli was conducted to estimate the effect size, which was determined to be 0.376. Based on this value and using a significance level of 5% and a statistical power of 80%, the sample size calculation was performed using the G*Power 3.1.9.4 software (Heinrich-Heine-Universität, Düsseldorf, Germany), considering a repeated-measures analysis of variance (ANOVA) model. The calculation indicated the need to include at least 12 participants (48 alveoli). To compensate for a potential loss to follow-up estimated at 20%, a minimum final sample of 15 participants (60 alveoli) was determined. Statistical analysis included one-way ANOVA with Bonferroni post hoc tests, or, when normality assumptions were not met, Kruskal-Wallis with Dunn or Sidak post hoc tests. Statistical significance was set at p < 0.05 (95% CI). Data were processed in STATA 15 (StataCorp LLC, College Station, TX, USA), and graphs were generated using STATISTICA 12.0 (StatSoft Inc., Tulsa, OK, USA).
3. Results
Fifteen subjects (7 women and 8 men), with a mean age of 56.7 ± 8.2 years, were analyzed, and sockets were randomly assigned to four treatment groups: CTR, AB, L-PRF, and LPRFHA. After eight weeks, histomorphometric analyses were performed on the biopsy samples, evaluating the New Bone Formation (BRA) in μm2 and as a percentage. The data showed a normal distribution, allowing valid statistical comparisons between groups, which presented a homogeneous distribution of BRA percentages: AB (25.0%), CTR (25.2%), LPRF (24.9%), and LPRFHA (25.0%).
Statistical analysis using one-way ANOVA revealed a significant difference between the CTR and the LPRFHA group (p = 0.014), which was confirmed by Bonferroni post hoc testing. The CTR group exhibited the highest mean NBF percentage (62.4 ± 18.6%), compared with the lowest in the LPRFHA group (55.8 ± 17.2%) (p = 0.012). No significant differences were observed between the groups receiving L-PRF alone or in combination with the synthetic graft (Table 1, Figure 4).

Table 1.
Mean (%) values and measures of dispersion for New Bone Formation (NBF) across groups.
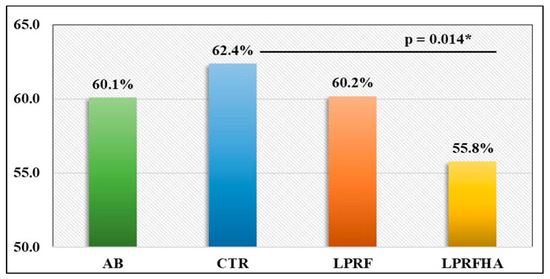
Figure 4.
Comparison of mean NBF (New Bone Formation) (%) rates between groups: AB (autogenous bone); CTR (control); LPRF (L-PRF membrane); LPRFHA (L-PRF combined with synthetic HA/β-TCP graft); * (significant difference).
Furthermore, the LPRF and AB groups showed similar NBF results, with values of 60.2% and 60.1%, respectively. These similarities are reflected in the multiple comparison analyses (Figure 5), where significant differences between groups are detailed (p = 0.012).
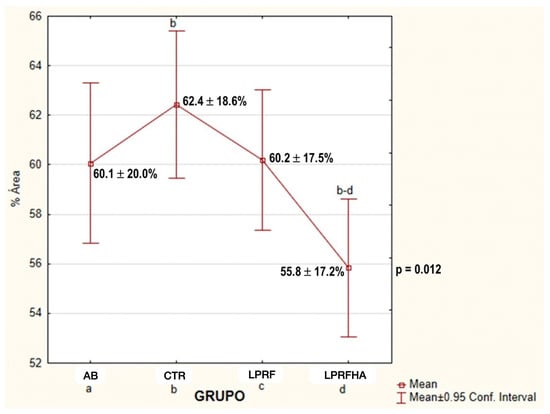
Figure 5.
Multiple comparison of mean NBF (%) between groups: AB (autogenous bone); CTR (control); LPRF (L-PRF membrane); LPRFHA (L-PRF combined with synthetic HA/β-TCP graft). a: AB (autogenous bone); b: CTR (control); c: LPRF (L-PRF membrane); d: LPRFHA (L-PRF combined with synthetic HA/β-TCP graft).
Regarding the descriptive analysis of the histological samples, notable morphological differences were observed in the organization of newly formed bone and tissue integration. In the CTR, AB, and L-PRF groups, mature lamellar bone was present, with densely eosinophilic trabeculae containing osteocytes within well-defined lacunae (indicated by yellow arrows), bordered by loose, vascularized fibrous connective tissue. In the AB group, autogenous bone exhibited an organized architecture, with well-formed Haversian systems and osteonal structures, indicative of active remodeling. In the L-PRF group, the interface between newly formed bone and soft tissue was continuous, with no signs of inflammation or residual particulate material, demonstrating favorable tissue integration.
In the LPRFHA/β-TCP group, the bone tissue exhibited an immature and fragmented arrangement, with lower trabecular density and areas of forming osteoid matrix. Multiple basophilic spherical particles corresponding to remnants of the synthetic HA/β-TCP biomaterial (green asterisks) were observed, partially integrated into the perivascular connective tissue (Figure 6).
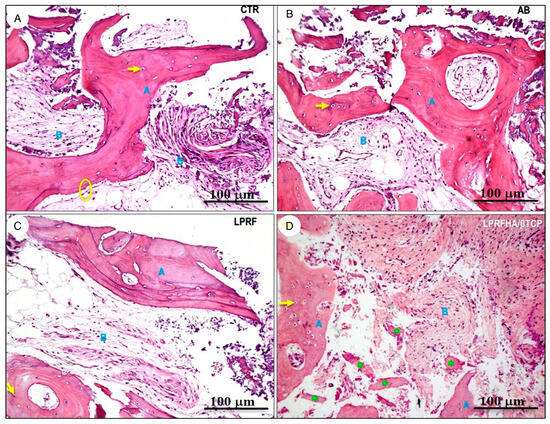
Figure 6.
Histological sections stained with hematoxylin and eosin (H&E, 10× magnification) show different experimental conditions: (A) control (CTR), (B) autogenous bone (AB), (C) L-PRF membrane (L-PRF), and (D) L-PRF combined with synthetic HA/β-TCP graft. The bone matrix (blue A) is clearly identified alongside connective tissue (blue B). Osteocytes are indicated with arrows (→); osteoblasts with circles (○); and residual bone graft particles with asterisks (*).
4. Discussion
This study allowed comparison of the materials’ influence on early bone development and their potential suitability for implant osseointegration. Synthetic bone substitutes are commercially available in various forms, exhibiting differences in physicochemical properties, including porosity, particle or block size and morphology, as well as degradation rates. Despite these variations, their capacity is limited to osteoconduction, lacking osteoinductive potential [18,19]. HA/β-TCP-based substitutes act as biocompatible, osteoconductive scaffolds that facilitate bone repair, with the aim of being progressively degraded and replaced by new bone at the defect site [20,21,22].
Although the growth factors present in L-PRF are gradually released over a period of up to 14 days, supported by a fibrillar matrix that acts as a natural biological scaffold, this fibrin matrix also restricts fibrous connective tissue infiltration and promotes cell migration and differentiation key processes for regeneration [23]. Several studies [23,24,25] report that L-PRF, when combined with bone biomaterials, functions as a biological connector that enhances cell adhesion, growth factor release, and vascularization at the graft site, thereby potentiating osteoinductive properties and accelerating new bone formation. The primary aim of this study was to evaluate the presence of bone and specific characteristics of the bone structure that would be in contact with the implant. After the 8-week healing period, bone formation was assessed in sockets treated as control, hydroxyapatite, or L-PRF, alone or in combination with HA/β-TCP. Results showed lower bone formation in the L-PRF + HA/β-TCP group, suggesting that the addition of an alloplastic material may have early reduction of osteogenesis, while no significant differences when compared to the use of L-PRF or autologous blood clot (AB).
In an experimental rabbit calvarial defect model, Acar et al. [23] reported that the group treated with the HA/β-TCP + L-PRF combination exhibited greater new bone formation at eight weeks, likely attributable to the sustained release of growth factors such as PDGF, TGF-β, and VEGF from L-PRF. Similarly, Nacopoulos et al. [14] evaluated bone regeneration in an animal model using defects treated with L-PRF, HA/β-TCP, and their combination. At three months, the combined group showed significantly higher bone density, demonstrating a synergistic effect that enhances angiogenesis and stimulates bone formation and remodeling. Baghele et al. [26] conducted a clinical study in patients undergoing tooth extractions, comparing bone regeneration using L-PRF alone and in combination with hydroxyapatite/β-tricalcium phosphate (HA/β-TCP). At six months, both groups showed significant improvements in bone volume and density, with no statistically relevant differences between them, suggesting that the addition of the biomaterial did not provide any further significant benefits.
In the present study, similar values were observed, with the groups treated with L-PRF alone (60.2%) and L-PRF + HA/β-TCP (55.8%) showing no statistically significant differences in bone formation. The lower proportion observed in the combined group could be explained by space occupation by the biomaterial, which requires a vascularization process for integration, as well as by the slow degradation of hydroxyapatite, thus limiting the osteogenic potential of L-PRF. Other factors, such as differences in biological study models, individual characteristics, alveolar defect morphology and microstructure, as well as variations in the bone substitutes used in other studies, may account for the observed differences.
Previous studies [27,28] have shown that the use of alveolar ridge preservation after tooth extraction whether using the clot, L-PRF, or growth factors without bone grafts or biomaterials does not result in significant differences in primary implant stability. However, during the secondary stability phase, the L-PRF-treated group exhibited higher ISQ values, suggesting that changes in bone response to implant loading may occur in the medium term.
A randomized clinical trial, Elsheikh et al. [29] independently compared the use of L-PRF, xenograft, and alloplastic material, followed by implant placement in premolar sockets. Results showed that during implant placement and at 6- and 18-month evaluations, there were no significant differences between groups; however, ISQ values were higher in the xenograft and alloplastic material groups compared with the L-PRF-only group. Similarly, Shahbaz-Alam et al. [30] reported comparable findings when comparing alloplastic biomaterial, L-PRF, and their combination, observing similar primary stability across the three groups. It is noteworthy that in both clinical trials, participants had residual bone heights between 2 and 5 mm and insertion torque values ranging from 35 to 40 Ncm. These findings suggest that the amount of residual bone is a determining factor for primary implant stability, whereas the use of bone grafts acts as a complementary measure that does not affect this initial phase but may contribute to enhanced secondary stability.
L-PRF promotes soft tissue healing and regeneration through the release of growth factors, improving keratinized mucosa thickness and gingival stability associated with favorable aesthetic outcomes in color and texture for root coverage or peri-implant areas [31]. Additionally, it reduces inflammation, postoperative pain, and morbidity by avoiding a donor site [32]. Hajibagheri et al. [33], through a meta-analysis, demonstrated that PRF enables faster and higher-quality soft tissue repair during the first two weeks post-intervention, resulting in improved aesthetic outcomes. Regarding hard tissues, L-PRF did not achieve vertical alveolar preservation but did help maintain bone width and prevent postoperative complications within the first three months [33]. These results are not directly comparable to those of the present study, as aesthetic or soft tissue parameters were not included; however, the authors agree that L-PRF contributes to the enhancement of peripheral soft tissues and that its use in alveolar ridge preservation may also support the maintenance and stability of peri-implant soft tissue.
L-PRF is an autologous material with regenerative properties, without the costs associated with other materials or membranes, eliminating the need for autologous donor sites [34,35]. A notable feature of L-PRF is its high leukocyte concentration, which plays a key role in pathogen inhibition [3,9]. In our study, no participants experienced postoperative clinical complications (pain, swelling, or infection), regardless of the treatment received. The highest percentage of bone repair was observed in the control group (CTR = 62.4%), followed by L-PRF (60.2%) and autogenous bone (AB, 60.1%), with no statistically significant differences between them. These findings are consistent with previous reports [23], which highlighted that, in addition to its hemostatic function, platelets release factors such as PDGF, VEGF, and TGF-β, stimulating the expression of osteogenic markers in osteoblasts. Given its bone repair capacity comparable to autogenous grafts, L-PRF can be considered a viable alternative.
In this study, the use of sticky bone demonstrated a significantly lower percentage of bone formation (55.8 ± 17.2%) compared with the control sockets (CTR: 62.4 ± 18.6%). Maintaining the blood clot alone in post-extraction sockets could be more efficient for new bone formation than using biomaterials, as it eliminates the need for degradation or remodeling and the formation is exclusively bone. Considering that in the present study a trephine was used to harvest bone from the implant surgical site, it is not possible to guarantee that using only the clot would maintain the alveolar volume, since only the trephine contents were analyzed. The presence of biomaterials within the socket may help preserve ridge volume without significantly limiting new bone formation when used in combination with L-PRF. Although the evaluated healing period is relatively short to fully assess bone consolidation, this approach remains a viable alternative to allow early loading while promoting stability in the vestibular bone plate and facilitating implant placement.
It was confirmed that after eight weeks, the particles of this material, like those of the autogenous graft, maintained their structure and did not impede early bone formation. In this context, the allograft and xenograft exhibit a shorter degradation time compared to alloplastic biomaterials, with β-TCP remaining in an active degradation phase for approximately 12 months [36]. In contrast, HA displays a longer degradation time than β-TCP, suggesting that HA/β-TCP incorporation may necessitate an extended period to achieve complete bone remodeling [37], although this process is also influenced by the material’s microstructure.
Eight weeks is a short period to expect complete bone repair and material degradation, but it is sufficient to identify new soft tissue formation to cover the socket and allow implant surgery with predictable aesthetic outcomes. The presence of biomaterial within the socket does not interfere with osseointegration and may help maintain the soft tissue support necessary for the future function of the implant in the anterior region. Thus, the use of biomaterials under these conditions contributes to preserving ridge volume and stabilizing the soft tissues that support esthetics [16]. Additionally, a six-month randomized controlled clinical trial [26] evaluated the use of an alloplastic graft (HA/β-TCP), applied either alone or in combination with L-PRF, in intraosseous defects of patients with periodontitis. The results showed that the combination of HA/β-TCP with L-PRF produced more favorable outcomes compared to the graft used alone, with improvements observed in radiographic bone fill parameters. Although these studies have different objectives, it is important to emphasize that bone remodeling is closely associated with the duration of the follow-up period. On the other hand, the study by Wei et al. [38] evaluated the clinical efficacy and safety of the bone substitute composed of ErhBMP-2/BioCaP/β-TCP through a proof-of-concept randomized clinical trial applied to a post-extraction socket-healing model. This investigation was conducted in a single premolar site per patient under controlled conditions, including vestibular and palatal bone thicknesses greater than in the anterior area, thereby providing consistent evidence regarding the performance of the biomaterial in a defined clinical setting. In contrast, the present study was carried out in four sites per individual, all located in the anterior region. This approach offers a broader and complementary methodological scope, allowing for a more comprehensive characterization of the tissue response in anatomical and functional scenarios.
Volumetric analysis of socket preservation using CBCT was not performed, as the two-month follow-up period would likely not provide a clear assessment of bone formation in this research.
L-PRF, both alone and in combination with the synthetic HA/β-TCP graft, can be used for alveolar ridge preservation in maxillary sockets. However, alveolar anatomy is a key determinant in bone healing due to variations in width, height, thickness, and density of the bone walls, as well as the possible presence of fenestrations or dehiscences, which complicates precise quantification of the graft’s effect on regeneration. In addition, graft location influences the pattern of bone formation, which begins at the lateral wall of the socket and progresses toward the center [39]. Despite the short follow-up period inherent to the early loading protocol, there is a recognized need to include assessments of vertical bone height using cone beam computed tomography to quantitatively determine the amount of vertical bone preserved, as well as to evaluate implant insertion torque and the Implant Stability Quotient in relation to different biomaterials.
This randomized clinical trial has certain limitations that should be considered when interpreting the results. The small sample size may have limited the statistical power and increased imprecision, particularly given that the effect size was not calculated for each group. Although proper randomization was applied, incomplete operator blinding and individual biological variability could have influenced the outcomes. An adjustment for multiplicity of comparisons was performed to reduce the risk of type I errors; however, future studies should incorporate effect size estimation for each outcome, with larger sample sizes and longer follow-up periods to strengthen the evidence.
Another limitation of this study was the absence of cellular and platelet counts via complete blood count to assess the cellular profile, as blood composition can influence tissue regeneration, including platelets, leukocytes, and erythrocytes. In this context, platelet count could be related to L-PRF membrane size, whereas higher peripheral erythrocyte counts may be associated with smaller L-PRF membranes [40]. Additionally, qualitative and quantitative analyses using specific histological collagen stains could reveal differences in tissue organization between experimental groups, and immunostaining would be useful to identify cell types characteristic of inflammatory granulation and to distinguish intrinsic tissue cellularity.
Finally, it is essential to advance controlled and randomized clinical trials with standardized protocols in humans to confirm whether the findings of this research support the use of L-PRF as a standalone biomaterial in fresh maxillary sockets, promoting bone repair within an eight-week period.
5. Conclusions
In conclusion, early implantation at eight weeks can be successfully achieved using L-PRF, either alone or in combination with a synthetic HA/β-TCP graft, for alveolar ridge preservation in the anterior maxilla. All treatments resulted in over 50% new bone formation, ensuring adequate bone volume for subsequent implant placement, with the blood clot alone demonstrating superior bone formation compared to the graft combination. Future research should focus on long-term implant stability and prosthetic outcomes.
Author Contributions
Conceptualization, P.d.S.G., V.R., S.O. and H.D.; methodology, P.d.S.G., C.P.d.S., A.C.N., V.R., S.O. and H.D.; software, P.d.S.G., C.P.d.S. and A.C.N.; validation, S.O. and H.D. formal analysis, P.d.S.G., V.R., S.O. and H.D.; investigation, P.d.S.G., C.P.d.S., A.C.N., V.R., S.O. and H.D.; resources, P.d.S.G., C.P.d.S., A.C.N. and H.D.; data curation, P.d.S.G., C.P.d.S. and A.C.N.; writing—original draft preparation, P.d.S.G., V.R., S.O. and H.D.; writing—review and editing, V.R., S.O. and H.D.; visualization, V.R., S.O. and H.D.; supervision, S.O. and H.D.; project administration, P.d.S.G. and H.D.; funding acquisition, P.d.S.G. and H.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declara-tion of Helsinki and approved by the Institutional Review Board under Federal University of Juiz de Fora, approval No. 4,875,915, 30 July 2021.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Alghamdi, H.S. Methods to Improve Osseointegration of Dental Implants in Low Quality (Type-IV) Bone: An Overview. J. Funct. Biomater. 2018, 9, 7. [Google Scholar] [CrossRef]
- Chappuis, V.; Engel, O.; Reyes, M.; Shahim, K.; Nolte, L.P.; Buser, D. Ridge alterations post-extraction in the esthetic zone: A 3D analysis with CBCT. J. Dent. Res. 2013, 92, 195–201. [Google Scholar] [CrossRef]
- De Angelis, P.; De Angelis, S.; Passarelli, P.C.; Liguori, M.G.; Manicone, P.F.; D’Addona, A. Hard and Soft Tissue Evaluation of Different Socket Preservation Procedures Using Leukocyte and Platelet-Rich Fibrin: A Retrospective Clinical and Volumetric Analysis. J. Oral Maxillofac. Surg. 2019, 77, 1807–1815. [Google Scholar] [CrossRef] [PubMed]
- Dipalma, G.; Inchingolo, A.M.; Colonna, V.; Marotti, P.; Carone, C.; Ferrante, L.; Inchingolo, F.; Palermo, A.; Inchingolo, A.D. Autologous and Heterologous Minor and Major Bone Regeneration with Platelet-Derived Growth Factors. J. Funct. Biomater. 2025, 16, 16. [Google Scholar] [CrossRef] [PubMed]
- Janjua, O.S.; Qureshi, S.M.; Shaikh, M.S.; Alnazzawi, A.; Rodriguez-Lozano, F.J.; Pecci-Lloret, M.P.; Zafar, M.S. Autogenous Tooth Bone Grafts for Repair and Regeneration of Maxillofacial Defects: A Narrative Review. Int. J. Environ. Res. Public Health 2022, 19, 3690. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone Grafts and Substitutes in Dentistry: A Review of Current Trends and Developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef]
- Yang, N.; Liu, Y. The Role of the Immune Microenvironment in Bone Regeneration. Int. J. Med. Sci. 2021, 18, 3697–3707. [Google Scholar] [CrossRef]
- Sohn, H.S.; Oh, J.K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part V: Histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, 299–303. [Google Scholar] [CrossRef]
- Choukroun, J.; Diss, A.; Simonpieri, A.; Girard, M.O.; Schoeffler, C.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Dohan, D.M. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part IV: Clinical effects on tissue healing. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e56–e60. [Google Scholar] [CrossRef]
- Zhao, J.H.; Tsai, C.H.; Chang, Y.C. Clinical application of platelet-rich fibrin as the sole grafting material in maxillary sinus augmentation. J. Formos. Med. Assoc. 2015, 114, 779–780. [Google Scholar] [CrossRef]
- Canellas, J.V.D.S.; da Costa, R.C.; Breves, R.C.; de Oliveira, G.P.; Figueredo, C.M.D.S.; Fischer, R.G.; Thole, A.A.; Medeiros, P.J.D.; Ritto, F.G. Tomographic and histomorphometric evaluation of socket healing after tooth extraction using leukocyte- and platelet-rich fibrin: A randomized, single-blind, controlled clinical trial. J. Craniomaxillofac. Surg. 2020, 48, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Walia, K.D.; Belludi, S.A.; Pradhan, N.; Jain, V.; Shaik, S. Evaluation of Platelet-Rich Fibrin Matrix as a Regenerative Material in the Surgical Management of Human Periodontal Intraosseous Defects—A Randomized Controlled Trial. Contemp. Clin. Dent. 2022, 13, 9–17. [Google Scholar] [CrossRef]
- Nacopoulos, C.; Dontas, I.; Lelovas, P.; Galanos, A.; Vesalas, A.M.; Raptou, P.; Mastoris, M.; Chronopoulos, E.; Papaioannou, N. Enhancement of bone regeneration with the combination of platelet-rich fibrin and synthetic graft. J. Craniofac. Surg. 2014, 25, 2164–2168. [Google Scholar] [CrossRef] [PubMed]
- Galav, S.; Chandrashekar, K.T.; Mishra, R.; Tripathi, V.; Agarwal, R.; Galav, A. Comparative evaluation of platelet-rich fibrin and autogenous bone graft for the treatment of infrabony defects in chronic periodontitis: Clinical, radiological, and surgical reentry. Indian J. Dent. Res. 2016, 27, 502–507. [Google Scholar] [CrossRef]
- Weingart, D.; Chen, S.T. International Team for Implantology (ITI). In Proceedings of the 4th International Team for Implantology (ITI) Consensus Conference, Stuttgart, Germany, 26–28 August 2008; Quintessence Publishing: Batavia, IL, USA, 2009; Volume 24, pp. 7–278. [Google Scholar]
- Caplanis, N.; Lozada, J.L.; Kan, J.Y. Extraction defect assessment, classification, and management. J. Calif. Dent. Assoc. 2005, 33, 853–863. [Google Scholar] [CrossRef]
- Sheikh, Z.; Sima, C.; Glogauer, M. Bone Replacement Materials and Techniques Used for Achieving Vertical Alveolar Bone Augmentation. Materials 2015, 8, 2953–2993. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef] [PubMed]
- Montero, J.; Becerro, A.; Dib, A.; Quispe-López, N.; Borrajo, J.; Benito Garzón, L. Preliminary results of customized bone graft made by robocasting hydroxyapatite and tricalcium phosphates for oral surgery. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2023, 135, 192–203. [Google Scholar] [CrossRef]
- Kucko, S.K.; Raeman, S.M.; Keenan, T.J. Current Advances in Hydroxyapatite- and β-Tricalcium Phosphate-Based Composites for Biomedical Applications: A Review. Biomed. Mater. Devices 2023, 1, 49–65. [Google Scholar] [CrossRef]
- Helaehil, J.V.; Huang, B.; Bartolo, P.; Santamaria, M., Jr.; Caetano, G.F. Bone regeneration: The influence of composite HA/TCP scaffolds and electrical stimulation on TGF/BMP and RANK/RANKL/OPG pathways. Injury 2025, 56, 112158. [Google Scholar] [CrossRef]
- Acar, A.H.; Yolcu, Ü.; Gül, M.; Keleş, A.; Erdem, N.F.; Altundag Kahraman, S. Micro-computed tomography and histomorphometric analysis of the effects of platelet-rich fibrin on bone regeneration in the rabbit calvarium. Arch. Oral Biol. 2015, 60, 606–614. [Google Scholar] [CrossRef]
- Oliveira, M.R.; deC Silva, A.; Ferreira, S.; Avelino, C.C.; Garcia, I.R., Jr.; Mariano, R.C. Influence of the association between platelet-rich fibrin and bovine bone on bone regeneration. A histomorphometric study in the calvaria of rats. Int. J. Oral Maxillofac. Surg. 2015, 44, 649–655. [Google Scholar] [CrossRef]
- Maia, P.W.; Teixeira, M.L.; Scavone de Macedo, L.G.; Aloise, A.C.; Passos Junior, C.A.; Aragoneses, J.M.; Calvo-Guirado, J.L.; Pelegrine, A.A. Use of Platelet-Rich Fibrin Associated with Xenograft in Critical Bone Defects: Histomorphometric Study in Rabbits. Symmetry 2019, 11, 1293. [Google Scholar] [CrossRef]
- Baghele, O.; Thorat, M.; Malpani, P. Clinical and radiographic evaluation of platelet-rich fibrin and bone graft material (β-tricalcium phosphate + hydroxyapatite) in the treatment of intrabony defects of periodontitis patients: A randomized controlled trial. Quintessence Int. 2023, 54, 472–483. [Google Scholar]
- Gaur, S.; Chugh, A.; Chaudhry, K.; Bajpayee, A.; Jain, G.; Chugh, V.K.; Kumar, P.; Singh, S. Efficacy and Safety of Concentrated Growth Factors and Platelet- Rich Fibrin on Stability and Bone Regeneration in Patients with Immediate Dental Implants: A Randomized Controlled Trial. Int. J. Oral Maxillofac. Implant. 2022, 37, 784–792. [Google Scholar] [CrossRef]
- Anapu, M.P.; Atluri, K.R.; Chandra Tripuraneni, S.; Issrani, R.; Bader, A.K.; Alkhalaf, Z.A.; Sghaireen, M.G.; Prabhu, N.; Rbea Dh Alshammari, R.; Khalid, G.; et al. Evaluation of effect on stability of implants with and without platelet rich fibrin using a resonance frequency analyzer—An in-vivo study. Heliyon 2024, 10, e27971. [Google Scholar] [CrossRef] [PubMed]
- Elsheikh, H.A.; Abdelsameaa, S.E.; Elbahnasi, A.A.; Abdel-Rahman, F.H. Comparison between platelet rich fibrin as space filling material versus xenograft and alloplastic bone grafting materials in immediate implant placement: A randomized clinical trial. BMC Oral Health 2023, 23, 977. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz-Alam, M.; Dhiman, A.; Jain, V.; Bhutia, O.; Pruthi, G. Vertical Bone Implant Contact Around Anterior Immediate Implants and Their Stability After Using Either Alloplast or L-PRF or Both in Peri-Implant Gap: A Prospective Randomized Trial. J. Maxillofac. Oral Surg. 2022, 21, 533–541. [Google Scholar] [CrossRef]
- Giammarinaro, E.; Baldini, N.; Covani, U.; Menini, M.; Pesce, P.; Marconcini, S. Does platelet-rich fibrin enhance the outcomes of peri-implant soft tissues? A systematic review. BMC Oral Health 2025, 25, 615. [Google Scholar] [CrossRef]
- Miron, R.J.; Moraschini, V.; Del Fabbro, M.; Piattelli, A.; Fujioka-Kobayashi, M.; Zhang, Y.; Saulacic, N.; Schaller, B.; Kawase, T.; Cosgarea, R.; et al. Use of platelet-rich fibrin for the treatment of gingival recessions: A systematic review and meta-analysis. Clin. Oral Investig. 2020, 24, 2543–2557. [Google Scholar] [CrossRef]
- Hajibagheri, P.; Basirat, M.; Tabari-Khomeiran, Z.; Asadi-Aria, A. The efficacy of platelet-rich fibrin (PRF) in post-extraction hard and soft tissue healing and associated complications: A systematic review and meta-analysis of split-mouth randomized clinical trials. BMC Oral Health 2025, 25, 869. [Google Scholar] [CrossRef]
- Castro, A.B.; Meschi, N.; Temmerman, A.; Pinto, N.; Lambrechts, P.; Teughels, W.; Quirynen, M. Regenerative potential of leucocyte- and platelet-rich fibrin. Part B: Sinus floor elevation, alveolar ridge preservation and implant therapy. A systematic review. J. Clin. Periodontol. 2017, 44, 225–234. [Google Scholar] [CrossRef]
- Ustaoğlu, G.; Göller Bulut, D.; Gümüş, K.Ç. Evaluation of different platelet-rich concentrates effects on early soft tissue healing and socket preservation after tooth extraction. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, W.A. Evaluation of bone regenerative capacity in rats claverial bone defect using platelet rich fibrin with and without beta tri calcium phosphate bone graft material. Saudi Dent. J. 2016, 28, 109–117. [Google Scholar] [CrossRef]
- Clark, D.; Rajendran, Y.; Paydar, S.; Ho, S.; Cox, D.; Ryder, M.; Dollard, J.; Kao, R.T. Advanced platelet-rich fibrin and freeze-dried bone allograft for ridge preservation: A randomized controlled clinical trial. J. Periodontol. 2018, 89, 379–387. [Google Scholar] [CrossRef]
- Wei, L.; Sun, Y.; Yu, D.; Pieterse, H.; Wismeijer, D.; Liu, Y.; Wu, Y. The Clinical Efficacy and Safety of ErhBMP-2/BioCaP/β-TCP as a Novel Bone Substitute Using the Tooth-Extraction-Socket-Healing Model: A Proof-of-Concept Randomized Controlled Trial. J. Clin. Periodontol. 2025, 52, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Kollati, P.; Koneru, S.; Dwarakanath, C.D.; Gottumukkala, S.N.V.S. Effectiveness of naturally derived bovine hydroxyapatite (Cerabone™) combined with platelet-rich fibrin matrix in socket preservation: A randomized controlled clinical trial. J. Indian Soc. Periodontol. 2019, 23, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, J.E.I.G.; van der Vorm, L.N.; Buis, S.; Haumann, R.; Karanzai, A.; Konings, J.; de Groot, P.G.; de Laat, B.; Remijn, J.A. Implant stability in patients treated with platelet-rich fibrin and bovine bone substitute for alveolar ridge preservation is associated with peripheral blood cells and coagulation factors. Clin. Exp. Dent. Res. 2020, 6, 236–243. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.