Controlling Sodium Titanate Crystal Size to Improve Wettability and Early Osseointegration of Titanium Implants: Insights from an Animal Model
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vitro Experiment Study
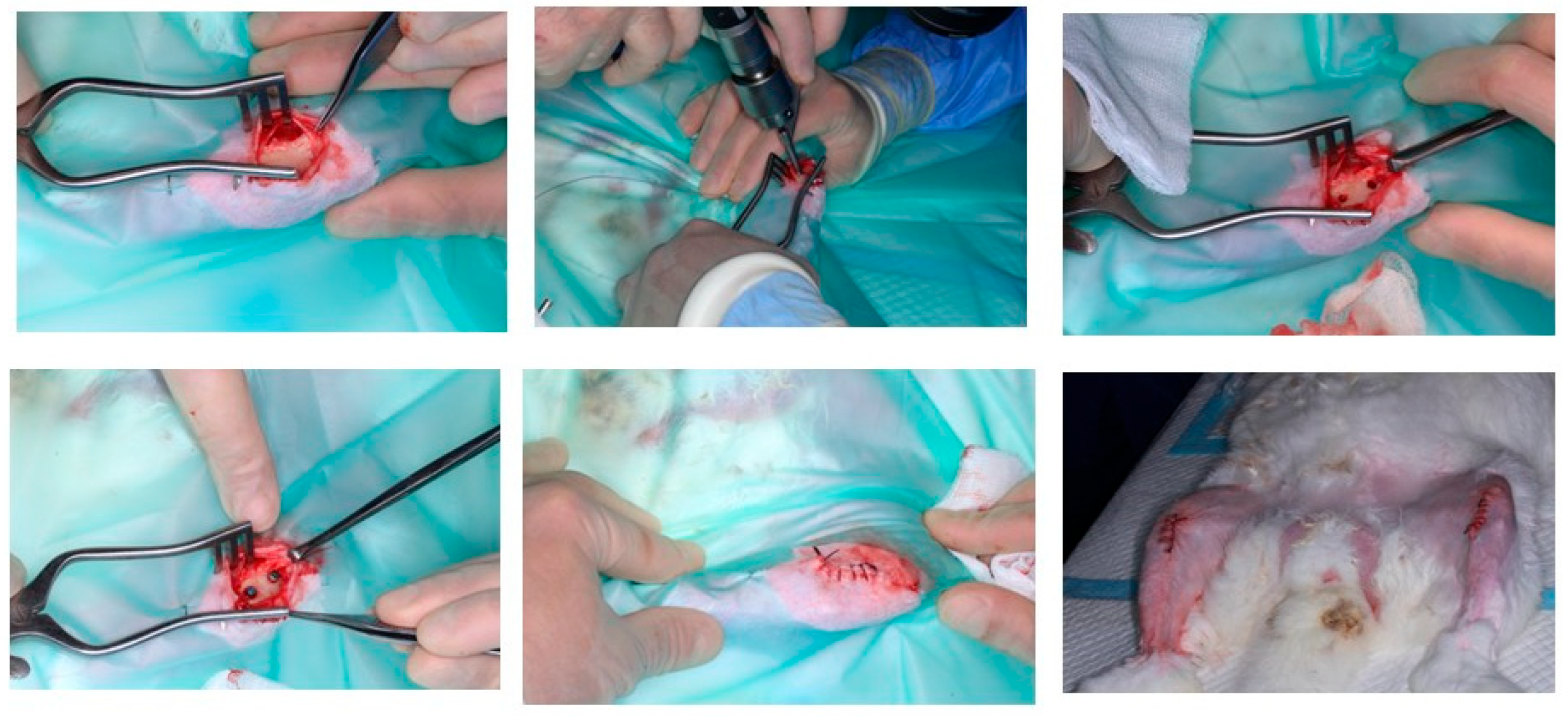
2.2. Animal Model Study
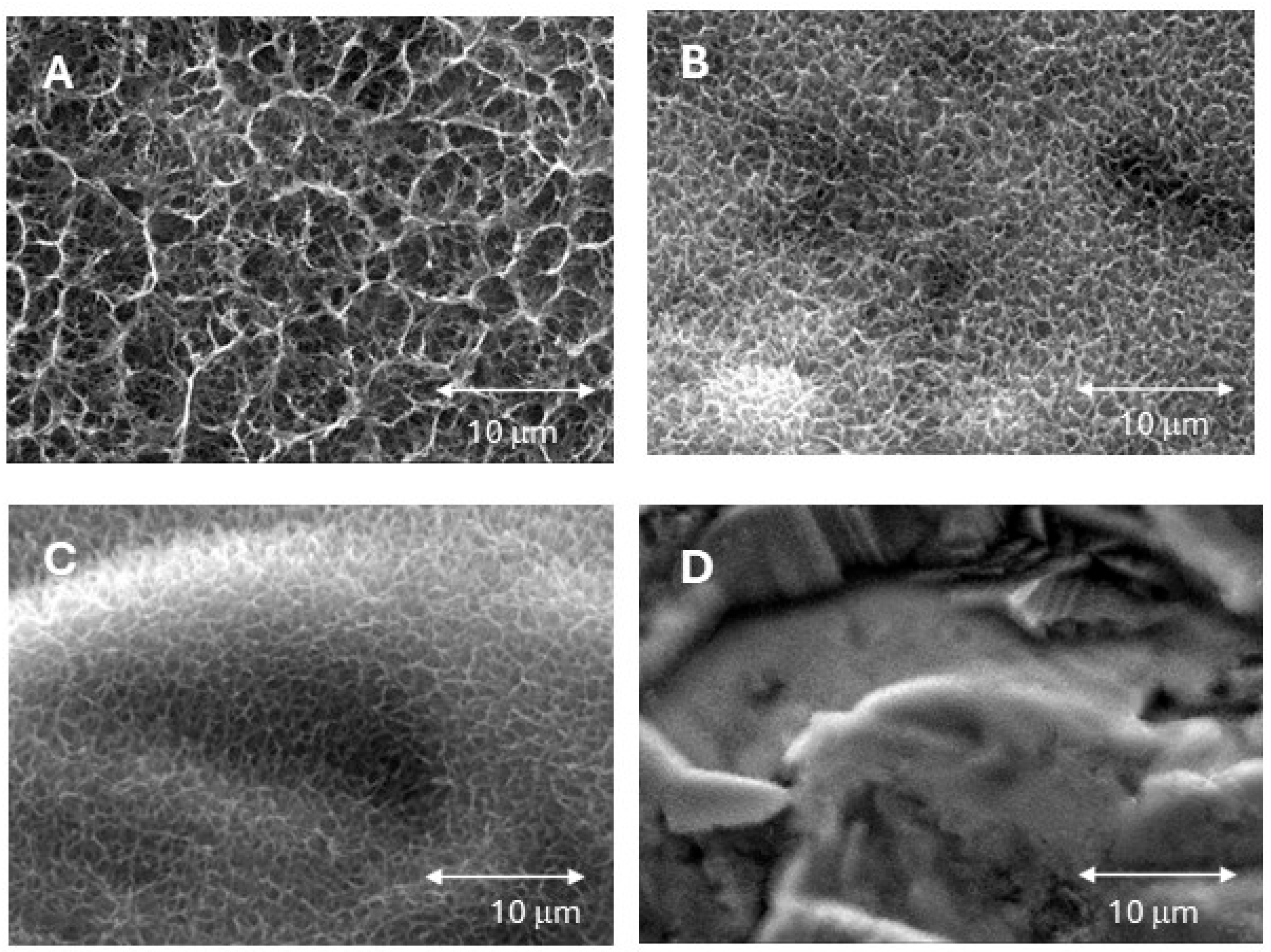
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schenk, R.K.; Buser, D. Osseointegration: A reality. Periodontology 2000 1998, 17, 22–35. [Google Scholar] [CrossRef]
- Nicholson, W.J. Titanium Alloys for Dental Implants: A Review. Prosthesis 2020, 2, 100–116. [Google Scholar] [CrossRef]
- Stanford, C.M.; Schneider, G.B. Functional behaviour around dental implants. Gerodontology 2004, 21, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.E. Understanding peri-implant endosseous healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, R.; Barhoum, A.; Uludag, H. A review of nanostructured surfaces and materials for dental implants: Surface coating, patterning and functionalization for improved performance. Biomater. Sci. 2018, 6, 1312–1338. [Google Scholar] [CrossRef]
- Calazans Neto, J.V.; Kreve, S.; Valente, M.L.D.C.; Reis, A.C.D. Protein absorption on titanium surfaces treated with a high-power laser: A systematic review. J. Prosthet. Dent. 2024, 131, 591–597. [Google Scholar] [CrossRef]
- Marie, P.J. Targeting integrins to promote bone formation and repair. Nat. Rev. Endocrinol. 2013, 9, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Bachhuka, A.; Han, S.; Wei, F.; Lu, S.; Visalakshan, R.M.; Vasilev, K.; Xiao, Y. Tuning Chemistry and Topography of Nanoengineered Surfaces to Manipulate Immune Response for Bone Regeneration Applications. ACS Nano 2017, 11, 4494–4506. [Google Scholar] [CrossRef]
- Pierschbacher, M.D.; Ruoslahti, E. Cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature 1984, 309, 30–33. [Google Scholar] [CrossRef]
- Popat, K.C.; Leoni, L.; Grimes, C.A.; Desai, T.A. Influence of engineered titania nanotubular surfaces on bone cells. Biomaterials 2007, 28, 3188–3197. [Google Scholar] [CrossRef]
- Annunziata, M.; Guida, L. The Effect of Titanium Surface Modifications on Dental Implant Osseointegration. Front. Oral Biol. 2015, 17, 62–77. [Google Scholar] [PubMed]
- López-Valverde, N.; Aragoneses, J.; López-Valverde, A.; Quispe-López, N.; Rodríguez, C.; Aragoneses, J.M. Effectiveness of biomolecule-based bioactive surfaces, on os-seointegration of titanium dental implants: A systematic review and meta-analysis of in vivo studies. Front. Bioeng. Biotechnol. 2022, 10, 986112. [Google Scholar] [CrossRef]
- Saini, P.; Sood, S.; Chahal, G.S.; Jain, A. Evaluation of bone apposition on surface modified titanium implant in experimental animal model: A systematic review and meta-analysis. J. Indian. Soc. Periodontol. 2024, 28, 43–74. [Google Scholar] [CrossRef]
- Albertini, M.; Fernandez-Yague, M.; Lázaro, P.; Herrero-Climent, M.; Rios-Santos, J.V.; Bullon, P.; Gil, F.J. Advances in surfaces and osseointegration in implantology. Biomimetic surfaces. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e316–e325. [Google Scholar] [CrossRef]
- Makowiecki, A.; Hadzik, J.; Błaszczyszyn, A.; Gedrange, T.; Dominiak, M. An evaluation of superhydrophilic surfaces of dental implants—A systematic review and meta-analysis. BMC Oral Health 2019, 19, 79. [Google Scholar] [CrossRef] [PubMed]
- Massaro, C.; Baker, M.A.; Cosentino, F.; Ramires, P.A.; Klose, S.; Milella, E. Surface and biological evaluation of hydroxiapatite-based coatings on titanium deposited by different techniques. J. Biomed. Mater. Res. 2001, 58, 651–657. [Google Scholar] [CrossRef]
- Kilpadi, D.V.; Lemons, J.E. Surface energy characterization of unalloyed titanium implants. J. Biomed. Mater. Res. 1994, 28, 1419–1425. [Google Scholar] [CrossRef]
- Telleman, G.; Albrektsson, T.; Hoffman, M.; Johansson, C.B.; Vissink, A.; Meijer, H.J.; Raghoebar, G.M. Peri-implant endosseous healing properties of dual acid-etched mini-implants with a nanometer-sized deposition of CaP: A histological and histomorphometric human study. Clin. Implant. Dent. Relat. Res. 2010, 12, 153–160. [Google Scholar] [CrossRef]
- Davies, J.E. Bone bonding at natural and biomaterial surfaces. Biomaterials 2007, 28, 5058–5067. [Google Scholar] [CrossRef]
- Smeets, R.; Stadlinger, B.; Schwarz, F.; Beck-Broichsitter, B.; Jung, O.; Precht, C.; Kloss, F.; Gröbe, A.; Heiland, M.; Ebker, T. Impact of Dental Implant Surface Modifications on Osseointegration. Biomed. Res. Int. 2016, 2016, 6285620. [Google Scholar] [CrossRef]
- Gil, F.J.; Manzanares, N.; Badet, A.; Aparicio, C.; Ginebra, M.P. Biomimetic treatment on dental implants for short-term bone regeneration. Clin. Oral Investig. 2014, 18, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef]
- Fernández-Hernández, S.; Gil, J.; Robles-Cantero, D.; Pérez-Pevida, E.; Herrero-Climent, M.; Brizuela-Velasco, A. Influence of the Sodium Titanate Crystal Size of Biomimetic Dental Implants on Osteoblastic Behavior: An In Vitro Study. Biomimetics 2025, 10, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, T.P.; Souza, F.Á.; Guastaldi, A.C.; Margonar, R.; Garcia-Júnior, I.R.; Hochuli-Vieira, E. Commercially pure titanium implants with surfaces modified by laser beam with and without chemical deposition of apatite. Biomechanical and topographical analysis in rabbits. Clin. Oral Implants Res. 2013, 24, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Chavarri-Prado, D.; Brizuela-Velasco, A.; Álvarez-Arenal, Á.; Dieguez-Pereira, M.; Pérez-Pevida, E.; Viteri-Agustín, I.; Estrada-Martínez, A. The Bone Buttress Theory: The Effect of the Mechanical Loading of Bone on the Osseointegration of Dental Implants. Biology 2020, 10, 12. [Google Scholar] [CrossRef]
- Asalzadeh, S.; Yasserian, K. The Effect of Various Annealing Cooling Rates on Electrical and Morphological Properties of TiO2 Thin Films. Semiconductors 2019, 53, 1603–1607. [Google Scholar] [CrossRef]
- Hamouda, I.M.; Enan, E.T.; Al-Wakeel, E.E.; Yousef, M.K.M. Alkali and heat treatment of titanium implant material for bioactivity. Int. J. Oral Maxillofac. Implants 2012, 27, 776–784. [Google Scholar] [PubMed]
- Kim, H.S.; Kim, Y.J.; Jang, J.H.; Park, J.W. Surface Engineering of Nanostructured Titanium Implants with Bioactive Ions. J. Dent. Res. 2016, 95, 558–565. [Google Scholar] [CrossRef]
- Kokubo, T.; Yamaguchi, S. Bioactive titanate layers formed on titanium and its alloys by simple chemical and heat treatments. Open Biomed. Eng. J. 2015, 9, 29–41. [Google Scholar] [CrossRef]
- Liu, Y.; Shelton, R.; Gbureck, U.; Barralet, J. Influence of calcium phosphate crystal morphology on the adhesion, spreading, and growth of bone derived cells. J. Biomed. Mater. Res. A 2009, 90, 972–980. [Google Scholar] [CrossRef]
- Dieguez-Pereira, M.; Brizuela-Velasco, A.; Chavarri-Prado, D.; Perez-Pevida, E.; deLlanos-Lanchares, H.; Álvarez-Arenal, A. The Utility of Implant-Supported Fixed Dental Prosthesis Material for Implant Micromovement and Peri-implant Bone Microstrain: A Study in Rabbit Tibia. Int. J. Oral Maxillofac. Implants 2020, 35, 1132–1140. [Google Scholar] [CrossRef]
- Gursoytrak, B.; Ataoglu, H. Use of resonance frequency analysis to evaluate the effects of surface properties on the stability of different implants. Clin. Oral Implants Res. 2020, 31, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, C.; Gil, F.J.; Thams, U.; Muñoz, F.; Padrós, A.; Planell, J.A. Osseointegration of Grit-Blasted and Bioactive Titanium Implants: Histomorphometry in Minipigs. KEM 2003, 254–256, 737–740. [Google Scholar] [CrossRef]
- Herrero-Climent, M.; Romero Ruiza, M.M.; Calvo, P.L.; Santos, J.V.R.; Perez, R.A.; Gil Mur, F.J. Effectiveness of a new dental implant bioactive surface: Histological and histomorphometric comparative study in minipigs. Clin. Oral Investig. 2018, 22, 1423–1432. [Google Scholar] [CrossRef]
- Romero-Ruiz, M.M.; Gil-Mur, F.J.; Ríos-Santos, J.V.; Lázaro-Calvo, P.; Ríos-Carrasco, B.; Herrero-Climent, M. Influence of a novel surface of bioactive implants on osseointegration: A comparative and histomorfometric correlation and implant stability study in minipigs. Int. J. Mol. Sci. 2019, 20, 2307–2321. [Google Scholar] [CrossRef]
- Stich, T.; Alagboso, F.; Křenek, T.; Kovářík, T.; Alt, V.; Docheva, D. Implant-bone-interface: Reviewing the impact of titanium surface modifications on osteogenic processes in vitro and in vivo. Bioeng. Transl. Med. 2021, 7, e10239. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, J.; Wu, R.; Wei, J. Construction of functional surfaces for dental implants to enhance osseointegration. IFront Bioeng. Biotechnol. 2023, 11, 1320307. [Google Scholar] [CrossRef]
- Almeida, D.; Sartoretto, S.C.; de Albuquerque Calasans-Maia, J.; Ghiraldini, B.; Bezerra, F.J.B.; Granjeiro, J.M.; Calasans-Maia, M.D.; Cui, W. In vivo osseointegration evaluation of implants coated with nanostructured hydroxyapatite in low density bone. PLoS ONE 2023, 18, e0282067. [Google Scholar] [CrossRef]
- Nevins, M.; Chen, C.-Y.; Khang, W.; Kim, D.M. Clinical and histological efficacy of a new implant surface in achieving early and stable osseointegration: An in vivo study. Int. J. Oral Implantol. 2024, 17, 297–306. [Google Scholar]
- Albertini, M.; Herrero-Climent, F.; Díaz-Castro, C.M.; Nart, J.; Fernández-Palacín, A.; Ríos-Santos, J.V.; Herrero-Climent, M. A radiographic and clinical comparison of immediate vs. Early loading (4 weeks) of implants with a new thermo-chemically treated surface: A randomized clinical trial. Int. J. Environ. Res. Public Health 2021, 18, 1223. [Google Scholar] [CrossRef]
- Carmo Filho, L.C.D.; Marcello-Machado, R.M.; Castilhos, E.D.; Del Bel Cury, A.A.; Faot, F. Can implant surfaces affect implant stability during osseointegration? A randomized clinical trial. Braz. Oral Res. 2018, 32, e110. [Google Scholar] [CrossRef]
- Pabst, A.; Asran, A.; Lüers, S.; Laub, M.; Holfeld, C.; Palarie, V.; Thiem, D.G.E.; Becker, P.; Hartmann, A.; Heimes, D.; et al. Osseointegration of a New, Ultrahydrophilic and Nanostructured Dental Implant Surface: A Comparative In Vivo Study. Biomedicines 2022, 10, 943. [Google Scholar] [CrossRef]
- Tallarico, M.; Baldini, N.; Martinolli, M.; Xhanari, E.; Kim, Y.J.; Cervino, G.; Meloni, S.M. Do the New Hydrophilic Surface Have Any Influence on Early Success Rate and Implant Stability during Osseointegration Period? Four-Month Preliminary Results from a Split-Mouth, Randomized Controlled Trial. Eur. J. Dent. 2019, 13, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Chambrone, L.; Shibli, J.A.; Mercúrio, C.E.; Cardoso, B.; Preshaw, P.M. Efficacy of standard (SLA) and modified sandblasted and acid-etched (SLActive) dental implants in promoting immediate and/or early occlusal loading protocols: A systematic review of prospective studies. Clin. Oral Implants Res. 2015, 26, 359–370. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Yamada, M.; Watanabe, J.; Tiskratok, W.; Ishibashi, M.; Kitaura, H.; Mizoguchi, I.; Egusa, H. Titanium nanotopography induces osteocyte lacunar-canalicular networks to strengthen osseointegration. Acta Biomater. 2022, 151, 613–627. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.F.; Quarrington, R.D.; Tsangari, H.; Starczak, Y.; Mulaibrahimovic, A.; Burzava, A.L.S.; Christou, C.; Barker, A.J.; Morel, J.; Bright, R.; et al. A Novel Nanostructured Surface on Titanium Implants Increases Osseointegration in a Sheep Model. Clin. Orthop. Relat. Res. 2022, 480, 2232–2250. [Google Scholar] [CrossRef]
- Nack, C.; Raguse, J.D.; Stricker, A.; Nelson, K.; Nahles, S. Rehabilitation of irradiated patients with chemically modified and conventional SLA implants: Five-year follow-up. J. Oral Rehabil. 2015, 42, 57–64. [Google Scholar] [CrossRef]
- Patel, R.; Patel, S.; Girgis, W.; Ahmed, W.; Barrak, F. A systematic assessment of the stability of SLA® vs. SLActive® implant surfaces over 12 weeks. Evid. Based Dent. 2025, 26, 67–68. [Google Scholar] [CrossRef]
- Ríos-Santos, J.V.; Menjívar-Galán, A.M.; Herrero-Climent, M.; Ríos-Carrasco, B.; Fernández-Palacín, A.; Perez, R.A.; Gil, F.J. Unravelling the effect of macro and microscopic design of dental implants on osseointegration: A randomised clinical study in minipigs. J. Mater. Sci. Mater. Med. 2018, 29, 99. [Google Scholar] [CrossRef]
- Schlegel, K.A.; Prechtl, C.; Möst, T.; Seidl, C.; Lutz, R.; von Wilmowsky, C. Osseointegration of SLActive implants in diabetic pigs. Clin. Oral Implants Res. 2013, 24, 128–134. [Google Scholar] [CrossRef]
- López-Valverde, N.; Flores-Fraile, J.; Ramírez, J.M.; Sousa, B.M.; Herrero-Hernández, S.; López-Valverde, A. Bioactive Surfaces vs. Conventional Surfaces in Titanium Dental Implants: A Comparative Systematic Review. J. Clin. Med. 2020, 9, 2047. [Google Scholar] [CrossRef]
- Nguyen, A.K.; Nelson, S.B.; Skoog, S.A.; Jaipan, P.; Petrochenko, P.E.; Kaiser, A.; Lo, L.; Moreno, J.; Narayan, R.J.; Goering, P.L.; et al. Effect of simulated body fluid formulation on orthopedic device apatite-forming ability assessment. J. Biomed. Mater. Res. B Appl. Biomater. 2023, 111, 987–995. [Google Scholar] [CrossRef] [PubMed]



| Cooling Rate (°C/h) | Crystal Size (μm) Mean ± SD |
|---|---|
| 20 | 0.77 ± 0.34 |
| 50 | 0.48 ± 0.25 |
| 75 | 0.39 ± 0.20 |
| 115 | 0.12 ± 0.09 |
| Cooling Rate (°C/h) | Contact Angle (°) Mean ± SD |
|---|---|
| Control (without treatment) | 91 ± 3 |
| 20 | 87 ± 7 |
| 50 | 72 ± 8 |
| 75 | 69 ± 6 |
| 115 | 80 ± 9 |
| Cooling Rate (°C/h) | BIC (%) Mean ± SD |
|---|---|
| Control (without treatment) | 37.5 ± 5.6 |
| 20 | 59.8 ± 7.0 * |
| 50 | 68.2 ± 5.2 * |
| 75 | 73.5 ± 9.0 * |
| 115 | 41.3 ± 9.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Hernández, S.; Gil, J.; Sanjuán-Álvarez, M.; Sanz, I.; Herrero-Climent, M.; Brizuela-Velasco, A. Controlling Sodium Titanate Crystal Size to Improve Wettability and Early Osseointegration of Titanium Implants: Insights from an Animal Model. J. Funct. Biomater. 2025, 16, 283. https://doi.org/10.3390/jfb16080283
Fernández-Hernández S, Gil J, Sanjuán-Álvarez M, Sanz I, Herrero-Climent M, Brizuela-Velasco A. Controlling Sodium Titanate Crystal Size to Improve Wettability and Early Osseointegration of Titanium Implants: Insights from an Animal Model. Journal of Functional Biomaterials. 2025; 16(8):283. https://doi.org/10.3390/jfb16080283
Chicago/Turabian StyleFernández-Hernández, Saray, Javier Gil, Marta Sanjuán-Álvarez, Ignacio Sanz, Mariano Herrero-Climent, and Aritza Brizuela-Velasco. 2025. "Controlling Sodium Titanate Crystal Size to Improve Wettability and Early Osseointegration of Titanium Implants: Insights from an Animal Model" Journal of Functional Biomaterials 16, no. 8: 283. https://doi.org/10.3390/jfb16080283
APA StyleFernández-Hernández, S., Gil, J., Sanjuán-Álvarez, M., Sanz, I., Herrero-Climent, M., & Brizuela-Velasco, A. (2025). Controlling Sodium Titanate Crystal Size to Improve Wettability and Early Osseointegration of Titanium Implants: Insights from an Animal Model. Journal of Functional Biomaterials, 16(8), 283. https://doi.org/10.3390/jfb16080283









