Impact of Pre- and Post-Dilatation on Long-Term Outcomes After Self-Expanding and Balloon-Expandable TAVI
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Otto, C.M.; Lind, B.K.; Kitzman, D.W.; Gersh, B.J.; Siscovick, D.S. Association of Aortic-Valve Sclerosis with Cardiovascular Mortality and Morbidity in the Elderly. N. Engl. J. Med. 1999, 341, 142–147. [Google Scholar] [CrossRef]
- Kodali, S.K.; Williams, M.R.; Smith, C.R.; Svensson, L.G.; Webb, J.G.; Makkar, R.R.; Fontana, G.P.; Dewey, T.M.; Thourani, V.H.; Pichard, A.D.; et al. Two-Year Outcomes after Transcatheter or Surgical Aortic-Valve Replacement. N. Engl. J. Med. 2012, 366, 1686–1695. [Google Scholar] [CrossRef]
- McInerney, A.; Vera-Urquiza, R.; Tirado-Conte, G.; Marroquin, L.; Jimenez-Quevedo, P.; Nuñez-Gil, I.; Pozo, E.; Gonzalo, N.; de Agustín, J.A.; Escaned, J.; et al. Pre-dilation and Post-dilation in Transcatheter Aortic Valve Replacement: Indications, Benefits and Risks. Interv. Cardiol. Lond. Engl. 2021, 16, e28. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Mei, Z.; Ge, X.; Li, Y.; Zhou, Q.; Meng, X.; An, G. Comparison of outcomes of self-expanding versus balloon-expandable valves for transcatheter aortic valve replacement: A meta-analysis of randomized and propensity-matched studies. BMC Cardiovasc. Disord. 2023, 23, 382. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.A.; Kazemian, S.; Gupta, T.; Patel, N.K.; Sakhuja, R.; Inglessis, I.; Jassar, A.; Langer, N.; Passeri, J.J.; Dauerman, H.L.; et al. Outcomes of Transcatheter Aortic Valve Replacement Using Third-Generation Balloon-Expandable Versus Self-Expanding Valves: A Meta-analysis. J. Soc. Cardiovasc. Angiogr. Interv. 2024, 3, 102146. [Google Scholar] [CrossRef] [PubMed]
- Conrotto, F.; D’Ascenzo, F.; Franchin, L.; Bruno, F.; Mamas, M.A.; Toutouzas, K.; Cuisset, T.; Leclercq, F.; Dumonteil, N.; Latib, A.; et al. Transcatheter Aortic Valve Implantation With or Without Predilation: A Meta-Analysis. J. Invasive Cardiol. 2022, 34, E104–E113. [Google Scholar] [CrossRef]
- Wang, N.; Lal, S. Post-dilation in transcatheter aortic valve replacement: A systematic review and meta-analysis. J. Intervent. Cardiol. 2017, 30, 204–211. [Google Scholar] [CrossRef]
- Nombela-Franco, L.; Rodés-Cabau, J.; DeLarochellière, R.; Larose, E.; Doyle, D.; Villeneuve, J.; Bergeron, S.; Bernier, M.; Amat-Santos, I.J.; Mok, M.; et al. Predictive factors, efficacy, and safety of balloon post-dilation after transcatheter aortic valve implantation with a balloon-expandable valve. JACC Cardiovasc. Interv. 2012, 5, 499–512. [Google Scholar] [CrossRef]
- Luengo-Fernandez, R.; Little, M.; Gray, A.; Torbica, A.; Maggioni, A.P.; Huculeci, R.; Timmis, A.D.; Vardas, P.; Leal, J. Cardiovascular disease burden due to productivity losses in European Society of Cardiology countries. Eur. Heart J.—Qual. Care Clin. Outcomes 2023, 10, 36–44. [Google Scholar] [CrossRef]
- Luengo-Fernandez, R.; Walli-Attaei, M.; Gray, A.; Torbica, A.; Maggioni, A.P.; Huculeci, R.; Bairami, F.; Aboyans, V.; Timmis, A.D.; Vardas, P.; et al. Economic burden of cardiovascular diseases in the European Union: A population-based cost study. Eur. Heart J. 2023, 44, 4752–4767. [Google Scholar] [CrossRef]
- Harrell, F.E. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis; Springer International Publishing: Cham, Germany, 2015; ISBN 978-3-319-19424-0. [Google Scholar]
- Cavanaugh, J.E.; Neath, A.A. The Akaike information criterion: Background, derivation, properties, application, interpretation, and refinements. WIREs Comput. Stat. 2019, 11, e1460. [Google Scholar] [CrossRef]
- Berkelmans, G.F.N.; Read, S.H.; Gudbjörnsdottir, S.; Wild, S.H.; Franzen, S.; van der Graaf, Y.; Eliasson, B.; Visseren, F.L.J.; Paynter, N.P.; Dorresteijn, J.A.N. Population median imputation was noninferior to complex approaches for imputing missing values in cardiovascular prediction models in clinical practice. J. Clin. Epidemiol. 2022, 145, 70–80. [Google Scholar] [CrossRef]
- Lanz, J.; Möllmann, H.; Kim, W.-K.; Burgdorf, C.; Linke, A.; Redwood, S.; Hilker, M.; Joner, M.; Thiele, H.; Conzelmann, L.; et al. Final 3-Year Outcomes of a Randomized Trial Comparing a Self-Expanding to a Balloon-Expandable Transcatheter Aortic Valve. Circ. Cardiovasc. Interv. 2023, 16, e012873. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Bruno, F.; Baldetti, L.; De Filippo, O.; Marengo, G.; Breviario, S.; Melillo, F.; Thyregod, H.G.H.; Thiele, H.; Sondergaard, L.; et al. Aortic valve replacement vs. balloon-expandable and self-expandable transcatheter implantation: A network meta-analysis. Int. J. Cardiol. 2021, 337, 90–98. [Google Scholar] [CrossRef]
- Nakase, M.; Tomii, D.; Maznyczka, A.; Heg, D.; Okuno, T.; Samim, D.; Stortecky, S.; Lanz, J.; Reineke, D.; Windecker, S.; et al. Five-year outcomes with self-expanding versus balloon-expandable TAVI in patients with left ventricular systolic dysfunction. Am. Heart J. 2025, 280, 18–29. [Google Scholar] [CrossRef]
- Hosseinpour, A.; Gupta, R.; Kamalpour, J.; Hosseinpour, H.; Chaturvedi, A.; Agrawal, A.; Patel, N.C.; Patel, C. Balloon-Expandable Versus Self-Expanding Transcatheter Aortic Valve Implantation in Patients With Small Aortic Annulus: A Meta-Analysis. Am. J. Cardiol. 2023, 204, 257–267. [Google Scholar] [CrossRef]
- Halapas, A.; Koliastasis, L.; Doundoulakis, I.; Antoniou, C.-K.; Stefanadis, C.; Tsiachris, D. Transcatheter Aortic Valve Implantation and Conduction Disturbances: Focus on Clinical Implications. J. Cardiovasc. Dev. Dis. 2023, 10, 469. [Google Scholar] [CrossRef]
- Ternacle, J.; Al-Azizi, K.; Szerlip, M.; Potluri, S.; Hamandi, M.; Blanke, P.; Leipsic, J.; Dahou, A.; Salaun, E.; Vincent, F.; et al. Impact of Predilation During Transcatheter Aortic Valve Replacement: Insights From the PARTNER 3 Trial. Circ. Cardiovasc. Interv. 2021, 14, e010336. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Potenza, D.R.; Casella, M.; Massaro, R.; Russo, G.; Braccio, M.; Russo, A.D.; Cassese, M. Rate and Predictors of Permanent Pacemaker Implantation After Transcatheter Aortic Valve Implantation: Current Status. Curr. Cardiol. Rev. 2019, 15, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Castelo, A.; Grazina, A.; Teixeira, B.; Mendonça, T.; Rodrigues, I.; Garcia Brás, P.; Vaz Ferreira, V.; Ramos, R.; Fiarresga, A.; Cacela, D.; et al. Outcomes and predictors of periprocedural stroke after transcatheter aortic valve implantation. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2023, 32, 107054. [Google Scholar] [CrossRef] [PubMed]
- Baron, S.J.; Arnold, S.V.; Wang, K.; Magnuson, E.A.; Chinnakondepali, K.; Makkar, R.; Herrmann, H.C.; Kodali, S.; Thourani, V.H.; Kapadia, S.; et al. Health Status Benefits of Transcatheter vs Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis at Intermediate Surgical Risk: Results From the PARTNER 2 Randomized Clinical Trial. JAMA Cardiol. 2017, 2, 837–845. [Google Scholar] [CrossRef]
- Adams, D.H.; Popma, J.J.; Reardon, M.J.; Yakubov, S.J.; Coselli, J.S.; Deeb, G.M.; Gleason, T.G.; Buchbinder, M.; Hermiller, J.; Kleiman, N.S.; et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis. N. Engl. J. Med. 2014, 370, 1790–1798. [Google Scholar] [CrossRef] [PubMed]
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef]
- Mack, M.J.; Leon, M.B.; Smith, C.R.; Miller, D.C.; Moses, J.W.; Tuzcu, E.M.; Webb, J.G.; Douglas, P.S.; Anderson, W.N.; Blackstone, E.H.; et al. 5-year outcomes of transcatheter aortic valve replacement or surgical aortic valve replacement for high surgical risk patients with aortic stenosis (PARTNER 1): A randomised controlled trial. Lancet Lond. Engl. 2015, 385, 2477–2484. [Google Scholar] [CrossRef] [PubMed]
- Gleason, T.G.; Reardon, M.J.; Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Lee, J.S.; Kleiman, N.S.; Chetcuti, S.; Hermiller, J.B.; Heiser, J.; et al. 5-Year Outcomes of Self-Expanding Transcatheter Versus Surgical Aortic Valve Replacement in High-Risk Patients. J. Am. Coll. Cardiol. 2018, 72, 2687–2696. [Google Scholar] [CrossRef] [PubMed]
- Thyregod, H.G.H.; Jørgensen, T.H.; Ihlemann, N.; Steinbrüchel, D.A.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; De Backer, O.; Olsen, P.S.; Søndergaard, L. Transcatheter or surgical aortic valve implantation: 10-year outcomes of the NOTION trial. Eur. Heart J. 2024, 45, 1116–1124. [Google Scholar] [CrossRef]
- Van Mieghem, N.M.; Deeb, G.M.; Søndergaard, L.; Grube, E.; Windecker, S.; Gada, H.; Mumtaz, M.; Olsen, P.S.; Heiser, J.C.; Merhi, W.; et al. Self-expanding Transcatheter vs Surgical Aortic Valve Replacement in Intermediate-Risk Patients: 5-Year Outcomes of the SURTAVI Randomized Clinical Trial. JAMA Cardiol. 2022, 7, 1000–1008. [Google Scholar] [CrossRef]
- Călburean, P.-A.; Grebenișan, P.; Nistor, I.-A.; Pal, K.; Vacariu, V.; Drincal, R.-K.; Ion, A.A.; Adorján, I.; Oltean, T.; Hadadi, L. Addition of eptifibatide and manual thrombus aspiration to ticagrelor does not improve long-term survival after STEMI treated with primary PCI. Front. Pharmacol. 2024, 15, 1415025. [Google Scholar] [CrossRef]
- Călburean, P.-A.; Grebenișan, P.; Nistor, I.-A.; Șulea, I.P.; Scurtu, A.-C.; Brinzaniuc, K.; Suciu, H.; Harpa, M.; Dobreanu, D.; Hadadi, L. High long-term mortality in ischaemic heart disease accentuated among ethnic minorities in Eastern Europe: Findings from a prospective all-comers percutaneous coronary intervention registry in Romania. J. Epidemiol. Community Health 2025, 79, 272–279. [Google Scholar] [CrossRef]
- Suciu, H.; Elkahlout, A.; Nicolae, V.; Tomșa, F.; Stan, A.; Al-Hussein, H.; Călburean, P.-A.; Scurtu, A.-C.; Aniței, D.E.; Hadadi, L.; et al. Outcomes and Cost-Effectiveness of Transcatheter Versus Surgical Aortic Valve Replacement in Patients with and Without Coronary Artery Disease. J. Cardiovasc. Dev. Dis. 2025, 12, 217. [Google Scholar] [CrossRef]
- Baron, S.J.; Wang, K.; House, J.A.; Magnuson, E.A.; Reynolds, M.R.; Makkar, R.; Herrmann, H.C.; Kodali, S.; Thourani, V.H.; Kapadia, S.; et al. Cost-Effectiveness of Transcatheter Versus Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis at Intermediate Risk. Circulation 2019, 139, 877–888. [Google Scholar] [CrossRef] [PubMed]
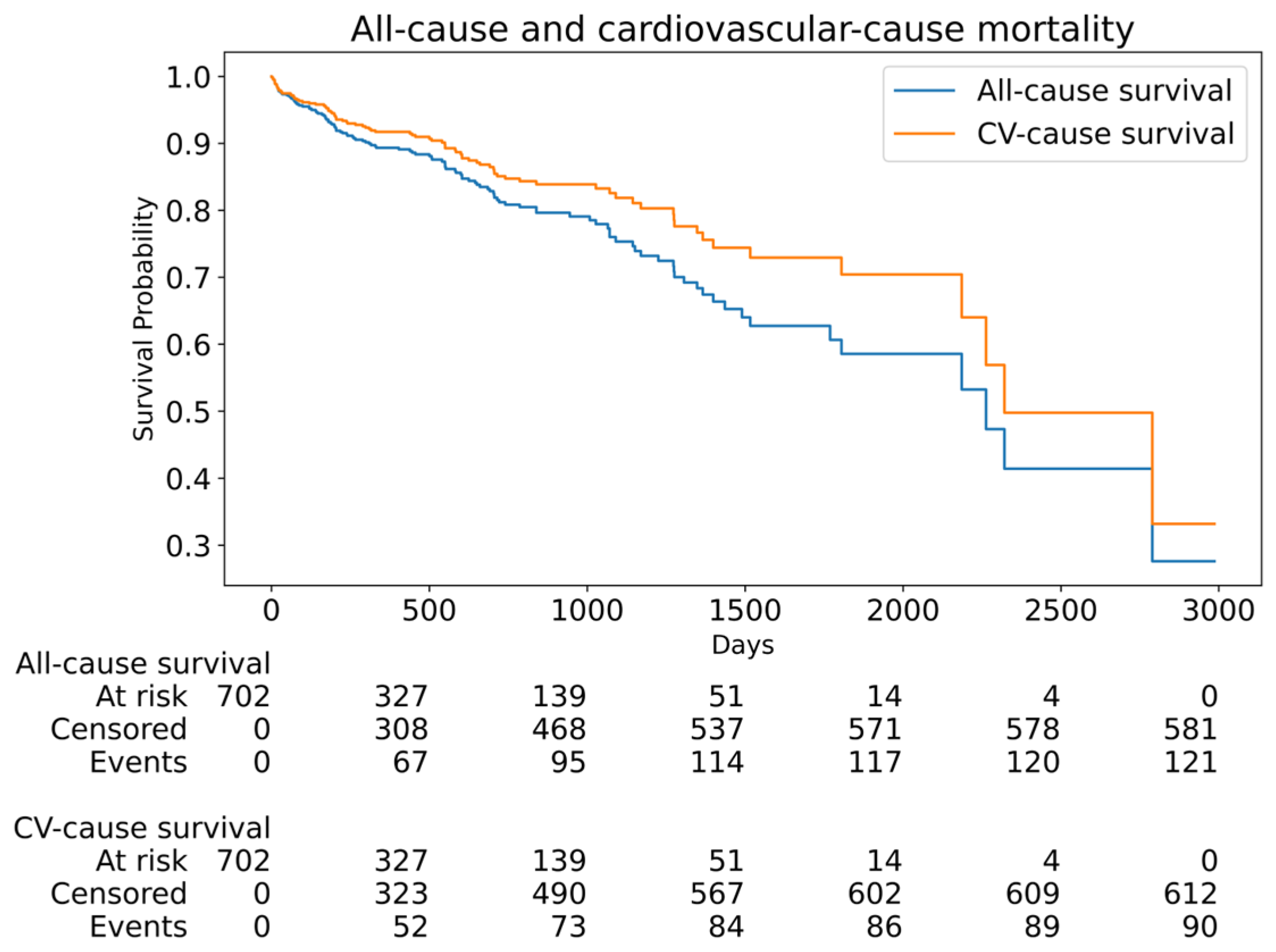
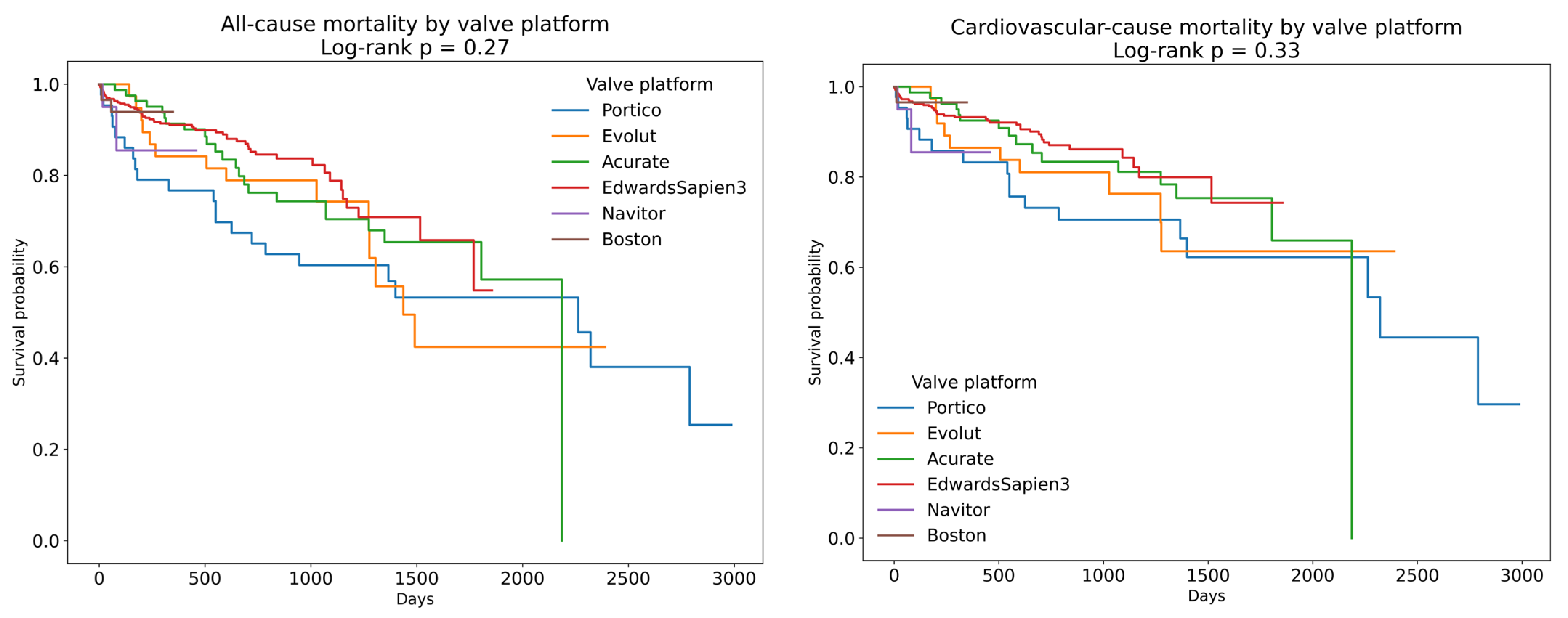
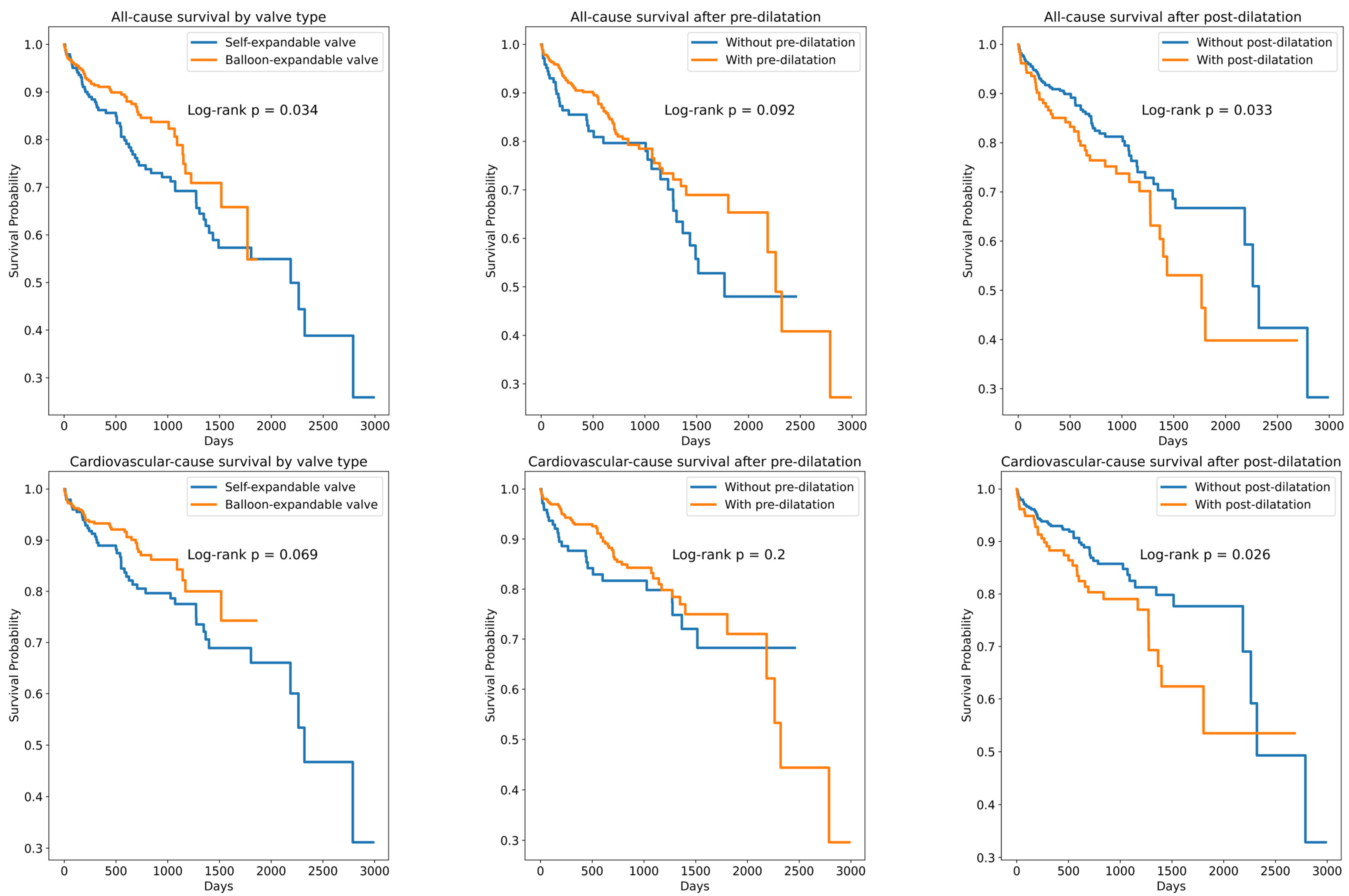


| Parameter | All Patients (n = 702) | Self-Expanding Valve (n = 245) | Balloon-Expanding Valve (n = 457) | p-Value |
|---|---|---|---|---|
| Age (years) | 79 (75–83) | 80 (76–84) | 78 (74–82) | 0.0001 |
| Male sex | 380 (54.1%) | 100 (40.5%) | 280 (61.5%) | 0.0001 |
| Hospitalization (days) | 6.88 (5.02–9.77) | 6.90 (5.20–9.91) | 6.87 (4.98–9.70) | 0.24 |
| Diabetes mellitus | 256 (36.4%) | 93 (37.5%) | 163 (35.7%) | 0.68 |
| Hypertension | 603 (85.7%) | 223 (89.9%) | 380 (83.3%) | 0.01 |
| Atrial fibrillation | 250 (35.5%) | 77 (31.0%) | 173 (37.9%) | 0.07 |
| Chronic kidney disease | 128 (18.2%) | 44 (17.7%) | 84 (18.4%) | 0.91 |
| Stroke | 48 (6.8%) | 17 (6.9%) | 31 (6.8%) | 1.00 |
| Prior MI | 77 (10.9%) | 27 (10.9%) | 50 (11.0%) | 1.00 |
| Prior CABG | 29 (4.1%) | 9 (3.6%) | 20 (4.4%) | 0.69 |
| LBBB | 92 (13.1%) | 32 (12.9%) | 60 (13.2%) | 1.00 |
| Active smoker | 21 (3.0%) | 6 (2.4%) | 15 (3.3%) | 0.64 |
| Dyslipidemia | 347 (49.3%) | 128 (51.6%) | 219 (48.0%) | 0.38 |
| COPD | 54 (7.7%) | 20 (8.1%) | 34 (7.5%) | 0.76 |
| CAD | 117 (16.6%) | 35 (14.1%) | 82 (18.0%) | 0.20 |
| DCM | 44 (6.2%) | 7 (2.8%) | 37 (8.1%) | 0.005 |
| Creatinine (mg/dl) | 1.06 (0.87–1.28) | 1.04 (0.84–1.24) | 1.07 (0.88–1.29) | 0.10 |
| Hemoglobin (g/dL) | 12.77 ± 1.77 | 12.55 ± 1.79 | 12.89 ± 1.75 | 0.01 |
| Leucocytes (×103/µL) | 6.93 (5.84–8.32) | 6.83 (5.81–8.16) | 6.99 (5.91–8.37) | 0.22 |
| Platelets (×103/µL) | 204 (166–244) | 203 (163–239.50) | 204 (167–247) | 0.15 |
| LVEF (%) | 50 (41.50–55) | 55 (45–55) | 50 (40–55) | 0.0003 |
| LV diameter (mm) | 51 (45–56) | 49.78 ± 7.64 | 51 (46–57) | 0.04 |
| Maximum gradient (mmHg) | 79 (65–97) | 84 (70.50–105.50) | 75 (63–90.50) | 0.0008 |
| Mean gradient (mmHg) | 49 (40–62) | 52.50 (43–67) | 45 (39.90–57) | 0.0001 |
| AVA (cm2) | 0.67 ± 0.16 | 0.60 (0.54–0.70) | 0.75 ± 0.16 | 0.009 |
| Annulus area (mm) | 463.8 (410.5–532.8) | 417.0 (365.0–463.0) | 477.2 (420.2–546.5) | 0.0001 |
| Annulus perimeter (mm) | 77.8 (72.95–83.35) | 73.8 ± 6.8 | 79.1 (74.0–84.3) | 0.0001 |
| Minimum annulus diameter (mm) | 21.6 (20.0–23.3) | 20.0(18.4–21.4) | 21.80 (20.4–23.4) | 0.0001 |
| Maximum annulus diameter (mm) | 27.7 (25.8–29.7) | 26.0 ± 2.4 | 28.1 ± 2.7 | 0.0001 |
| Sinus of Valsalva diameter (mm) | 30.3 (27.9–33.0) | 27.7 (26.2–30.2) | 30.9 ± 3.3 | 0.0001 |
| Sinotubular junction diameter (mm) | 28.6 (25.8–31) | 26.6 (24.8–30.0) | 28.9 (26.1–31.0) | 0.0016 |
| LCA height (mm) | 14.0(12.0–16.5) | 12.8 ± 3.2 | 14.5 (12.4–16.7) | 0.0001 |
| RCA height (mm) | 17.9 (15.5–20.0) | 16.5 (15.0–18.1) | 18.0 (16.0–20.0) | 0.0001 |
| Sinotubular junction height (mm) | 23.5 (21.5–25.6) | 22.0 ± 2.9 | 24.0 (22.0–26.0) | 0.0001 |
| EuroSCORE I (%) | 8.52 (5.69–14.49) | 8.75 (5.43–14.46) | 8.35 (5.72–14.49) | 0.58 |
| EuroSCORE II (%) | 3.83 (2.41–7.70) | 3.74 (2.21–6.39) | 4.12 (2.74–8.42) | 0.14 |
| Maximum transprosthetic gradient (mmHg) | 19 (13–27) | 19 (14–26) | 20 (13–28) | 0.64 |
| Mean transprosthetic gradient (mmHg) | 10 (7–15) | 10 (7–13) | 11 (8–15) | 0.21 |
| Variable | Number | Percentage (%) | HR | CI Lower 95% | CI Upper 95% | p-Value * |
|---|---|---|---|---|---|---|
| All-cause survival prediction | ||||||
| Pre-dilatation | 515 | 73.1 | 0.71 | 0.48 | 1.04 | 0.08 |
| Post-dilatation | 190 | 26.9 | 1.51 | 1.05 | 2.19 | 0.03 |
| ES3 | 457 | 64.7 | 0.67 | 0.46 | 0.96 | 0.03 |
| Accurate | 81 | 11.5 | 1.07 | 0.67 | 1.7 | 0.77 |
| Boston | 59 | 8.5 | 0.91 | 0.28 | 2.91 | 0.87 |
| Portico | 43 | 6.1 | 1.58 | 0.96 | 2.6 | 0.07 |
| Evolut | 38 | 5.4 | 1.29 | 0.73 | 2.26 | 0.38 |
| Navitor | 26 | 3.6 | 2.03 | 0.64 | 6.48 | 0.23 |
| Cardiovascular-cause survival prediction | ||||||
| Pre-dilatation | 515 | 73.1 | 0.72 | 0.46 | 1.14 | 0.16 |
| Post-dilatation | 190 | 26.9 | 1.63 | 1.07 | 2.50 | 0.02 |
| ES3 | 457 | 64.7 | 0.66 | 0.43 | 1.02 | 0.06 |
| Accurate | 81 | 11.5 | 1.04 | 0.60 | 1.80 | 0.88 |
| Boston | 59 | 8.5 | 0.74 | 0.18 | 3.08 | 0.68 |
| Portico | 43 | 6.1 | 1.66 | 0.92 | 2.96 | 0.08 |
| Evolut | 38 | 5.4 | 1.24 | 0.64 | 2.42 | 0.51 |
| Navitor | 26 | 3.6 | 2.54 | 0.79 | 8.19 | 0.11 |
| Permanent pacemaker implantation | ||||||
| Pre-dilatation | 38 | 7.69 | 0.79 | 0.43 | 1.43 | 0.43 |
| Post-dilatation | 18 | 9.94 | 1.34 | 0.74 | 2.42 | 0.33 |
| ES3 | 31 | 7.16 | 0.69 | 0.39 | 1.20 | 0.19 |
| Accurate | 5 | 6.41 | 0.74 | 0.28 | 1.92 | 0.54 |
| Boston | 5 | 8.77 | 1.08 | 0.41 | 2.84 | 0.86 |
| Portico | 7 | 16.2 | 2.35 | 1.00 | 5.57 | 0.05 |
| Evolut | 2 | 5.41 | 0.62 | 0.14 | 2.68 | 0.52 |
| Navitor | 5 | 20.8 | 3.14 | 1.12 | 8.75 | 0.02 |
| Predictors of Balloon-Expandable Versus Self-Expandable Valve | |||
|---|---|---|---|
| Variable | OR | 95% CI | p-value * |
| LVEF (%) | 0.85 | 0.75–0.99 | 0.03 |
| Aortic valve area (cm2) | 0.97 | 0.96–0.99 | 0.01 |
| Aortic regurgitation | 4.08 | 2.04–6.05 | 0.02 |
| Annulus area (mm2) | 0.71 | 0.59–0.86 | 0.0006 |
| Sinotubular junction diameter (mm) | 1.01 | 1.00–1.01 | 0.0002 |
| LCA height (mm) | 0.89 | 0.83–0.97 | 0.004 |
| Predictors of pre-dilatation | |||
| Variable | OR | 95% CI | p-value * |
| Dilated cardiomyopathy | 0.35 | 0.18–0.67 | 0.001 |
| Maximum aortic velocity (m/s) | 1.01 | 1.00–1.02 | 0.003 |
| Predictors of post-dilatation | |||
| Variable | OR | 95% CI | p-value * |
| Coronary artery bypass graft | 2.53 | 1.17–5.48 | 0.01 |
| Mean aortic gradient (mmHg) | 1.01 | 1.00–1.02 | 0.004 |
| Aortic regurgitation | 1.20 | 1.00–1.44 | 0.05 |
| Sinotubular junction height (mm) | 0.91 | 0.85–0.98 | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stan, A.; Elkahlout, A.; Harpa, M.M.; Pop, M.; Veres, M.; Stan, A.D.; Călburean, P.-A.; Scurtu, A.-C.; Brînzaniuc, K.; Suciu, H. Impact of Pre- and Post-Dilatation on Long-Term Outcomes After Self-Expanding and Balloon-Expandable TAVI. J. Funct. Biomater. 2025, 16, 282. https://doi.org/10.3390/jfb16080282
Stan A, Elkahlout A, Harpa MM, Pop M, Veres M, Stan AD, Călburean P-A, Scurtu A-C, Brînzaniuc K, Suciu H. Impact of Pre- and Post-Dilatation on Long-Term Outcomes After Self-Expanding and Balloon-Expandable TAVI. Journal of Functional Biomaterials. 2025; 16(8):282. https://doi.org/10.3390/jfb16080282
Chicago/Turabian StyleStan, Alexandru, Ayman Elkahlout, Marius Mihai Harpa, Marian Pop, Mihaly Veres, Antonela Delia Stan, Paul-Adrian Călburean, Anda-Cristina Scurtu, Klara Brînzaniuc, and Horatiu Suciu. 2025. "Impact of Pre- and Post-Dilatation on Long-Term Outcomes After Self-Expanding and Balloon-Expandable TAVI" Journal of Functional Biomaterials 16, no. 8: 282. https://doi.org/10.3390/jfb16080282
APA StyleStan, A., Elkahlout, A., Harpa, M. M., Pop, M., Veres, M., Stan, A. D., Călburean, P.-A., Scurtu, A.-C., Brînzaniuc, K., & Suciu, H. (2025). Impact of Pre- and Post-Dilatation on Long-Term Outcomes After Self-Expanding and Balloon-Expandable TAVI. Journal of Functional Biomaterials, 16(8), 282. https://doi.org/10.3390/jfb16080282







